Abstract
Introduction
Urinary bladder carcinoma ranks the fourth position in malignancy incidence rates in men (6.1%) and the 17th position in women (1.6%). In general, neoplastic diseases should be approached from two perspectives: prevention with implementation of prophylactic measures and early diagnostics. Prophylactics is possible in the preclinical phase of neoplasm, being both justified and plausible in patients from high–risk groups. Thus, it is particularly important to select such groups, not only by referring to environmental carcinogenic factors (occupational and extra–occupational) but also from genetic predisposition, which may be conductive for neoplasm formation. The mutations / polymorphisms of CHEK2 and CYP1B1 genes predispose to neoplasm via multiorgan mechanisms, while the human papilloma virus (HPV) may participate in the neoplastic transformation as an environmental factor.
Material and methods
131 patients with diagnosed urinary bladder cancer were qualified to the study. Mutations/polymorphisms of CHEK2 (IVS2 + 1G > A gene, 1100delC, del5395, I157T) and CYP1B1– 355T/T were identified by the PCR in DNA isolated directly from the tumor and from peripheral blood. The ELISA test was used for the studies of 37 HPV genotypes in DNA, isolated tumour tissue.
Results
11 mutations of CHEK2 gene were found, 355T/T polymorphism if CYP1B1 gene occurred in 18 patients (12.9%). Oncogenic HPV was found in 36 (29.3%), out of 123 examined patients.
Conclusions
The concomitance of CHEK2 gene mutations or 355T/T polymorphism of CYP1B1 gene and the presence of oncogenic HPV types statistically significantly correlates with histological malignancy grades of urinary bladder carcinoma.
Keywords: urinary bladder cancer, CHEK2, CYP1B1, HPV, histological malignancy grade
INTRODUCTION
In Poland, urinary bladder carcinoma ranks 4th among malignant neoplasms in men (6.9%) and 13th (2.0%) in women (data from 2008) [1].
Regarding mortality rates, malignant neoplasms are the second cause of death in Poland, being responsible for 26% of deaths in men and 23% in women. The risk factors for the occurrence of urinary bladder carcinoma include occupational exposures. Cigarette smokers reveal a two to four times higher risk for urinary bladder cancer than non–smokers [2].
Mutations of suppressor genes that are involved in cellular cycle control induce changes in the process, which results in an accumulation of gene mutations in cells secondary to lost mechanisms of DNA control and repair. The gene mutations that accumulate in cells remain ‘latent’ and rise to the surface only in favorable conditions, e.g., in result of some environmental (HPV, exposure, etc.) or genetic (the second impact effect) factors [3]. CHEK2, a suppressor gene, is localized at band 12.1 on the long arm of chromosome 22 and encodes the CHEK2 protein – a protein kinase, which is activated by DNA damage. The protein undergoes rapid phosphorylation and activation as a result of DNA damage and the fact that this replication blocks suppression. It then inhibits CDC25 phosphatase by serine phosphorylation in position 216. All the above–mentioned processes result in suppression of the CDK2–cycline E complex, which in turn prevents the cell from mitotic division [4]. Following the activity of the DNA damaging factor, the CHEK2 protein also controls the functions of the BRCA1 protein by phosphorylating serine in position 988. Serine phosphorylation in BRCA1 protein allows releasing the protein from the complex, made together with the CHEK2 protein, and plays an important role in the BRCA1 protein ability to restore normal cell functions after DNA damage [5].
It is estimated that mutations of the CHEK2 gene occur in approximately 2.2 million Poles, in whom four mutations of the gene are founding alleles in the Polish population. These included three protein shortening mutations and one missense type mutation (IVS2 + 1G > A gene, 1100delC, del5395, and I157T respectively) [6, 7]. The process of cellular cycle control involves the suppressor genes: Rb1 and p53. The loss of function by Rb1 protein or its abnormal phosphorylation may result in disturbances of the cellular cycle, an example of which may be the shortened G1 phase of the cellular cycle. An uncontrolled cellular cycle may bring about genomic instability [3].
The CYP1B1 gene is localized on chromosome–2 at p22–21 region and contains three exons. In result of translation beginning from the 5’–end of exon–2, a protein containing 543 amino acids is formed. In patients suffering from glaucoma, 17 mutations and several single nucleotide polymorphisms (SNPs) have been identified in the CYP1B1 gene–encoding region. Regarding the Caucasian population, four SNPs have been recognized, which induce changes in CYP1B1 protein in the following loci: Arg48Gly (SNP called m1), Ala119Ser (SNP – m2), Leu432Val (SNP – m3), and Asn453Ser (SNP – m4). Ala119Ser polymorphism is localized at the substrate recognition site one (SRS1), while m3 and m4 variants possess their polymorphism at the heme–binding site [8]. The CYP1B1 protein, besides its participation in metabolic changes of xenobiotics, plays a big role in estradiol transformations. Estradiol is a natural sex hormone, which is produced by maturing Graafian follicles. In case of excessive estradiol production or a higher than therapeutic estradiol dose, this hormone may induce formation of breast or uterine neoplasms. The CYP1B1 protein catalyzes the transformation of 17b–estradiol into 4–hydroxyoestradiol and, to a small degree, is involved in the transformation of 17b–estradiol into 2–hydroxyoestradiol. The 4–hydroxyoestradiol is a catecholic derivative of estradiol and is transformed – via a hydroperoxidase–dependent oxidation process – into semi–chinones and chinones. This process causes a release of free radicals that may injure DNA. The formed derivatives, i.e., chinones, are reactive metabolites and may cause damages to proteins and DNA by production of convalescent bindings [9, 10].
The human papilloma virus is a small non–enveloped virus containing two DNA strands; its genome consists of 8,000 nucleotides [11] and codes two oncoproteins that perform transformational activities: E6 and E7. In case of high–risk HPV types, the E6 protein mainly functions in the creation of a combined complex with the p53 protein, which results in ubiquitination and degradation of this protein. The repeatedly ubiquitinated p53 protein undergoes proteasomic degradation by the controlling subunit of protease, followed by hydrolysis into small polypeptides. Epithelial cells of the uterine cervix that are infected with high risk HPV undergo transition into a cellular cycle that does not depend on exogenous growth factors, but on the contrary is stimulated by the viral E7 protein, which binds to and degrades the RB protein and enables the expression of proteins that are necessary to enter the EdF–dependent S–phase of the cellular cycle. Despite the growing p16 protein levels, which – in normal conditions – acts on a feedback principle and controls D/Cdk4/6 cyclin levels, the inhibiting action of this protein is now neglected as a consequence of the HPV–dependent proliferation of cells. The growth of the p14ARF level, which occurs in case of nil p16–dependent activity, leads to an inactivation of the MDM protein and to higher levels of the p53 protein. This protein is bound with E6 protein and undergoes degradation, which prevents cell growth inhibition and/or apoptosis [12].
Nowadays, the possibility of diagnosing the neoplasm in its early stage, associated, among others, with the development of molecular cytogenetics (the UroVysion test), offers much better prognoses for the treatment process and outcome [13, 14].
With the above–mentioned in mind, this paper aimed to:
search for a correlation between the occurrence oncogenic HPV form and CHEK2 and CYP1B1 mutations/polymorphisms – i.e., genetic and environmental factors, predisposing to neoplastic transformation in material obtained from patients with urinary bladder cancer; and
evaluate the effects of a simultaneous occurrence of HPV and the above–mentioned mutations on clinical stage and histopathological grade of urinary bladder carcinoma.
MATERIAL AND METHODS
Study group
The study group comprised 131 patients with urinary bladder cancer, diagnosed for the first time and demonstrating different clinical stages (Ta, T1, T2, T3, and T4) and histopathological malignancy grades (G1, G2, and G3). The procedures were carried out after obtaining official consent from the Local Bioethical Commission of the Medical University in Lodz, Poland, No. RNN/154/10/KE, issued on September 7, 2010. All the patients declared their informed consent to participate in the studies.
Tumor tissue and peripheral blood
DNA from tumor cells and DNA isolated from peripheral blood made the material for studies. In order to find out whether the searched mutations were of somatic (being limited to neoplastic cells) or constitutional character, detection procedures first concentrated on the DNA from the tumor tissue and then, in case of identified CHEK2 gene mutation or CYP1B1 gene polymorphism, an analogous study was repeated on DNA isolated from peripheral blood. Regarding the study group, HPV DNA was identified in tumor cells. The study was performed on 123 patients of the study group with a limited volume of the DNA isolated from the tumor tissue. The UroVysion assay was performed using cytological preparations of urine samples collected before cystoscopy. Urine from 102 patients was sampled.
Study group – DNA isolation from tumor tissue
DNA was isolated from tissue fragments with a commercial kit for genomic DNA isolation of the A&A Biotechnology Company (www.aabiot.com).
Study group – DNA isolation from peripheral blood leukocytes
DNA was isolated from lymphocyte sediments with a commercial kit for genomic DNA isolation of the A&A Biotechnology Company (www.aabiot.com).
Detection of CHEK2 gene mutations (IVS2 + 1G > A, 1100delC, del5395, and I157T) and CYP1B1 gene polymorphism (355T/T)
Molecular analyses were run on the studied and control DNA to identify four mutations of CHEK2 gene (IVS2 + 1G > A, 1100delC, del5395, I157T) and one CYP1B1 gene polymorphism (355T/T). The genetic tests used to identify the above–mentioned mutations/polymorphism were designed at the Department of Genetics and Pathomorphology of the Pomeranian Medical University in Szczecin, Poland (www.genetyka.com).
HPV detection
HPV DNA was detected with the LINNEAR ARRAY Human Papilloma Virus GENOTYPING Test of Roche (www.roche.pl), which enables a simultaneous amplification of the target HPV DNA and of beta–globin DNA – the latter providing an internal cell control. The test employs pairs of primers, which detect nucleotide sequences within L1 polymorphic region of HPV genome. A pool of HPV primers, included in the test, encompasses 37 HPV genotypes, including 13 high–risk genotypes: 6, 11, 16, 18, 26, 31, 33, 35, 39, 40, 42, 45, 51, 52, 53, 54, 55, 56, 58, 59, 61, 62, 64, 66, 67, 68, 69, 70, 71, 72, 73, 81, 82, 83, and 84.
Test UroVysion
UroVysion is a FISH–based assay (Fluorescence In Situ Hybridization). It utilizes three centrometric probes (Chromosome Enumeration Probes CEP3, CEP7, and CEP17) and a unique locus specific probe for the 9p21. It is designed to detect aneuploidy for chromosomes 3, 7, 17, and deletion or polysomy of the 9p21 locus. The assay was performed in interphase of cell nuclei in urine sediment. Cell morphology was assessed and 25 large cell nuclei were selected in the first stage of the analysis. The number of signals from the molecular probe fluorochromes was calculated. When large, morphologically abnormal nuclei were not present, one hundred cells were assayed. The UroVysion results were considered positive based on two criteria:
„n1” criterion: more than 10 of the 25 cell nuclei showed the loss of the p21 locus on one or both chromosomes 9 or polysomy of one of the chromosomes 3, 7, or 17;
„n2” criterion: four or more cell nuclei per 25 cell nuclei or analogously 16 or more per 100 showed polysomy of at least two chromosomes of 3, 7, or 17 [13].
Statistical evaluation of results
The R computer software (The R Project for Statistical Computing) was used for statistical analysis and the obtained results were evaluated by calculating the odds ratio (OR). Data failing to meet the assumptions of the chi–square test were analyzed using Fisher's exact test.
RESULTS
Studies of the four CHEK2 gene mutations (IVS2 + 1G > A, 1100delC, del5395, I157T) and of the 355T/T polymorphism were carried out on the study group, which included 131 patients in whom urothelial carcinoma of different histopathological malignancy grades (G) and different clinical stages (T) was diagnosed. Molecular studies on 123 patients were attempted in order to identify oncogenic HPV types in tumor cell DNA. In eight patients, tumor DNA was not evaluated for oncogenic HPV because of limited material volume. The UroVysion assay was performed in cytological preparations of urine samples collected before cystoscopy. Urine was sampled from 102 patients.
The study group included 17 women (13.0%) and 114 men (87.0%); 11 non–smokers (8.4%) and 120 smokers (91.6%) (active or passive). Fifty–six (56) subjects (42.7%) reported occupational exposure in their history, while no occupational exposure was reported in 75 (57.3%) subjects. Ta non–invasive papillary carcinoma was diagnosed in 77 patients, a bladder submucosa infiltrating T1 tumor was found in 30 patients, and 23 patients were diagnosed with 3 T2 bladder cancer. Sixty–nine (69) patients demonstrated a well–differentiated G1 tumor, 39 patients had a moderately differentiated G2 tumor, and 23 patients had a G3 tumor.
In the study group there were 11 mutations of the CHEK2 gene, including nine (81.8%) I157T missense type mutations and two (18.2%) CHEK2 protein shortening changes: 1100delC and IVS2 + 1G > A splicing mutation. The 355T/T polymorphism of CYP1B1 gene occurred in 18 (12.9%) patients of the study group. Oncogenic HPV types were found in 36 (29.3%) out of the 123 examined patients. All the positive results of CHEK2 mutations and CYP1B1 polymorphism were also constitutionally confirmed (in blood) in subjects of the study group. UroVysion was positive in 68 patients of a total of 102 informative results. Table 1 summarizes the demographic and clinical characteristics of the study group, including evaluation of the known risk factors of the urinary bladder cancer. Table 2 reviews the results of virological, genetic, and cytogenetic (UroVysion) tests.
Table 1.
Demographical and clinical characteristics of patients group with urinary bladder cancer with risk factors data
| Noumber of patients | 131 | |
|---|---|---|
| Sex | Females | 16 |
| Males | 115 | |
| Age at diagnosis | mean ±s.d.: (max–min) | 66.4 ±10.8 (32–88) |
| Smoking | Smokers | 120 |
| Non–smokers | 11 | |
| Occupational exposure | Positive | 56 |
| Negative | 75 | |
| Stage | Ta | 77 |
| T1 | 31 | |
| T2 | 20 | |
| T3 | 2 | |
| T4 | 1 | |
| Grading | G1 | 69 |
| G2 | 39 | |
| G3 | 23 |
Table 2.
Results of molecular, cytogenetic and virological testings
| Grading | ||||
|---|---|---|---|---|
| G1 | G2 | G3 | ||
| CHEK2 mutations: | I157T | 5 | 2 | 2 |
| 1100delC | 1 | 0 | 0 | |
| IVS2 +1G > A | 1 | 0 | 0 | |
| Total | 7 | 2 | 2 | |
| CYP1B1 variant | G/G | 33 | 14 | 9 |
| G/T | 26 | 21 | 10 | |
| T/T | 10 | 4 | 4 | |
| Total | 69 | 39 | 23 | |
| Oncogenic HPV | Positive | 14 | 13 | 9 |
| Negative | 53 | 23 | 11 | |
| Total | 67 | 36 | 20 | |
| Not–performed | 2 | 3 | 3 | |
| UroVysion | Positive | 25 | 23 | 20 |
| Negative | 28 | 5 | 1 | |
| Total | 53 | 28 | 21 | |
| Not–performed | 16 | 11 | 2 | |
Statistical analysis
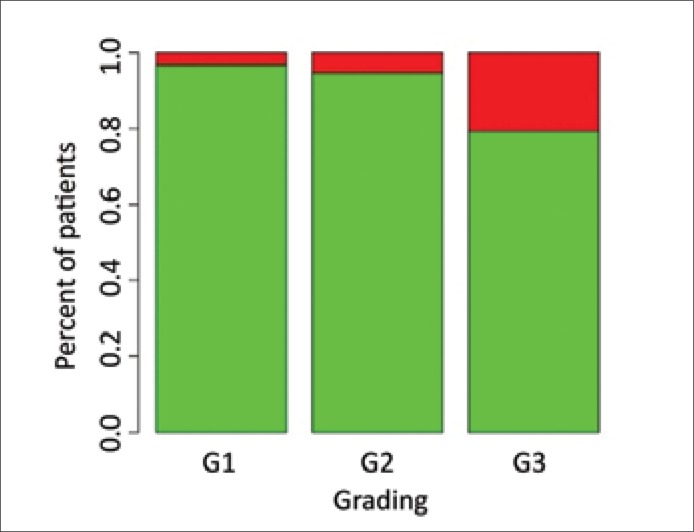
A statistical analysis of the study results revealed a statistically significant relationship between the occurrence of HPV infection, CHEK2 gene mutations, or CYP1B1 polymorphism on one side and the histological grade of tumor malignancy on the other, OR = 6.136602 (Table 2 and Fig. 1).
Figure 1.
The percent of examined patients with concomitance of HPV infection and mutations of studied genes (red color) and without such concomitance (green color) in patients with determined tumor grading.
DISCUSSION
The reported study demonstrated a statistically significant effect of concomitance of the three factors – infection with oncogenic HPV, CHEK2 gene mutations, and CYP1B1 gene polymorphism – on the histopathological malignancy grade of urinary bladder carcinoma.
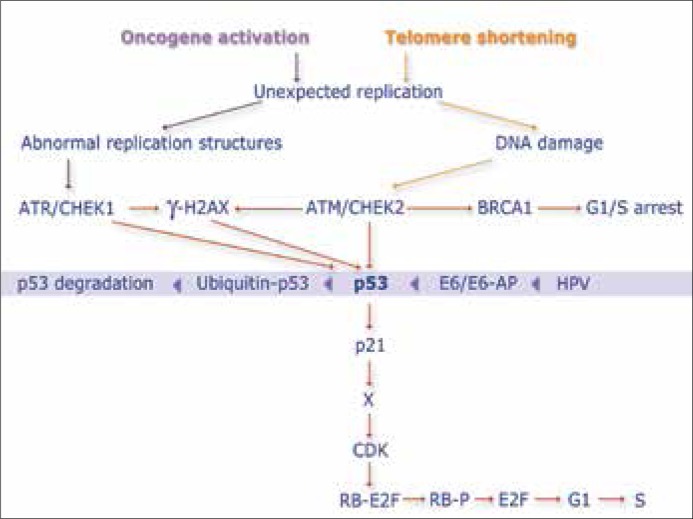
The transformation of a normal cell into a neoplastic cell is a multi–stage process. One of the factors that may play some role in this process is the integration of HPV DNA with DNA of the infected cell, a process that disturbs the E2 reading frame and causes an overexpression of E6 and E7 oncoproteins. Respective of published literature data [12, 15], E6 and E7 oncoproteins produced by oncogenic HPV types come into interactions with endogenous proteins, which are responsible for cellular cycle control: p53 and Rb proteins. These interactions lead to a loss of cellular cycle control by degrading p53 and Rb proteins and dysconfiguration of intracellular control points (Figure 2). The presence of HPV in the cell is an additional biological factor, which may favor a deeper penetration of the already existing mutation of DNA in the infected cell. The 1100del mutation of the CHEK2 gene entirely impairs protein functions while the missense mutation encodes a protein that seems to have the potential of CDC25C phosphorylation and inactivation that leads to G2 control point. This reveals a decreased catalytic ability with regards to CDC25A and an impaired ability of binding p53 and BRCA1 [16]. Literature data quote a model of response to DNA damage in early carcinogenesis based on activation of the ATM/ATR pathway in which the CHEK2 protein plays a big role. A mutation, which either completely switches off protein activity or disturbs its course, may lead to pathway disorders that normally suppress the growth of cells with damaged genetic material [17].
Figure 2.
Model of response to DNA damage in early carcinogenesis based on ATM/ATR path activation – modified.
Following the activity of a DNA damaging factor, the normal CHEK2 protein controls BRCA1 protein functions via serine phosphorylation in position 988. Any disorders in this protein function may lead to pathway disorders, suppressing the growth of the cell that has the damaged genetic material [5, 6, 7, 17]. The literature data mention the formation of free radicals and reactive metabolites, both of which may cause DNA damage during changes catalyzed by enzymes of P450 cytochrome [9, 10]. The literature data provide also the incidence of 355T/T polymorphism in the Polish population (control group) at 8.4% vs. 11.4% in patients with breast cancer (OR 1.4, p = 0.0005). These studies involved 2,033 breast cancer cases and a control group of 3,353 subjects. Statistically significant differences were observed in case of neoplasms of the following organs: lungs, prostate, and larynx, OR: 1.4 (95% CI 1.0–1.9), 1.4 (95% CI 1.0–1.8), 1.5 (95% CI 1.1–2.2), respectively [18]. In turn, studies of the 355T/T variant in male subjects with positive familial history towards prostate cancer 14.3% revealed the variant to have occurred in 14 out of 98 examined subjects in the study group, which gave a prevalence level of 14.3%. Among the identified carriers of the CYP1B1 gene 355T/T variant, the majority (71.4%) were patients with family–confirmed neoplasms of other organs: breast, uterus, stomach, colon, ovaries, lungs, larynx, urinary bladder, pancreas, and patients with melanoma [19]. The 355T/T polymorphism – Ala119Ser – in codon 119 is located at an important region of the gene, namely at the site of SRS1 substrate identification [20]. An increased catalytic activity of the CYP1B1 enzyme may cause amino acid replacement: Ala119Ser in codon 119 [21] may induce formation of a higher number of DNA damaging molecules.
A concomitance of two mechanisms in a cell, which may compromise the control of repair mechanisms, may prompt transformation of a normal cell into a neoplastic cell during a considerably shorter time period and/or enhance genomic instability or malignancy development of higher histological grade.
Studies of other authors indicate a correlation between urinary bladder cancer grading and the presence of oncogenic HPV types. De Gaetani et al., in order to evaluate the sensitivity of the in situ hybridization (ISH) technique for HPV detection and for comparison of the correlations between HPV infection and the level of antibodies in serum, studied 43 paraffin sections from patients with urinary bladder cancer and obtained a positive result in 39.5% (17/43). Out of the 17 positive cases, 12 demonstrated the presence of antibodies against HPV in serum. In 15 of the negative cases of ISH evaluations of blood serum samples from the patients, a positive result was obtained when evaluated with ELISA methods [22]. The authors found that HPV infection correlated with tumor staging and grading. HPV–positive tumors presented, in their majority (53%), with G3 staging and were also invasive (T2–T4) in 77% [22].
Also, LaRue's studies confirmed the correlation between HPV and tumor grading. Out of 11 patients with diagnosed G1 tumor, only two samples revealed the presence of viral DNA; out of 31 studied G2 tumors, 11 samples were positive; and out of 28 diagnosed G3 tumors, the presence of viral DNA was identified in 15 cases [23].
In patients who had a single genetic change (CHEK2 gene mutation or CYP1B1 polymorphism) together with the simultaneous occurrence of HPV infection, a positive correlation of the above–mentioned parameters was observed with positive UroVysion test results. Regarding those patients (eight) who obtained UroVysion test results, six were positive. The rather small group of patients did not allow for a statistical analysis, but the observed tendency in the obtained results is a reason to continue research in the same direction. Numerous literature reports from performed studies confirm the strong correlation among neoplastic transformations, disturbed cell repair mechanisms, and genomic hemostasis disorders. Endogenous or environmental structural damage to the cell genome may lead to a stabilization of mutations that may affect the integrity of the cellular DNA [3]. The obtained results suggest that a concomitance of such mechanisms in a cell, which either compromise the cellular cycle control – an infection with oncogenous HPV – or lead to ineffective repair processes in the cell – CHEK2 gene mutations or an excessive production of genetic material injuring free radicals (CYP1B1 gene 355T/T polymorphism) – may trigger cell chromosome aberrations and/or enhance genomic instability, which may lead to the development of a neoplasm with a higher grade of histological malignancy.
In the performed studies, a statistically significant and positive correlation was found between the simultaneous occurrences of: genetic (CHEK2/CYP1B1 mutations/polymorphisms) and environmental (HPV) factors on one hand and the histopathological grade of urinary bladder carcinoma on the other, regardless of pT. In this way it was possible to find out that non–invasive cancers of the urinary bladder, in which the above–mentioned factors coexisted, were associated with an increased risk of progression towards invasive changes. To our knowledge other authors have not yet performed such studies; however, the pioneer conclusions drawn from this study are rather significant, not only from the scientific but also from the clinical point of view. The relatively small number of the study population was an unquestionable limitation. For this reason, this study requires continuation in order to put research questions into clinical context. The techniques used in this study should eventually become the diagnostic method of choice for urologists and pathologists, helping them identify the risk of progression of superficial urinary bladder cancers.
References
- 1.Wojciechowska U, Didkowska J, Zatoński W. Warszawa: Centrum Onkologii – Instytut im. M. Skłodowskiej–Curie Zakład Epidemiologii i Prewencji Nowotworów; Malignant neoplasms in Poland in 2006. [Google Scholar]
- 2.Jung I, Messing E. Molecular mechanisms and pathways in bladder cancer development and progression. Cancer Control. 2011;7:325–334. doi: 10.1177/107327480000700401. [DOI] [PubMed] [Google Scholar]
- 3.Coleman WB, Tsongalis GJ. Multiple mechanisms account for genomic instability and molecular mutation in neoplastic transformation. Clin Chem. 1995;41:644–657. [PubMed] [Google Scholar]
- 4.Matsuoka S, Huang M, Elledge SJ. Linkage of ATM to cell cycle regulation by the Chk2 protein kinase. Science. 1998;282:1893–1897. doi: 10.1126/science.282.5395.1893. [DOI] [PubMed] [Google Scholar]
- 5.Lee J–S, Collins KM, Brown AL, Cahng-Hun Lee, Chund Jay H. hCds1–mediated phosphorylation of BRCA1 regulates the DNA damage response. Nature. 2000;404:201–204. doi: 10.1038/35004614. [DOI] [PubMed] [Google Scholar]
- 6.Cybulski C, Wokołorczyk D, Kładny J, Kurzawski G, Suchy J, Grabowska E, et al. Germline CHEK2 mutations and colorectal cancer risk: different effects of a missense and truncating mutations? Eur J Human Genet. 2007;15:237–241. doi: 10.1038/sj.ejhg.5201734. [DOI] [PubMed] [Google Scholar]
- 7.Bell DW, Varley JM, Szydlo TE, Kang DH, Wahrer DC, Shanon KE, et al. Heterozygous germ line hCHK2 mutations in Li–Fraumeni syndrome. Science. 1999;286:2528–2531. doi: 10.1126/science.286.5449.2528. [DOI] [PubMed] [Google Scholar]
- 8.Chang BL, Zheng SL, Isaacs SD, Turner A, Hawkins GA, Wiley KE, et al. Polymorphisms in the CYP1B1gene are associated with increased risk of prostate cancer. Brit J Canc. 2003;89:1524–1529. doi: 10.1038/sj.bjc.6601288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsuchiya Y, Nakajima M, Kyo S, Taro K, Inoue M, Yokoi T. Human CYP1B1 is regulated by estradiol via estrogen receptor. Cancer Res. 2004;64:3119–3125. doi: 10.1158/0008-5472.can-04-0166. [DOI] [PubMed] [Google Scholar]
- 10.Liehr JG. Is estradiol a genotoxic mutagenic carcinogen? Endocrine Reviews. 2000;21:40–54. doi: 10.1210/edrv.21.1.0386. [DOI] [PubMed] [Google Scholar]
- 11.Walboomers JMM, Jacobs MV, Manos M, Bosch FX, Kummer JA, Shah KV, et al. Human Papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Path. 1999;189:12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 12.Doorbar J. Molecular biology of human papillomavirus infection and cervical cancer. Clin Sc. 2006;110:525–541. doi: 10.1042/CS20050369. [DOI] [PubMed] [Google Scholar]
- 13.Binka–Kowalska A, Constantinou M, Kałużewski B. The use of the UroVysion assay in bladder cancer diagnosis. Urol Pol. 2006;59:249–254. [Google Scholar]
- 14.Rojewska J, Musierowicz A. Epidemiologia i badania przesiewowe dla onkologicznych chorych w urologii [Epidemiology and screening investigations for oncological patients] Urol Pol. 1994;47:262–268. [Google Scholar]
- 15.Szyfter K, Wierzbicka M. Rola wirusa brodawczaka (HPV) w nowotworach głowy i szyi [The role of the human papillary virus (HPV) in head and neck neoplasms] Postępy w chirurgii głowy i szyi [Progr. Head & Neck Surg] 2008;2:41–50. [Google Scholar]
- 16.Bell DW, Kim SH, Godwin AK, Schiripo TA, Harris PL, Haserlat SM, et al. Genetic and functional analysis of CHEK2 (CHK2) variants in multiethnic cohorts. Int J Cancer. 2007;15(121):2661–2667. doi: 10.1002/ijc.23026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bartkova J, Horejsí Z, Koed K, Krämer A, Tort F, Zieger K, et al. DNA damage response as a candidate anti–cancer barrier in early human tumorigenesis. Nature. 2005;14:864–870. doi: 10.1038/nature03482. [DOI] [PubMed] [Google Scholar]
- 18.Matyjasik J, Cybulski C, Górski B. The 355 T/T variant of CYP1B1 predisposes to breast cancer in Poland. conference proceedings: Nowotwory Dziedziczne – profilaktyka, diagnostyka, leczenie [Hereditary malignancies: prophylaxis, diagnostics, treatment]; Szczecin. 2005. [Google Scholar]
- 19.Schab M, Janiszewska H, Jarzemski P, Bak A, Junkiert–Czarnecka A, Pilarska M, et al. Frequency of CYP1B1 homozygous genotype 355T/T in prostate cancer families from Poland. Eur J Cancer Prev. 2010;19:31–34. doi: 10.1097/CEJ.0b013e32832f9ac6. [DOI] [PubMed] [Google Scholar]
- 20.Watanabe J, Shimada T, Gillam EM, Ikuta T, Suemasu K, Yasuhiro H, et al. Association of CYP1B1 genetic polymorphism with incidence to breast and lung cancer. Pharmacogenetics. 2000;10:25–33. doi: 10.1097/00008571-200002000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Shimada T, Watanabe J, Kawajiri K, Sutter TR, Guengerich FP, Gillam EMJ, et al. Catalytic properties of polymorphic human cytochrome P450 1B1 variants. Carcinogenesis. 1999;20:1607–1613. doi: 10.1093/carcin/20.8.1607. [DOI] [PubMed] [Google Scholar]
- 22.De Gaetani C, Ferrari G, Righi E, Bettelli S, Migaldi M, Ferrari P, Trentini GP. Detection of human papillomavirus DNA in urinary bladder carcinoma by in situ hybridisation. J Clin Pathol. 1999;52:103–106. doi: 10.1136/jcp.52.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.LaRue H, Simoneau M, Fradet Y. Human Papillomavirus in Transitional Cell Carcinoma of the Urinary Bladder. Clin Cancer Res. 1995;1:435–440. [PubMed] [Google Scholar]