Abstract
Introduction
Chronic prostatitis is a widespread urological disease with a lengthy course and a propensity to frequent recurrences. Adequate response to anti–inflammatory therapy is lacking in a high percentage of patients, which causes them to seek medical advice from different doctors. Thus, the physicians are challenged to look for other reasons causing the pathological symptoms.
Material and methods
We have reviewed the patients with treatment–resistant chronic bacterial prostatitis (CBP) from the perspective of psychosomatic medicine. For the evaluation of primary mental status and treatment control we used standard approved questionnaires. All 337 CBP patients initially underwent therapy aimed at pathogen eradication. If psychopathological symptoms were evident and dominated over urological ones, the patients were referred to psychiatric evaluation and treatment.
Results
The frequency of concomitant psychosomatic disorders (PSD) in patients with CBP was 28.2% and neurotic disorders – 26.4%. Adequate multimodal anti–inflammatory therapy followed by a few sessions of psychotherapy decreased the manifestations of PSD in 30.5%, neurotic disorders in 51.7%, and premature ejaculation in 60.5% of patients with CBP. The addition of pharmacotherapy to psychotherapy is effective in treatment–resistant cases. However, after multimodal treatment, 31.5% of pts. with PSD and 13.5% of pts. with neurotic disorders still remain treatment–resistant and required in–depth long–term psychiatric care.
Conclusions
A significant portion of CBP patients were diagnosed with neurotic, psychosomatic, and/or depressive disorders.
Antibacterial and anti–inflammatory therapy, when followed by appropriate psychotherapy and pharmacotherapy, significantly decrease the manifestations of mental disorders in CBP patients.
Keywords: chronic bacterial prostatitis, mental status, psychosomatic disorders, premature ejaculation
INTRODUCTION
For a long time mental status in patients with chronic prostatitis was a topic for investigation. Green M.R. and Dean A.L. (1954) were among the first authors to describe patients with the combination of chronic prostatitis and various mental disorders (referred to as ”psychoneurosis”) [1]. Sometime later, in 1973 Mellan J. et al. coined the term “prostatic neurosis” to describe this patient population [2]. According to Jansen P.L. et al. (1983), “neurotic” disorders, such as sensation of anxiety, insecurity, fear, and depression are present in a significant number of chronic bacterial prostatitis (CBP) patients regardless of urological findings [3]. Until now, the studies of psychological condition in patients with chronic prostatitis have been uninterrupted. The main mental disorders among the CBP patients have been considered as neurotic and/or psychosomatic disorders accompanied by depression [4–8].
Our aim was to estimate the incidence of mental disorders in patients with CBP, to evaluate the efficacy of their multimodal therapy, and to determine the principles of comprehensive medical aid in patients with such pathology.
MATERIALS AND METHODS
Over the last five years a total of 337 patients with CBP had been observed. The duration of disease persistence ranged from two to 11.5 years. The choice of diagnostic and therapeutic methods was made according to approved clinical protocols that adhered to European Association of Urology Guidelines and European Psychiatric Association Guidance, and did not digress from the principles of the local ethical committee.
The diagnosis of CBP was confirmed if prostatic fluid contained more than 10 leucocytes per high–power microscopic field and a pathogen was concomitantly identified [9]. The intensity of clinical symptoms associated with CBP was measured using the CPSI pain domain score (0–21) from the National Institute of Health–Chronic Prostatitis Symptom Index (NIH–CPSI) scale [10].
Initially, all patients were subjected to multimodal therapy with antibiotics, immunomodulators, and physiotherapy that included prostatic massages. Recommendations for diet and lifestyle changes were also expressed, which also included the need to assess patients’ sexual partner(s) and treat if indicated.
In cases in which sexually transmitted and/or non–specific pathogens were detected, antibacterial therapy was continued until all traces of the pathogen(s) were eradicated. Antibacterial regimens were directed by the sensitivity of pathogens (Table 1).
Table 1.
Pathogens implicated in chronic bacterial prostatitis and the suitable treatment
| Pathogen | Treatment |
|---|---|
| Non–specific pathogens (E.coli, Staphylococcus sp., Streptococcus sp., Enterococcus sp., Klebsiella pneumoniae, Pseudomonas aeruginosa, Proteus sp., etc.) | Antibiotics according to sensitivity of pathogen |
| Chlamydia trachomatis | Doxycycline hydrochloride, Azithromycin, Josamycin |
| Trichomonas vaginalis | Metronidazole, Ornidazole, Tinidazole |
| Ureaplasma urealitycum | Doxycycline hydrochloride, Ofloxacin, Levofloxacin |
| Mycoplasma hominis, Mycoplasma henitalium | Tetracycline, Doxycycline hydrochloride, Erythromycin, Azithromycin |
| Gardnerella vaginalis | Metronidazole, Clindamycin, |
If, after pathogen eradication, psychopathological symptoms dominated over urological and/or lack of efficiency of long–term CBP–specific therapy occurred, the patients were referred to psychiatric consultation.
For the evaluation of primary mental status and treatment control we used our local as well as standard approved questionnaires included a Symptom Checklist Scale, a Diagnostic and Statistical Manual IV Text Revision (DSM–IV–TR), depression inventories: the Beck Depression Inventory II (BDI–II) as a self–report scale for depressed mood and the Montgomery–Åsberg Depression Rating Scale (MADRS) as a clinician–administered measure for depression and a guide to evaluate recovery [11–13].
After confirming the mental disorder in our patients, initially 4–5 sessions of psychotherapy (Rational Emotive Behavior Therapy (REBT)) were arranged upon completion of antibacterial treatment. If psychiatric symptoms still persisted then pharmacotherapy was prescribed on a per case basis according to the diagnosis, dynamics, and severity of symptoms and their regression/progression together with consideration given to the tolerance of adverse effects to drug therapy (Table 2). Psychotherapy was continued, even if changing its form was necessary.
Table 2.
Pharmacotherapy of mental disorders
| Mental disorder | Treatment |
|---|---|
| PSD | Trazodonum, Flupenthixol, Quetiapine fumarate |
| Neurotic disorders | Sertraline, Citalopram, Escitalopram, Diazepam, Gidazepam |
| Depressions | Venlafaxine, Paroxetine, Gidazepam, Oxazepam, Alprazolam |
RESULTS
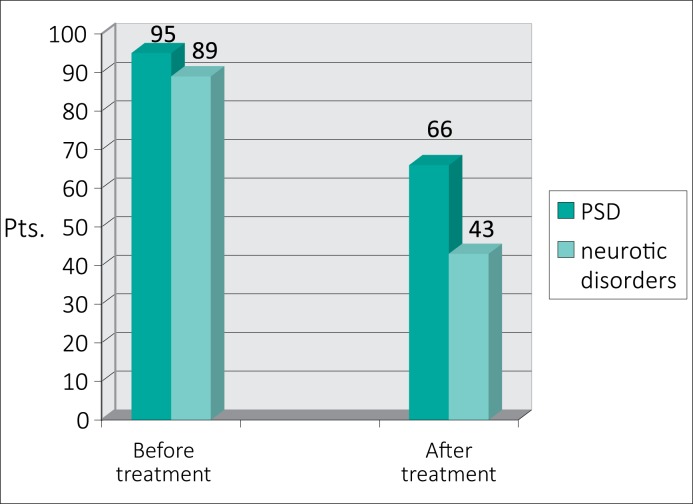
At the time of CBP primary diagnostics or confirming, different mental disorders were registered in 137 (40.7%) pts. Among them were concomitant psychosomatic disorders at the onset of CBP treatment that were noted in 95 patients (28.2%), namely: panic attacks in 44 patients (13.1%), irritable bowel syndrome in 35 patients (10.4%), paroxysmal tachycardia in 38 (11.3%) pts., tension headaches in 29 (8.6%), anorexia nervosa in 21 (6.2%) pts., hyperhidrosis in 16 (4.7%) pts., and multiform parasthesias in 75 (22.2%) pts. In most CBP patients, the abovementioned disorders were present in various combinations. Neurotic disorders were registered in 89 (26.4%) pts. as well independently in 42 (12.5%) pts. as in combination with PSD in 47 (13.9%) patients. All of the neurotic and PSD patients also demonstrated different depressive disorders, which were predominantly mild or moderate with only a few severe cases.
After normalization of the parameters of prostatic fluid and urinalysis, eradication of CBP causative pathogens, and 4–5 sessions of psychotherapy (REBT), the mental status spontaneously returned to normal in 29 pts. with PSD (30.5% of this subgroup) and in 46 pts. with neurotic disorders (51.7% of this subgroup). This fact demonstrates that the application of rational emotive behavior therapy plays an important role in treatment strategy for patients with CBP and mental disorders.
Another 66 patients with PSD (66.3% of this subgroup) and 43 patients with neurotic disorders (48.3% of this subgroup), after normalization of prostate fluid parameters and a few sessions of REBT, did not demonstrate a subjective response to the standard treatment and required additional specialized treatment. This specialized treatment included the continuation of psychotherapy with possible alteration of its form combined with pharmacotherapy including the use of tranquilizers, antidepressants, and/or neuroleptics (Table 2).
In cases of PSD we used antidepressants and/or neuroleptics. The effect of such pharmacotherapy usually became evident only after two to three weeks treatment, which is why patients were informed in advance about the necessity the to follow the prescribed drug regimes.
Tranquilizers were predominantly prescribed in cases of neurotic disorders and sometimes in patients with mild depressions. Their effect became subjectively appreciable by the end of the first or second day of use.
After application of the abovementioned psychopharmacologic treatment to the 66 patients with refractory PSD:
36 (54.5%) pts. showed significant improvement (partial or complete elimination of the pathological symptoms);
24 (36.4%) pts. showed moderate improvement;
six (9.1%) pts. did not demonstrate a subjective response to the treatment at all and, thus, a severe concomitant psychiatric pathology was appreciated during a more in depth psychiatric assessment (hypochondria in three pts., major depressive disorder in two pts., and schizophrenia in one pt.) that required prolonged medication therapy and further observation.
After adding the pharmacotherapy to psychotherapy in 43 treatment–resistant patients with CBP and concomitant neurotic disorders, a significant improvement was observed in 31 cases (72.1% of this subgroup) – a finding that supports such a therapeutic approach (Fig. 1).
Figure 1.
Efficacy of anti–inflammatory treatment combined with psychotherapy (REBT) in patients with CBP and mental disorders.
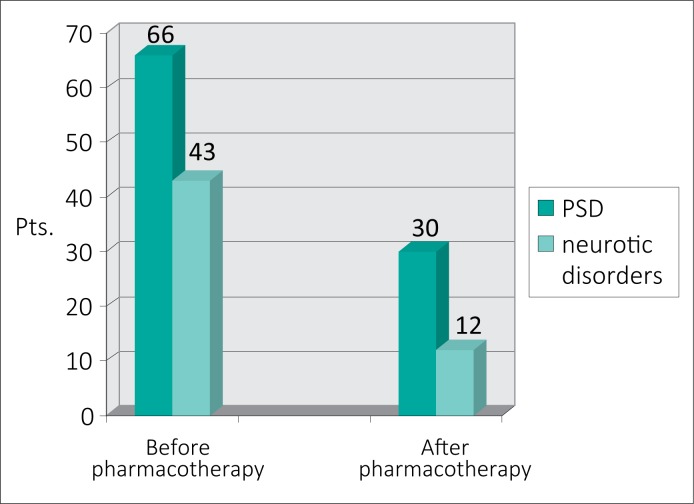
The addition of pharmacotherapy to psychotherapy in patients with CBP and mental disorders was found to significantly decrease the manifestation of neurotic disorders (Fig. 2).
Figure 2.
Efficacy of previous anti–inflammatory treatment followed by psychotherapy and pharmacotherapy in patients with CBP and mental disorders.
Obtained results reflected by inventory scores are presented in Table 3.
Table 3.
Efficacy of multimodal treatment in 137 patients with CBP and mental disorders that reflected by inventory scores
| CPSI pain domain (NIH–CPSI scale), pts. | Depression grade (MADRS score), pts. | Expressive symptoms manifestation* (DSM–IV–TR), pts. | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Mild 0–7 | Moderate 8–13 | Severe 14–21 | None 0–6 | Mild 7–19 | Moderate 20–34 | Severe >34 | PSD | Neurotic disorders | |
| Before treatment | 28 | 66 | 43 | – | 94 | 39 | 4 | 95 | 89 |
| After anti–inflammatory treatment and 4–5 sessions of REBT | 69 | 46 | 22 | 52 | 60 | 21 | 4 | 66 | 43 |
| After pharmacotherapy and psychotherapy | 103 | 26 | 8 | 98 | 20 | 17 | 2 | 30 | 12 |
In 47 pts. before treatment were present both psychosomatic and neurotic disorders
However, after anti–inflammatory therapy combined with psychotherapy and pharmacotherapy, 30 (31.5%) pts. with PSD and 12 (13.5%) pts. with neurotic disorders were still treatment–resistant. Moreover, both PSD and neuroses were registered in eight of them, which required in–depth long–term psychiatric assistance.
A separate subgroup included 114 (33.8%) pts. with CBP and premature ejaculation. Abnormalities of orgasm and ejaculation are among the main symptoms of CBP and appear due to increased tone of alpha–1A–receptors against the background of prostatic inflammation [14]. Patients with premature ejaculation and without CBP were not included into our study because such patients require a different therapeutic strategy.
The presence of premature ejaculation more often than not put a patient into a mood of anxiety and could be the trigger factor provoking the development of mental disorders. Such disorders have a significant psychological burden on men, their partners, and the male/female relationship [15, 16]. The primary treatment should involve psychological/sexual therapy. Despite the etiology, psychosexual treatment can play an important role in the management of premature ejaculation [17].
After multimodal anti–inflammatory therapy of CBP followed by several sessions of REBT, various prolongations in terms of intercourse time were observed in 69 patients (60.5%). Another 45 patients (39.5%) required applications of local anesthetics and/or oral administration of selective serotonin reuptake inhibitors (SSRIs). By prolonging the intercourse time, these therapeutic options consequently improved the mental status in a large group of patients suffering from both CBP and premature ejaculation.
The results presented herein are indicative of a substantial portion of concomitant PSD (28.2%), neurotic disorders (26.4%), and premature ejaculation (33.8%) in patients with chronic prostatitis. In a large fraction of patients, mental disorders and/or premature ejaculation reverse spontaneously with adequate antibacterial therapy followed by a few sessions of REBT. However, some patients demonstrate resistance to the conventional multimodal treatment for CBP, which challenges the experts to identify other causes of pathological symptoms and their respective treatment.
DISCUSSION
Very often in daily practice, urologists encounter the situation in which the application of long–term antibacterial therapy in patients with chronic prostatitis does not yield adequate results. Some patients still complain of a variety of disorders despite the use of all standard treatment options, some of them even lose faith in the ability to remedy after years of futile treatment. Such disappointment hurts the patient's mentality and even confuses urologists to the point of perplexity, which is why a deeper insight into the essence of the problem is required for a more complete understanding of psychological state of patients with CBP, as well as the mechanisms that influence it.
The chronic pain syndrome, caused by antibiotic–resistant prostatic inflammation, reduces the working capacity of patients and worsens their quality of life and their ability to interact socially [18].
Dysuria, incomplete bladder emptying, and especially lengthy pain syndrome with frequent exacerbations deplete the psyche of patients with CBP, contributing to a negative impact on the patient's quality of life that may trigger the development of mental disorders [19, 20]. Almost a third of men experiencing urogenital pain or prostatitis–like symptoms would be less than satisfied if this was to be ongoing for the rest of their life [21]. The symptoms of chronic prostatitis are strongly associated with psychosocial risk factors such as pain catastrophizing, depression, and anxiety [22].
According to Keltikangas–Järvinen L. et al. (1982), there is a correlation between the duration of somatic irritative symptoms and the intensity of psychiatric manifestations [23]. Zhou Q. et al. (2007) have also registered that there is a close correlation between the severity of psychological symptoms and the clinical symptoms in patients with chronic prostatitis. [24]. Symptoms of depression in patients with chronic prostatitis are closely associated with clinical symptom intensity. According to Jiang S. et al. (2012), pain or discomfort in the genital area and urination disorders have an impact on symptoms and total score in the NIH–CPSI scale. These authors showed a significant positive correlation of the NIH–CPSI score with the symptoms of depression with a simultaneously negative correlation with erectile function in patients with chronic prostatitis (r = 0.32, 0.31, 0.35, 0.38, and –0.36, P <0.05). [25].
Intellectualization, redefinition, and flexibility in healthy individuals are higher in frequency in descending order comparing to prostatitis patients – fatalism, externalization, and self–pity are correspondingly lower in frequency. Patients with chronic prostatitis often suffer from depression, anxiety, and a higher perception of stress. In particular, these disorders were closely related to the pain and subsequently decreased the quality of life in such patients [26].
The manifestations of complex neuroendocrine abnormalities are usually present in patients with CBP that exert a remarkable negative influence. Statistically significant changes of serum cortisol levels were found in patients with chronic prostatitis/chronic pelvic pain syndrome [4]. Patients with chronic prostatitis have abnormally high levels of progesterone and androstenedione, and abnormally low levels of corticosterone, aldosterone, and 11–deoxycortisol compared to healthy volunteers. Furthermore, lower levels of cortisol and higher levels of aldosterone were associated with higher pain and CPSI scores. These clinical correlates provide tantalizing insights into the systemic nature of CP/CPPS [27]. The above–mentioned systemic hormonal violations objectively have a bad affect on a patient's behavior and resistance in stressful situations.
A significant part of mental disorders in our CBP patients was presented by psychosomatic disorders, which are a group of somatic diseases or conditions in which the predominant role belongs to psychoemotional factors (imagination, emotions, and fantasies).
The founder of psychosomatic medicine, Prof. Franz Alexander, considered that classical PSDs include the following conditions: peptic ulcers of stomach and duodenum, bronchial asthma, hypertension, rheumatoid arthritis, neurodermitis, hyperthyroidism, and ulcerative colitis [28]. This list is currently expanded by the inclusion of erosive bulbitis, cystalgia, irritable bowel syndrome, paroxysmal tachycardia, and tension headaches among others. The complaints of somatic origin are interpreted as manifestations of the ‘symbolic language’ of internal organs, reflecting the occult libido–related trends, displaced complexes, and negative emotions (anger, jealousy, hatred, remorse etc.). Depending on the type of emotions and their duration and intensity, disturbances of certain organs and systems occur.
According to Prof. F. Alexander, the development of PSD requires three preconditions [28]:
The patient should have a psychological conflict–specific attitude;
A certain specific situation serves as a PSD trigger; e.g., peptic ulcer disease is frequently caused by a loss of the patient's significant other;
The patient must have a constitutional mental vulnerability and a certain biological X–factor increasing the propensity to PSD.
The developments of a hypochondriac personality with the projection of fear upon various bodily functions also facilitate the development of PSD. The patient's perceptions of multiform paresthesias and/or premature ejaculation, typical for chronic prostatitis, are extremely harmful; the patients usually focus excessive attention upon such disorders. The concomitant psychogenic erectile dysfunction that often develops by the ‘vicious circle’ mechanism in patients with CBP also negatively influences their mental status.
The presence of a traumatic factor (situation) and the patient's predisposition to PSD are decisive factors in PSD development. Since the presentation of chronic prostatitis is directly associated with the genital area and sexual behavior disorders, this condition is a powerful traumatic factor in patients prone to PSD – psychosomatic disorders in CBP are noted for their particular intensity and duration. The verified fact of prostatitis and/or STD being diagnosed can affect emotional balance and become a trigger of negative changes in the patient's mental status, thereby inducing the development of the subsequent mental disorder.
The risk of psychosomatic or another mental disorder development and progression depends on:
duration and intensity of the traumatic factor;
presence or absence of patient's predisposition to mental disorders;
inducibility of mental disorders as a result of communication with doctors and other patients;
incorrect interpretation of information obtained from the Internet and/or literature;
In turn, a patient's propensity to develop of mental disorders is increased under the following circumstances:
unfavorable heredity;
unstable social status;
the presence of any form of addictive behavior (drug and alcohol abuse, addiction to gambling etc.); or
the presence of concomitant psychiatric and severe somatic disorders.
Based on our experience and the results obtained from inventories, we believe that a mental disorder should be suspected in a patient if, in addition to the typical clinical presentation of CBP, the following signs and/or symptoms are present:
distinctive signs of psychological discomfort: complaints of irrational anxiety, insomnia, nervous breakdowns, and accelerated or slow speech;
signs of social maladjustment (it is important to pay close attention to the patient's history and appearance);
intense and lasting negative emotions in the patient;
changes in eating preferences, such as reducing/absence of appetite or on the contrary – overeating;
patients are often uneasy with their surroundings and their opinion of the way they look;
discrepancy between usual clinical manifestations of the disease and somatic symptoms described by patient;
patients are avoiding mirrors or quite the opposite, constantly checking themselves in a mirror;
patient's propensity to describe symptoms in figurative terms (‘goose skin’, ‘wobbly feet’, ‘mist in the eyes’, etc.) that are pronounced with pained facial expressions;
lengthy and ineffective treatment with medical professionals of various specialties – the patient demands multiple diagnostic tests to be performed and then treats the test results as ‘collectibles’;
the patient has a profound knowledge of his condition and frequently engages in disputes with his urologist, during which he subconsciously seeks to gain an advantage; he also commonly considers that his doctor has made a mistake by wrongfully diagnosing the real disease and cause of his disturbing symptoms;
etiotropic and pathogenesis–targeted therapies produce brief and/or controversial effects, the most effective agents eliminating ‘organic’ symptoms being psychotropic remedies, tranquilizers, and/or antidepressants;
temporary relief is produced by either alternative therapies (‘sorcerers’, acupuncture, homeopathy, ‘modern computer treatment technologies’, etc.) or sometimes different minimally invasive procedures.
As long as one or more of the abovementioned signs are found in a patient by the urologist, a meticulous comparison of the clinical presentation and the intensity of the symptoms described should be performed. Then, upon consideration of patient's history, the patient should be referred to a psychiatrist or a qualified psychotherapist for validation of the complete diagnosis.
Out of deontological considerations, a single appropriate way of persuading the importance of psychiatric consultation should be carefully selected for each patient with suspected mental disorder. Keeping in mind the fragile psyche typical of most patients with CBP, the use of the following wording is recommended during conversation: psychoneurologist, psychotherapist, and/or neurologist. This is because the patient is likely to be biased against being referred to a ‘psychiatrist’, which is a common finding in post–Soviet society, and may cause rejection in a patient and, thus, affecting the impartiality of his decisions concerning further assessment and treatment.
In our opinion to preserve mental health, the patient should adhere to certain practices during the therapy of CBP:
First of all, the treatment should be aimed at eliminating the manifestations of the disease (such as urethral discharge and genital rash) and subjective symptoms that bother the patient (pain, itching, dysuria, concomitant ED, and/or premature ejaculation etc.). If the findings of tests improve in the course of treatment without the elimination of the abnormal symptoms, the patient may develop distrust in his physician and may be inclined to partake in inadequate alternative therapies.
Sufficient attention should be paid to each patient since trusting the doctor is an important factor of recovery and could influence the appearance of psycho–emotional complications in the future.
Taking into consideration the high degree of trust to the physician, during communications with neurotic patients the urologist should convey his/her confidence in positive treatment outcomes whenever treatment is feasible and its success can be confidently predicted. Conversely, if achievement of expected treatment results is dubious, the patient should not be given vain hopes since this will eventually cause mistrust of the physician and may provoke the emergence of exacerbated mental disorders.
For effective treatment, all patients with CBP should be informed of the necessity to assess and treat sexual partners as needed.
In cases in which psychopathological manifestations dominate over urological symptoms, the patients should be referred to a psychiatrist for consultation.
The population of patients with CBP and mental disorders is heterogeneous in their personality traits. In terms of personality traits, these patients can be divided into four distinctive personality types [23]:
Psychosomatic type. The prostatic problems are of a secondary nature with the primary disruptions being those of the nervous system;
Alexithymic type. The patients are unable to adequately verbalize their emotions, concerns, and complaints;
Borderline type, between neurotic type (health–related anxiety and depression) and psychotic type (delusional symptoms, persistent demands for frequent additional assessments; developing their own theories concerning the origin and treatment of CBP);
Narcissistic type (self–admiration with attention increasingly focused on bodily sensations and physiological manifestations, such as urination, urethral discharges, urethral itching, etc.).
Each patient with mental disorder requires a customized individual therapeutic approach; however, the general direction of psychological correction during psychotherapy should be chosen considering the above types of aberrant personalities.
In diagnosis of mental disorder and selection of therapeutic tactics, it should be taken into consideration that such patients predominantly have concomitant depression (with anxiety, occult or neurosis–like), as well as other pathologic mental conditions, which may require additional long–term psychotherapy and pharmacotherapy. There are some publications on the efficacy of antidepressants in the treatment of symptoms of chronic prostatitis/chronic pelvic pain syndrome in patients who are resistant to conventional anti–inflammatory therapy [29, 30].
Taking into consideration the obtained results and the abovementioned opinions of authorities, it is certain that pathologic manifestations and hormonal violations in patients with chronic prostatitis, especially when long–lasting, are capable of a negative influence on their mental status. On the other hand, a part of CBP patients are presented by persons with different mental disorders at the time of visiting their urologist. This is why the institution of long–tern antibacterial treatment with prostatic massages must be revised carefully in treatment–resistant patients. The timely reappraisal of diagnosis should be appropriate by making up a psychiatric investigation before the changes in mental status and their complications may become irreversible [31].
In cases in which the pathogen is fully eradicated, but CBP patient's condition has not improved by a prolonged treatment, it is contraindicated to continue antibiotics use and prostatic massages. Such patient should be thoroughly examined by a psychiatrist to exclude/confirm mental disorders with subsequent treatment considered thereafter.
CONCLUSIONS
According to our data, the frequency of concomitant psychosomatic disorders in patients with chronic bacterial prostatitis is 28.2% – neurotic disorders were registered in 26.4% pts. Among the neurotic and PSD patients, mild or moderate depressive disorders were also demonstrated.
Adequate antibacterial and anti–inflammatory therapy followed by a few sessions of psychotherapy decreased the manifestations of psychosomatic disorders in 30.5%, neurotic disorders in 51.7%, and premature ejaculation in 60.5% patients with CBP.
A substantial portion of patients with CBP required in–depth psychiatric assistance with pharmacotherapy. The addition of pharmacotherapy in treatment–resistant patients improved treatment results.
Urologists should be able to identify patients with concomitant mental disorders. When the signs of such disorders are present, the patient with chronic prostatitis must be examined by a psychiatrist.
References
- 1.Green MR, Dean AL. Some psychiatric aspects of symptoms of genitourinary disease. J Urol. 1954;72:742–747. doi: 10.1016/S0022-5347(17)67659-6. [DOI] [PubMed] [Google Scholar]
- 2.Mellan J, Raboch J, Kohlicec J. The problem of prostatic neurosis. Cesk Psychiatr. 1972;68:145–149. [PubMed] [Google Scholar]
- 3.Jansen PL, Kukahn R, Spieler KH, et al. Psychosomatische Untersuchungen zur Chronische Prostatitis. Z Psychosom Med Psychoanal. 1983;29:253–269. [PubMed] [Google Scholar]
- 4.Anderson RU, Orenberg EK, Chan CA, Morey A, Flores V. Psychometric Profiles and Hypothalamic–Pituitary–Adrenal Axis Function in Men With Chronic Prostatitis/Chronic Pelvic Pain Syndrome. J Urol. 2008;179:956–960. doi: 10.1016/j.juro.2007.10.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De la Rosette JJ, Ruijgrok MC, Jeuken JM, Karthaus HF, Debruyne FMJ. Personality variables involved in chronic prostatitis. Urology. 1993;42:654–662. doi: 10.1016/0090-4295(93)90529-j. [DOI] [PubMed] [Google Scholar]
- 6.Egan KJ, Krieger JN. Psychological problems in chronic prostatitis patients with pain. Clin J Pain. 1994;113:218–226. doi: 10.1097/00002508-199409000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Ku JH, Jeon YS, Kim ME, Lee NK, Park YH. Psychological problems in young men with chronic prostatitis–like symptoms. Scand J Urol Nephrol. 2002;36:296–301. doi: 10.1080/003655902320248272. [DOI] [PubMed] [Google Scholar]
- 8.Wu LX, Liang CZ, Hao ZY, Guo QK, Tang ZG. Epidemiological study of chronic prostatitis patients with depression symptoms. Zhonghua Nan Ke Xue. 2006;12:583–586. [PubMed] [Google Scholar]
- 9.Betts RF, Chapman SW, Penn RL. Reese and Betts’ a practical approach to infectious diseases. 5th edition. Lippincott Williams & Wilkins; 2003. p. 518. [Google Scholar]
- 10.Litwin MS, McNaughton–Collins MM, Fowler FJ, Nickel JC, Calhoun EA, Potari MA, et al. The National Institutes of Health Chronic Prostatitis Symptom Index (NIH–CPSI): Development and validation of a new outcome measure. J Urol. 1999;162:369–375. doi: 10.1016/s0022-5347(05)68562-x. [DOI] [PubMed] [Google Scholar]
- 11.Spitzer RL, Gibbon M, Skodol AE, Williams JBW, First MB. Diagnostic and Statistical Manual of Mental Disorders DSM–IV–TR Fourth Edition (Text Revision) Arlington: American Psychiatric Pubisching; 2000. [Google Scholar]
- 12.Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory–II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 13.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psych. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 14.Barbalias GA. Why alpha–blockers in prostatitis? Eur Urol Suppl. 2003;2:27–29. [Google Scholar]
- 15.Rowland DL, Patrick DL, Rothman M, Gagnon DD. The psychological burden of premature ejaculation. J Urol. 2007;177:1065–1070. doi: 10.1016/j.juro.2006.10.025. [DOI] [PubMed] [Google Scholar]
- 16.Rosen RC, Althof S. Impact of premature ejaculation: the psychological, quality of life, and sexual relationship consequences. J Sex Med. 2008;5:1296–1307. doi: 10.1111/j.1743-6109.2008.00825.x. [DOI] [PubMed] [Google Scholar]
- 17.Althof S. The psychology of premature ejaculation: therapies and consequences. J Sex Med. 2006;3(Suppl 4):324–331. doi: 10.1111/j.1743-6109.2006.00308.x. [DOI] [PubMed] [Google Scholar]
- 18.Tripp DA, Curtis Nickel J, Landis JR, Wang YL, Knauss JS. Predictors of quality of life and pain in chronic prostatitis/chronic pelvic pain syndrome: findings from the National Institutes of Health Chronic Prostatitis Cohort Study. BJU Int. 2004;94:1279–1282. doi: 10.1111/j.1464-410X.2004.05157.x. [DOI] [PubMed] [Google Scholar]
- 19.Walz J, Perrotte P, Hutterer G, et al. Impact of chronic prostatitis–like symptoms on the quality of life in a large group of men. BJU Int. 2007;100:1307–1311. doi: 10.1111/j.1464-410X.2007.07250.x. [DOI] [PubMed] [Google Scholar]
- 20.Wagenlehner FM, van Till JW, Magri V, Perletti G, Houbiers JG, Weidner W, Nickel JC. National Institutes of Health Chronic Prostatitis Symptom Index (NIH–CPSI) Symptom Evaluation in Multinational Cohorts of Patients with Chronic Prostatitis/Chronic Pelvic Pain Syndrome. Eur Urol. 2012 doi: 10.1016/j.eururo.2012.10.042. [DOI] [PubMed] [Google Scholar]
- 21.Ferris JA, Pitts MK, Richters J, Simpson JM, Shelley JM, Smith AM. National prevalence of urogenital pain and prostatitis–like symptoms in Australian men using the National Institutes of Health Chronic Prostatitis Symptoms Index. BJU Int. 2010;105:373–379. doi: 10.1111/j.1464-410X.2009.08708.x. [DOI] [PubMed] [Google Scholar]
- 22.Tripp DA, Nickel JC, Katz L. A feasibility trial of a cognitive–behavioural symptom management program for chronic pelvic pain for men with refractory chronic prostatitis/chronic pelvic pain syndrome. Can Urol Assoc J. 2011;5:328–332. doi: 10.5489/cuaj.10201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keltikangas–Järvinen L, Ruokolainen J, Lehtonen T. Personality pathology underlying chronic prostatitis. Psychother Psychosom. 1982;37:87–95. doi: 10.1159/000287558. [DOI] [PubMed] [Google Scholar]
- 24.Zhou Q, Li LQ, Wang CH, Liu CY, Zhao LM. The severity of psychic symptoms closely correlated with that of clinical ones in chronic prostatitis patients. Zhonghua Nan Ke Xue. 2007;13:531–534. [PubMed] [Google Scholar]
- 25.Jiang S, Zhu CY, Ma J, Tao R. Predictors of depression symptoms in patients with chronic prostatitis. Zhonghua Nan Ke Xue. 2012;18:212–215. [PubMed] [Google Scholar]
- 26.Ahn SG, Kim SH, Chung KI, Park KS, Cho SY, Kim HW. Depression, anxiety, stress perception, and coping strategies in Korean military patients with chronic prostatitis/chronic pelvic pain syndrome. Korean J Urol. 2012;53:643–648. doi: 10.4111/kju.2012.53.9.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dimitrakov J, Joffe HV, Soldin SJ, Bolus R, Buffington CAT, Nickel JC. Adrenocortical Hormone Abnormalities in Men with Chronic Prostatitis/Chronic Pelvic Pain Syndrome. Urology. 2008;71:261–266. doi: 10.1016/j.urology.2007.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alexander Fr. Psychosomatic Medicine. Its Principles and Applications. New York: W. W. Norton & Co., Inc; 1950. p. 300. [Google Scholar]
- 29.Schaeffer A. Chronic prostatitis and the chronic pelvic pain syndrome. N Eng J Med. 2006;355:1690–1698. doi: 10.1056/NEJMcp060423. [DOI] [PubMed] [Google Scholar]
- 30.Xia D, Wang P, Chen J, Wang S, Jiang H. Fluoxetine ameliorates symptoms of refractory chronic prostatitis/chronic pelvic pain syndrome. Chin Med J. 2011;124:2158–2161. [PubMed] [Google Scholar]
- 31.Mendlewicz J, Schulman CC, de Schutter B, Wilmotte J. Chronic Prostatitis: Psychosomatic Incidence. Psychother Psychosom. 1971;19:118–125. doi: 10.1159/000286313. [DOI] [PubMed] [Google Scholar]