Abstract
Oxidative stress results from the imbalance between production of the reactive oxygen species (ROS) and the protective effect of the antioxidant system responsible for their neutralization and removal. An excess of ROS causes a pathological reaction resulting in damage to cells and tissues. Spermatozoa are particularly vulnerable to the harmful effects of ROS. Oxidative stress affects their activity, damages DNA structure, and accelerates apoptosis, all of which consequently decrease their numbers, hinders motility and development of normal morphology, and impairs function. This leads to disturbances in fertility or embryo development disorder. The main cellular source of ROS in the semen are immature sperm cells and white blood cells. The increase in the number of leukocytes may be due to infection and inflammation, but can also be secondary to harmful environmental factors, long sexual abstinence, or varicocele. The protective antioxidant system in the semen is composed of enzymes, as well as nonenzymatic substances, which closely interact with each other to ensure optimal protection against ROS. Non–enzymatic antioxidants include vitamins A, E, C, and B complex, glutathione, pantothenic acid, coenzyme Q10 and carnitine, and micronutrients such as zinc, selenium, and copper. It seems that a deficiency of any of them can cause a decrease in total antioxidant status. In vitro and in vivo that studies demonstrate many antioxidants possess a beneficial effect on fertility and, therefore, their use is recommended as supportive therapy for the treatment of infertility in men.
Keywords: male, sperm, fertility, oxidative stress, antioxidants, infertility treatment
INTRODUCTION
The World Health Organization (WHO) defines infertility as the inability to achieve pregnancy within 12 months of regular sexual intercourse for couples in conception. Infertility affects 13–20% of couples in Poland and around the world, regardless of race or ethnicity [1–4]. It is estimated that male factor of couple infertility is between 25% to 50% [2, 5]. The lower reference limits for semen parameters according to WHO (2010) are shown in Table 1 [6].
Table 1.
Lower reference limits for basic semen parameters according to WHO manual (2010)
| Semen parameter | Lower reference value |
|---|---|
| Liquefaction | <60 min |
| Volume (mL) | ≥1.5 |
| Ph | ≥7.2 |
| Total sperm number | ≥39 mln /ejaculate |
| Sperm concentration | ≥15 mln/ml |
| Total sperm motility | ≥40% ≥15.6 mln/ejaculate |
| Progressive sperm motility | ≥32% ≥12.5 mln/ejaculate |
| Vitality | 58% ≥22.6 mln/ejaculate |
| Normal morphology | ≥4% ≥1.6 mln/ejaculate |
| Peroxidase–positive cells (leukocytes) | <1 mln/ml |
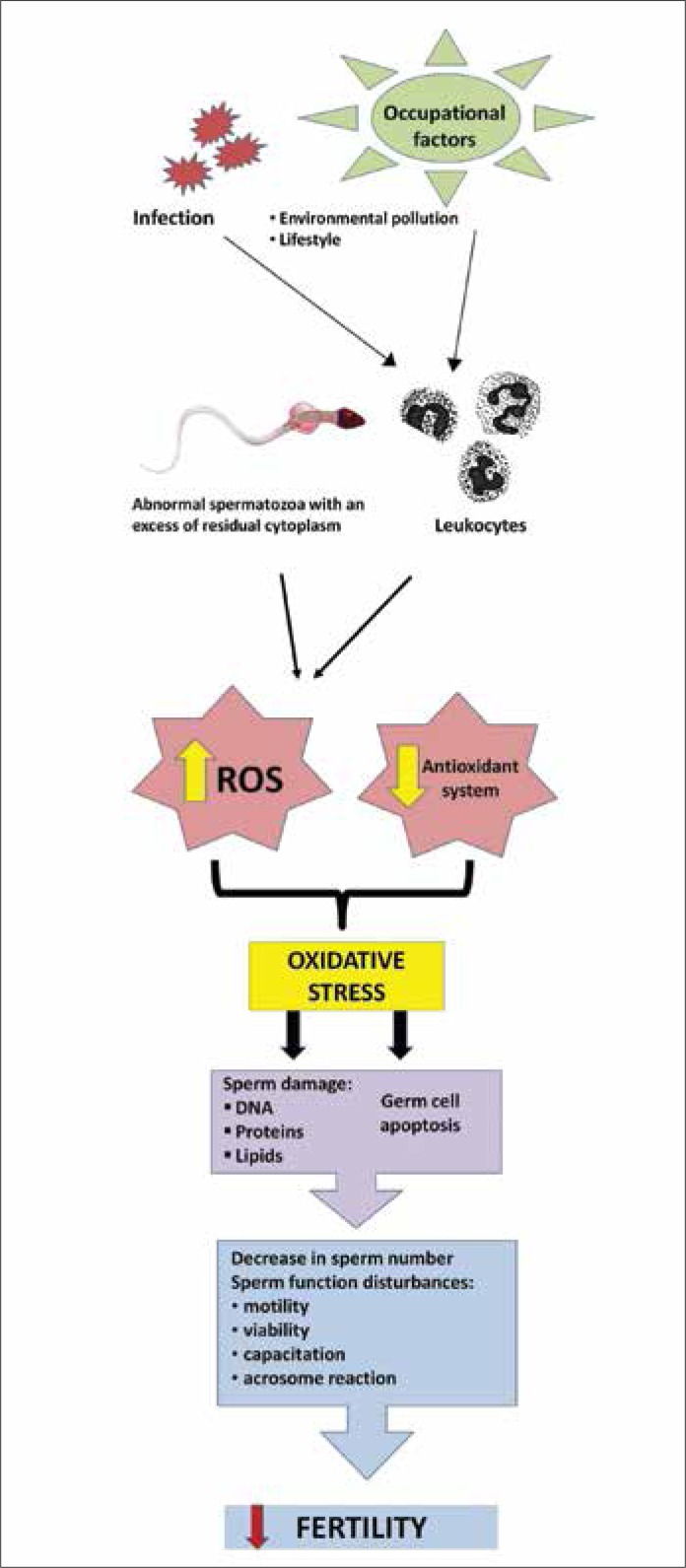
Although causes of male infertility can be identified as anatomical abnormalities, such as varicocele, semen outflow tract obstruction or neurological disorders of ejaculation, most of the cases are caused by abnormal spermatogenesis and failure in sperm function [1]. Despite the development of science and increasingly sophisticated diagnostic methods, in some cases, the etiology and pathogenesis of male infertility are still unknown and constitute the group of idiopathic infertility. Male fertility disorder is attributed to environmental factors such as exposure to certain chemicals, heavy metals, pesticides, and heat, or electromagnetic radiation [7, 8, 9]. Smoking, alcohol abuse, chronic stress, obesity, urogenital trauma, and inflammation in the male reproductive system are also associated with decreased male fertility [10, 11, 12]. The consequence of most of these factors is oxidative stress. Oxidative stress is caused by the imbalance between the production of so–called reactive oxygen species (ROS) and the protective action of antioxidant system responsible for their neutralization and removal. An excess of ROS causes a pathological response leading to damage of cells and tissues. Spermatozoa are particularly susceptible to the damaging effects of ROS, because their cell membrane contains large amounts of unsaturated fatty acids, which can be oxidized (lipid peroxidation), and the cytoplasm has only small concentrations of the enzyme able to neutralize ROS. The lipid oxidation process leads to a loss of membrane integrity and an increase in its permeability, inactivation of cellular enzymes, structural DNA damage, and cell apoptosis. The consequence is reduced sperm count and activity, decreased motility and abnormal morphology [13, 14, 15]. It is estimated that approximately 25% of infertile men present elevated levels of ROS in the semen [16, 17, 18] and often lower antioxidant capacity of semen [19, 20, 21]. The scheme of harmful effects of ROS is shown in Figure 1. Therefore, it seems reasonable to try to support the treatment of male infertility with supplementation having the ability to neutralize ROS i.e. antioxidants [22].
Figure 1.
Scheme of harmful effects of reactive oxygen species (ROS) on male fertility.
Cellular source of ROS in semen
One of the major cellular sources of ROS in the semen are sperm cells. From the earliest stages of the development of male germ cells they are able to produce small amounts of ROS [23]. ROS are involved in the sperm chromatin condensation, adjusting the number of germ cells by induction of apoptosis or proliferation of spermatogonia [24]. In mature sperm, ROS play an important role in the capacitation, acrosome reaction, mitochondrial sheath stability and sperm motility. ROS can also function as signaling molecules (second messengers of cell signaling). There are at least two positions and mechanisms of their production. The first is the membrane NADPH oxidase, an enzyme complex that is contained in the cell membrane, and the second are the mitochondria, which leak electrons from the respiratory chain. It was found also that the production of the superoxide anion involves NADH oxidoreductase (diaphorase), an enzyme located in the region of the sperm cell midpiece, which is in close conjunction with the mitochondrial chain and xanthine oxidase present in both sperm and seminal plasma [25]. Sperm cells with impaired morphology, mainly with cytoplasmic residues indicating their immaturity and reduced fertility potential, produce higher amounts of ROS than spermatozoa with normal structure [26, 27]. There is also a difference between the amounts of ROS produced by spermatozoa in different stage of maturation [13].
The second source of ROS in the semen are leukocytes that, under physiological conditions, produce up to 1,000 times more ROS than spermatozoa [28]. This high production of ROS by leukocytes plays an important role in the cellular defense mechanism against infections and inflammation. In such cases activated leukocytes infiltrate the affected organ secreting large amounts of ROS that lead to the elimination of infectious agents [28]. However, this imbalance of oxidants and antioxidants can damage the cells. The increase in the number of leukocytes in the semen can also be a result of environmental factors, long sexual abstinence, or varicocele [11, 25].
The antioxidant system in semen
The protective antioxidant system in the semen is made up of both enzymatic and non–enzymatic factors and low molecular weight compounds with antioxidant capacity, all of which closely interact with each other in order to ensure optimum protection against ROS. It seems that the deficiency of any of them can cause a decrease in total plasma antioxidant capacity. The main antioxidant enzyme system in the semen is called an enzyme triad comprising superoxide dismutase, catalase, and glutathione peroxidase.
Superoxide dismutases (superoxide oxidoreductases – SOD) are metaloenzymes catalyzing dismutation reactions of the superoxide anion. They are present in both intra– and extracellular forms. Two of the intracellular forms are copper–zinc SOD, which are localized mainly in the cytoplasm and contains copper and zinc (Cu, ZnSOD, SOD–1) in the active center, and manganese SOD, which is located mainly in the mitochondrial matrix with manganese at its active center (MnSOD, SOD–2). The extracellular form of SOD (EC–SOD, SOD–3) acts in the extracellular space. It is related to the surface polysaccharides although it may also be present in a free form. It is similar to SOD–2 in construction, but instead of manganese it has zinc and copper in its active center [25, 29]. SOD demonstrates high activity in seminal plasma with 75% of its activity related to the activity of SOD–1 and the remaining 25% to SOD–3. It was demonstrated that these two isoenzymes are probably derived from the prostate [30].
Catalase (CT) catalyzes the decomposition of hydrogen peroxide to molecular oxygen and water. A characteristic feature of its structure is a heme system with a centrally–located iron atom. Its activity has been demonstrated in peroxisomes, mitochondria, endoplasmic reticulum and the cytosol in many types of cells [31]. In semen, it was found in the sperm cells of humans and rats, as well as seminal plasma, where its source is the prostate [25]. Catalase activates the sperm cell capacitation induced by nitric oxide, which is a complicated mechanism using hydrogen peroxide [32].
Another enzyme of the antioxidant system in the semen is glutathione peroxidase (GPX), which catalyzes the reduction of hydrogen peroxide and organic peroxides, including the peroxides of phospholipids [29]. In its active site, it contains selenium in the form of selenocysteine. In sperm it is located mainly in the mitochondrial matrix [30], but a nuclear form that protects sperm DNA from oxidative damage and participates in the process of chromatin condensation has also been found [33]. The presence of GPX has also been demonstrated in the seminal plasma, suggesting its origin from the prostate [34].
Besides the enzymes neutralizing the overproduction of ROS, an important role in the antioxidant protective system is played by the low molecular weight, non–enzymatic antioxidants that assist enzyme activity. These include, among others, glutathione, pantothenic acid, coenzyme Q10, carnitine, vitamins A, E, C and B complex and minerals, such as zinc, selenium, and copper [18, 35]. Chrome also seems to be another essential micronutrient that is a component of a number of enzymes involved in carbohydrate metabolism. In rats its supplementation reduces fat deposition, thus preventing obesity and consequently prevents the initiation of inflammation and, therefore, oxidative stress [36].
Non–enzymatic antioxidants
Carotenoids are a group of fat–soluble organic compounds found mainly in yellow, red, orange, and pink vegetable dyes. These retinoids are precursors of vitamin A. Retinal is formed from them in the gastrointestinal tract, before being converted to retinol, the most important component of vitamin A. Carotenoids are natural antioxidants, responsible for the integrity of cell membranes and regulating epithelial cell proliferation, and are involved in the regulation of spermatogenesis [37]. Their deficiency in the diet can lead to a reduction in sperm motility.
Vitamin E (α–tocopherol) is an organic chemical compound soluble in fats localized mainly in cell membranes. Its antioxidant activity is mainly based on quenching lipid peroxidation initiated by ROS and the capture of free hydroxyl radicals and superoxide. Thus, vitamin E mainly protects the sperm cell membrane components from damage and, to a lesser extent, decreases the production of ROS. In vitro studies have shown that vitamin E prevents reduced motility of sperm and also improves their ability to fertilize in the hamster egg penetration test [38]. In vivo studies have shown that in male infertility, supplementation with vitamin E was effective in cases with a reduced number and motility of sperm cells (oligoasthenozoospermia) caused by oxidative stress [39, 40]. Its oral administration significantly increased sperm motility by decreasing sperm production of malonic dialdehyde (MDA), which is the end product of lipid peroxidation and is, hence, an indirect indicator of the intensity of the process in the cell [41]. It was found that the concentration of MDA in the semen is twice as high in men with asthenozoospermia as in men with normozoospermia, and its reduction correlates well with the percentage of successful attempts to achieve pregnancy. Vitamin E was observed to have an enhanced effect on sperm motility when its administration was combined with selenium [40]. It was also found that selenium alone prevents oxidative damage to sperm DNA. Selenium is an essential micronutrient for normal testicular development, spermatogenesis, sperm motility, and function [42]. Lack of selenium leads to atrophy of the seminiferous epithelium, disorders of spermatogenesis and maturation of spermatozoa in the epididymis, and testis volume reduction [43]. Also, an increased percentage of sperm cells with abnormal morphology (mainly the head and midpiece) and poor sperm motility can be observed in the semen [44]. Not only selenium, but also zinc and copper are trace elements that are important for the normal function of testes, including the process of spermatogenesis. Zinc is a component of over 200 enzymes that are involved in the biosynthesis of nucleic acids and proteins as well as in the process of cell division. The concentrations of zinc, copper, and selenium in seminal plasma have correlated well with sperm quality in men [45, 46].
Vitamin C (ascorbic acid) is a water–soluble substance which concentration in the seminal plasma is about 10 times higher than in blood serum. This vitamin has high antioxidant potency, which is valuable in protecting sperm DNA from the harmful effects of ROS [47]. Reduced levels of vitamin C and elevated ROS levels have been found in the seminal plasma of men with asthenozoospermia [20] and a dose–dependent improvement in sperm motility has been found to be associated with vitamin C level, especially in smokers [48]. It seems that supplementation with hydrophilic vitamin C and lipophilic vitamin E can act synergistically in reducing the impact of sperm oxidative stress [49].
Glutathione is the most abundant intracellular thiol, that is, a sulfur–containing component, and hence, is present in the largest amounts. It has anti–oxidant properties by reconstruction of thiol groups (–SH) in proteins, which can be eliminated during oxidative stress. Glutathione also protects the cell membranes from lipid oxidation and prevents the formation of free oxygen. A glutathione deficit leads to instability of the midpiece of the spermatozoa, which results in a motility disorder [50]. Glutathione supplementation in infertile men with unilateral varicocele or inflammation of the urogenital system leads to a significant improvement in sperm parameters [51]. A glutathione precursor is N–acetyl–cysteine, which also improves sperm motility and prevents oxidative damage to sperm DNA [52]. A factor increasing the level of glutathione is pantothenic acid, which by doing so also protects tissues against oxidative stress [53].
Antioxidant therapy for infertile men
Over the last two decades numerous clinical studies have been carried out to establish the possible beneficial effects of treatment with antioxidants on improvement of sperm parameters in men as well as fertilization or pregnancy rates in their partners. However, there is a lack of uniformity between most of them and thus it is difficult to compare their results and draw the unambiguous conclusion as for establishment of the optimal dietary supplementation. Most of the trials are small in size (usually less than 100 men), with no homogenous target population. Moreover, the threshold for study inclusion differed among studies for the same target group as well as for study exclusions criteria or outcomes analyzed. A significant number of studies lack randomization and placebo–controlled arms. The type, dose, and duration of the antioxidant therapy also differ substantially. In some of the studies only one from a dozen of known antioxidants was tested while in others different combinations of them – the therapy duration ranged from 4 to 48 weeks. The most commonly studied antioxidants were vitamin C, vitamin E, selenium, zinc, glutathione, L–carnitine and N–acetyl–cysteine (for ref. see [54–58]).
However, only recently a few reviews summarizing the results of carefully selected clinical trials were published trying to establish current medical evidence on the effect of oral antioxidants on sperm quality and pregnancy rate in infertile men. Ross et al. [54] analyzed 17 trials, including a total 1,665 men. All of them had a randomized design, the target population was infertile men and the therapeutic intervention was oral antioxidant(s) compared with placebo or no treatment. Outcome measures were semen parameters (sperm concentration, motility, and morphology) and/or occurrence of pregnancy. Although there was the methodological and clinical heterogeneity concerning the studied population, as well as type, dosage, and duration of antioxidant therapy, 14 of the 17 (82%) trials showed an improvement in sperm quality, mainly motility (63%) but also sperm concentration (33%) and/or morphology (17%). In six of the 10 trials, increased pregnancy rates were found. Gharagoozloo and Aitken [55] selected 20 trials that assessed the effect of oral antioxidants against a measure of sperm oxidative stress or DNA damage. Again, the studies were small in size and heterogeneous, but the analysis showed that 95% of them reported a significant reduction of oxidative stress or DNA damage after treatments with antioxidants. Moreover, among semen parameters, motility was improved in 10 of 16 studies, but it is worth to note that this improvement was seen in all the studies where the asthenozoospermic men were a target group (seven groups). Less common, however, was an improvement in sperm concentration (15%). Zinni and Al–Hathal [56] compared 24 randomized controlled trials evaluating the effects of antioxidants such as vitamin C, vitamin E, selenium, zinc, folic acid, N–acetyl–cysteine, L–carnitine, L–acetyl carnitine, and N–acetyl–cysteine either taken alone or in different combinations on different semen variables. An improvement was observed in 18 studies in contrast to six where nil effect was reported. Further comparison of eight studies evaluating the effect of the therapies on sperm DNA damage showed the beneficial effects of antioxidants on sperm DNA integrity and pregnancy rates after assisted reproduction. However, as the authors reported that none of them estimated seminal oxidative stress, seminal vitamin levels, or used oxidative DNA damage (e.g. by estimation of 8–hydroxy–2’–deoxyguanosine) as a selection criterion for monitoring the response to the therapy, the precise mechanism of action of these antioxidants on sperm DNA quality is still unclear. Additionally, authors also summarized the results from articles evaluating the role of in vitro antioxidants in protecting spermatozoa from the loss of motility and DNA damage due to 1) exogenous ROS, 2) semen processing, or 3) cryopreservation and thawing and they concluded a beneficial effect of in vitro antioxidant supplements in protecting spermatozoa from exogenous oxidants and cryopreservation with subsequent thawing. Showell et al. [57] published the Cochrane review on the basis of meta–analysis of 34 trials that included 2,876 couples evaluating the effect of oral supplementation with antioxidants for male partners of couples undergoing assisted reproduction techniques. The outcomes were live birth, pregnancy, miscarriage, stillbirth, sperm DNA damage, sperm motility, sperm concentration, and adverse effects. The authors concluded that taking oral antioxidants had an associated statistically significant increase in live birth rate and pregnancy rate in subfertile couples undergoing assisted reproduction techniques cycles.
Although the presented reviews generally demonstrate a beneficial effect of antioxidants on infertile men, the optimal type and dose of antioxidants is still unknown together with the precise mechanism of their action. The dosage and type of antioxidants should probably be adjusted to the level of oxidative stress in the semen. It can be studied with the use of direct methods such as chemiluminescence, cytochrome C reduction, flow cytometry, or indirect methods that measure levels of biomarkers of oxidative stress. Measurement of ROS levels could help to identify patients who could benefit from supplementation with antioxidants. However, no reliable, cheap and easy to perform tests that would apply in routine practice have been developed so far.
The authors unanimously emphasized the need for larger, carefully designed clinical trials in the future. The best trials being multi–centered, double–blind, randomized cross–over with strict selection criteria targeting patients with moderate to severe sperm oxidative stress and those with many measurable outcomes, such as pregnancy and miscarriages rates. Moreover, in a very recent review concerning over–the–counter (OTC) supplements, such as herbs, vitamins, and nutritional supplements for the treatment of male infertility, one more point is stressed by the authors – as many OTC studies fail to control the baseline dietary intake by patients of the analyzed substances the authors caution against the widespread use of the OTC supplements above and beyond the recommended daily allowance in order to avoid triggering any possible side effects [58].
Antioxidants and assisted reproductive technologies
Semen preparation methods used in assisted reproductive technologies (ART) have an impact on the results of the different procedures. Washing, centrifugation, and the incubation of sperm in different culture media may result in increased production of ROS. This is due to the separation of sperm from seminal plasma, which is rich in antioxidants. One of the main consequences of applying these methods is a reduction in sperm motility. It has been shown that the use of antioxidants such as vitamin E, glutathione, N–acetyl–cysteine, and catalase in the semen preparation reduces ROS levels and prevents the reduction of sperm motility [52, 59, 60]. It seems that the semen samples of men with reduced fertility potential are more susceptible to the oxidative stress that occurs during semen preparation and may require greater antioxidant protection in comparison with semen samples obtained from fertile men.
Another important issue is to protect against the loss of sperm motility and DNA damage after cryopreservation and subsequent thawing. It appears that another major antioxidant is pentoxifylline, which was observed to improve sperm motility and function after thawing [61, 62]. Other tested antioxidants, such as vitamins E and C, have a much smaller impact on improving sperm motility [63, 64]. On the other hand, it has been shown that vitamin C, catalase, and genistein can protect sperm DNA from damage due to oxidative stress induced by cryopreservation and subsequent thawing [65–68].
In addition, supplementation of culture media with N–tert–butyl hydroxylamine (NTBH) and mimetics of SOD / catalase also blocks sperm chromatin damage. There are also reports that the production of ROS increases with the prolonged incubation of sperm with oocyte, thus it is recommended that the incubation period is as short as possible [69]. Composition of media used for IVF (in vitro fertilization) also has a significant impact on the status of oocytes and embryos before the implantation phase. Recently, it has been shown that the amount of ROS in sperm correlates significantly with the fertilization rate obtained after IVF. This suggests that the level of ROS in the semen is an important predictor of the success of this technique. Media supplementation with antioxidants may therefore be beneficial to the development of embryos and increase the number of pregnancies [70].
CONCLUSIONS
Over the past 25 years, there have been many experimental and clinical studies on the pathophysiology of oxidative stress and its impact on fertility disorders, both in men and women [57]. There is now little doubt that oxidative stress causes damage to the function of sperm, causing structural damage to DNA and accelerating cell apoptosis, which consequently lead to the inability to achieve conception or lack of development of the embryo. In vitro and in vivo studies have demonstrated the beneficial effect of many antioxidant factors on sperm, pregnancy rate and live birth rates.
The controversial issue is, however, the effectiveness of antioxidants on sperm, which has not yet been confirmed by other studies [57]. This may be primarily due to the multitude of causes of male fertility problems and the paucity of diagnostic capabilities currently available. In addition, as these studies are not usually based on an evaluation of oxidative stress intensity and the only criterion for inclusion in the study is a decreased number of spermatozoa, the groups of men surveyed in such studies tend to be nonhomogenic.
Another issue of concern is the dose of antioxidant preparations. It appears that in the case of oxidative stress, doses of antioxidants should be higher than usual daily dose and used for at least three months, because the development of a mature sperm from spermatogonia is 72 ±4 days. Determination of the most preferred active doses of antioxidants requires further research. Although products available without prescription include safe doses of the individual components, an overdose of some of them such as vitamin A, can have side effects. The daily requirement of these components is presented in Table 2 [71].
Table 2.
Recommended daily allowance (RDA) of vitamins and minerals with antioxidant effect according to the European Commission Directive 2008/100/EC from 28 October 2008
| Vitamins | RDA | Minerals | RDA |
|---|---|---|---|
| A (carotenoids) | 800 µg | zinc | 10 mg |
| E (tocopherols) | 12 mg | selenium | 55 µg |
| C (ascorbic acid) | 80 mg | copper | 1 mg |
| B1 (thiamine) | 1.1 mg | chrome | 40 µg |
| B2 (riboflavin) | 1.4 mg | magnesium | 2 mg |
| B3 (niacin) | 16 mg | iron | 14 mg |
| B5 (pantothenic acid)) | 6 mg | ||
| B6 (pyridoxine) | 1.4 mg | ||
| B7 (biotin) | 50 µg | ||
| B12 (cobalamine) | 2.5 µg |
ACKNOWLEDGEMENT
This work was supported by Medical University of Łódź grant nos 503/1–1089–02/503–1 and 503/1–1089–03/503–1. The authors thank MSc E. Lowczowski, a native speaker for language correction.
References
- 1.Hull MG, Glazener CM, Kelly NJ, Conway DI, Foster PA, Hinton RA, et al. Population study of causes, treatment, and outcome of infertility. Br Med J (Clin Res Ed) 1985;291:1693–1697. doi: 10.1136/bmj.291.6510.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bablok L, Dziadecki W, Szymusik I, Wolczynski S, Kurzawa R, Pawelczyk L, et al. Patterns of infertility in Poland – multicenter study. Neuro Endocrinol Lett. 2011;32:799–804. [PubMed] [Google Scholar]
- 3.Sanocka D, Kurpisz M. Infertility in Poland – present status, reasons and prognosis as a reflection of Central and Eastern Europe problems with reproduction. Med Sci Monit. 2003;9:SR16–20. [PubMed] [Google Scholar]
- 4.Irvine DS. Epidemiology and aetiology of male infertility. Hum Reprod. 1998;13(Suppl 1):33–44. doi: 10.1093/humrep/13.suppl_1.33. [DOI] [PubMed] [Google Scholar]
- 5.Safarinejad MR. Infertility among couples in a population – based study in Iran: prevalence and associated risk factors. Int J Androl. 2008;31:303–314. doi: 10.1111/j.1365-2605.2007.00764.x. [DOI] [PubMed] [Google Scholar]
- 6.WHO laboratory manual for the examination and processing of human semen; Geneva: World Health Organization; 2010. [Google Scholar]
- 7.Lahdetie J. Occupation – and exposure–related studies on human sperm. J Occup Environ Med. 1995;37:922–930. [PubMed] [Google Scholar]
- 8.Thonneau P, Bujan L, Multigner L, Mieusset R. Occupational heat exposure and male fertility: a review. Hum Reprod. 1998;13:2122–2125. doi: 10.1093/humrep/13.8.2122. [DOI] [PubMed] [Google Scholar]
- 9.Slowikowska–Hilczer J. Xenobiotics with estrogen or antiandrogrn action – disruptors of the male reproductive system. CEJM. 2006;3:205–227. [Google Scholar]
- 10.De Celis R, Pedron–Nuevo N, Feria–Velasco A. Toxicology of male reproduction in animals and humans. Arch Androl. 1996;37:201–218. doi: 10.3109/01485019608988523. [DOI] [PubMed] [Google Scholar]
- 11.Agarwal A, Sharma RK, Desai NR, Prabakaran S, Tavares A, Sabanegh E. Role of oxidative stress in pathogenesis of varicocele and infertility. Urology. 2009;73:461–469. doi: 10.1016/j.urology.2008.07.053. [DOI] [PubMed] [Google Scholar]
- 12.Tunc O, Bakos HW, Tremellen K. Impact of body mass index on seminal oxidative stress. Andrologia. 2011;43:121–128. doi: 10.1111/j.1439-0272.2009.01032.x. [DOI] [PubMed] [Google Scholar]
- 13.Henkel R, Schill WB. Sperm separation in patients with urogenital infections. Andrologia. 1998;30(Suppl 1):91–97. doi: 10.1111/j.1439-0272.1998.tb02832.x. [DOI] [PubMed] [Google Scholar]
- 14.Sanocka–Maciejewska D, Ciupinska M, Kurpisz M. Bacterial infection and semen quality. J Reprod Immunol. 2005;67:51–56. doi: 10.1016/j.jri.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 15.Schuppe HC, Meinhardt A, Allam JP, Bergmann M, Weidner W, Haidl G. Chronic orchitis: a neglected cause of male infertility? Andrologia. 2008;40:84–91. doi: 10.1111/j.1439-0272.2008.00837.x. [DOI] [PubMed] [Google Scholar]
- 16.Zini A, San Gabriel M, Baazeem A. Antioxidants and sperm DNA damage: a clinical perspective. J Assist Reprod Genet. 2009;26:427–432. doi: 10.1007/s10815-009-9343-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aitken RJ, De Iuliis GN, Finnie JM, Hedges A, McLachlan RI. Analysis of the relationships between oxidative stress, DNA damage and sperm vitality in a patient population: development of diagnostic criteria. Hum Reprod. 2010;25:2415–2426. doi: 10.1093/humrep/deq214. [DOI] [PubMed] [Google Scholar]
- 18.Agarwal A, Nallella KP, Allamaneni SS, Said TM. Role of antioxidants in treatment of male infertility: an overview of the literature. Reprod Biomed Online. 2004;8:616–627. doi: 10.1016/s1472-6483(10)61641-0. [DOI] [PubMed] [Google Scholar]
- 19.Smith R, Vantman D, Ponce J, Escobar J, Lissi E. Total antioxidant capacity of human seminal plasma. Hum Reprod. 1996;11:1655–1660. doi: 10.1093/oxfordjournals.humrep.a019465. [DOI] [PubMed] [Google Scholar]
- 20.Lewis SE, Sterling ES, Young IS, Thompson W. Comparison of individual antioxidants of sperm and seminal plasma in fertile and infertile men. Fertil Steril. 1997;67:142–147. doi: 10.1016/s0015-0282(97)81871-7. [DOI] [PubMed] [Google Scholar]
- 21.Sanocka D, Miesel R, Jedrzejczak P, Kurpisz MK. Oxidative stress and male infertility. J Androl. 1996;17:449–454. [PubMed] [Google Scholar]
- 22.Agarwal A, Sekhon LH. The role of antioxidant therapy in the treatment of male infertility. Hum Fertil (Camb) 2010;13:217–225. doi: 10.3109/14647273.2010.532279. [DOI] [PubMed] [Google Scholar]
- 23.Fisher HM, Aitken RJ. Comparative analysis of the ability of precursor germ cells and epididymal spermatozoa to generate reactive oxygen metabolites. J Exp Zool. 1997;277:390–400. doi: 10.1002/(sici)1097-010x(19970401)277:5<390::aid-jez5>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 24.Aitken RJ. The Amoroso Lecture. The human spermatozoon–a cell in crisis? J Reprod Fertil. 1999;115:1–7. doi: 10.1530/jrf.0.1150001. [DOI] [PubMed] [Google Scholar]
- 25.Fraczek M, Kurpisz M. The redox system in human semen and peroxidative damage of spermatozoa. Postepy Hig Med Dosw (online) 2005;59:523–534. [PubMed] [Google Scholar]
- 26.Gomez E, Buckingham DW, Brindle J, Lanzafame F, Irvine DS, Aitken RJ. Development of an image analysis system to monitor the retention of residual cytoplasm by human spermatozoa: correlation with biochemical markers of the cytoplasmic space, oxidative stress, and sperm function. J Androl. 1996;17:276–287. [PubMed] [Google Scholar]
- 27.Aziz N, Saleh RA, Sharma RK, et al. Novel association between sperm reactive oxygen species production, sperm morphological defects, and the sperm deformity index. Fertil Steril. 2004;81:349–354. doi: 10.1016/j.fertnstert.2003.06.026. [DOI] [PubMed] [Google Scholar]
- 28.Plante M, de Lamirande E, Gagnon C. Reactive oxygen species released by activated neutrophils, but not by deficient spermatozoa, are sufficient to affect normal sperm motility. Fertil Steril. 1994;62:387–393. doi: 10.1016/s0015-0282(16)56895-2. [DOI] [PubMed] [Google Scholar]
- 29.Galecka E, Jacewicz R, Mrowicka M, Florkowski A, Galecki P. Enzymy antyoksydacyjne – budowa, właściwości, funkcje [Antioxidative enzymes – structure, properties, functions] Pol Merkur Lekarski. 2008;25:266–268. [PubMed] [Google Scholar]
- 30.Peeker R, Abramsson L, Marklund SL. Superoxide dismutase isoenzymes in human seminal plasma and spermatozoa. Mol Hum Reprod. 1997;3:1061–1066. doi: 10.1093/molehr/3.12.1061. [DOI] [PubMed] [Google Scholar]
- 31.Scibior D, Czeczot H. Katalaza – budowa, właściwości, funkcje [Catalase: structure, properties, functions] Postepy Hig Med Dosw (Online) 2006;60:170–180. [PubMed] [Google Scholar]
- 32.de Lamirande E, Leclerc P, Gagnon C. Capacitation as a regulatory event that primes spermatozoa for the acrosome reaction and fertilization. Mol Hum Reprod. 1997;3:175–194. doi: 10.1093/molehr/3.3.175. [DOI] [PubMed] [Google Scholar]
- 33.Pfeifer H, Conrad M, Roethlein D, Kyriakopoulos A, Brielmeier M, Bronkamm GW, et al. Identification of a specific sperm nuclei selenoenzyme necessary for protamine thiol cross–linking during sperm maturation. FASEB J. 2001;15:1236–1238. [PubMed] [Google Scholar]
- 34.Yeung CH, Cooper TG, De Geyter M, De Geyter C, Rolf C, Kamischke A, Nieschlag E. Studies on the origin of redox enzymes in seminal plasma and their relationship with results of in–vitro fertilization. Mol Hum Reprod. 1998;4:835–839. doi: 10.1093/molehr/4.9.835. [DOI] [PubMed] [Google Scholar]
- 35.Wolski JK. Rola mikroelementów i witamin w niepłodności męskiej [Role of trace elements and vitamins in male infertility] Przegl Urol. 2011;(Suppl 4):1–4. [Google Scholar]
- 36.Park S, Park NY, Valacchi G, Lim Y. Calorie restriction with a high–fat diet effectively attenuated inflammatory response and oxidative stress–related markers in obese tissues of the high diet fed rats. Mediators Inflamm. 2012 doi: 10.1155/2012/984643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hogarth CA, Griswold MD. The key role of vitamin A in spermatogenesis. J Clin Invest. 2010;120:956–962. doi: 10.1172/JCI41303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Lamirande E, Gagnon C. Reactive oxygen species and human spermatozoa. I. Effects on the motility of intact spermatozoa and on sperm axonemes. J Androl. 1992;13:368–378. [PubMed] [Google Scholar]
- 39.Kessopoulou E, Powers HJ, Sharma KK, Pearson MJ, Russel JM, CookeID , et al. A double–blind randomized placebo cross–over controlled trial using the antioxidant vitamin E to treat reactive oxygen species associated male infertility. Fertil Steril. 1995;64:825–831. doi: 10.1016/s0015-0282(16)57861-3. [DOI] [PubMed] [Google Scholar]
- 40.Keskes–Ammar L, Feki–Chakroun N, Rebai T, et al. Sperm oxidative stress and the effect of an oral vitamin E and selenium supplement on semen quality in infertile men. Arch Androl. 2003;49:83–94. doi: 10.1080/01485010390129269. [DOI] [PubMed] [Google Scholar]
- 41.Suleiman SA, Ali ME, Zaki ZM, el–Malik EM, Nasr MA. Lipid peroxidation and human sperm motility: protective role of vitamin E. J Androl. 1996;17:530–537. [PubMed] [Google Scholar]
- 42.Boitani C, Puglisi R. Selenium, a key element in spermatogenesis and male fertility. Adv Exp Med Biol. 2008;636:65–73. doi: 10.1007/978-0-387-09597-4_4. [DOI] [PubMed] [Google Scholar]
- 43.Camejo MI, Abdala L, Vivas–Acevedo G, Lozano–Hernandez R, Angeli–Greaves M, Greaves ED. Selenium, copper and zinc in seminal plasma of men with varicocele, relationship with seminal parameters. Biol Trace Elem Res. 2011;143:1247–1254. doi: 10.1007/s12011-011-8957-5. [DOI] [PubMed] [Google Scholar]
- 44.Ursini F, Heim S, Kiess M, Maiorino M, Roveri A, Wissing J, Flohe L. Dual function of the selenoprotein PHGPx during sperm maturation. Science. 1999;285:1393–1396. doi: 10.1126/science.285.5432.1393. [DOI] [PubMed] [Google Scholar]
- 45.Colagar AH, Marzony ET, Chaichi MJ. Zinc levels in seminal plasma are associated with sperm quality in fertile and infertile men. Nutr Res. 2009;29:82–88. doi: 10.1016/j.nutres.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 46.Mankad M, Sathawara NG, Doshi H, Saiyed HN, Kumar S. Seminal plasma zinc concentration and alpha–glucosidase activity with respect to semen quality. Biol Trace Elem Res. 2006;110:97–106. doi: 10.1385/BTER:110:2:97. [DOI] [PubMed] [Google Scholar]
- 47.Fraga CG, Motchnik PA, Shigenaga MK, Helbock HJ, Jacob RA, Ames BN. Ascorbic acid protects against endogenous oxidative DNA damage in human sperm. Proc Natl Acad Sci USA. 1991;88:11003–11006. doi: 10.1073/pnas.88.24.11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dawson EB, Harris WA, Teter MC, Powell LC. Effect of ascorbic acid supplementation on the sperm quality of smokers. Fertil Steril. 1992;58:1034–1039. [PubMed] [Google Scholar]
- 49.Baker HW, Brindle J, Irvine DS, Aitken RJ. Protective effect of antioxidants on the impairment of sperm motility by activated polymorphonuclear leukocytes. Fertil Steril. 1996;65:411–419. doi: 10.1016/s0015-0282(16)58109-6. [DOI] [PubMed] [Google Scholar]
- 50.Lenzi A, Picardo M, Gandini L, Lombardzo F, Terminali O, Passi S, Dondero F. Glutathione treatment of dyspermia: effect on the lipoperoxidation process. Hum Reprod. 1994;9:2044–2050. doi: 10.1093/oxfordjournals.humrep.a138391. [DOI] [PubMed] [Google Scholar]
- 51.Irvine DS. Glutathione as a treatment for male infertility. Rev Reprod. 1996;1:6–12. doi: 10.1530/ror.0.0010006. [DOI] [PubMed] [Google Scholar]
- 52.Oeda T, Henkel R, Ohmori H, Schill WB. Scavenging effect of N–acetyl–L–cysteine against reactive oxygen species in human semen: a possible therapeutic modality for male factor infertility? Andrologia. 1997;29:125–131. doi: 10.1111/j.1439-0272.1997.tb00305.x. [DOI] [PubMed] [Google Scholar]
- 53.Etensel B, Ozkisacik S, Ozkara E, Karul A, Oztan O, Yazici M, Gursoy H. Dexpanthenol attenuates lipid peroxidation and testicular damage at experimental ischemia and reperfusion injury. Pediatr Surg Int. 2007;23:177–181. doi: 10.1007/s00383-006-1781-x. [DOI] [PubMed] [Google Scholar]
- 54.Ross C, Morriss A, Khairy M, Khalaf Y, Braude P, Coomarasamy A, El–Toukhy T. A systematic review of the effect of oral antioxidants on male infertility. Reprod Biomed Online. 2010;20:711–723. doi: 10.1016/j.rbmo.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 55.Gharagozloo P, Aitken RJ. The role of sperm oxidative stress in male infertility and the significance of oral antioxidant therapy. Hum Reprod. 2011;26:1628–1240. doi: 10.1093/humrep/der132. [DOI] [PubMed] [Google Scholar]
- 56.Zini A, Al–Hathal N. Antioxidant therapy in male infertility: fact or fiction? Asian J Androl. 2011;13:374–381. doi: 10.1038/aja.2010.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Showell MG, Brown J, Yazdani A, Stankiewicz MT, Hart RJ. Antioxidants for male subfertility. Cochrane Database Syst Rev. 2011:CD007411. doi: 10.1002/14651858.CD007411.pub2. [DOI] [PubMed] [Google Scholar]
- 58.Ko EY, Sabanegh ES., Jr The role of over–the–counter supplements for the treatment of male infertility – fact or fiction? J Androl. 2012;33:292–308. doi: 10.2164/jandrol.111.013730. [DOI] [PubMed] [Google Scholar]
- 59.Griveau JF, Le Lannou D. Effects of antioxidants on human sperm preparation techniques. Int J Androl. 1994;17:225–231. doi: 10.1111/j.1365-2605.1994.tb01247.x. [DOI] [PubMed] [Google Scholar]
- 60.Verma A, Kanwar KC. Effect of vitamin E on human sperm motility and lipid peroxidation in vitro. Asian J Androl. 1999;1:151–154. [PubMed] [Google Scholar]
- 61.Esteves SC, Sharma RK, Thomas AJ, Jr, Agarwal A. Cryopreservation of human spermatozoa with pentoxifylline improves the post–thaw agonist–induced acrosome reaction rate. Hum Reprod. 1998;13:3384–3389. doi: 10.1093/humrep/13.12.3384. [DOI] [PubMed] [Google Scholar]
- 62.Bell M, Wang R, Hellstrom WJ, Sikka SC. Effect of cryoprotective additives and cryopreservation protocol on sperm membrane lipid peroxidation and recovery of motile human sperm. J Androl. 1993;14:472–478. [PubMed] [Google Scholar]
- 63.Park NC, Park HJ, Lee KM, Shin DG. Free radical scavenger effect of rebamipide in sperm processing and cryopreservation. Asian J Androl. 2003;5:195–201. [PubMed] [Google Scholar]
- 64.Askari HA, Check JH, Peymer N, Bollendorf A. Effect of natural antioxidants tocopherol and ascorbic acids in maintenance of sperm activity during freeze–thaw process. Arch Androl. 1994;33:11–15. doi: 10.3109/01485019408987797. [DOI] [PubMed] [Google Scholar]
- 65.Li Z, Lin Q, Liu R, Xiao W, Liu W. Protective effects of ascorbate and catalase on human spermatozoa during cryopreservation. J Androl. 2010;31:437–444. doi: 10.2164/jandrol.109.007849. [DOI] [PubMed] [Google Scholar]
- 66.Martinez–Soto JC, de DiosHourcade J, Gutierrez–Adan A, Landeras JL, Gadea J. Effect of genistein supplementation of thawing medium on characteristics of frozen human spermatozoa. Asian J Androl. 2010;12:431–441. doi: 10.1038/aja.2009.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Taylor K, Roberts P, Sanders K, Burton P. Effect of antioxidant supplementation of cryopreservation medium on post–thaw integrity of human spermatozoa. Reprod Biomed Online. 2009;18:184–189. doi: 10.1016/s1472-6483(10)60254-4. [DOI] [PubMed] [Google Scholar]
- 68.Nallella KP, Sharma RK, Allamaneni SS, Aziz N, Agarwal A. Cryopreservation of human spermatozoa: comparison of two cryopreservation methods and three cryoprotectants. Fertil Steril. 2004;82:913–918. doi: 10.1016/j.fertnstert.2004.02.126. [DOI] [PubMed] [Google Scholar]
- 69.Kattera S, Chen C. Short coincubation of gametes in in vitro fertilization improves implantation and pregnancy rates: a prospective, randomized, controlled study. Fertil Steril. 2003;80:1017–1021. doi: 10.1016/s0015-0282(03)01154-3. [DOI] [PubMed] [Google Scholar]
- 70.Agarwal A, Allamaneni SS, Nallella KP, George AT, Mascha E. Correlation of reactive oxygen species levels with the fertilization rate after in vitro fertilization: a qualified meta–analysis. Fertil Steril. 2005;84:228–231. doi: 10.1016/j.fertnstert.2004.12.057. [DOI] [PubMed] [Google Scholar]
- 71.Commission Directive 2008/100/EC of 28 October 2008 amending Council Directive 90/496/EEC on nutrition labelling for foodstuffs as regards recommended daily allowance, energy conversion factors and definitions. Official Journal of the European Union. 2008 [Google Scholar]