Abstract
Chronic cough is a common reason for patients to seek medication attention. Over the last few decades, we have experienced significant clinical success by applying the paradigm of 'evaluating and treating the causes for chronic cough'. However, we still ask ourselves 'what underlies chronic cough. Indeed in a considerable proportion of patients cough is idiopathic, or unexplained despite vigorous evaluation. Commonly associated conditions such as rhinitis, eosinophilic bronchitis, asthma, or gastroesophageal acidic reflux may not be fundamental to cough, and thus may be triggers rather than causes. The cardinal feature of chronic cough is persistent upregulation the cough reflex, which may be driven by complex interactions between biologic, neurologic, immunologic, genetic, comorbid, and environmental factors. We suggest the new paradigm 'cough hypersensitivity syndrome' should finally bring us further advances in understanding and management of chronic cough.
Keywords: Cough, Respiratory hypersensitivity, Etiology, Pathophysiology
INTRODUCTION
Chronic cough is a centuries-old puzzle [1]. Cough is an essential defense mechanism for protecting self from harmful inhalation, or aspiration [2]; but cough is also one of the most troublesome symptom for which patients seek medical attention [3]. In particular, chronic cough is a significant health issue for high prevalence (3-33%) in general populations [4, 5], considerable impacts on quality of life [6], and clinical challenges [7].
In the past, chronic cough had been considered just as a consequent symptom resulting from respiratory infection [1] or chronic bronchitis [8], related to poor hygiene and high pollution/smoke exposure. However, chronic cough patients were found not to be confined to these factors, and clinical pictures did not correspond well.
In 1977, the first clinical insight was made for managing chronic cough. Irwin et al. [9] introduced the anatomical diagnostic protocol, leading to significant advances in the management of the patients. The protocol was based on the anatomical distribution of cough receptors and afferent pathways [10], to provide rationale for systematic approach for 'finding the cause and treat it'. The approach has produced significant success in various regions [11]. Therefore, until now, chronic cough has mostly been considered as the results from following disease triad: rhinitis (postnasal drip syndrome or upper airway cough syndrome), eosinophilic airway inflammation (asthma and nonasthmatic eosinophilic bronchitis [NAEB]), and gastroesophageal reflux diseases (GERD).
However, a clinical discrepancy was also frequently experienced; not a few patients (12-42%) did not fit into any categories despite vigorous diagnostic and therapeutic efforts, thus named as idiopathic or refractory cough [12]. Another confusion was that only a minority of patients with these common conditions suffered from cough [13, 14]. Moreover, in many patients cough does not easily improve despite specific treatment for the diagnosed cause. Current diagnostic term 'chronic cough' has been based on the assumption that 'different duration' indicates 'different etiologies' [15]; then, the term may be misleading, as their causal relationships could not be fully explained by the terminology.
More recently, a different paradigm viewing chronic cough from more mechanistic perspective has evolved [16, 17]. In this view, chronic cough is a single syndrome with a common intrinsic mechanism of 'cough hypersensitivity', wherein the common diseases which had been previously thought as causes are rather triggers. 'Cough hypersensitivity syndrome (CHS)' is still an evolving conceptual entity, and its clinical relevance should be further proven [18], however, emerging evidence provides clinical characters and mechanistic insights into the disease [19, 20]. We believe that the new paradigm of 'cough hypersensitivity' should finally bring us further advances in understanding and management of chronic cough.
COUGH REGULATION: PERIPHERAL REFLEX AND BRAIN CONTROL
To understand chronic cough from the viewpoint of 'cough hypersensitivity', let us start with brief reviews on the mechanisms of cough regulation. Cough is an expulsive motor act with a characteristic sound [21], which can be provoked voluntarily or induced reflexively (consciously or unconsciously). That is, cough is the summation act from complex interactions between central and peripheral nervous systems [22].
Primarily, the cough reflex is mediated by peripheral sensory nerves in the airways. Different airway sensory nerves are involved in cough, mostly originating from the vagal nodose and jugular ganglia. The sensory nerve fibers terminate in and under the airway epithelium, and sense the irritant signals coming into the airways and get activated. The activation is mediated by various ion channels, and the generated action potential is conducted along afferent pathways to the nucleus tractus solitaries (nTS) in the convergence center. Afferent signals are then integrated, and efferent signals for cough act are decided [23].
However, there are different kinds of vagal afferent fiber subtypes, depending on how they respond to different stimuli [23]. The sensation of mechanical stimuli is mainly mediated by Aδ cough receptors; it is also responsive to rapid change in pH but not to capsaicin or bradykinin (BK) [24, 25]. It is fast conducting, and may be thought as to mediate the immediate protection against acid or foreign body aspiration.
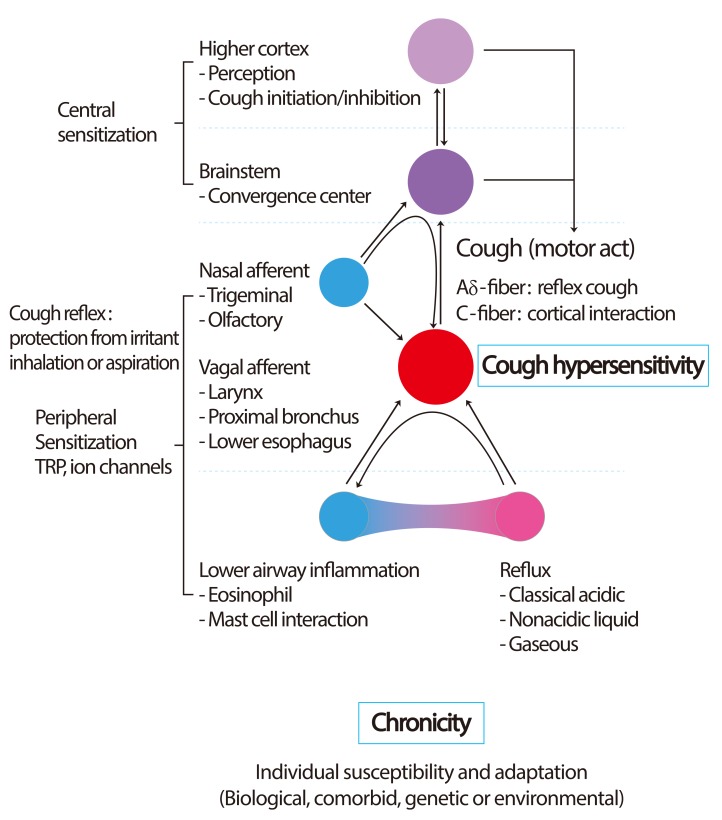
The recognition of chemical irritants and endogenous inflammatory mediators is mostly mediated by C-fibers [23]. Interestingly, C-fibers are functionally divided into two types, according to their innervation sites (bronchial vs. pulmonary). Bronchial C-fibers innervate the upper airway, and the activation readily initiates the cough reflex. However, pulmonary C-fibers innervate the lower airways, and their activation is thought to inhibit cough but induce apnea [23]. The paradoxical actions of two common but differently acting pathways may not be explained by peripheral levels, but may be the evolutionary adaptation to protect lower airways. These airway C-fibers exert chemosensitive functions by expression of various sensory receptors. Transient receptor potential vanilloid 1 (TRPV1) is one of well-known nociceptors relevant to cough, which responds to high temperature, low pH and capsaicin [26]. TRPA1 (ankyrin 1) is another recently identified cough receptors, which readily senses cold temperature and various irritants including cigarette smoke [27]. The upregulation or activation of these TRPs may be a major component subserving cough hypersensitivity (Fig. 1).
Fig. 1.
Schematic presentation on development of cough hypersensitivity. Key event may be the development of vagal neuronal hypersensitivity located in the airways. Commonly associated diseases like rhinitis, eosinophilic airway inflammation, or classical acidic reflux may be triggers to lower thresholds for peripheral cough reflex activation. Nasal afferent stimulation may not directly initiate cough reflex, but modulate (sensitize or desensitize) the cough reflex depending on the type of nasal stimulus. Gaseous reflux has been hypothesized to be a common factor to develop cough hypersensitivity. TRP, transient receptor potential.
Rapidly adapting receptors and slowly adapting receptors are another sensory fibers for mechanical stimuli; however, they mainly terminate in the intrapulmonary airways, and are thought as being less involved in cough reflex [23].
However, not every cough is reflex; we can also voluntarily initiate and suppress coughing. This indicates the involvement of higher brain circuit that controls cough consciously [22]. Interestingly, Aδ fiber stimulation induces cough reflex regardless of general anesthesia [28]; however, the effect of C-fiber is maintained only when we are conscious [24]. It means that peripheral cough reflexes are differently associated with central nervous system; the function of mechanosensitive Aδ fiber is fundamental and instinctive for airway protection, whereas the chemosensitive C-fiber function is slower but more complexly related to perception by higher cortex level. In practice, we see many cough patients have abnormal sensation or irritation in the throat that leads to 'urge-to-cough' and cough act [29]. The 'urge-to-cough' sensation may be experimentally induced by C-fiber stimulation or capsaicin inhalation [30, 31], which is a TRPV1 agonist. The presence of 'urge-to-cough' is a clinical evidence to suggest the connection between peripheral and central cough pathways.
In addition, many cough patients may be seen, who cannot suppress cough as wanted. Recent capsaicin cough challenge studies by Hilton et al. [32] have suggested evidence that chronic cough patients have potential defects in inhibitory mechanisms of cough regulation. Functional magnetic resonance imaging (MRI) studies by Mazzone et al. [22] may provide objective evidence for the presence of suprapontine pathways involved in cough regulation. The interesting interactions between higher brain circuits and peripheral afferent fibers warrant further elucidation.
COUGH HYPERSENSITIVITY: DISEASE ASSOCIATIONS
Clinically, chronic cough patients commonly do cough in response to just trivial stimulant such as perfume, temperature change, or phonation. As previously introduced, the upregulation of the TRP receptors in the airway afferents may be one of the pathophysiological basis [16].
Cough hypersensitivity is a common intrinsic component in various diseases associated with chronic cough (Fig. 2). Not every subject with disease triad (asthma, rhinitis, or GERD) does cough, which indicates that these diseases are not fundamental to cough but in some patients are associated. Features of cough hypersensitivity are the cardinal common link. These may vary between individuals, which suggests the role of individual susceptibility. Therefore, it warrants further elucidation which individual susceptibility is related to the hypersensitivity. Here we briefly discuss roles of cough hypersensitivity in commonly associated diseases.
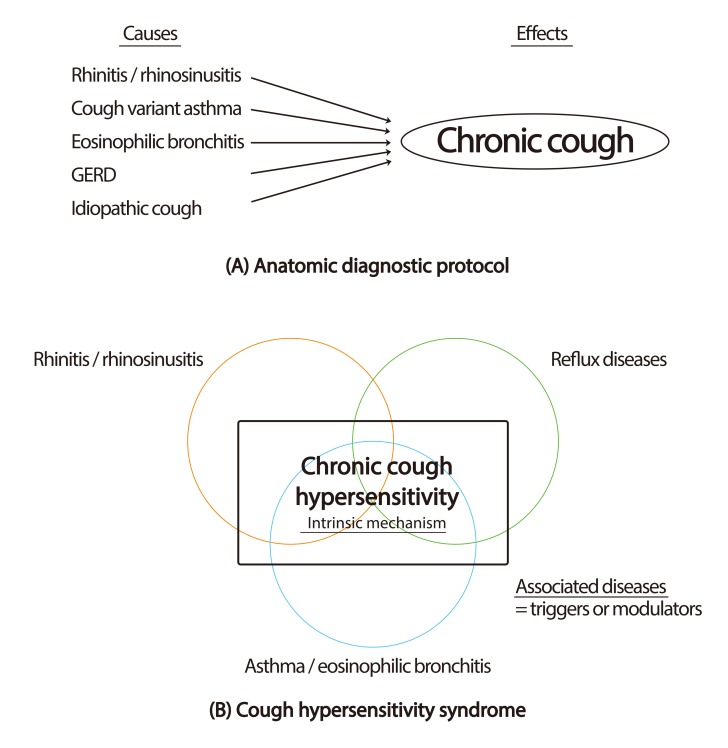
Fig. 2.
Paradigms for chronic cough. (A) Anatomic diagnostic protocol: chronic cough is considered as the outcome from causative diseases affecting anatomically relevant cough reflex pathways. (B) Cough hypersensitivity syndrome: chronic cough hypersensitivity is a common intrinsic mechanism for chronic cough. Commonly associated diseases are triggers or modulators for cough reflex pathways. GERD, gastroesophageal reflux disease.
Asthma and eosinophilic bronchitis
Cough is a common presentation of asthma and bronchitis, but it was in 1979 that chronic cough began to be recognized as the possible sole symptom of asthma, so-called cough variant asthma [33]. Later, NAEB was identified as a major cause for chronic cough [34], promoting the immunologic research for chronic cough; the difference between asthma and NAEB was suggested as the anatomical distribution of mast cell infiltration within the airways (such as smooth muscle) [35]. Japanese groups suggested the term 'atopic cough' to indicate a subtype with cough hypersensitivity and general atopic tendency but no asthma [36]. However, Birring et al. [37] demonstrated that cough reflex activation was a common phenomenon within the airways of chronic cough patients regardless of whether they were asthmatic, eosinophilic bronchitic, or idiopathic cough. Methacholine airway hyperresponsiveness (AHR) is independent from cough sensitivity [38]. Moreover, eosinophilic reduction by mepolizumab did not resolve cough in asthma [39], which could raise questions on the causal relationships between eosinophil and cough. In this regard, mast cell has been recently tipped as the key effector cell mediating cough hypersensitivity in eosinophilic diseases, on the basis of accumulating evidence [40].
However, despite the uncertainty regarding their mechanisms, it is plausible enough that eosinophilic/allergic airway inflammation is significantly associated with cough hypersensitivity. In eosinophilic airways, endogenous inflammatory mediators like prostaglandins (PG) or BK were frequently elevated [37, 41]; and recently, Grace et al. [42] have found that TRPA1 and TRPV1 channels are key regulators to mediate tussive responses provoked by PGE2 and BK. It is also notable that endogenous lipoxygenase products such as hydroperoxyeicosatetraenoic acids and leukotriene B4 were potent agonists for TRPV1 channel [43]. In animal models, TRPA1 channels have been associated with ovalbumin-induced allergic asthma [44], hypochlorite-induced nonallergic AHR [45], or formaldehyde-promoted asthma [46]. As these channels are also expressed in nonneuronal cells like airway epithelium or bronchial smooth muscle cells [47, 48], their clinical relevance should be further explored. Very recently, TRPV1 has been implicated in refractory asthma [49].
Upper airway diseases
Detailed mechanisms of associations between chronic cough and upper airway diseases have been previously reviewed in this journal [50]. Briefly, in the past, the mechanical stimulation by postnasal drip was thought as the likely mechanism, as named as 'postnasal drip syndrome'. However, postnasal drip is a physiologic finding, and is not of itself an adequate explanation [13]. Now the main mechanisms are considered to be the up- or down-regulation by nasal afferent nerves [50]. Nasal afferents express various cough receptors and sense different kinds of stimuli. Although direct nasal stimulation did not directly provoke cough but only sneeze reflex, intranasal histamine or capsaicin stimulation significantly regulated cough responses (or induced cough hypersensitivity) to tussigen inhalation [51]. Pollen allergic rhinitis patients are cough sensitized during pollen seasons [52]. The nasal trigeminal afferent stimulation also induced c-fos expression in the nTS, indicating the potential contribution of upper airway neurogenic inflammation in central sensitization of cough [53]. Recent findings on potential interactions between different sensory afferents (trigeminal, olfactory, and vagal) and receptors (TRPA1, TRPV1, and TRPM8 [melastatin 8]) in the airways are expected to bring us new clinical application for cough control by nasal afferent modulation [50, 54].
Reflux diseases
GER has long been described as a cause of chronic cough [11]. In those presenting with the classic acid related symptoms such as heartburn, then the diagnosis can appear obvious, particularly when there are mechanical abnormalities of the upper gastrointestinal (GI) tract known to be associated with reflux disease including hiatus hernia and esophageal dysmotility. Greater difficulty is encountered when acid related symptoms are absent. Patients may have an excessive degree of acid reflux when tested with an esophageal pH study, and the close temporal relationship between episodes of acid reflux and the coughing - the so-called symptom association probability or SAP confirms that relationship [55]. Advances in technology have led to the appreciation that not all gastro-esophageal reflux is acidic. Impedance studies demonstrate that reflux is not necessarily acidic [56] and may even be gaseous in nature [57]. This form of gaseous nonacid reflux is a natural phenomenon occurring in everyone and is an important physiological mechanism preventing intestinal gas bloat and excessive flatulence. In patients with chronic cough, it is neuronal hypersensitivity to this natural reflux phenomenon which causes a relatively innocuous and low intensity stimulus to provoke the irritation leading to cough. Unfortunately, few if any objective measures are capable of recording episodes of nonacid reflux. Electronic capture by methodologies such as impedance is difficult because recording in the upper esophagus is problematical [58]. Recently, salivary pepsin estimation shows promise [59]. Clinicians however are left to rely on the questionnaires such as reflux symptom index [60] or the cough specific Hull Airway Reflux Questionnaire [61].
Whilst it is obvious that a liquid or gaseous bolus impacting on the larynx or upper airway could provoke paroxysmal coughing, it is less clear how reflux could lead to the phenomenon of cough hypersensitivity. Damage to the epithelium from pepsin bile or low pH in gastric juice could result in an inflammatory reaction. More recently, it has been suggested that that macrophages could be responsible for activating the inflammatory cascade releasing mediators like the family of the nerve growth factors responsible for the neuronal proliferation and upregulation of nociceptors such as those of the TRP receptor family [62].
Inflammation produced by reflux may also produce some of the other associated features seen in patients with chronic cough. This reaction is dependent on the site of inflammation and the genetic characteristics of the individual suffering the reflux. Thus in the phenotype of cough associated with rhinitis, reflux driven upper airways inflammation may predominant. In the phenotype of asthmatic cough, lower airways involvement through aspiration may be the cardinal feature. The type of inflammation may also vary according to the "soil" on which the "seed" of a reflux lands. In an atopic person, eosinophilic inflammation of the TH2 type may be the major feature. The unifying characteristic of CHS is that whatever features are caused by airway reflux (a more inclusive the term for extraesophageal reflux) then the symptom of a cough may be explicable by the afferent neuronal hypersensitivity common to all phenotypes [16].
Viral infection
Viral infection is a frequent cause for acute cough and subacute cough (duration: 3-8 weeks, also named as postinfectious cough) [63]. We experience bouts of cough attacks during acute respiratory viral illness, and cough more easily in response to minor triggers like temperature change, speaking, or positional changes. This is a typical picture of cough hypersensitivity which will also be seen in chronic cough patients. Clinical studies also demonstrated that subjects with respiratory viral infection had lower threshold in capsaicin cough challenge tests [64, 65]. Then, what is the role of cough hypersensitivity in viral infection? It may be speculated that respiratory viruses have evolved to induce cough reflex hypersensitivity to enable themselves to be disseminated further. Specific mechanisms how rhinovirus regulates cough hypersensitivity have been recently identified [66]; in in vitro experiments, rhinovirus infects human neuronal cells and induces the up-regulation of TRPA1 and TRPV1 by mediating soluble factor production from the infected cells. As atopy is a risk factor for rhinovirus infection and subsequent asthma [67], these interactions could possibly be a factor to explain the frequent associations between airway eosinophilia and cough hypersensitivity.
WHAT DRIVES CHRONICITY?
We have briefly reviewed the associations of cough hypersensitivity with well-known conditions. Then, what drives this 'chronicity', or the persistence of cough hypersensitivity? Unfortunately, the mechanisms related to chronicity are still unclear. Here we suggest some clues pertaining to this question, based on recent clinical, experimental and epidemiological findings.
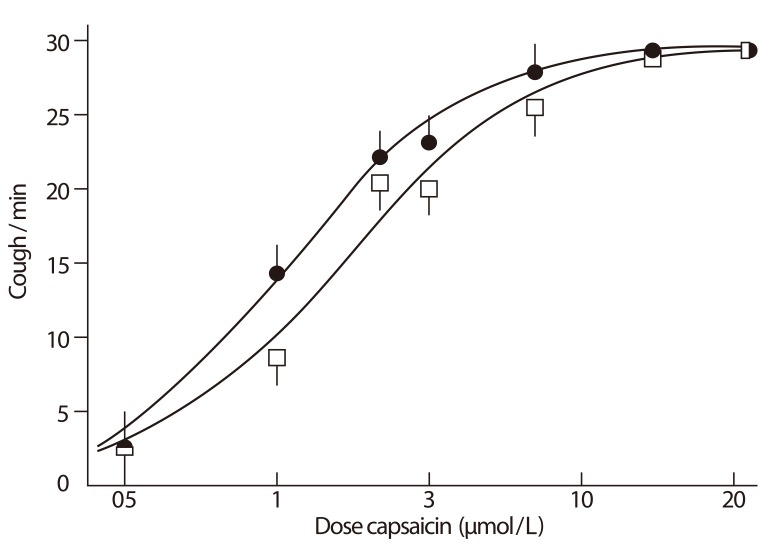
Clinically, chronic cough is observed to be predominant among middle-aged women, with homogeneity across various regions; peak age of onset appears to be perimenopausal, and the female ratio is twice that of male [68]. We also found that the demographic characteristics were significantly associated with capsaicin cough sensitivity, but not with eosinophilic bronchitis or rhinitis, in an unselected sample of Korean adult patients [29]. These findings could suggest that the development of cough sensitivity is a biologic phenomenon and also an intrinsic factor to determine cough response to any given stimulation. Higher prevalence of angiotensin converting enzyme inhibitor (ACEi) induced cough among females [69] also supports the notion. ACEi medication induces left-shifts in tussigen-induced cough responses (Fig. 3) [70], by promoting the accumulation of endogenous TRP agonists such as PG, BK or substance P [71]. Then, why do females develop more cough hypersensitivity than males?
Fig. 3.
Captopril-induced cough reflex sensitization. Dose-response curve for capsaicin challenge up to a maximum response of 30 coughs/min; round, captopril; square, placebo. Values shown are mean and standard error of the mean. Reprinted from Morice AH, et al. Lancet 1987;2:1116-8, with permission of Elsevier [70].
One mechanistic explanation may be the estrogen-TRPV1 association [72]. Female sex hormones have been implicated in the pathogenesis of various diseases involving ion channels, such as drug-induced arrhythmias or pain [73, 74]. In cough challenge tests, gender differences in cough sensitivity are not observed during prepuberty [75], but become evident after puberty [76] or being older [29]. Teleologically, the development of cough sensitivity may provide advantages to prevent aspiration during pregnancy in females. Acute estrogen effects are unlikely to be a sole factor, as chronic cough and capsaicin sensitivity is also prevalent among elderly females [20, 29].
Another hypothetical explanation is the gender difference in cerebral or perceptive responses. Chronic pain is a clinical entity with considerable pathophysiological homologies with chronic cough [77]. Female predominance is consistently found in pain epidemiologic surveys, and the gender difference in pain sensitivity to capsaicin is also similarly observed [78]. In experimental studies using functional MRI or positron emission tomography, females showed different patterns of brain activation, compared to males, in response to physical or thermal stimulation [79-81]. These gender discrepancies are supposed to be similar in chronic cough [77]. In addition, defective changes in counter-regulatory mechanisms such as descending inhibitory pathways may also play a part for chronicity [82], as chronic cough patients had increased maximal (or uninhibited) cough responses to capsaicin challenge [32].
Several disease associations may also be reviewed. Irritable bowel syndrome (IBS) was traditionally a functional GI disease without evident pathology, but now is considered as having complex pathophysiology involving mast cells, food allergy, or neuro-immune interaction [83]. The mechanisms of IBS are beyond the scope of the present review, but appear to have several mechanisms in common with chronic cough, such as mast cell involvement, persistent low-grade allergic inflammation, sensory hypersensitivity, and possible involvement of higher cortex. The female predominance is also consistent for IBS [84]. The significant associations between IBS and frequent cough have been well reported in United Kingdom adult population surveys with an odds ratio of 2 [85].
As older adults have more frequent cough but also more comorbidity, we recently analyzed the associations between chronic cough and various comorbid conditions in Korean elderly community samples [5]. We hypothesized that they could contribute to 'chronicity' of cough. Interestingly, we found previously unrecognized associations with uncontrolled diabetes mellitus and constipation. Although the mechanisms are unclear, their potential links could be reflux and cough sensitization [86-90]. Untreated classical triads of asthma, rhinitis, or acidic reflux may also contribute to 'chronicity'. Successful relief of cough by specific treatment of underlying conditions is the well-supporting evidence [11]. Thus persistent neurogenic inflammation in sensory afferents may lead to the changes in the convergence center [53].
Genetic susceptibility may also be a pathophysiologic background. Recent analyses by using European community population samples have found the genetic associations of TRPV1 single nucleotide polymorphisms (SNP) with cough in non-asthmatic adults [91]. In addition, SNPs in neurokinin receptors have been also associated with capsaicin sensitivity [92].
Collectively, 'chronicity' may be driven by interactions between biologic, neurologic, genetic, and immunologic systems, and also be affected by environmental conditions such as temperature, infection, irritant, or pollutant. The development of cough sensitivity may have some advantages for protecting airways in evolutions, but also have detrimental health effects particularly when combined with comorbid conditions.
DOES THE 'HYPERSENSITIVITY' CONCEPT AID OUR CLINICAL PRACTICE?
By applying the concept of cough hypersensitivity, we aim to control of cough reflex, or rather normalize it. Chronic refractory cough is the disease of particular interest, in which traditional diagnostic and therapeutic approach has failed to identify and remove the triggers.
Antitussive agents are classified based on their location of action, as centrally-acting or peripherally-acting [93]; however, most of traditional agents were centrally-acting nonselective ones. Possibly the most frequent class of antitussive agent is an opiate. However, despite the long clinical experience, the exact roles of opiates have not been elucidated for cough therapy; the mechanisms are not clear, but have been just hypothesized to be sedation, or modulation of opioid receptors. Opiates do not appear to suppress capsaicin or citric acid-induced cough responses [93]. More problems can be found in their side-effects, and in the questionable interindividual variability particularly in the metabolism of codeine. Morphine remains however one of the few agents to have been shown to have efficacy in patients with chronic cough [94]. Neuroleptic agents are another class of centrally-acting antitussive agents. Amongst them, gabapentin has been recently highlighted for clinical efficacy in chronic refractory cough, particularly among the subgroup with evidence of central cough sensitization (laryngeal hypersensitivity), supporting the concept of cough hypersensitivity [95]. However, gabapentin did not effectively suppress peripheral capsaicin cough sensitivity, and had side effects in 31%. Pregabalin, baclofen and amitriptyline are other agents with potential antitussive activity, but their indications warrant further convincing evidence from controlled trials [93].
Therefore, selective blockade of specific receptors at periphery is expected to bring out therapeutic breakthroughs in the future. Several clinical trials are currently on the progress [93]. However, a preliminary report of a specific TRPV1 antagonist in chronic cough failed to demonstrate any efficacy, despite blocking the capsaicin induced cough [96], which is not only disappointing from the clinical point of view but also questions whether antagonism of the specific nociceptors is a correct therapeutic strategy. A more promising target appears to be the P2X3 receptor and specific antagonists have shown very promising efficacy for idiopathic cough [97]. The randomized, double-blind, placebo-controlled, crossover phase-II trial with oral P2X3 receptor antagonists, AF-219, has demonstrated that the 2-week treatment was quite effective as reducing daytime cough frequency by 75%, and also improving secondary outcomes (cough severity, urge to cough, and quality of life score). The results suggested the significant role of this receptor in peripherally-mediated cough hypersensitivity. P2X3 is one of purinergic receptors for adenosine 5'-triphosphate, located in peripheral C-fibers but not in the brain, and has been known to be important for peripheral nociception [98]. Selective inhibitors for TRPV1, TRPA1, and other ion channels also continue to be targets for novel treatment [93].
The introduction of 'cough hypersensitivity' concept is expected to fill a gap in the relationships between previous 'common etiologies' and chronic cough. It would help reduce unnecessary classifications of overlapping and similar diseases, and also aid the discovery of biomarkers and new indications of traditional therapeutic agents.
CONCLUSIONS
One disease may have several different faces; but the intrinsic mechanism may be common. Persistent cough may present with several disease associations depending on individual susceptibility, but the key mechanism may commonly be the persistent upregulation of cough reflex, 'chronic cough hypersensitivity'. Our efforts are being exerted to develop methodologies for clinical, experimental and epidemiological studies. We hope that the new paradigm of 'cough hypersensitivity' would bring us further advances in understanding and clinical practice of chronic cough.
References
- 1.Voorsanger WC, Firestone F. Etiological factors in chronic cough: an analysis of one hundred cases-preliminary report. Cal West Med. 1927;26:48–50. [PMC free article] [PubMed] [Google Scholar]
- 2.Brooks SM. Perspective on the human cough reflex. Cough. 2011;7:10. doi: 10.1186/1745-9974-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morice AH. Epidemiology of cough. Pulm Pharmacol Ther. 2002;15:253–259. doi: 10.1006/pupt.2002.0352. [DOI] [PubMed] [Google Scholar]
- 4.Chung KF, Pavord ID. Prevalence, pathogenesis, and causes of chronic cough. Lancet. 2008;371:1364–1374. doi: 10.1016/S0140-6736(08)60595-4. [DOI] [PubMed] [Google Scholar]
- 5.Song WJ, Morice AH, Kim MH, Lee SE, Jo EJ, Lee SM, Han JW, Kim TH, Kim SH, Jang HC, Kim KW, Cho SH, Min KU, Chang YS. Cough in the elderly population: relationships with multiple comorbidity. PLoS One. 2013;8:e78081. doi: 10.1371/journal.pone.0078081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Birring SS, Prudon B, Carr AJ, Singh SJ, Morgan MD, Pavord ID. Development of a symptom specific health status measure for patients with chronic cough: Leicester Cough Questionnaire (LCQ) Thorax. 2003;58:339–343. doi: 10.1136/thorax.58.4.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McGarvey L. The difficult-to-treat, therapy-resistant cough: why are current cough treatments not working and what can we do? Pulm Pharmacol Ther. 2013;26:528–531. doi: 10.1016/j.pupt.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 8.Phillips AM, Phillips RW, Thompson JL. Chronic cough: analysis of etiologic factors in a survey of 1,274 men. Ann Intern Med. 1956;45:216–231. doi: 10.7326/0003-4819-45-2-216. [DOI] [PubMed] [Google Scholar]
- 9.Irwin RS, Corrao WM, Pratter MR. Chronic persistent cough in the adult: the spectrum and frequency of causes and successful outcome of specific therapy. Am Rev Respir Dis. 1981;123(4 Pt 1):413–417. doi: 10.1164/arrd.1981.123.4.413. [DOI] [PubMed] [Google Scholar]
- 10.Irwin RS, Rosen MJ, Braman SS. Cough. A comprehensive review. Arch Intern Med. 1977;137:1186–1191. doi: 10.1001/archinte.137.9.1186. [DOI] [PubMed] [Google Scholar]
- 11.Morice AH, Fontana GA, Sovijarvi AR, Pistolesi M, Chung KF, Widdicombe J, O'Connell F, Geppetti P, Gronke L, De Jongste J, Belvisi M, Dicpinigaitis P, Fischer A, McGarvey L, Fokkens WJ, Kastelik J ERS Task Force. The diagnosis and management of chronic cough. Eur Respir J. 2004;24:481–492. doi: 10.1183/09031936.04.00027804. [DOI] [PubMed] [Google Scholar]
- 12.McGarvey LP. Does idiopathic cough exist? Lung. 2008;186(Suppl 1):S78–S81. doi: 10.1007/s00408-007-9048-4. [DOI] [PubMed] [Google Scholar]
- 13.O'Hara J, Jones NS. "Post-nasal drip syndrome": most patients with purulent nasal secretions do not complain of chronic cough. Rhinology. 2006;44:270–273. [PubMed] [Google Scholar]
- 14.Jaspersen D, Kulig M, Labenz J, Leodolter A, Lind T, Meyer-Sabellek W, Vieth M, Willich SN, Lindner D, Stolte M, Malfertheiner P. Prevalence of extra-oesophageal manifestations in gastro-oesophageal reflux disease: an analysis based on the ProGERD Study. Aliment Pharmacol Ther. 2003;17:1515–1520. doi: 10.1046/j.1365-2036.2003.01606.x. [DOI] [PubMed] [Google Scholar]
- 15.Irwin RS, Madison JM. The diagnosis and treatment of cough. N Engl J Med. 2000;343:1715–1721. doi: 10.1056/NEJM200012073432308. [DOI] [PubMed] [Google Scholar]
- 16.Morice AH. The cough hypersensitivity syndrome: a novel paradigm for understanding cough. Lung. 2010;188(Suppl 1):S87–S90. doi: 10.1007/s00408-009-9185-z. [DOI] [PubMed] [Google Scholar]
- 17.Chung KF. Chronic 'cough hypersensitivity syndrome': a more precise label for chronic cough. Pulm Pharmacol Ther. 2011;24:267–271. doi: 10.1016/j.pupt.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 18.Morice AH, McGarvey LP, Dicpinigaitis PV. Cough hypersensitivity syndrome is an important clinical concept: a pro/con debate. Lung. 2012;190:3–9. doi: 10.1007/s00408-011-9351-y. [DOI] [PubMed] [Google Scholar]
- 19.Dicpinigaitis PV, Fontana GA, Lee LY, Tatar M. Summary of papers presented at the 2012 seventh international cough symposium. Cough. 2013;9:13. doi: 10.1186/1745-9974-9-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morice AH. Chronic cough hypersensitivity syndrome. Cough. 2013;9:14. doi: 10.1186/1745-9974-9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morice AH. Rebuttal: cough is an expiratory sound. Lung. 2008;186(Suppl 1):S7–S9. doi: 10.1007/s00408-007-9039-5. [DOI] [PubMed] [Google Scholar]
- 22.Mazzone SB, McGovern AE, Yang SK, Woo A, Phipps S, Ando A, Leech J, Farrell MJ. Sensorimotor circuitry involved in the higher brain control of coughing. Cough. 2013;9:7. doi: 10.1186/1745-9974-9-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grace MS, Dubuis E, Birrell MA, Belvisi MG. Pre-clinical studies in cough research: role of Transient Receptor Potential (TRP) channels. Pulm Pharmacol Ther. 2013;26:498–507. doi: 10.1016/j.pupt.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Canning BJ, Mazzone SB, Meeker SN, Mori N, Reynolds SM, Undem BJ. Identification of the tracheal and laryngeal afferent neurones mediating cough in anaesthetized guinea-pigs. J Physiol. 2004;557(Pt 2):543–558. doi: 10.1113/jphysiol.2003.057885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Canning BJ, Mori N, Mazzone SB. Vagal afferent nerves regulating the cough reflex. Respir Physiol Neurobiol. 2006;152:223–242. doi: 10.1016/j.resp.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 26.Morice AH, Geppetti P. Cough. 5: The type 1 vanilloid receptor: a sensory receptor for cough. Thorax. 2004;59:257–258. doi: 10.1136/thx.2003.013482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Birrell MA, Belvisi MG, Grace M, Sadofsky L, Faruqi S, Hele DJ, Maher SA, Freund-Michel V, Morice AH. TRPA1 agonists evoke coughing in guinea pig and human volunteers. Am J Respir Crit Care Med. 2009;180:1042–1047. doi: 10.1164/rccm.200905-0665OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Canning BJ. Functional implications of the multiple afferent pathways regulating cough. Pulm Pharmacol Ther. 2011;24:295–299. doi: 10.1016/j.pupt.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 29.Song WJ, Kim JY, Jo EJ, Lee SE, Kim MH, Yang MS, Kang HR, Park HW, Chang YS, Min KU, Cho SH. Capsaicin cough sensitivity is related to the older female predominant feature in chronic cough patients. Allergy Asthma Immunol Res Forthcoming. 2014 doi: 10.4168/aair.2014.6.5.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee LY. Respiratory sensations evoked by activation of bronchopulmonary C-fibers. Respir Physiol Neurobiol. 2009;167:26–35. doi: 10.1016/j.resp.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davenport PW, Bolser DC, Vickroy T, Berry RB, Martin AD, Hey JA, Danzig M. The effect of codeine on the Urge-to-Cough response to inhaled capsaicin. Pulm Pharmacol Ther. 2007;20:338–346. doi: 10.1016/j.pupt.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hilton EC, Baverel PG, Woodcock A, Van Der Graaf PH, Smith JA. Pharmacodynamic modeling of cough responses to capsaicin inhalation calls into question the utility of the C5 end point. J Allergy Clin Immunol. 2013;132:847–855. e1–e5. doi: 10.1016/j.jaci.2013.04.042. [DOI] [PubMed] [Google Scholar]
- 33.Corrao WM, Braman SS, Irwin RS. Chronic cough as the sole presenting manifestation of bronchial asthma. N Engl J Med. 1979;300:633–637. doi: 10.1056/NEJM197903223001201. [DOI] [PubMed] [Google Scholar]
- 34.Brightling CE, Ward R, Goh KL, Wardlaw AJ, Pavord ID. Eosinophilic bronchitis is an important cause of chronic cough. Am J Respir Crit Care Med. 1999;160:406–410. doi: 10.1164/ajrccm.160.2.9810100. [DOI] [PubMed] [Google Scholar]
- 35.Brightling CE, Bradding P, Symon FA, Holgate ST, Wardlaw AJ, Pavord ID. Mast-cell infiltration of airway smooth muscle in asthma. N Engl J Med. 2002;346:1699–1705. doi: 10.1056/NEJMoa012705. [DOI] [PubMed] [Google Scholar]
- 36.Fujimura M, Ogawa H, Yasui M, Matsuda T. Eosinophilic tracheobronchitis and airway cough hypersensitivity in chronic non-productive cough. Clin Exp Allergy. 2000;30:41–47. doi: 10.1046/j.1365-2222.2000.00698.x. [DOI] [PubMed] [Google Scholar]
- 37.Birring SS, Parker D, Brightling CE, Bradding P, Wardlaw AJ, Pavord ID. Induced sputum inflammatory mediator concentrations in chronic cough. Am J Respir Crit Care Med. 2004;169:15–19. doi: 10.1164/rccm.200308-1092OC. [DOI] [PubMed] [Google Scholar]
- 38.Ternesten-Hasseus E, Farbrot A, Lowhagen O, Millqvist E. Sensitivity to methacholine and capsaicin in patients with unclear respiratory symptoms. Allergy. 2002;57:501–507. doi: 10.1034/j.1398-9995.2002.23380.x. [DOI] [PubMed] [Google Scholar]
- 39.Haldar P, Brightling CE, Hargadon B, Gupta S, Monteiro W, Sousa A, Marshall RP, Bradding P, Green RH, Wardlaw AJ, Pavord ID. Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med. 2009;360:973–984. doi: 10.1056/NEJMoa0808991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Niimi A, Brightling CE, Dicpinigaitis PV. Cough in asthma Is due to eosinophilic airway inflammation: a Pro/Con Debate. Lung. 2013 Dec 14; doi: 10.1007/s00408-013-9543-8. [Epub]. http://dx.doi.org/10.1007/s00408-013-9543-8. [DOI] [PubMed] [Google Scholar]
- 41.Profita M, Sala A, Bonanno A, Riccobono L, Siena L, Melis MR, Di Giorgi R, Mirabella F, Gjomarkaj M, Bonsignore G, Vignola AM. Increased prostaglandin E2 concentrations and cyclooxygenase-2 expression in asthmatic subjects with sputum eosinophilia. J Allergy Clin Immunol. 2003;112:709–716. doi: 10.1016/s0091-6749(03)01889-x. [DOI] [PubMed] [Google Scholar]
- 42.Grace M, Birrell MA, Dubuis E, Maher SA, Belvisi MG. Transient receptor potential channels mediate the tussive response to prostaglandin E2 and bradykinin. Thorax. 2012;67:891–900. doi: 10.1136/thoraxjnl-2011-201443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hwang SW, Cho H, Kwak J, Lee SY, Kang CJ, Jung J, Cho S, Min KH, Suh YG, Kim D, Oh U. Direct activation of capsaicin receptors by products of lipoxygenases: endogenous capsaicin-like substances. Proc Natl Acad Sci U S A. 2000;97:6155–6160. doi: 10.1073/pnas.97.11.6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Caceres AI, Brackmann M, Elia MD, Bessac BF, del Camino D, D'Amours M, Witek JS, Fanger CM, Chong JA, Hayward NJ, Homer RJ, Cohn L, Huang X, Moran MM, Jordt SE. A sensory neuronal ion channel essential for airway inflammation and hyperreactivity in asthma. Proc Natl Acad Sci U S A. 2009;106:9099–9104. doi: 10.1073/pnas.0900591106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hox V, Vanoirbeek JA, Alpizar YA, Voedisch S, Callebaut I, Bobic S, Sharify A, De Vooght V, Van Gerven L, Devos F, Liston A, Voets T, Vennekens R, Bullens DM, De Vries A, Hoet P, Braun A, Ceuppens JL, Talavera K, Nemery B, Hellings PW. Crucial role of transient receptor potential ankyrin 1 and mast cells in induction of nonallergic airway hyperreactivity in mice. Am J Respir Crit Care Med. 2013;187:486–493. doi: 10.1164/rccm.201208-1358OC. [DOI] [PubMed] [Google Scholar]
- 46.Wu Y, You H, Ma P, Li L, Yuan Y, Li J, Ye X, Liu X, Yao H, Chen R, Lai K, Yang X. Role of transient receptor potential ion channels and evoked levels of neuropeptides in a formaldehyde-induced model of asthma in BALB/c mice. PLoS One. 2013;8:e62827. doi: 10.1371/journal.pone.0062827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nassini R, Pedretti P, Moretto N, Fusi C, Carnini C, Facchinetti F, Viscomi AR, Pisano AR, Stokesberry S, Brunmark C, Svitacheva N, McGarvey L, Patacchini R, Damholt AB, Geppetti P, Materazzi S. Transient receptor potential ankyrin 1 channel localized to non-neuronal airway cells promotes non-neurogenic inflammation. PLoS One. 2012;7:e42454. doi: 10.1371/journal.pone.0042454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sadofsky LR, Ramachandran R, Crow C, Cowen M, Compton SJ, Morice AH. Inflammatory stimuli up-regulate transient receptor potential vanilloid-1 expression in human bronchial fibroblasts. Exp Lung Res. 2012;38:75–81. doi: 10.3109/01902148.2011.644027. [DOI] [PubMed] [Google Scholar]
- 49.McGarvey LP, Butler CA, Stokesberry S, Polley L, McQuaid S, Abdullah H, Ashraf S, McGahon MK, Curtis TM, Arron J, Choy D, Warke TJ, Bradding P, Ennis M, Zholos A, Costello RW, Heaney LG. Increased expression of bronchial epithelial transient receptor potential vanilloid 1 channels in patients with severe asthma. J Allergy Clin Immunol. 2013 Nov 07; doi: 10.1016/j.jaci.2013.09.016. [Epub]. http://dx.doi.org/10.1016/j.jaci.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 50.Plevkova J, Song WJ. Chronic cough in subjects with upper airway diseases: analysis of mechanisms and clinical applications. Asia Pac Allergy. 2013;3:127–135. doi: 10.5415/apallergy.2013.3.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tatar M, Plevkova J, Brozmanova M, Pecova R, Kollarik M. Mechanisms of the cough associated with rhinosinusitis. Pulm Pharmacol Ther. 2009;22:121–126. doi: 10.1016/j.pupt.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 52.Pecova R, Vrlik M, Tatar M. Cough sensitivity in allergic rhinitis. J Physiol Pharmacol. 2005;56(Suppl 4):171–178. [PubMed] [Google Scholar]
- 53.Plevkova J, Poliacek I, Antosiewicz J, Adamkov M, Jakus J, Svirlochova K, Tatar M. Intranasal TRPV1 agonist capsaicin challenge and its effect on c-fos expression in the guinea pig brainstem. Respir Physiol Neurobiol. 2010;173:11–15. doi: 10.1016/j.resp.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 54.Buday T, Brozmanova M, Biringerova Z, Gavliakova S, Poliacek I, Calkovsky V, Shetthalli MV, Plevkova J. Modulation of cough response by sensory inputs from the nose - role of trigeminal TRPA1 versus TRPM8 channels. Cough. 2012;8:11. doi: 10.1186/1745-9974-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weusten BL, Roelofs JM, Akkermans LM, Van Berge-Henegouwen GP, Smout AJ. The symptom-association probability: an improved method for symptom analysis of 24-hour esophageal pH data. Gastroenterology. 1994;107:1741–1745. doi: 10.1016/0016-5085(94)90815-x. [DOI] [PubMed] [Google Scholar]
- 56.Patterson N, Mainie I, Rafferty G, McGarvey L, Heaney L, Tutuian R, Castell D, Johnston BT. Nonacid reflux episodes reaching the pharynx are important factors associated with cough. J Clin Gastroenterol. 2009;43:414–419. doi: 10.1097/MCG.0b013e31818859a3. [DOI] [PubMed] [Google Scholar]
- 57.Faruqi S, Sedman P, Jackson W, Molyneux I, Morice AH. Fundoplication in chronic intractable cough. Cough. 2012;8:3. doi: 10.1186/1745-9974-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zerbib F, Roman S, Bruley Des Varannes S, Gourcerol G, Coffin B, Ropert A, Lepicard P, Mion F Groupe Francais De Neuro-Gastroenterologie. Normal values of pharyngeal and esophageal 24-hour pH impedance in individuals on and off therapy and interobserver reproducibility. Clin Gastroenterol Hepatol. 2013;11:366–372. doi: 10.1016/j.cgh.2012.10.041. [DOI] [PubMed] [Google Scholar]
- 59.Crossfield GL, Jackson W, Burke J, Woodcock AD, Strugala V, Ward C, Pearson JP, Dettmar PW, Morice AH. Pepsin detection despite the use of acid suppressant medication in patients with airway reflux related chronic cough. Thorax. 2013;68:A19. [Google Scholar]
- 60.Belafsky PC, Postma GN, Koufman JA. Validity and reliability of the reflux symptom index (RSI) J Voice. 2002;16:274–277. doi: 10.1016/s0892-1997(02)00097-8. [DOI] [PubMed] [Google Scholar]
- 61.Morice AH, Faruqi S, Wright CE, Thompson R, Bland JM. Cough hypersensitivity syndrome: a distinct clinical entity. Lung. 2011;189:73–79. doi: 10.1007/s00408-010-9272-1. [DOI] [PubMed] [Google Scholar]
- 62.Gu Q, Lee LY. Airway irritation and cough evoked by acid: from human to ion channel. Curr Opin Pharmacol. 2011;11:238–247. doi: 10.1016/j.coph.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kwon NH, Oh MJ, Min TH, Lee BJ, Choi DC. Causes and clinical features of subacute cough. Chest. 2006;129:1142–1147. doi: 10.1378/chest.129.5.1142. [DOI] [PubMed] [Google Scholar]
- 64.Dicpinigaitis PV, Bhat R, Rhoton WA, Tibb AS, Negassa A. Effect of viral upper respiratory tract infection on the urge-to-cough sensation. Respir Med. 2011;105:615–618. doi: 10.1016/j.rmed.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 65.O'Connell F, Thomas VE, Studham JM, Pride NB, Fuller RW. Capsaicin cough sensitivity increases during upper respiratory infection. Respir Med. 1996;90:279–286. doi: 10.1016/s0954-6111(96)90099-2. [DOI] [PubMed] [Google Scholar]
- 66.Abdullah H, Heaney LG, Cosby SL, McGarvey LP. Rhinovirus upregulates transient receptor potential channels in a human neuronal cell line: implications for respiratory virus-induced cough reflex sensitivity. Thorax. 2014;69:46–54. doi: 10.1136/thoraxjnl-2013-203894. [DOI] [PubMed] [Google Scholar]
- 67.Kusel MM, de Klerk NH, Kebadze T, Vohma V, Holt PG, Johnston SL, Sly PD. Early-life respiratory viral infections, atopic sensitization, and risk of subsequent development of persistent asthma. J Allergy Clin Immunol. 2007;119:1105–1110. doi: 10.1016/j.jaci.2006.12.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Morice AH, Jakes AD, Faruqi S, Birring S, McGarvey L, Canning B, Smith JA, Parker S, Chung KF, Lai KF, Mazzone S, Pavord ID, Dicpinigaitis PV. Age and sex distribution of patients presenting to specialist cough clinics [abstract]; American Thoracic Society 2013 International Conference; 2013 May 17-22; Philadelphia, USA. New York: American Thoracic Society; 2013. p. A1943. [Google Scholar]
- 69.Visser LE, Stricker BH, van der Velden J, Paes AH, Bakker A. Angiotensin converting enzyme inhibitor associated cough: a population-based case-control study. J Clin Epidemiol. 1995;48:851–857. doi: 10.1016/0895-4356(94)00231-e. [DOI] [PubMed] [Google Scholar]
- 70.Morice AH, Lowry R, Brown MJ, Higenbottam T. Angiotensin-converting enzyme and the cough reflex. Lancet. 1987;2:1116–1118. doi: 10.1016/s0140-6736(87)91547-9. [DOI] [PubMed] [Google Scholar]
- 71.Dicpinigaitis PV. Angiotensin-converting enzyme inhibitor-induced cough: ACCP evidence-based clinical practice guidelines. Chest. 2006;129(1 Suppl):169S–173S. doi: 10.1378/chest.129.1_suppl.169S. [DOI] [PubMed] [Google Scholar]
- 72.Patberg KW. The female preponderance to cough hypersensitivity syndrome: another clue pointing to the role of TRPV1 in cough. Lung. 2011;189:257–258. doi: 10.1007/s00408-011-9295-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pham TV, Rosen MR. Sex, hormones, and repolarization. Cardiovasc Res. 2002;53:740–751. doi: 10.1016/s0008-6363(01)00429-1. [DOI] [PubMed] [Google Scholar]
- 74.Yan T, Liu B, Du D, Eisenach JC, Tong C. Estrogen amplifies pain responses to uterine cervical distension in rats by altering transient receptor potential-1 function. Anesth Analg. 2007;104:1246–1250. doi: 10.1213/01.ane.0000263270.39480.a2. [DOI] [PubMed] [Google Scholar]
- 75.Chang AB, Phelan PD, Sawyer SM, Del Brocco S, Robertson CF. Cough sensitivity in children with asthma, recurrent cough, and cystic fibrosis. Arch Dis Child. 1997;77:331–334. doi: 10.1136/adc.77.4.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Varechova S, Plevkova J, Hanacek J, Tatar M. Role of gender and pubertal stage on cough sensitivity in childhood and adolescence. J Physiol Pharmacol. 2008;59(Suppl 6):719–726. [PubMed] [Google Scholar]
- 77.Chung KF, McGarvey L, Mazzone SB. Chronic cough as a neuropathic disorder. Lancet Respir Med. 2013;1:414–422. doi: 10.1016/S2213-2600(13)70043-2. [DOI] [PubMed] [Google Scholar]
- 78.Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley JL., 3rd Sex, gender, and pain: a review of recent clinical and experimental findings. J Pain. 2009;10:447–485. doi: 10.1016/j.jpain.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Derbyshire SW, Nichols TE, Firestone L, Townsend DW, Jones AK. Gender differences in patterns of cerebral activation during equal experience of painful laser stimulation. J Pain. 2002;3:401–411. doi: 10.1054/jpai.2002.126788. [DOI] [PubMed] [Google Scholar]
- 80.Paulson PE, Minoshima S, Morrow TJ, Casey KL. Gender differences in pain perception and patterns of cerebral activation during noxious heat stimulation in humans. Pain. 1998;76:223–229. doi: 10.1016/s0304-3959(98)00048-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Moulton EA, Keaser ML, Gullapalli RP, Maitra R, Greenspan JD. Sex differences in the cerebral BOLD signal response to painful heat stimuli. Am J Physiol Regul Integr Comp Physiol. 2006;291:R257–R267. doi: 10.1152/ajpregu.00084.2006. [DOI] [PubMed] [Google Scholar]
- 82.Yarnitsky D. Conditioned pain modulation (the diffuse noxious inhibitory control-like effect): its relevance for acute and chronic pain states. Curr Opin Anaesthesiol. 2010;23:611–615. doi: 10.1097/ACO.0b013e32833c348b. [DOI] [PubMed] [Google Scholar]
- 83.Philpott H, Gibson P, Thien F. Irritable bowel syndrome - an inflammatory disease involving mast cells. Asia Pac Allergy. 2011;1:36–42. doi: 10.5415/apallergy.2011.1.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lovell RM, Ford AC. Effect of gender on prevalence of irritable bowel syndrome in the community: systematic review and meta-analysis. Am J Gastroenterol. 2012;107:991–1000. doi: 10.1038/ajg.2012.131. [DOI] [PubMed] [Google Scholar]
- 85.Ford AC, Forman D, Moayyedi P, Morice AH. Cough in the community: a cross sectional survey and the relationship to gastrointestinal symptoms. Thorax. 2006;61:975–979. doi: 10.1136/thx.2006.060087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hamdan AL, Jabbour J, Barazi R, Korban Z, Azar ST. Prevalence of laryngopharyngeal reflux disease in patients with diabetes mellitus. J Voice. 2013;27:495–499. doi: 10.1016/j.jvoice.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 87.Wang X, Pitchumoni CS, Chandrarana K, Shah N. Increased prevalence of symptoms of gastroesophageal reflux diseases in type 2 diabetics with neuropathy. World J Gastroenterol. 2008;14:709–712. doi: 10.3748/wjg.14.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lee JH, McCarty R. Pain threshold in diabetic rats: effects of good versus poor diabetic control. Pain. 1992;50:231–236. doi: 10.1016/0304-3959(92)90167-A. [DOI] [PubMed] [Google Scholar]
- 89.Bair MJ, Brizendine EJ, Ackermann RT, Shen C, Kroenke K, Marrero DG. Prevalence of pain and association with quality of life, depression and glycaemic control in patients with diabetes. Diabet Med. 2010;27:578–584. doi: 10.1111/j.1464-5491.2010.02971.x. [DOI] [PubMed] [Google Scholar]
- 90.Herbert MS, Varley AL, Andreae SJ, Goodin BR, Bradley LA, Safford MM. Association of pain with HbA1c in a predominantly black population of community-dwelling adults with diabetes: a cross-sectional analysis. Diabet Med. 2013;30:1466–1471. doi: 10.1111/dme.12264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Smit LA, Kogevinas M, Anto JM, Bouzigon E, Gonzalez JR, Le Moual N, Kromhout H, Carsin AE, Pin I, Jarvis D, Vermeulen R, Janson C, Heinrich J, Gut I, Lathrop M, Valverde MA, Demenais F, Kauffmann F. Transient receptor potential genes, smoking, occupational exposures and cough in adults. Respir Res. 2012;13:26. doi: 10.1186/1465-9921-13-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Park HK, Oh SY, Kim TB, Bahn JW, Shin ES, Lee JE, Oh HB, Kim YK, Park T, Cho SH, Min KU, Kim YY. Association of genetic variations in neurokinin-2 receptor with enhanced cough sensitivity to capsaicin in chronic cough. Thorax. 2006;61:1070–1075. doi: 10.1136/thx.2005.054429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Morjaria JB, Dickinson RS, Morice AH. Novel antitussive strategies. Drug Discov Today. 2013;18:380–388. doi: 10.1016/j.drudis.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 94.Morice AH, Menon MS, Mulrennan SA, Everett CF, Wright C, Jackson J, Thompson R. Opiate therapy in chronic cough. Am J Respir Crit Care Med. 2007;175:312–315. doi: 10.1164/rccm.200607-892OC. [DOI] [PubMed] [Google Scholar]
- 95.Ryan NM, Birring SS, Gibson PG. Gabapentin for refractory chronic cough: a randomised, double-blind, placebo-controlled trial. Lancet. 2012;380:1583–1589. doi: 10.1016/S0140-6736(12)60776-4. [DOI] [PubMed] [Google Scholar]
- 96.Smith JA, Murdoch RD, Newlands A, Smart K, Khalid S, Kelsall A, Holt K, Dockry R, Woodcock A. The impact of a selective oral TRPV1 antagonist in patients with chronic cough. Thorax. 2012;67:A128. doi: 10.1016/j.jaci.2014.01.038. [DOI] [PubMed] [Google Scholar]
- 97.Abdulqawi R, Dockry R, Holt K, Woodcock A, Layton G, McCarthy B, Ford A, Smith J. Inhibition of ATP-gated P2X3 channels by AF-219: An effective anti-tussive mechanism in chronic cough [oral presentation]; Annual Cogress Barcelona 2013; 2013 Sep 7-11; Barcelona, Spain. Lausanne (CH): European Respiratory Society; 2013. [Google Scholar]
- 98.Ford AP. In pursuit of P2X3 antagonists: novel therapeutics for chronic pain and afferent sensitization. Purinergic Signal. 2012;8(Suppl 1):3–26. doi: 10.1007/s11302-011-9271-6. [DOI] [PMC free article] [PubMed] [Google Scholar]