Abstract
Asthma, a chronic inflammatory disorder of the airway, has features of both heritability as well as environmental influences which can be introduced in utero exposures and modified through aging, and the features may attribute to epigenetic regulation. Epigenetic regulation explains the association between early prenatal maternal smoking and later asthma-related outcomes. Epigenetic marks (DNA methylation, modifications of histone tails or noncoding RNAs) work with other components of the cellular regulatory machinery to control the levels of expressed genes, and several allergy- and asthma-related genes have been found to be susceptible to epigenetic regulation, including genes important to T-effector pathways (IFN-γ, interleukin [IL] 4, IL-13, IL-17) and T-regulatory pathways (FoxP3). Therefore, the mechanism by which epigenetic regulation contributes to allergic diseases is a critical issue. In the past most published experimental work, with few exceptions, has only comprised small observational studies and models in cell systems and animals. However, very recently exciting and elegant experimental studies and novel translational research works were published with new and advanced technologies investigating epigenetic mark on a genomic scale and comprehensive approaches to data analysis. Interestingly, a potential link between exposure to environmental pollutants and the occurrence of allergic diseases is revealed recently, particular in developed and industrialized countries, and endocrine disrupting chemicals (EDCs) as environmental hormone may play a key role. This review addresses the important question of how EDCs (nonylphenol, 4 octylphenol, and phthalates) influences on asthma-related gene expression via epigenetic regulation in immune cells, and how anti-asthmatic agents prohibit expression of inflammatory genes via epigenetic modification. The discovery and validation of epigenetic biomarkers linking exposure to allergic diseases might lead to better epigenotyping of risk, prognosis, treatment prediction, and development of novel therapies.
Keywords: Epigenetics, Allergy, Asthma, Acetylation, Methylation, Histone
INTRODUCTION
Asthma and allergic diseases are the most common chronic inflammatory disease in child and cause a substantial morbidity and mortality burden in severe cases [1]. Evidences indicate that etiology of asthma and allergic diseases is complex and has strong genetic and environmental components. Environment influence can start as early as in utero exposure and continue through aging, and affects the development, clinical phenotype, exacerbation and outcomes of asthma. Epigenetic mechanisms provide a new understanding of gene versus environment interactions. Modifications to the epigenome mediate endogenous or exogenous environmental exposures on immune development [2]. The processes provide regulatory control of gene expression independently of genomic sequence, and vary in response to environmental cues. Epigenetic control of gene expression plays an important role in development, differentiation and immune regulation in the immune system [3]. The genetic factor of asthma and allergic diseases synergistically interacts with prenatal and early-life exposures (e.g., tobacco smoke, endotoxins and air pollution) to affect asthma and allergic diseases risk [4]. While the enthusiasm in and expectations from genome-wide association studies have been slowly fading in the scientific community, findings that environmental exposures affects epigenetic profile have brought a new era in asthma and allergic disease research by examining epigenetic mechanisms as mediators of these exposures for occurrence and clinical course of asthma and allergic diseases. Epigenetic modifications (DNA methylation, histone modification and miRNA) can affect transcriptional activity in multiple genetic pathways relevant for the development of asthma and allergic diseases. However limited work has been carried out so far to examine the role of epigenetic variations on asthma and allergic diseases development and management.
Exposure to allergens induces an immune response that triggers the differentiation of T cells toward Th2 cells which expressed cytokines interleukin (IL) 4, IL-5, as well as IL-13, and are responsible for the allergic diseases. Decreased DNA methylation and increased association with activating histone marks conjointly establish and maintain a euchromatin structure at the Th2 locus of Th2 cells, allowing recruitment of the transcriptional machinery to this region for a rapid and coordinated expression of the Th2-related cytokines [5-7]. The hypermethylation of the first exon is correlated with promoter hypermethylation resulting in transcriptional silencing. The early response is marked by increases in IL-4 expression because the GATA-3 transcriptional factor binding sites within the first intron of the gene loses CpG methylation and the IL 4 locus gains H3K9 acetylation and trimethylation of H3K4 [8]. Th2 polarization is associated with loss of interferon (IFN)-g expression, which is thought to be mediated by methylation of specific CpGs in its promoter region [9, 10].
EPIGENOMIC STUDY DESIGN
The most common epigenetic mechanisms include DNA methylation, histone modifications, and noncoding RNAs. All can affect gene transcription through effects on DNA structure and induction of gene silencing. Microarrays can be the tool of choice for profiling epigenetic marks on a genomic scale, with several platforms and protocols available for DNA methylation [11]. The above technologies have been widely used for the study of histone marks (ChIP-seq) and miRNAs (miRNA-seq) because of providing superb-quality data compared with array platforms. The majority of methylation profiling is still performed on array platforms because bisulfite-converted DNA sequencing on the genomic scale is more costly [12]. However, several techniques that examine only regions of the genome enriched for methylation marks have been developed and are being increasingly used [12, 13]. Recent advances in the development of techniques for epigenomic profilings include try to define genome-wide patterns of DNA hydroxymethylation and to study DNA methylation and histone modifications in one experiment [13-15].
ENVIRONMENTAL TRIGGERS FOR ASTHMA AND EPIGENETIC REGULATIONS
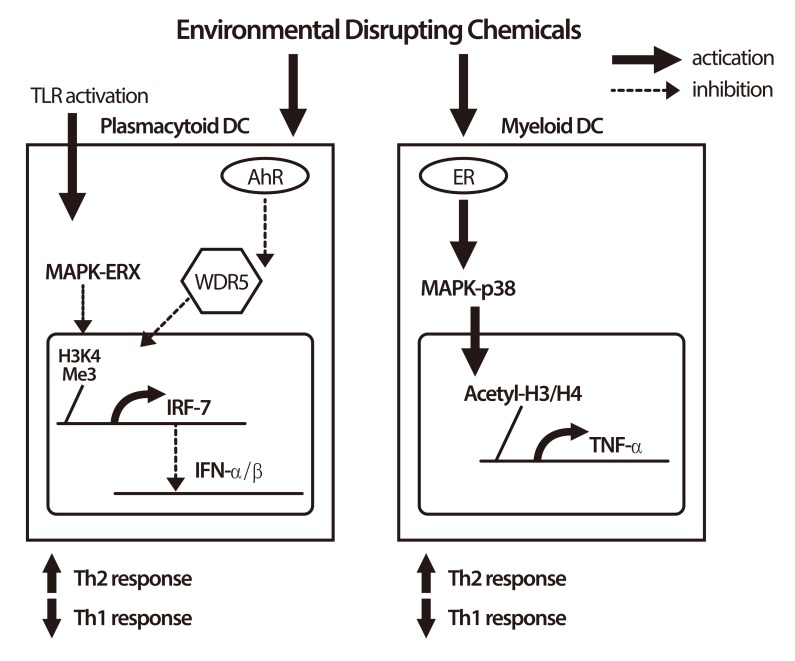
The rapid increase in the prevalence of asthma in the world was found over only the past few decades. The huge variations can be observed among populations with a similar racial/ethnic background but different environmental exposures [16], and there is a strong correlation between exposure to environmental pollutants and the occurrence of allergic diseases. Exposure to environmental endocrine-disrupting chemicals (EDCs) is associated with allergy, asthma, chronic inflammation and immunodeficiency via immunomodulatory mechanisms and epigenetic regulations. One of the possible mechanisms for the effect of EDCs on the allergic diseases may result from their impact on antigen-presenting cells and subsequently direct Th2 polarization. For example, nonylphenol and 4 octylphenol may have functional effects on the response of myeloid dendritic cells (DCs) via, in part, the estrogen receptor, MKK3/6-p38 MAPK signaling pathway, and histone modifications, with subsequent influence on the T cell-associated cytokine responses in our study [17]. Phthalates, the common environmental hormone used in plastic industry, may act as adjuvants to disrupt immune system and enhance allergy. Phthalates may interfere with immunity against infection and promote the deviation of Th2 response to increase allergy by acting on human plasmacytoid DCs via suppressing IFN-α/IFN-β expression and modulating the ability to stimulate T-cell responses with promotion toward Th2 polarization and suppression toward Th1 polarization [18]. A schematic figure on the effects of EDCs on immune cells is provided (Fig. 1).
Fig. 1.
Immunomodulatory effects of endocrine disrupting chemicals (EDCs) on immune cells. In plasmacytoid dendritic cells (DCs), EDCs activate acryl hydrocarbon receptor (AhR) and inhibit toll-like receptor (TLR)-activated mitogen-activated protein kinases (MAPK)-ERK phosphorylation. EDCs also inhibit H3K4 methyltransferase WDR5 to suppress H3K4 trimethylation at interferon regulatory factor 7 (IRF-7) promoter region and finally suppress the expression of IRF-7, and subsequently inhibit the production of type 1 interferon (IFN)-α and IFN-β. In myeloid DCs, EDCs activate estrogen receptor (ER) and increase phosphorylation of MAPK-p38. EDCs increase H3 and H4 acetylation at tumor necrosis factor alpha (TNF-α) promoter region and increase the production of TNF-α. EDCs enhance the T-cell stimulatory ability of both plasmacytoid and myeloid DCs toward Th2 polarization. On the contrary, EDCs attenuate Th1 polarization. These effects of EDCs on immune cells suggest that EDCs may promote allergic reaction and suppress the immunity against invaded pathogens.
ASTHMA MEDICATIONS AND EPIGENETIC REGULATIONS
Modifications on histones, such as acetylation or trimethylation at H3K4, H3K36 and H3K79, are associated with gene activation, and these modifications are usually carried out by a variety of histone acetyltransferases or methyltransferases. Some anti-asthmatic medication, such as steroid or theophylline, can exert the function via altering HAT or HDAC activity. Recently, histone modification has become a novel target for anti-asthmatic drug development. In asthma treatment, some potent anti-asthmatic medications influence the immune cells at epigenetic level. Prostaglandin I2 (PGI2) analog is recently suggested as a candidate for treating asthma [19, 20]. Iloprost (PGI2 analog) enhanced H3 acety-lation in MDC/CCL22 (Th2-related chemokine) promoter area and suppressed H3 acetylation, H3K4, and H3K36 trimethylation in IP-10 (Th1-related chemokine) promoter area. PGI2 analogs enhanced MDC expression via the I prostanoid (IP)-receptor-cyclic adenosine monophosphate (cAMP), peroxisome proliferator-activated receptor (PPAR)-α and PPAR-γ, NFκB-p65, MAPK-p38-ATF2 pathways and increasing histone acetylation, and suppressed IP-10/CXCL-10 expression via the IP-receptor-cAMP, PPAR-γ, MAPK-ERK-ELK1 pathways and inhibiting histone acetylation and trimethylation in LPS-stimulated monocytes [21]. In circulating mDCs, PGI2 analogs enhance IL-10 and suppress TNF-α expression through the IP/EP2/EP4 receptors-cAMP and EP1 receptor-Ca2+ pathway. Iloprost suppressed TNF-α expression via the MAPK-p38-ATF2 pathway and epigenetic regulation by downregulation of histone H3K4 trimethylation [22]. These evidences may partly explain the therapeutic efficacy of anti-asthmatic medication to alter the clinical outcome of asthma.
CONCLUSIONS
The gradual evidence has solidified for implicating epigenetic regulation as a mediator of a complex gene by environment interactions relevant to the development of asthma and allergic diseases. Several advances have been linking environmental hormone, air pollution and smoking exposure with atopy and asthma via epigenetic mechanisms. Asthma and allergy-related medications can also influence the function of immune cells at epigenetic level. Despite the acceptance of epigenetic regulation in the pathogenesis of complex diseases, the extent of environmental epigenetics in the pathogenesis of asthma and allergies is just being realized. Large sample size cohort studies are needed to examine the time course and time period of susceptibility to epigenetic regulation following environmental exposures and their contribution to allergic disease. Ultimately, an individual's epigenome early in life may be helpful in determining later risk of asthma and atopy and initiating an early intervention or treatment. Studying epigenetics as a mediating compound for the associations between environmental exposures, medications and pathogenesis of allergic disease may promise to find novel study pathways. The potentially modifiable of epigenetics may identify the approaches to decrease the risk of allergic disease and asthma and improve their nature history in the future.
References
- 1.Davies DE, Wicks J, Powell RM, Puddicombe SM, Holgate ST. Airway remodeling in asthma: new insights. J Allergy Clin Immunol. 2003;111:215–225. doi: 10.1067/mai.2003.128. [DOI] [PubMed] [Google Scholar]
- 2.Esteller M. Epigenetics in evolution and disease. Lancet. 2008;372:S90–S96. [Google Scholar]
- 3.Schoenborn JR, Dorschner MO, Sekimata M, Santer DM, Shnyreva M, Fitzpatrick DR, Stamatoyannopoulos JA, Wilson CB. Comprehensive epigenetic profiling identifies multiple distal regulatory elements directing transcription of the gene encoding interferon-gamma. Nat Immunol. 2007;8:732–742. doi: 10.1038/ni1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eder W, Ege MJ, von Mutius E. The asthma epidemic. N Engl J Med. 2006;355:2226–2235. doi: 10.1056/NEJMra054308. [DOI] [PubMed] [Google Scholar]
- 5.Lee DU, Agarwal S, Rao A. Th2 lineage commitment and efficient IL-4 production involves extended demethylation of the IL-4 gene. Immunity. 2002;16:649–660. doi: 10.1016/s1074-7613(02)00314-x. [DOI] [PubMed] [Google Scholar]
- 6.Tykocinski LO, Hajkova P, Chang HD, Stamm T, Sözeri O, Löhning M, Hu-Li J, Niesner U, Kreher S, Friedrich B, Pannetier C, Grutz G, Walter J, Paul WE, Radbruch A. A critical control element for interleukin-4 memory expression in T helper lymphocytes. J Biol Chem. 2005;280:28177–28185. doi: 10.1074/jbc.M502038200. [DOI] [PubMed] [Google Scholar]
- 7.Wei G, Wei L, Zhu J, Zang C, Hu-Li J, Yao Z, Cui K, Kanno Y, Roh TY, Watford WT, Schones DE, Peng W, Sun HW, Paul WE, O'Shea JJ, Zhao K. Global mapping of H3K4me3 and H3K27me3 reveals specificity and plasticity in lineage fate determination of differentiating CD4+ T cells. Immunity. 2009;30:155–167. doi: 10.1016/j.immuni.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fields PE, Kim ST, Flavell RA. Cutting edge: changes in histone acetylation at the IL-4 and IFN-gamma loci accompany Th1/Th2 differentiation. J Immunol. 2002;169:647–650. doi: 10.4049/jimmunol.169.2.647. [DOI] [PubMed] [Google Scholar]
- 9.White GP, Watt PM, Holt BJ, Holt PG. Differential patterns of methylation of the IFN-gamma promoter at CpG and non-CpG sites underlie differences in IFN-gamma gene expression between human neonatal and adult CD45RO-T cells. J Immunol. 2002;168:2820–2827. doi: 10.4049/jimmunol.168.6.2820. [DOI] [PubMed] [Google Scholar]
- 10.Jones B, Chen J. Inhibition of IFN-gamma transcription by site-specific methylation during T helper cell development. EMBO J. 2006;25:2443–2452. doi: 10.1038/sj.emboj.7601148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schones DE, Zhao K. Genome-wide approaches to studying chromatin modifications. Nat Rev Genet. 2008;9:179–191. doi: 10.1038/nrg2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harris RA, Wang T, Coarfa C, Nagarajan RP, Hong C, Downey SL, Johnson BE, Fouse SD, Delaney A, Zhao Y, Olshen A, Ballinger T, Zhou X, Forsberg KJ, Gu J, Echipare L, O'Geen H, Lister R, Pelizzola M, Xi Y, Epstein CB, Bernstein BE, Hawkins RD, Ren B, Chung WY, Gu H, Bock C, Gnirke A, Zhang MQ, Haussler D, Ecker JR, Li W, Farnham PJ, Waterland RA, Meissner A, Marra MA, Hirst M, Milosavljevic A, Costello JF. Comparison of sequencing-based methods to profile DNA methylation and identification of monoallelic epigenetic modifications. Nat Biotechnol. 2010;28:1097–1105. doi: 10.1038/nbt.1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brinkman AB, Gu H, Bartels SJ, Zhang Y, Matarese F, Simmer F, Marks H, Bock C, Gnirke A, Meissner A, Stunnenberg HG. Sequential ChIP-bisulfite sequencing enables direct genome-scale investigation of chromatin and DNA methylation cross-talk. Genome Res. 2012;22:1128–1138. doi: 10.1101/gr.133728.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Statham AL, Robinson MD, Song JZ, Coolen MW, Stirzaker C, Clark SJ. Bisulfite sequencing of chromatin immunoprecipitated DNA (BisChIP-seq) directly informs methylation status of histone-modified DNA. Genome Res. 2012;22:1120–1127. doi: 10.1101/gr.132076.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song CX, Clark TA, Lu XY, Kislyuk A, Dai Q, Turner SW, He C, Korlach J. Sensitive and specific single-molecule sequencing of 5-hydroxymethylcytosine. Nat Methods. 2011;9:75–77. doi: 10.1038/nmeth.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alati R, Al Mamun A, O'Callaghan M, Najman JM, Williams GM. In utero and postnatal maternal smoking and asthma in adolescence. Epidemiology. 2006;17:138–144. doi: 10.1097/01.ede.0000198148.02347.33. [DOI] [PubMed] [Google Scholar]
- 17.Hung CH, Yang SN, Kuo PL, Chu YT, Chang HW, Wei WJ, Huang SK, Jong YJ. Modulation of cytokine expression in human myeloid dendritic cells by environmental endocrine-disrupting chemicals involves epigenetic regulation. Environ Health Perspect. 2010;118:67–72. doi: 10.1289/ehp.0901011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuo CH, Hsieh CC, Kuo HF, Huang MY, Yang SN, Chen LC, Huang SK, Hung CH. Phthalates suppress type I interferon in human plasmacytoid dendritic cells via epigenetic regulation. Allergy. 2013;68:870–879. doi: 10.1111/all.12162. [DOI] [PubMed] [Google Scholar]
- 19.Idzko M, Hammad H, van Nimwegen M, Kool M, Vos N, Hoogsteden HC, Lambrecht BN. Inhaled iloprost suppresses the cardinal features of asthma via inhibition of airway dendritic cell function. J Clin Invest. 2007;117:464–472. doi: 10.1172/JCI28949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hung CH, Chu YT, Suen JL, Lee MS, Chang HW, Lo YC, Jong YJ. Regulation of cytokine expression in human plasmacytoid dendritic cells by prostaglandin I2 analogues. Eur Respir J. 2009;33:405–410. doi: 10.1183/09031936.00070008. [DOI] [PubMed] [Google Scholar]
- 21.Kuo CH, Ko YC, Yang SN, Chu YT, Wang WL, Huang SK, Chen HN, Wei WJ, Jong YJ, Hung CH. Effects of PGI2 analogues on Th1- and Th2-related chemokines in monocytes via epigenetic regulation. J Mol Med (Berl) 2011;89:29–41. doi: 10.1007/s00109-010-0694-2. [DOI] [PubMed] [Google Scholar]
- 22.Kuo CH, Lin CH, Yang SN, Huang MY, Chen HL, Kuo PL, Hsu YL, Huang SK, Jong YJ, Wei WJ, Chen YP, Hung CH. Effect of prostaglandin I2 analogs on cytokine expression in human myeloid dendritic cells via epigenetic regulation. Mol Med. 2012;18:433–444. doi: 10.2119/molmed.2011.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]