Abstract
Introduction
Despite the introduction of better diagnostic tools, very large kidney tumors are still not so rare in our country. The paper presents our experience in the treatment of 12 patients with kidney tumors larger than 14 cm in size.
Material and methods
Between spring 2009 and autumn 2011, radical nephrectomies were performed in 12 patients due to a large kidney tumor (larger than 14 cm in size). Symptoms (hematuria, weight loss, anemia, etc.) were not present in all the patients, but the kidney tumor was confirmed by imaging studies (ultrasound, CT, MRI) in all of them.
Results
Full recovery was observed with no severe complications in all of the patients treated with radical nephrectomy. Pathological staging was correctly established by imaging studies in all of them. After a few months, five of patients (41.6%) required systemic therapy due to lymph node involvement.
Conclusions
Patients with large kidney tumors should be treated in selected medical centers that have experience in the treatment of such cases. Radical nephrectomy has to be the method of choice in the treatment of patients with this kind of tumor and its diameter should not disqualify from surgical treatment, which is still the only chance for the patients to be cured, as no adjuvant chemotherapy treatment has proved to be significantly effective.
Keywords: kidney tumor, nephrectomy, RCC, upper urinary tract, kidney masses
INTRODUCTION
Epidemiological data has indicated the increase in detection of kidney tumors in recent years [1, 2]. It is probably bound to a better availability of imaging techniques, as the majority of tumors are found incidentally, without any symptoms. Despite this, however, some of the detected tumors are larger than 14 cm in diameter. Surprisingly, a palpable abdominal mass is not an alarming symptom for some patients and does not force them to seek medical help. Other symptoms of classic Virchow triad like flank pain and gross hematuria are rare not always present [1, 2]. Paraneoplastic syndromes i.e., weight loss, hypertension, pyrexia or anemia are often linked with other conditions.
Removing a large kidney tumor creates a considerable challenge, even for a skilled urologist. The infiltration of adjacent organs, presence of neoplasmatic thrombus in vena cava, or distant metastases might be found [1, 2, 3]. All of these increase the perioperative risk. On the other hand, only surgical treatment when followed by administration of TK inhibitors gives the patient a chance for cure [4]. Work by Schrader showed that currently available chemotherapy is not effective as a neoadjuvant treatment used in order to reduce tumor size and stage [5].
The aim of this study is to analyze the result of surgical treatment in patients with very large (≥14 cm) kidney tumor as well as the perioperative complications on the basis of own experience and literature.
MATERIAL AND METHODS
Between spring 2009 and autumn 2011, 12 patients with kidney tumor ≥14 cm were operated in our department.
The group consisted of eight men and four women, aged 46–80 (mean 60). BMI was 21–38 (mean 27). On presentation, five out of the 12 patients (42%) suffered from hematuria, weight loss, and malaise. The remaining patients were asymptomatic. Lab tests did not reveal abnormal kidney parameters (creatinine levels were <1.3 mg/dl and GFR was >60 ml/min/1.73 m2) nor low hemoglobin concentration (<11 g/dl). When done precisely, palpation revealed abdominal mass in all of the patients (6/12 left sided, 6/12 right sided).
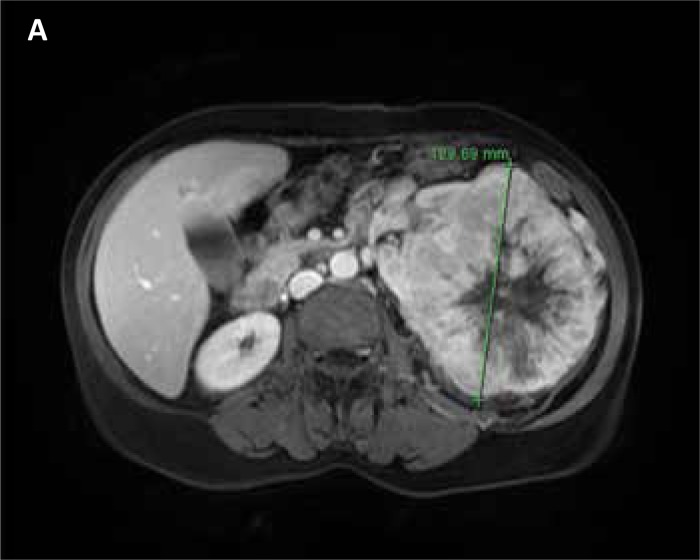
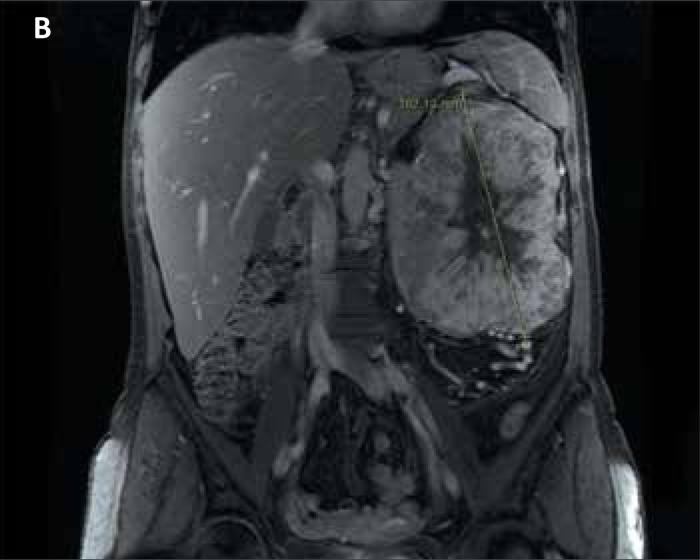
Kidney tumors were diagnosed by ultrasound (gross hypo–echoic tumor mass blurring normal kidney shape) and confirmed by CT (11/12) or MR (1/12) imaging – gross kidney lesions with heterogenous contrast enhancement (Figs. 1A, 1B). In six of the 12 patients, the kidney mass extended into the renal vein. In one patient the kidney cancer thrombus grossly extended into vena cava inferior below the diaphragm. Imaging modalities (CT/MR) indicted periaortic lymph nodes suspicious for metastases in five patients (41.6%). In four patients (33%), the kidney tumor was the only finding. Clinical details are presented in Tab. 1.
Figure 1A.
MRI scan – transverse section of a tumor (diameter – 12 cm).
Figure 1B.
MRI scan – crossection through the tumor (longitudinal length of tumor – 16 cm).
Table 1.
Clinical details (F – female, M – male)
| No. | Sex | Age | BMI | cTNM | Tumor diameter in cm | Symptoms | Metastases /thrombus /infiltration |
|---|---|---|---|---|---|---|---|
| 1 | M | 80 | 24 | cT2 | 17 | Varicocele | None |
| 2 | M | 78 | 24 | cT3 | 15 | Weakness, pyrexia | Perinephric fat infiltration, renal vein thrombus, lymphadenopathy |
| 3 | M | 67 | 21 | cT3 | 19 | Hematuria, weakness | Lung and lymph nodes metastases Perinephric fat infiltration, renal vein thrombus |
| 4 | M | 61 | 31 | cT2 | 15 | Varicocele | None |
| 5 | M | 52 | 28 | cT2 | 17 | Hematuria | None |
| 6 | M | 61 | 23 | cT3 | 14 | Weaknessi | Perinephric fat infiltration, renal vein thrombus |
| 7 | F | 55 | 26 | cT3 | 20 | None | Perinephric fat infiltration, renal vein thrombus |
| 8 | M | 62 | 37 | cT3 | 14 | Weakness, pyrexia | Renal vein thrombus, perinephric fat infiltration, lymph nodes metastases |
| 9 | M | 62 | 33 | cT3 | 14 | Pyrexia | Perinephric fat infiltration, lymph nodes metastases, renal vein thrombus |
| 10 | F | 46 | 21 | cT3 | 14 | None | Vena cava thrombus |
| 11 | F | 50 | 38 | cT2 | 14 | Weight loss | Lymph nodes metastases |
| 12 | F | 54 | 23 | cT2 | 15 | None | None |
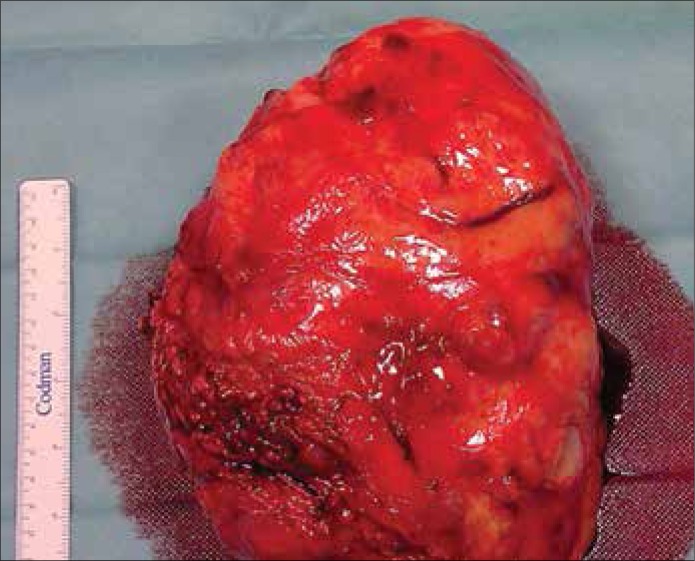
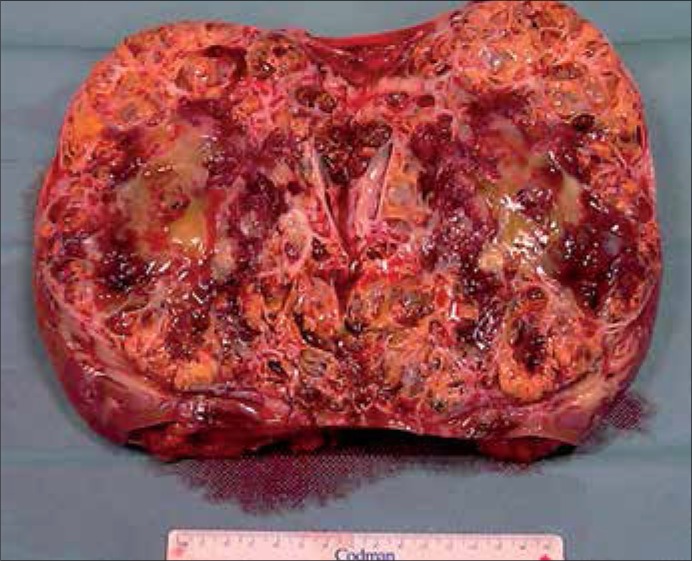
Radical nephrectomy including lymphadenectomy and adrenalectomy was performed in all patients due to good performance status facilitating planning of the additional systemic therapy. Transperitoneal medial incision was done in 11 patients (91.6%) and extraperitoneal lumbar approach in one patient (8.4%). Splenectomy was necessary in three cases due to hemorrhage after kidney dissection was completed. (Figs. 2 & 3).
Figure 2.
Kidney with tumor after excision (Line has a length of 15 cm).
Figure 3.
Kidney with tumor after excision (Line has a length of 15 cm).
RESULTS
Mean operation time was 2 h 45’ (2 h 15’ – 4 h) and mean blood loss 700 ml (300–1800 ml). Blood transfusions were necessary in five patients because of hemoglobin level <8 g/dl. Mean hospital stay was six days (5–8).
RCC pT2–pT3 was confirmed in 11 cases (91.6%). T2 oncocytoma was diagnosed in one patient (8.4%). Detailed pathological findings are disclosed in Table 2 with the TNM staging system and Fuhrman classification. Clinical and pathological T staging was completely concordant in all 12 cases. Lymph node metastases and tumor thrombus size were correctly assessed in all cases using imaging studies.
Table 2.
Pathological results in accordance with the 2009 TNM staging system
| No. | Tumor size | Pathology | TNM | Fuhrman | Stage |
|---|---|---|---|---|---|
| 1 | 17 x 13 x 13 cm | Carcinoma nephrogenes typus papillaris 2 | pT2bN0Mx | 2 | II |
| 2 | 15 x 11 x 9 cm | Carcinoma nephrogenes male differentiatum | pT3aN2Mx | 4 | III |
| 3 | 19 x 12 x 11 cm | Carcinoma nephrogenes typus clarocellularis | pT3aN2Mx | 3 | III |
| 4 | 15 x 13 x 11 cm | Carcinoma chromophobes renis | pT2bN0Mx | 2 | II |
| 5 | 17 x 13.5 x 11 cm | Carcinoma clarocellulare renis | pT2bNxMx | 2 | II |
| 6 | 14 x 8 x 7 cm | Carcinoma clarocellulare renis | pT3aNxMx | 2 | III |
| 7 | 20 x 10 x 8 cm | Carcinoma clarocellulare renis | pT3aN0Mx | 3 | III |
| 8 | 14 x 12 x 12 cm | Carcinoma nephrogenes typus clarocellularis | pT3aN2Mx | 3 | III |
| 9 | 14 x 12 x 10 cm | Carcinoma nephrogenes typus clarocellularis | pT3aN1Mx | 3 | III |
| 10 | 14 x 11.5 x 9 cm | Carcinoma clarocellulare renis | pT3bNxMx | 3 | III |
| 11 | 14 x 9 x 7 cm | Carcinoma clarocellulare renis | pT2bN1Mx | 4 | III |
| 12 | 15 x 13 x 11 cm | Renal oncocytoma | cT2bNxMx | None | II |
Urological and oncological care of all patients treated is continued in the outpatient setting. In the six months after nephrectomy, five patients previously diagnosed with lymph node involvement required systemic therapy because of probable non–radical lymphadenectomy. No additional treatment was needed in the remaining patients (58.4%).
DISCUSSION
The most common malignant lesion in the kidney is renal cell carcinoma (RCC), which represents 85–90% of all kidney tumors. The remaining 10–15% of renal tumors are more rare cancers, unclassified lesions, and some types of benign tumors [1]. Among the causes of death in men from cancer, RCC occupies the 7th place. Statistically, the number of cases is higher in men than in women by a ratio of 1.5:1 with a peak of incidence between the 6th and 7th decade of life [1, 2].
Epidemiological data has indicated the increase in detection of kidney tumors in recent years. Mainly it is caused due to better availability of imaging techniques, as the majority of tumors are found incidentally without any symptoms.
The factors that increase the incidence rate include smoking, obesity, hypertension, and chemical agents. RCC is also present in some genetically determined syndromes such as: Von Hippel–Lindau, Birt–Hogg–Dubé, hereditary papillary RCC, familial leiomyomatosis, and Bourneville–Pringle among others [1, 2, 6].
Radical nephrectomy is the method of choice in the treatment of lager tumors. However, tumor size, location, local advancement, and presence of thrombus in vena cava may cause that some patients may not be qualified for the treatment because of a high risk of complications.
From the literature we also know that tumor size is an independent prognostic factor influencing the result of the treatment [7]. In our opinion tumor size alone should not be a disqualifying factor from surgical treatment. The currently used diagnostic tools allow for precisely determining the stage of the tumor, which was confirmed in our study group. Radiological results in most patients were confirmed by intraoperative examination and pathology results [8, 9].
The presence of a very large tumor undoubtedly increases the difficulty of surgery especially in obese patients, which was observed at the time of surgery in patients with BMI greater than 31 [10].
In the literature there are some case report studies suggesting that neoadjuvant chemotherapy with targeted therapy may reduce the stage of the tumor and thus make the surgery easier. However, currently used drugs have proven not to be very effective in larger studies, and this option in Poland and other countries is yet neither registered nor recommended [5].
An accurate preoperative diagnosis, proper preparation of the patient and experienced operating team allow the removal of the tumor without serious complications. In our opinion the most suitable surgical approach is a median incision because it allows better control over the vascular pedicle [3, 11, 12]. In experienced hands, operating time, blood loss, and the risk of complications should not be significantly greater than reported for smaller tumors [13, 14, 15]. The qualifications for surgery depend on the presence of infiltrating of adjacent organs, especially liver and spleen, which greatly affect the course of the treatment. The presence of metastasis is also important. It is not directly associated with the tumor size, but undoubtedly is important when planning surgery. The removal of the tumor in such cases is only justified in patients in good condition and with favorable prognostic factors enabling adjuvant treatment with targeted chemotherapy [1, 14, 16]. Radical removal of even very large tumors provides similar cancer–specific survival as the smaller lesions with the same stage [17].
CONCLUSIONS
In our opinion:
radical nephrectomy is the method of choice in the treatment of large renal tumors in Poland due to healthcare system requirements;
there is no scientific proof that neoadjuvant targeted therapy may significantly reduce the stage and size of a tumor;
tumor size alone should not affect the qualification of the patient for surgery;
currently used imaging modalities enable precise evaluation of clinical stage of the tumor and are basic instruments for surgical planning;
the removal of the tumor in disseminated disease is only justified in patients with a condition suile for targeted adjuvant treatment.
References
- 1.Ljungberg B, Cowan N, Hanbury DC, Hora M, Kuczyk MA, Merseburger AS, Mulders PFA, Patard J-J, Sinescu IC. Guidelines on Renal Cell Carcinoma. European Association of Urology. 2010 Apr; doi: 10.1016/j.eururo.2010.06.032. [DOI] [PubMed] [Google Scholar]
- 2.Wein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CA. 10th Edition. 2012. Campbell–Walsh Urology; pp. 1419–1449. [Google Scholar]
- 3.Robson CJ. Radical nephrectomy for renal cell carcinoma. J Urol. 1963;89:37–42. doi: 10.1016/S0022-5347(17)64494-X. [DOI] [PubMed] [Google Scholar]
- 4.Powles T, Kayani I, Blank C, Chowdhury S, Horenblas S, Peters J, et al. The safety and efficacy of sunitinib before planned nephrectomy in metastatic clear cell renal cancer. Ann Oncol. 2011;22:1041–1047. doi: 10.1093/annonc/mdq564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schrader AJ, Steffens S, Schnoeller TJ, Schrader M, Kuczyk MA. Neoadjuvant therapy of renal cell carcinoma: A noveltreatment option in the era of targeted therapy? Int J Urol. 2012;19:903–907. doi: 10.1111/j.1442-2042.2012.03065.x. [DOI] [PubMed] [Google Scholar]
- 6.Sudarshan S, Linehan WM. Genetic basis of cancer of the kidney. Semin Oncol. 2006;3:544–551. doi: 10.1053/j.seminoncol.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 7.Karakiewicz PI, Suardi N, Capitanio U, Jeldres C, Ficcara V, Cinddo L, et al. A preoperative prognostic model for patients treatedwith nephrectomy for renal cell carcinoma. Eur Urol. 2009;55:287–295. doi: 10.1016/j.eururo.2008.07.037. [DOI] [PubMed] [Google Scholar]
- 8.Kinouchi T, Saiki S, Meguro O, Maed O, Kuroda M, Usami M, Kotake T. Impact of tumor size on the clinical outcomes of patients with Robson stage I renal cell carcinoma. Cancer. 1999;85:689–695. doi: 10.1002/(sici)1097-0142(19990201)85:3<689::aid-cncr19>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 9.Guinan PD, Vogelzang NJ, Fremgen AM, Chmiel JS, Sylvester JL, Sener SF, Imperato JP. Renal cell carcinoma: tumor size, stage and survival. Members of the Cancer Incidence and End Results Committee. J Urol. 1995;153:901–903. [PubMed] [Google Scholar]
- 10.Raman JD, Smith B, Messer J, Rohner TJ, Harpster LE, Reese CT. Preoperative predictors of surgical approach for partial nephrectomy. Can J Urol. 2011;18:5896–5902. [PubMed] [Google Scholar]
- 11.Libertino JA. Renal cell cancer with extension into vena cava. In: McDougal WS, editor. Rob & Smiths Operative Surgery: Urology. 4th edn. London: Butterworths; 1986. [Google Scholar]
- 12.Bissada NK, Yakout HH, Babanouri A, Elsalamony T, Fahmy W, Gunham M, Hull GW, Chaudhary UB. Long–term experience with management of renal cell carcinoma involving the inferior vena cava. Urology. 2003;61:89–92. doi: 10.1016/s0090-4295(02)02119-2. [DOI] [PubMed] [Google Scholar]
- 13.Mazzucchi E, Nahas WC, Antonopoulos IM, Piovesan AC, Ianhez LE, Arap S. Surgical complications of graft nephrectomy in the modern transplant era. J Urol. 2003;170:734–737. doi: 10.1097/01.ju.0000080566.42381.94. [DOI] [PubMed] [Google Scholar]
- 14.Tan HJ, Hafez KS, Ye Z, Wei JT, Miller DC. Postoperative complications and long–term survival among patients treated surgically for renal cell carcinoma. J Urol. 2012;187:60–66. doi: 10.1016/j.juro.2011.09.031. [DOI] [PubMed] [Google Scholar]
- 15.Sun M, Bianchi M, Trinh QD, Abdollah F, Schmitges J, Jeldres C, Shariat SF, Graefen M, Montorsi F, Perrotte P, Karakiewicz PI. Hospital volume is a determinant of postoperative complications, blood transfusion and length of stay after radical or partial nephrectomy. J Urol. 2012;187:405–410. doi: 10.1016/j.juro.2011.10.025. [DOI] [PubMed] [Google Scholar]
- 16.Gouttefangeas C, Stenzl A, Stevanonic S, Rammensee HG. Immunotherapy of renal cell carcinoma. Cancer Immunol Imunother. 2007;56:117–128. doi: 10.1007/s00262-006-0172-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shulyak A, Banyra O. Radical or simple nephrectomy in localized renal cell carcinoma: what is a choice? CEJU. 2011;64:152–155. doi: 10.5173/ceju.2011.03.art12. [DOI] [PMC free article] [PubMed] [Google Scholar]