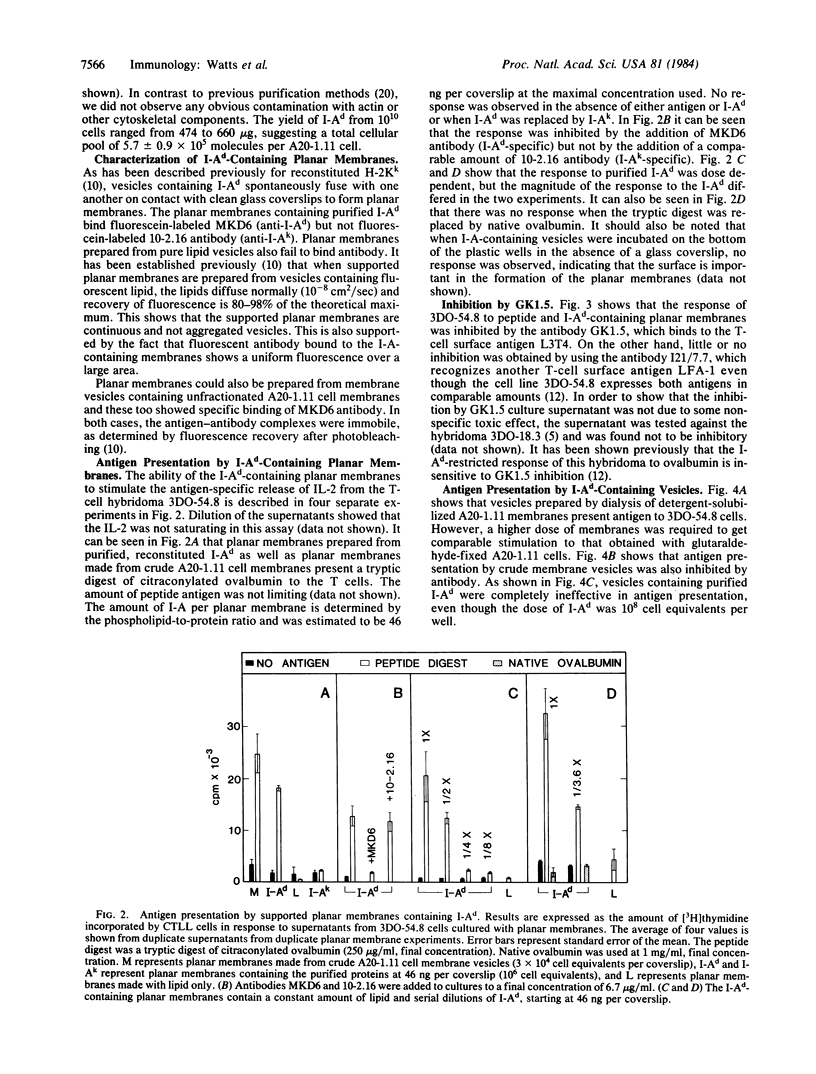
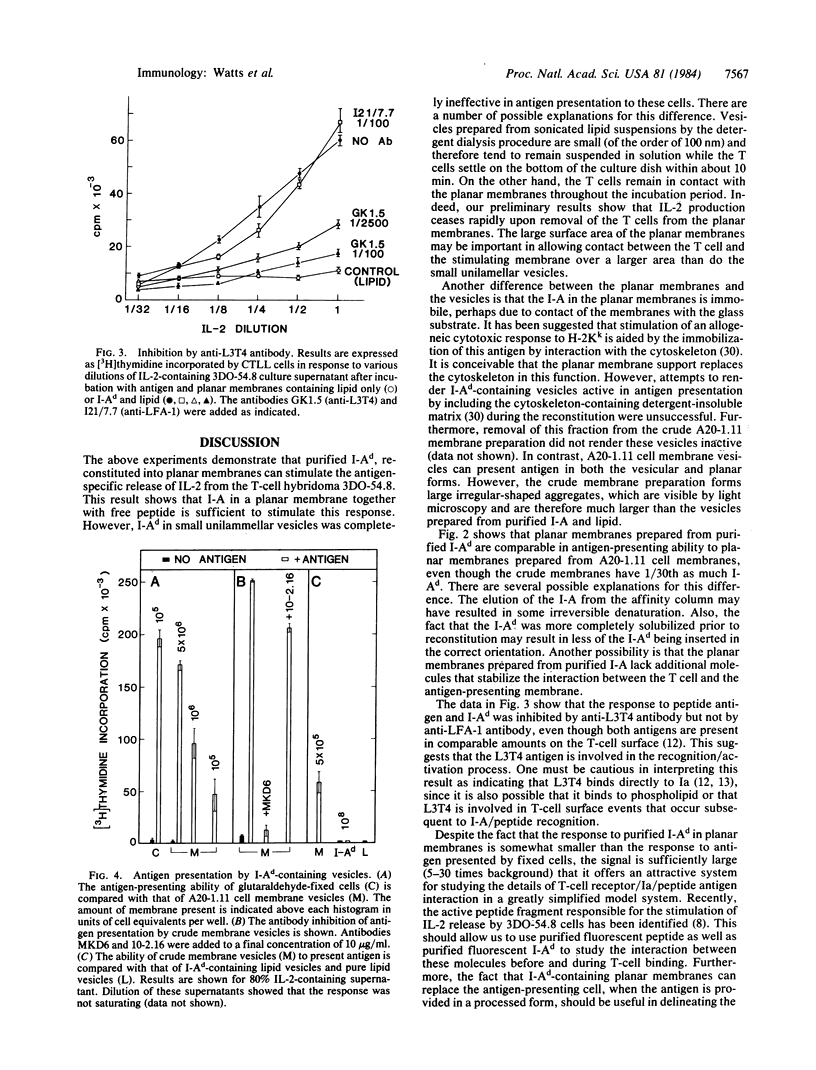
Abstract
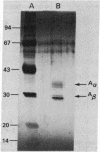
I-Ad, purified from A20-1.11 cells by affinity chromatography, was incorporated into supported planar membranes by incubation of I-Ad-containing phospholipid vesicles with clean glass coverslips. Such planar membranes present a peptide digest of ovalbumin to the ovalbumin-specific, I-Ad-restricted T-cell hybridoma 3DO-54.8, resulting in the antigen-specific release of interleukin 2. However, when the same material was provided in the form of small unilamellar vesicles, no response was obtained. Antigen presentation by the I-Ad-containing planar membranes was inhibited by the monoclonal antibody MKD6 (anti-I-Ad) but not by the antibody 10-2.16 (anti-I-Ak). The antibody GK1.5, which recognizes the T-cell surface antigen L3T4, was also inhibitory. In contrast to the results with purified I-Ad, crude membrane preparations from A20-1.11 cells were effective in antigen presentation in both planar and vesicular forms.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker P. E., Gillis S., Smith K. A. Monoclonal cytolytic T-cell lines. J Exp Med. 1979 Jan 1;149(1):273–278. doi: 10.1084/jem.149.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benacerraf B. A hypothesis to relate the specificity of T lymphocytes and the activity of I region-specific Ir genes in macrophages and B lymphocytes. J Immunol. 1978 Jun;120(6):1809–1812. [PubMed] [Google Scholar]
- Brian A. A., McConnell H. M. Allogeneic stimulation of cytotoxic T cells by supported planar membranes. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6159–6163. doi: 10.1073/pnas.81.19.6159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dialynas D. P., Wilde D. B., Marrack P., Pierres A., Wall K. A., Havran W., Otten G., Loken M. R., Pierres M., Kappler J. Characterization of the murine antigenic determinant, designated L3T4a, recognized by monoclonal antibody GK1.5: expression of L3T4a by functional T cell clones appears to correlate primarily with class II MHC antigen-reactivity. Immunol Rev. 1983;74:29–56. doi: 10.1111/j.1600-065x.1983.tb01083.x. [DOI] [PubMed] [Google Scholar]
- Gibbons I., Perham R. N. The reaction of aldolase with 2-methylmaleic anhydride. Biochem J. 1970 Mar;116(5):843–849. doi: 10.1042/bj1160843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenstein J. L., Kappler J., Marrack P., Burakoff S. J. The role of L3T4 in recognition of Ia by a cytotoxic, H-2Dd-specific T cell hybridoma. J Exp Med. 1984 Apr 1;159(4):1213–1224. doi: 10.1084/jem.159.4.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann S. H., Mescher M. F. Secondary cytolytic T lymphocyte stimulation by purified H-2Kk in liposomes. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2488–2492. doi: 10.1073/pnas.78.4.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappler J. W., Skidmore B., White J., Marrack P. Antigen-inducible, H-2-restricted, interleukin-2-producing T cell hybridomas. Lack of independent antigen and H-2 recognition. J Exp Med. 1981 May 1;153(5):1198–1214. doi: 10.1084/jem.153.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K. J., Kanellopoulos-Langevin C., Merwin R. M., Sachs D. H., Asofsky R. Establishment and characterization of BALB/c lymphoma lines with B cell properties. J Immunol. 1979 Feb;122(2):549–554. [PubMed] [Google Scholar]
- Klein J., Juretic A., Baxevanis C. N., Nagy Z. A. The traditional and a new version of the mouse H-2 complex. Nature. 1981 Jun 11;291(5815):455–460. doi: 10.1038/291455a0. [DOI] [PubMed] [Google Scholar]
- Marrack P., Endres R., Shimonkevitz R., Zlotnik A., Dialynas D., Fitch F., Kappler J. The major histocompatibility complex-restricted antigen receptor on T cells. II. Role of the L3T4 product. J Exp Med. 1983 Oct 1;158(4):1077–1091. doi: 10.1084/jem.158.4.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oi V. T., Jones P. P., Goding J. W., Herzenberg L. A., Herzenberg L. A. Properties of monoclonal antibodies to mouse Ig allotypes, H-2, and Ia antigens. Curr Top Microbiol Immunol. 1978;81:115–120. doi: 10.1007/978-3-642-67448-8_18. [DOI] [PubMed] [Google Scholar]
- Rao A., Ko W. W., Faas S. J., Cantor H. Binding of antigen in the absence of histocompatibility proteins by arsonate-reactive T-cell clones. Cell. 1984 Apr;36(4):879–888. doi: 10.1016/0092-8674(84)90037-0. [DOI] [PubMed] [Google Scholar]
- Rock K. L., Benacerraf B. Inhibition of antigen-specific T lymphocyte activation by structurally related Ir gene-controlled polymers. Evidence of specific competition for accessory cell antigen presentation. J Exp Med. 1983 May 1;157(5):1618–1634. doi: 10.1084/jem.157.5.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salacinski P. R., McLean C., Sykes J. E., Clement-Jones V. V., Lowry P. J. Iodination of proteins, glycoproteins, and peptides using a solid-phase oxidizing agent, 1,3,4,6-tetrachloro-3 alpha,6 alpha-diphenyl glycoluril (Iodogen). Anal Biochem. 1981 Oct;117(1):136–146. doi: 10.1016/0003-2697(81)90703-x. [DOI] [PubMed] [Google Scholar]
- Schwartz R. H., Yano A., Stimpfling J. H., Paul W. E. Gene complementation in the T-lymphocyte proliferative response to poly (Glu55Lys36Phe9)n. A demonstration that both immune response gene products must be expressed in the same antigen-presenting cell. J Exp Med. 1979 Jan 1;149(1):40–57. doi: 10.1084/jem.149.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevach E. M., Rosenthal A. S. Function of macrophages in antigen recognition by guinea pig T lymphocytes. II. Role of the macrophage in the regulation of genetic control of the immune response. J Exp Med. 1973 Nov 1;138(5):1213–1229. doi: 10.1084/jem.138.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimonkevitz R., Colon S., Kappler J. W., Marrack P., Grey H. M. Antigen recognition by H-2-restricted T cells. II. A tryptic ovalbumin peptide that substitutes for processed antigen. J Immunol. 1984 Oct;133(4):2067–2074. [PubMed] [Google Scholar]
- Shimonkevitz R., Kappler J., Marrack P., Grey H. Antigen recognition by H-2-restricted T cells. I. Cell-free antigen processing. J Exp Med. 1983 Aug 1;158(2):303–316. doi: 10.1084/jem.158.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung E., Jones P. P. The invariant chain of murine Ia antigens: its glycosylation, abundance and subcellular localization. Mol Immunol. 1981 Oct;18(10):899–913. doi: 10.1016/0161-5890(81)90013-4. [DOI] [PubMed] [Google Scholar]
- Trowbridge I. S., Omary M. B. Molecular complexity of leukocyte surface glycoproteins related to the macrophage differentiation antigen Mac-1. J Exp Med. 1981 Nov 1;154(5):1517–1524. doi: 10.1084/jem.154.5.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkewitz A. P., Sullivan C. P., Mescher M. F. Large-scale purification of murine I-Ak and I-Ek antigens and characterization of the purified proteins. Mol Immunol. 1983 Nov;20(11):1139–1147. doi: 10.1016/0161-5890(83)90137-2. [DOI] [PubMed] [Google Scholar]
- Walker E., Warner N. L., Chesnut R., Kappler J., Marrack P. Antigen-specific. I region-restricted interactions in vitro between tumor cell lines and T cell hybridomas. J Immunol. 1982 May;128(5):2164–2169. [PubMed] [Google Scholar]
- Weis R. M., Balakrishnan K., Smith B. A., McConnell H. M. Stimulation of fluorescence in a small contact region between rat basophil leukemia cells and planar lipid membrane targets by coherent evanescent radiation. J Biol Chem. 1982 Jun 10;257(11):6440–6445. [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]
- Ziegler H. K., Unanue E. R. Decrease in macrophage antigen catabolism caused by ammonia and chloroquine is associated with inhibition of antigen presentation to T cells. Proc Natl Acad Sci U S A. 1982 Jan;79(1):175–178. doi: 10.1073/pnas.79.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]