Abstract
Introduction
The assessment of risk of recurrence and progression of bladder cancer (BC) is still rather difficult. We decided to check the rates of the changes mentioned above in the group of the Polish patients after a year–long observation and next to compare them with the results calculated in the European Organisation of Research and Treatment of Cancer (EORTC) risk tables.
Methods
The tested group consisted of 91 patients who underwent transurethral resection of bladder tumour (TURBT). When being diagnosed, 60 cases were in the pTa clinical stage, whereas 30 cases were in T1. The coexisting carcinoma in situ (CIS) was observed in four cases. On the basis of the scores obtained from the EORTC tables, the patients were divided into the groups of low, intermediate or high risk of disease recurrence and progression.
Results
Recurrence was noticed in 23 patients (25%), while progression was observed in 11 patients (12.1%). The rate of the observed recurrences proved to be lower than it had been predicted in all the groups, except for one of the intermediate–risk group (score 1– 4). Moreover, the rate of the progressions predicted according to the EORTC risk tables was higher in all the risk groups.
Conclusions
It can be noticed that the rate of real recurrences is lower than expected, whereas the rate of the observed progressions is overestimated. Partly, it could be the result of using a relatively small group of patients for observation and applying a different method of treatment.
Keywords: bladder cancer, EORTC risk tables, recurrence, progression
INTRODUCTION
Bladder Cancer (BC) is the nineth most common cancer in the world. In Europe, the highest rates are observed in the south, west and north, whereas the lowest ones are in the eastern part [1]. Poland belongs to the countries of very high rates of morbidity and mortality from this cancer, especially among men (the third reason for morbidity and the sixth one for mortality) [2]. Undoubtedly, smoking cigarettes is one of the most serious risk factors leading to the development of this disease (in comparison with non–smokers, the risk is 2–4 times higher) [3]. According to the epidemiologic research results, 50% of men suffering from this disease are or were active smokers, whereas among women, the percentage is 35 [1, 4]. Quitting smoking causes the decrease in the risk of BC development by 30%, after 1–4 years, and by about 60%, after 25 years [5]. Another factor increasing the risk of contracting this disease is the exposure to aromatic amines and polycyclic aromatic hydrocarbons.
According to the histopathological classification, the definite majority of BC cases are papillary cancers, while, according to the disease clinical stage, they are non–muscle invasive cases (stage Ta, T1). Despite applying some treatment, about 50% of these cases suffer recurrence of non–invasive changes, whereas in about 20% of these cases, there is progression to higher clinical stages. Muscle invasive tumours have a poor prognosis (50% 5–year survival rates) and require radical therapy if cure is to be achieved. In non–muscle invasive bladder cancer, approximately 70% present as Ta lesion, 20% as T1 lesion and 10% as carcinoma in situ (CIS) [6]. NMIBC constitutes a very heterogeneous group of cancers with different disease courses [2]. For example, low–grade changes are characterized by the low recurrence rate and by low progression. On the other hand, the quite considerable recurrence rate, high progression and worse outcome are observed with high–grade changes. Staging, grading and risk stratification are essential for determining the most appropriate management strategies for NMIBC based on recurrence and progression.
The system of risk assessment by means of the The European Organisation for Research and Treatment of Cancer (EORTC) tables was based on six clinical and histopathological parameters: T category, grade, concomitant of CIS, the number of tumours, tumour size and prior recurrence rate [7]. It was described in detail by Sylvester et al. in 2006. It involves classifying patients into one of the low–, intermediate– or high–risk groups on the basis of the scores given in the six parameters mentioned above. The electronic version of the system is also available on: http://www.eortc.be/tools/bladdercalculator/.
The purpose of this paper was to compare the rate of the observed disease recurrence and progression cases (after a one–year treatment) with the scores obtained from the EORTC risk tables as well as proving the usefulness of this tool.
MATERIALS AND METHODS
Our tests were of the retrospective type. The group of 91 patients included 83 men and 8 women. The detailed description of the clinical and histopathological data used in assessing recurrence and progression risk is shown in Table 1. Tumours was excised at the Urological Department of the John Paul II Regional Hospital in Belchatow between 2006 and 2009. Patients were treated with transurethral resection of the bladder tumour (TURBT) and diagnosed with stage Ta or stage T1 urothelial carcinoma of the bladder. All studied tumours were graded according to The World Health Organisation (WHO) classification and staged according to the TNM pathological staging system respectively by two histopathologists [8]. The population studied was typical of BC populations with the majority of patients being male and having history of smoking (Table 2, Figure 1). Follow–up cystoscopies were done every 3 months for the first 2 years in all of the patients. The size of the tumour was estimated by means of measurement taken by resector loop. Patients with multifocal tumours (diameter more than 3 cm) or patients with changes G2, G3 were qualified for chemotherapy (from 6 to 8 doxorubicin applications, which usually started 2–3 days after the first TURBT). Later the therapy were continued on the result. Recurrence was determined by the cystoscopy and UroVysis test (Vysis) with a possible biopsy for the first two years [9, 10]. The multitarget (multicolor) Fluorescence in situ hybridization (FISH) urine assay has been shown to be more sensitive than urine cytology [9, 10]. The presence of CIS was assessed on the base of the biopsy of suspicious changes in the cystoscopy and TURBT. All patients have given written informed consent. The ethical committee of the Medical University of Łódź approved the project (permission No: RNN/99/11/KE).
Table 1.
Predicted versus actual 1-year recurrence and progression rates
| Recurrence group | EORTC's recurrence prediction (score) | No. of patients | Observed 1-year recurrence proportion no. (%) | Progression group | EORTC's progression prediction (score) | No. of patients | Observed 1-year progression proportion no. (%) |
|---|---|---|---|---|---|---|---|
| Low | 15% risk (0) | 29 | 4 (13.7%) | Low | 0.2% risk (0) | 37 | 1 (2.7%) |
| Intermediate | 24% risk (1-4) | 40 | 12 (30%) | Intermediate | 1% risk (2-6) | 35 | 5 (14.3%) |
| Intermediate | 38% risk (5-9) | 20 | 6 (30%) | Intermediate | 5% risk (7-13) | 16 | 4 (25%) |
| High | 61% risk (10-17) | 2 | 1 (50%) | High | 17% risk (14-23) | 3 | 1 (33.3%) |
| Total | 91 | Total | 91 |
Table 2.
Patients characteristics, recurrence and progression rates
| No. of patients (%) | No. of recurrence (%) | No. of progression (%) | |
|---|---|---|---|
| Total number of patients | 91 | 23 | 11 |
| Age | |||
| <60 | 33 (36.3) | 5 (15.2) | 1 (3.0) |
| 61-70 | 20 (22.0) | 7 (35.0) | 4 (20.0) |
| 71-80 | 25 (27.5) | 9 (36.0) | 5 (20.0) |
| >80 | 13 (14.2) | 2 (15.4) | 1 (7.7) |
| Unknown | |||
| Gender | |||
| Male | 83 (91.2) | 22 (26.5) | 11 (13.3) |
| Female | 8 (8.8) | 1 (12.5) | 0 |
| Number of tumors | |||
| Single | 64 (71.4) | 6 (9.4) | 10 (15.6) |
| 2 up to 7 | 25 (27.5) | 17 (68.0) | 1 (4.0) |
| More than 8 | 1 (1.1) | 0 | 0 |
| Size of tumors | |||
| < 3 cm | 59 (64.9) | 17 (28.8) | 8 (13.6) |
| > 3 cm | 32 (35.1) | 6 (18.8) | 3 (9.4) |
| PT category | |||
| Ta | 60 (65.9) | 17 (28.3) | 8 (13.3) |
| T1 | 31 (34.1) | 6 (19.4) | 3 (9.7) |
| Presence of CIS | |||
| No | 87 (95.6) | 21 (24.1) | 3 (75.0) |
| Yes | 4 (4.4) | 2 (50.0) | 8 (9.2) |
| Grade of tumor | |||
| G1 | 49 (53.8) | 10 (20.4) | 4 (8.2) |
| G2 | 25 (27.5) | 9 (36.0) | 4 (16.0) |
| G3 | 17 (18.7) | 4 (23.5) | 3 (17.7) |
Figure 1.
Clinical and pathological features of the individual tumors in this report.
RESULTS
The mean age of patients was 65 years (median 66 ±12), and their median follow–up duration was 32 months (range, 28 to 46 months). Tumours characteristics are summarized in Tables 2 and 3. After a one–year treatment, recurrence was observed in 23 patients (25%), whereas progression in 11 patients (12.1%). These data were different from the predicted scores taken from the EORTC risk tables. As far as recurrence is concerned, the predicted score was higher in all the groups, except for the low–risk group (results presented in Table 1). As for progression, the predicted rates were higher in all of the groups after 1–year treatment (2.2% vs. 0.2%, 14.3% vs. 1%, 25% vs. 5% and 33.3% vs. 17%).
Table 3.
Patient and tumor characteristics compering to EORTC and other group results
| Study Group No. (%) | EORTC Group No. (%) [7] | CUETO Group No. (%) [19] | UK Group No. (%) [21] | |
|---|---|---|---|---|
| Total number of patients | 91 | 2596 | 1062 | 109 |
| Age | ||||
| <60 | 33 (36.3) | 859 (33.1) | 331 (33.2) | 29 (26.6) |
| 61–70 | 20 (22.0) | 890 (34.3) | 394 (37.1) | 35 (32.1) |
| 71–80 | 25 (27.5) | 690 (26.6) | 301 (28.3) | 31 (28.4) |
| >80 | 13 (14.2) | 118 (4.5) | 36 (3.4) | 13 (11.9) |
| unknown | 39 (1.5) | 1 (0.9) | ||
| Gender | ||||
| Male | 83 (91.2) | 2044 (78.7) | – | 84 (77.1) |
| Female | 8 (8.8) | 561 (19.8) | – | 25 (22.9) |
| Number of tumors | ||||
| single | 64 (71.4) | 1465 (56.4) | 535 (50.4) | 64 (58.7) |
| 2 up to 7 | 25 (27.5) | 836 (32.2) | 438 (41.3) | 32 (29.3) |
| more than 8 | 1 (1.1) | 255 (9.8) | 89 (8.4) | 13 (11.9) |
| Size of tumors | ||||
| < 3 cm | 59 (64.9) | 2087 (80.4) | 581 (57.4) | 64( 58.6) |
| > 3 cm | 32 (35.1) | 464 (17.9) | 481 (45.3) | 43 (39.4) |
| PT category | ||||
| Ta | 60 (65.9) | 1451 (55.9) | 214 (20.2) | 78 (71.5) |
| T1 | 31 (34.1) | 1108 (42.7) | 848 (79.8) | 31 (28.5) |
| Presence of CIS | ||||
| No | 87 (95.6) | 2440 (94.0) | 982 (92.5) | 100 (91.7) |
| Yes | 4 (4.4) | 113 (4.4) | 80 (7.5) | 9 (8.3) |
| Grade of tumor | ||||
| G1 | 49 (53.8) | 1121 (43.2) | 167 (15.7) | 98 (89.9) |
| G2 | 25 (27.5) | 1139 (43.9) | 629 (59.2) | |
| G3 | 17 (18.7) | 271 (10.4) | 266 (25) | 11 (10.1) |
| Follow–up | ||||
| median | 2.8 | 3.9 | – | 5 |
| maximum | 3.8 | 14.8 | – | 5 |
| Recurrence | ||||
| No | 68 (74.7) | 1356 (52.2) | 706 (66.5) | 40 (36.7) |
| Yes | 23 (25.3) | 1240 (47.8) | 356 (33.5) | 69 (63.3) |
| Progression | ||||
| No | 80 (87.9) | 2317 (89.3) | – | 95 (87.2) |
| Yes | 11 (12.1) | 279 (10.7) | – | 14 (12.8) |
| Survival | ||||
| Alive | 84 (89.0) | 1743 (67.1) | – | 98 (90) |
| Dead | 10 (11.0) | 279 (32.9) | – | 11 (10) |
DISCUSSION
In recent years, a lot of attention has been focused on finding good markers for predicting recurrence and progression of bladder cancer [11, 12]. Taking the clinical parameters into account, the most important indicators are multiplicity, tumour stage, tumour grade and tumour size [13]. Also, the result of the first control cystoscopy, carried out 3 months after the diagnosis, must be carefully considered as incomplete resection, oversight of changes in the carcinoma in situ stage, reimplantation of tumour cells as well as a new change influence the further disease course [14]. In low–risk NMIBC the primary treatment which should be applied after transurethral resection (TUR) is immediate instillation, whereas in the intermediate–risk group (40–50% of all NMIBC), a series of intravesical instillation is given postoperatively. A second TUR has to be performed in the case of high–grade malignancy or incomplete resection. When the one single installation of chemotherapeutic agent is given in 24h after resection almost 50% of decrease in recurrence rate is observed, however, this effect does not last if we take a five–year observation period into consideration [15, 16].
It needs to be emphasized that the analysis which was made showed the rates of the observed recurrences only vary slightly in the group of the lowest risk of recurrence. In the other groups the differences are at the level of 11% and they grow bigger in groups of higher risk. In recent studies van Rhijn et al. successfully validated the EORTC risk tables in the group of the Dutch patients with NMIBC (230 patients) [17]. Similarly, Seo et al. and Fernandez–Gomez et al. validated the tables, respectively in the group of 251 Korean patients and in the group of 417 Spanish patients (the last group confirmed the accuracy of the EORTC tables only in predicting recurrence) [18, 19]. However, like in our analysis, the most common problem here is that the majority of patients are classified as the intermediate–risk group (60 out of 91 patients in the case of recurrence and 51 out of 91 patients in the case of progression in the Polish patients’ group). Some similar results were observed by the Japanese scientists. In a cohort of 592 patients, over 90% of them were put into the intermediate–risk group [20]. This overwhelming majority would indicate that all those patients should be treated in the same way. It does not reflect the reality and it poses a serious limitation in the use of the EORTC tables for assessing the risk of BC recurrence and progression. As for progression, the discrepancies between rates are also considerable (from 2.5% to 20% in our cohort). Pillali et al. also noticed the overestimation of the rate of predicted progression in intermediate and high risk group after 5 years (10% vs. 6%, 5.7% vs. 1%, 38.5% vs. 5% and 33.3% vs. 17%) [21]. Our differences after one year are bigger. It needs to be mentioned that poor prognosis depends on a bigger number of factors than just the six ones included in the EORTC risk tables. Others factors like: concomitant CIS, deep lamnia propria invasion, prostatic involvement, large or multifocal tumours, persistent T1G3 on repeat TUR, and persistent T1G3 or CIS after bacillus Calmette–Guerin (BCG) treatment should also be considered [6, 22]. The retrospective analysis of high–grade T1 patients treated with initial intravesical therapy proved that about 30% of the patients ultimately need cystectomy, and about 30% of them die of BC, regardless of cystectomy being applied or not [14]. Progression risk continues throughout patients’ lives and late progression is not infrequent. High–risk NMIBC tumours should be treated effectively in order to prevent frequent recurrences. BCG instillation is the primary treatment as chemotherapy does not prevent progression [23].
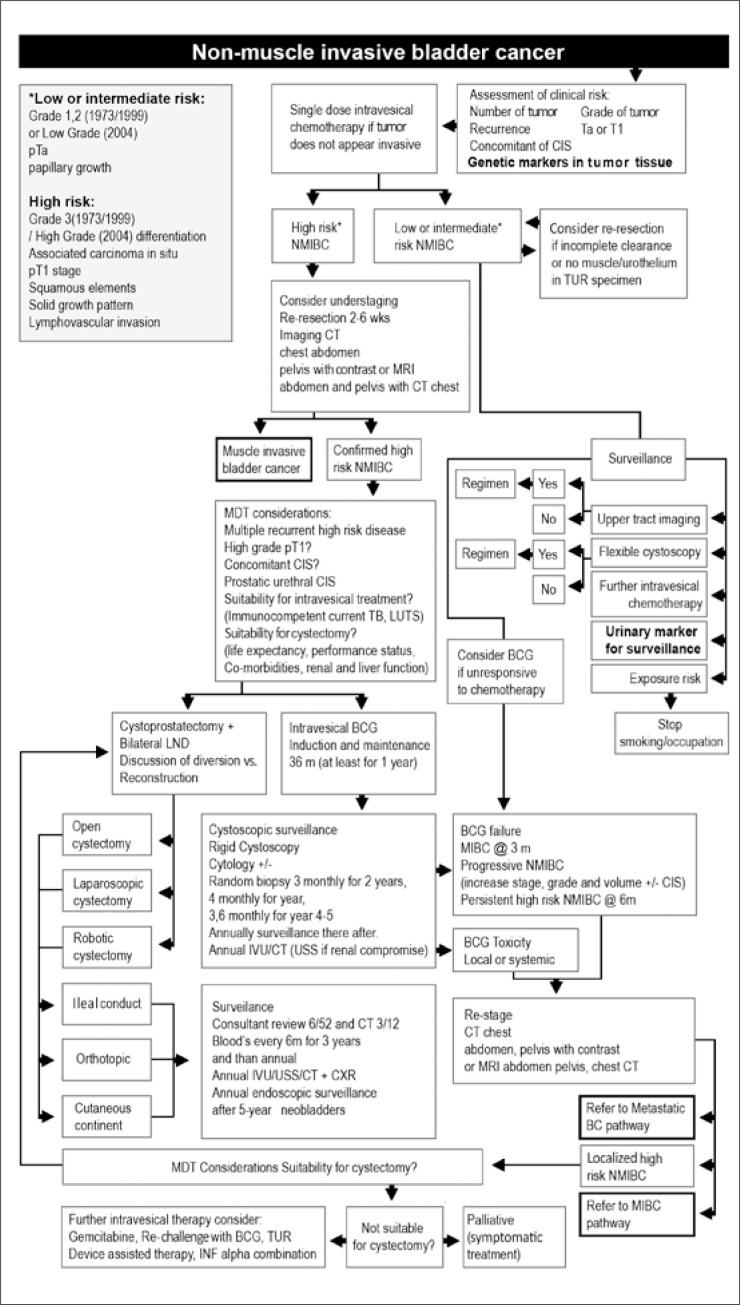
Recurrence, progression to higher clinical stages and metastases are the biggest problems related to cancers. At present, the decision about how a patient should be treated is mainly dependent on the results of histopathological tests. In 2004, the WHO proposed a new tumour grade classification. Unfortunately, the new system has been accepted despite of the lack of clinical evidence for its usefulness and proper studies with long–term follow–up to asses prognostic value and reproducibility. Therefore, according to the European Association of Urology (EAU) guidelines, both systems (the old one from 1973 and the new one from 2004) can be used in practice. It is known that the same histopathological assessment is likely to be repeated if made by the same person, but assessments sometimes tend to differ considerably when a number of people assess the same sample. The poor reproducibility of pathologic stage and grade is a recognized problem. Even in the grade case results can be different in 40–60% of cases [6, 24, 25]. Several nomograms have been estabilished to provide individualized risk assessment but the biological potential of bladder tumour is not sufficiently described by its histological classification [24]. Early recurrence is connected with a worse prognosis, which was pointed out in one of the scientific dissertations [26]. Therefore, more and more attention is given to biological markers with which there are no such big differences. Recently, there has been more and more information about biological markers which, together with the current research, would enable easier differentiation of patients for disease recurrence and progression [6, 7, 11, 12, 22, 23]. One of the study clearly demonstrated superior reproducibility of them (100%) [17]. Fibroblast growth factor receptor 3 (FGFR3) gene mutations are ones of the most frequently tested molecular biology markers in BC, in DNA taken both from tumour and from urine sediment cells. It has been shown that there is a link between these mutations in tumour and a relatively better prognosis [27, 28]. However, the information about this issue is still not very clear as the combination with a different marker showed the correlation with a worse prognosis [17]. The influence on recurrence rate is also controversial. Recent studies presented only slightly higher risk of recurrence with the presence of the mutation whereas in previous results, FGFR3 mutations was assumed to increase the recurrence rate [18, 29]. Another well tested marker are 9 chromosome deletions and loss of heterozygosity. These are mainly the changes in NMIBC and it is possible to determine their appearance both in tumour DNA and in DNA isolated from urine sediment [30, 31, 32]. They are observed even in 60% of cases (also confirmed by our earlier research) and can be used for detection and monitoring of the disease. On the other hand, a definitely negative outcome appears in the case of TP53 gene mutation [22, 33]. From our point of view, detection of these well tested markers, where correlation with the disease course was observed, should be included in the treatment plan for BC cases, as we propose in Figure 2. Costs of such analyses are currently much lower than several years ago, and certainly they are much lower than cystoscopy costs. Moreover, unlike cystoscopy, examinations carried out for urine analyses are noninvasive ones, which is an additional advantage.
Figure 2.
Non–muscle invasive bladder cancer pathways adapted from World J Urol. 2011 29: 291–301. Abbreviations: BCG – bacillus calmette–guérin, CIS – carcinoma in situ, CXR – chest X–ray, INF – interferon–alpha, IVU – intravenous urogram, LUTS – lower urinary tract symptoms, LND – lymph node dissection, MIBC – muscle invasive bladder.cancer, NMIBC – non–muscle invasive bladder cancer, Prostatic TCC – transitional cell carcinoma of the prostate, TB – tuberculosis [34].
CONCLUSIONS
To sum up, the analysis showed that recurrence risk rates were overestimated and progression risk rates were underestimated in almost all risk groups among the Polish patients with NMIBC. The large majority of patients were also classified as the intermediate–risk group, which is not helpful if we have to decide about further treatment and about disease monitoring. In our view, the usefulness of the tables for risk assessment is limited. However, we have to admit that there were limitations of our research such as a small number of patients in our tested group and the assessment which was made after only one–year treatment. It is necessary to mention that the tables were prepared using the group of patients treated with the old type of chemotherapy, which could have resulted in appearance of the observed differences. The treatment methods like instillation immediately after TUR or BCG therapy, which we mentioned, were not taken into consideration while working on these tables.
ACKNOWLEDGEMENT
This work was supported by the Ministry of Science and Higher Education, Poland (Grant No 617/MOB/2011/0) and by the State Committee for Scientific Research, Poland (Grant No 2P05C 076 30).
References
- 1.Burger M, Catto JWF, Dalbagni G, Grossman HB, Herr H, Karakiewicz P, et al. Epidemiology and risk factors of urothelial bladder cancer. Eur Urol. 2013;63:234–241. doi: 10.1016/j.eururo.2012.07.033. [DOI] [PubMed] [Google Scholar]
- 2.Didkowska J, Wojciechowska U, Zatoński W. Polish National Cancer Registry. Warsaw: Department of Epidemiology and Cancer Prevention; 2011. Cancer in Poland in 2009; p. 13. [Google Scholar]
- 3.Kirkali Z, Chan T, Manoharan M, Algaba F, Busch C, Cheng L, et al. Bladder cancer: epidemiology, staging and grading, and diagnosis. Urology. 2005;66:4–34. doi: 10.1016/j.urology.2005.07.062. [DOI] [PubMed] [Google Scholar]
- 4.Thomas F, Rosario DJ, Rubin N, Goepel JR, Abbod MF, Catto JW. The long term outcome of treated high–risk nonmuscle invasive bladder cancer. Cancer. 2012;118:5525–5534. doi: 10.1002/cncr.27587. [DOI] [PubMed] [Google Scholar]
- 5.Brennan P, Bogillot O, Cordier S, Greiser E, Schill W, Vineis O, et al. Cigarette smoking and bladder cancer in men: a pooled analysis of 11 casecontrol studies. Int J Cancer. 2000;86:289–294. doi: 10.1002/(sici)1097-0215(20000415)86:2<289::aid-ijc21>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 6.Van Rhijn B, Burger M, Lotan Y, Solsona E, Stief CG, Sylvester RJ, et al. Recurrence and progression of disease in non–muscle–invasive bladder cancer: from epidemiology to treatment. Eur Urol. 2009;56:430–442. doi: 10.1016/j.eururo.2009.06.028. [DOI] [PubMed] [Google Scholar]
- 7.Sylvester RJ, van der Meijden APM, Oosterlinck W, Witjes JA, Bouffioux C, Denis L, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol. 2006;49:466–477. doi: 10.1016/j.eururo.2005.12.031. [DOI] [PubMed] [Google Scholar]
- 8.Mostofi K, Sobin LH. International histological classification of tumors. Geneva, Switzerland: World Health Organization; 1973. Histologic typing of urinary bladder tumors. [Google Scholar]
- 9.Hajdinjak T. UroVysion FISH test for detecting urothelial cancers: Meta–analysis of diagnostic accuracy and comparison with urinary cytology testing. Urol Oncol. 2008;26:646–651. doi: 10.1016/j.urolonc.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 10.Kipp BR, Tanasescu M, Else TA, Bryant SC, Karnes RJ, Sebo TJ, et al. Quantitative fluorescence in situ hybridization and its ability to predict bladder cancer recurrence and progression to muscle–invasive bladder cancer. J Mol Diagn. 2009;11:148–154. doi: 10.2353/jmoldx.2009.080096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Rhijn BWG, Liu L, Vis AN, Bostrom PJ, Zuiverloon, Fleshner NE, et al. Prognostic value of molecular markers, substage and European Organisation for the Research and Treatment of Cancer risk scores in primary T1 bladder cancer. BJUInt. 2012;110:1169–1176. doi: 10.1111/j.1464-410X.2012.10996.x. [DOI] [PubMed] [Google Scholar]
- 12.Zuiverloon TCM, Tjin SS, Busstra M, Bangma CH, Boevé ER, Zwarthoff WE. Optimization of Nonmuscle Invasive Bladder Cancer Recurrence Detection Using a Urine Based FGFR3 Mutation Assay. J Urol. 2011;186:707–712. doi: 10.1016/j.juro.2011.03.141. [DOI] [PubMed] [Google Scholar]
- 13.Shariat SF, Palapattu GS, Karakiewicz PI, Rogers CG, Vazina A, Bastian PJ, et al. Concomitant carcinoma in situ is a feature of aggressive disease in patients with organ confined TCC at radical cystectomy. Eur Urol. 2007;51:152–160. doi: 10.1016/j.eururo.2006.08.037. [DOI] [PubMed] [Google Scholar]
- 14.Solsona E, Iborra I, Dumont R, Rubio–Briones J, Casanova J, Almenar S. The 3–month clinical response to intravesical therapy as a predictive factor for progression in patients with high risk superficial bladder cancer. J Urol. 2000;164:685–689. doi: 10.1097/00005392-200009010-00016. [DOI] [PubMed] [Google Scholar]
- 15.Oosterlinck W, Kurth KH, Schroder F, Bultinck J, Hammond B, Sylvester R. A prospective European Organization for Research and Treatment of Cancer Genitourinary Group randomized trial comparing transurethral resection followed by a single intravesical instillation of epirubicin or water in single stage Ta, T1 papillary carcinoma of the bladder. J Urol. 1993;149:749–752. doi: 10.1016/s0022-5347(17)36198-0. [DOI] [PubMed] [Google Scholar]
- 16.Burger M, van der Aa MNM, van Oers JMM, van der Kwast TH, Steyeberg EC, et al. Prediction of progression of non–muscle–invasive bladder cancer by WHO 1973 and 2004 grading and by FGFR3 mutation status: a prospective study. Eur Urol. 2008;54:835–844. doi: 10.1016/j.eururo.2007.12.026. [DOI] [PubMed] [Google Scholar]
- 17.Van Rhijn BWG, Zuiverloon TCM, Vis AN, Radvanyi F, van Leenders G, Ooms BCM, et al. Molecular grade (FGFR3/MIB–1) and EORTC risk score are predictive in primary non–muscle– invasive bladder cancer. Eur Urol. 2010;58:433–441. doi: 10.1016/j.eururo.2010.05.043. [DOI] [PubMed] [Google Scholar]
- 18.Seo KW, Kim BH, Park CH, Kim C, Chang HS. The Efficacy of the EORTC Scoring System and Risk Tables for the Prediction of Recurrence and Progression of Non–Muscle–Invasive Bladder Cancer after Intravesical Bacillus Calmette–Guerin Instillation. Korean J Urol. 2010;51:165–170. doi: 10.4111/kju.2010.51.3.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fernandez-Gomez J, Madero R, Solsona E, Unda M, Martinez–Piňeirol, Ojea A, et al. The EORTC Tables Overestimate the Risk of Recurrence and Progression in Patients with Non–Muscle–Invasive Bladder Cancer Treated with Bacillus Calmette–Guerin: External Validation of the EORTC Risk Tables. Eur Urol. 2011;60:423–430. doi: 10.1016/j.eururo.2011.05.033. [DOI] [PubMed] [Google Scholar]
- 20.Sakano S, Matsuyama H, Takai K, Yoshihiro S, Kamiryo Y, Shirataki S, et al. Risk group stratification to predict recurrence after transurethral resection in Japanese patients with stage Ta and T1 bladder tumors: validation study on the European Association of Urology guidelines. BJU Int. 2010;107:1598–1604. doi: 10.1111/j.1464-410X.2010.09850.x. [DOI] [PubMed] [Google Scholar]
- 21.Pillai R, Wang D, Mayer EK, Abel P. Do Standardised Prognostic Algorithms Reflect Local Practice? Application of EORTC Risk Tables for Non–Muscle Invasive (pTa/pT1) Bladder Cancer Recurrence and Progression in a Local Cohort. Scientific World Journal. 2011;11:751–759. doi: 10.1100/tsw.2011.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hernandez S, Lopez–Knowles E, Lloreta J, Kogevinas M, Jaromillo R, Amorós, et al. FGFR3 and Tp53 mutations in T1G3 transitional bladder carcinomas: independent distribution and lack of association with prognosis. Clin Cancer Res. 2005;11:5444–5450. doi: 10.1158/1078-0432.CCR-05-0122. [DOI] [PubMed] [Google Scholar]
- 23.Malmstrom P-U, Sylvester RJ, Crawford DE, Frierdich M, Krege S, Rinrala E, et al. An individual patient data meta–analysis of the long–term outcome of randomized studies comparing intravesical mitomycin C versus bacillus Calmette–Gue′ rin for non–muscle–invasive bladder cancer. Eur Urol. 2009;56:247–256. doi: 10.1016/j.eururo.2009.04.038. [DOI] [PubMed] [Google Scholar]
- 24.van Rhijn BWG, van Leenders GJLH, Ooms ECM, Kirkels WJ, Zlotta AR, Boevé, et al. The pathologist's mean grade is constant and individualizes the prognostic value of bladder cancer grading. Eur Urol. 2010;57:1052–1057. doi: 10.1016/j.eururo.2009.09.022. [DOI] [PubMed] [Google Scholar]
- 25.MacLennan GT, Kirkali Z, Cheng L. Histologic grading of noninvasive papillary urothelial neoplasms. Eur Urol. 2007;51:889–898. doi: 10.1016/j.eururo.2006.10.037. [DOI] [PubMed] [Google Scholar]
- 26.Nuhn P, May M, Sun M, Fritsce HM, Brookman–May S, Buchner A, et al. External Validation of Postoperative Nomograms for Prediction of All–Cause Mortality, Cancer–Specific Mortality, and Recurrence in Patients With Urothelial Carcinoma of the Bladder. Eur Urol. 2012;61:58–64. doi: 10.1016/j.eururo.2011.07.066. [DOI] [PubMed] [Google Scholar]
- 27.van Rhijn BWG, van der Kwast TH, Liu L, Fleshner NE, Bostrom PJ, Vis AN, et al. The FGFR3 Mutation is Related to Favorable pT1 Bladder Cancer. J Urol. 2012;187:310–314. doi: 10.1016/j.juro.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 28.Zuiverloon TC, van der Aa MN, van der Kwast TH, Steyeberg EW, Lingsma HF, Bangma CH, Zwarthoff WE. Fibroblast growth factor receptor 3 mutation analysis on voided urine for surveillance of patients with low–grade non–muscle–invasive bladder cancer. Clin Cancer Res. 2010;16:3011–3018. doi: 10.1158/1078-0432.CCR-09-3013. [DOI] [PubMed] [Google Scholar]
- 29.Van Oers JMM, Zwarthoff EC, Rehman I, Azzouzi A-R, Cussenot O, Meuth M, et al. FGFR3 mutations indicate better survival in invasive upper urinary tract and bladder tumors. Eur Urol. 2009;55:650–658. doi: 10.1016/j.eururo.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 30.Collin–Chavagnac D, Marçais C, Billon S, Descotes F, Piaton E, Decassin M, et al. Quantitative Loss of Heterozygosity Analysis for Urothelial Carcinoma Detection and Prognosis. Urology. 2010;76:515. doi: 10.1016/j.urology.2009.11.046. [DOI] [PubMed] [Google Scholar]
- 31.Traczyk M, Borkowska E, Jędrzejczyk A, Pietrusiński M, Rożniecki M, Marks P, Kałużeski B. Detection of loss of heterozygosity in patients with urinary bladder carcinoma: neoplastic tissue vs. urine sediment cells. CEJU. 2011;64:164–167. doi: 10.5173/ceju.2011.03.art16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van der Aa MN, Zwarthoff EC, Steyerberg EW, Boogaard MW, Nijsen Y, van der Keur KA, et al. Microsatellite analysis of voided–urine samples for surveillance of low–grade nonmuscle– invasive urothelial carcinoma: feasibility and clinical utility in a prospective multicenter study (Cost–Effectiveness of Follow–Up of Urinary Bladder Cancer trial [CEFUB]) Eur Urol. 2009;55:659–67. doi: 10.1016/j.eururo.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 33.Netto GJ. Molecular biomarkers in urothelial carcinoma of the bladder: are we there yet? Nature Rev Urol. 2012;9:41–51. doi: 10.1038/nrurol.2011.193. [DOI] [PubMed] [Google Scholar]
- 34.MacLennan SJ, MacLennan SJ, Imamura M, Omar IM, Vale L, Lam T, et al. Urological cancer care pathways: development and use in the context of systematic reviews and clinical practice guidelines. World J Urol. 2011;29:291–301. doi: 10.1007/s00345-011-0660-9. [DOI] [PMC free article] [PubMed] [Google Scholar]