Abstract
Both the reticulospinal and corticospinal systems are known to control recruitment of upper limb muscles, yet no known studies have attempted to assess their combined effects in the same experiment in the awake, behaving primate. The purpose of this study is to present an approach for the analysis of the cooperative control from these two motor systems. Muscle responses to electrical stimulation in the reticulospinal system and corticospinal system alone or in combination were studied. The responses were categorized based on simple neural circuits that could explain the interactions of these systems. Five such circuits were identified that could explain 86% of the observed patterns of combined recruitment during stimulation. Improved understanding of the cooperation between these motor systems could provide insight for development of better rehabilitation approaches for stroke patients and others with movement disorders.
Keywords: Corticospinal system, reticulospinal system, electrical stimulation, neural circuit, upper limb
INTRODUCTION
The reticulospinal system is a major descending system for motor control, and is perhaps best known for its role in control of whole body movements such as posture and locomotion [1–4]. Data currently in the literature show that the reticulospinal system recruits both proximal and distal muscles of the upper limb (UL) bilaterally [5–7] and can also influence hand muscles [8;9]. Originating in the ponto-medullary reticular formation (PMRF) (Fig. 1) in the brainstem, the reticulospinal system descends to the spinal cord and influences motor pools directly and through spinal neural networks [10]. In addition to direct recruitment during movement, neurons in the reticulospinal system display increased neural activity during preparation for movement [11;12].
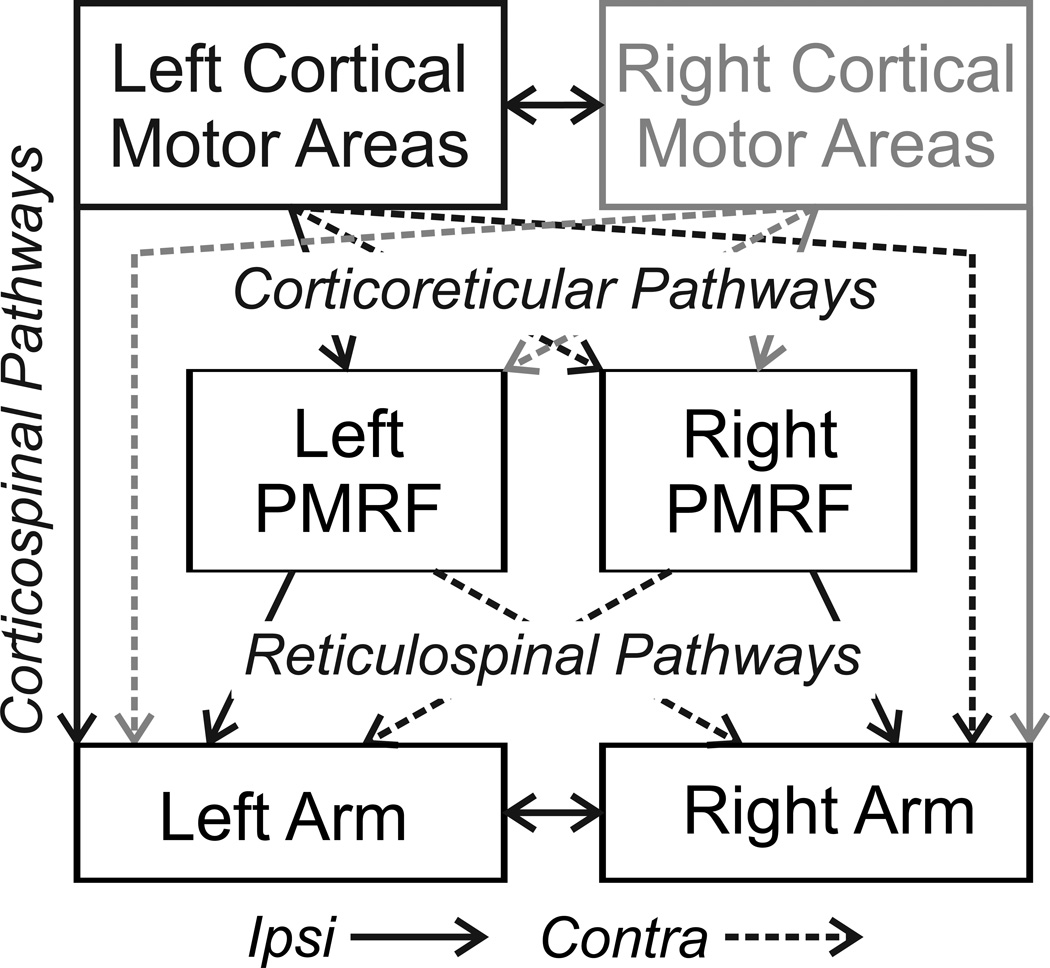
Figure 1.
Diagram of connections between cortical motor areas, the pontomedullary reticular formation (PMRF), and spinal cord segments for control of the upper limbs. Solid lines represent ipsilateral pathways, dashed lines contralateral. In the present study, stimulation was performed in the left cortical motor areas and in left and right sides of the PMRF. The grey connections show comparable projections from the right cortex.
In contrast to the reticulospinal system, the corticospinal system is perhaps the best understood system for motor control. With a substantial number of monosynaptic connections to motoneurons in the spinal cord, the corticospinal system is a significant driver of muscle recruitment and fine control [13;14]. In addition to this monosynaptic projection, it is well known that the corticospinal system also works through spinal neural networks [13;15;16]. The muscle recruitment resulting from the corticospinal system has been observed to be contralateral in most cases, but bilateral and ipsilateral effects can be observed with varying frequency depending on position of the muscle (proximal/distal) and cortical area stimulated [16;17].
Both the reticulospinal and corticospinal systems are known to work together in the recruitment of muscles [16], yet no known studies have attempted to assess their effects and interactions under the same experimental design in the awake, behaving primate. The purpose of this study is to present an initial approach for the analysis of the cooperative effects of these two motor systems. This initial attempt categorized muscle responses based on simple neural circuits. An understanding of how these two systems cooperate (or compete) is essential for a better understanding of motor control and could provide improved insight for development of rehabilitation approaches for patient’s recovery from stroke and other forms of damage to the motor system.
MATERIAL AND METHODS
Subjects and Task
Three male monkeys (Macaca fascicularis) identified as N, H, and O were trained to perform a reaching task previously reported [12]. The goal of this task was the generation of muscle activity in both upper limbs through a bilateral reach task that involved reaching a touch sensitive screen with one hand, while the other rested on a pressure sensitive sensor. The subjects were cared for as required by the NIH Guide for the Care and Use of Laboratory Animals and the laboratory protocol approved by the Institutional Animal Care and Use Committee at The Ohio State University. Every surgical procedure was supervised by a veterinarian in aseptic conditions. Further details of surgical procedures are published [17].
Stimulation (Cortex and PMRF)
For cortical stimulation, between two to four glass coated electrodes (Alpha Omega, Alpharetta, GA) were placed in the cortex, specifically in areas with strong relation to arm and shoulder activity. Three areas were stimulated in the cortex (CX): primary motor (M1), supplementary motor area (SMA), and pre-motor area (PMA). These areas are associated with the generation and planning of movements [18;19]. Similarly, polyimide/epoxy insulated tungsten electrodes (Frederick Haer & Co, Brunswick, ME) were placed in the PMRF, the source of the reticulospinal tracts, as previously described [20]. Both areas were stimulated with 36 biphasic pulses (200 us per phase) at 333 Hz delivered by using a digital stimulus controller (A.M.P.I. Master 8, Jerusalem, Israel) connected to a current-controlled stimulus isolator (AM-Systems model 2200, Sequim, WA, USA). Stimulation threshold currents were determined by the lowest visual response from the muscles (observed as a twitch) with threshold determined to within ±5 µA. Once the thresholds were noted, stimulus trains were applied either to one area at a time, a cortical electrode or the PMRF electrode, or jointly to a cortical electrode in conjunction with the PMRF electrode, at a current equal to the threshold. For joint stimulation, three different arrangements were created: RF followed by CX, CX followed by RF, and both at the same time. Four different stimulations were applied at each electrode site (e.g. 1: RF only, 2: CX only, 3: RF followed by CX, and 4: CX followed by RF). The initiation of the second stimulation (in a dual stimulation paradigm) varied from both initiated at the same time, to initiated within the middle of the other electrode’s train (at .033 ms of the .1 ms duration). In cases with multiple cortical electrodes, the joint patterns and cortical stimulation alone were conducted for each cortical electrode. For every electrode combination, stimulation was repeated 10 times in order to average an overall effect of the stimulation and avoid any outlier observations. The exception to this paradigm was subject O, being the first on the experiment, this subject was exposed to only three stimulation paradigms (1: RF only, 2: CX only, and 3: RF followed by CX).
EMG Recording
For electromyographic (EMG) recording, electrodes were implanted subcutaneously in 12 different muscles on each side of the UL region (24 total) according to established techniques (Davidson and Buford 2006). The muscles studied across all subjects were these: flexor carpi ulnaris, extensor carpi radialis, biceps, triceps, middle deltoid, latissimus dorsi, supraspinatus, upper trapezius, pectoralis major, and cervical paraspinal. A few muscles were unique to particular subjects, as follows: lumbar paraspinal (subjects H and O), brachialis (subject H), teres major (subject H), and sternocleidomastoid (subjects H and O). The 24 muscles were connected to an amplifier and sampled by Power 1401 CED data acquisition system (CED, Cambridge, UK). The data was filtered using a bandpass of 20 Hz to 3 kHz and sampled at 5 kHz. The EMG recordings were used to observe facilitation/suppression patterns resulting from stimulation. For a given set of stimulation sites (CX, RF, or joint), the responses were evaluated visually in comparison to voltage thresholds representing the mean level prior to stimulation and a level +/− 2 S.D. (standard deviation) from the mean in order to recognize significant responses.
Data Analysis
To simplify observations in the EMG recordings, a special set of notations were used to categorize the patterns. For each muscle, the response was coded as facilitation (+), suppression (−) or no response (/) (Fig. 2). The patterns across stimulation conditions were coded by the type of response and were based on visual inspection. For example, if biceps was facilitated by PMRF stimulation alone, showed no response to cortical stimulation, and did respond to combined stimulation of the PMRF and Cortex in either combination, the notation was “+,/,+,+”.
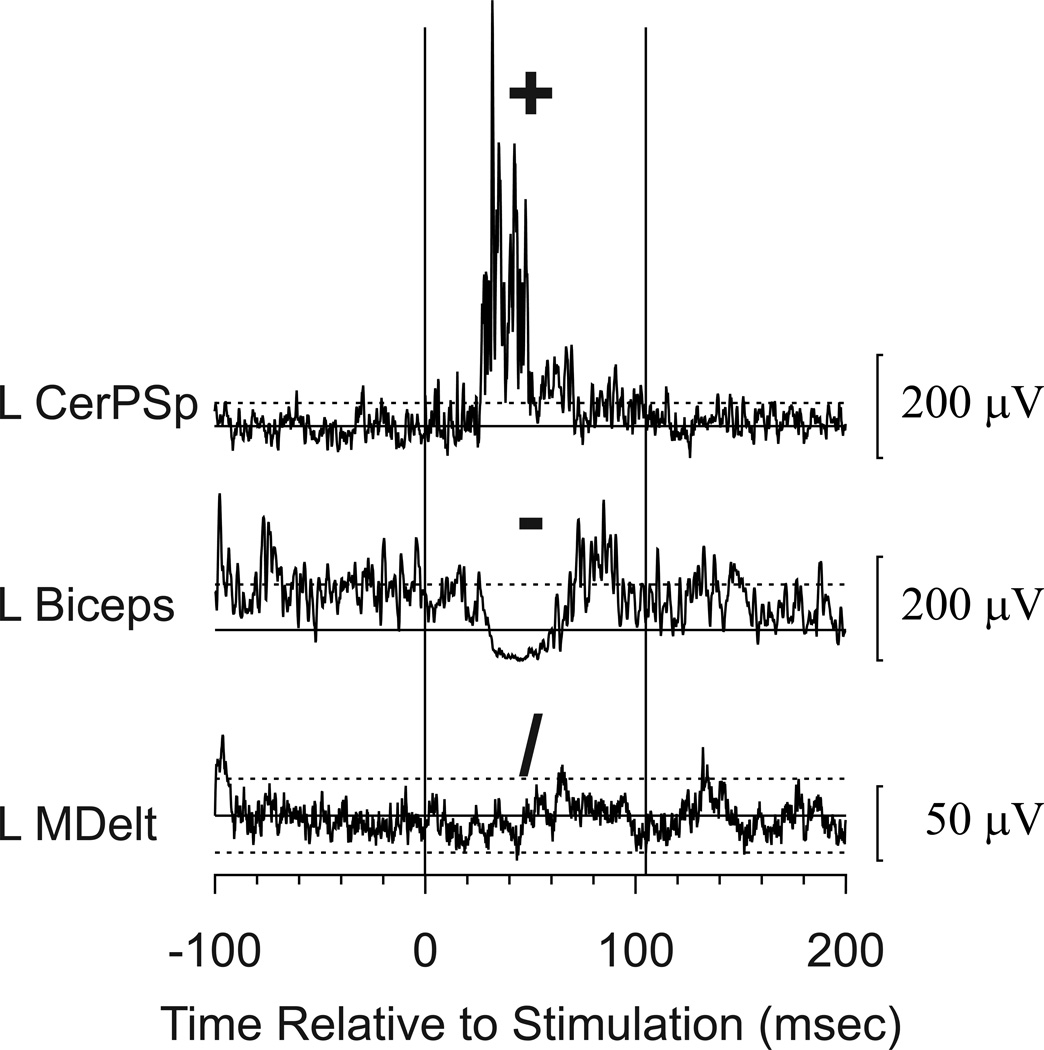
Figure 2.
Sample responses to stimulation and symbols used to code the responses. These responses were recorded during combined stimulation to the cortex and the PMRF and represent the mean EMG response to 10 stimulus trains. The solid horizontal line indicates the mean during the control period, and dashed lines show ± 2 standard deviations above or below the mean. Facilitation (coded +) was noted in the left cervical paraspinal muscle (L CerPSp), suppression (−) was noted in left Biceps (L Biceps), and there was no response (/) in the left middle deltoid (L MDelt). Several other muscles also responded to stimulation at this site, but only these examples are shown for this illustration. Calibration bars show response amplitudes.
A set of theoretical circuits were used to classify the patterns based on known or expected neurophysiological pathways from the PMRF and Cortex to the spinal cord [15;16;21].
RESULTS
Observed Patterns
The observed patterns were classified in two categories: predictable and unexpected (Table 1). Predictable patterns could be explained by one of the simple theoretical circuits, unexpected patterns could not. For the predictable patterns, the three most frequent patterns, which were observed consistently across subjects, were (+,/,+,/), (+,/,+,+), and (+,+,+,+). For the unexpected patterns the most frequent in two of the subjects was (+,/,/,+), while for subject O it was (+,+,/). For frequently observed patterns, 86% were predictable and the rest were classified as unexpected. About twice as many different combinations of unique patterns were found in the unexpected group compared to the predictable group. Given that so many more responses were classified as predictable, this emphasizes the high variability of patterns found in the unexpected responses.
Table 1.
Summary of comparison between observed patterns and neural circuits.
| Category | N | % | O | % | H | % | Total | Total % |
|---|---|---|---|---|---|---|---|---|
| Fequently Observed Patterns | ||||||||
| Predictable | 865 | 90% | 288 | 89% | 848 | 81% | 2001 | 86% |
| Unexpected | 101 | 10% | 35 | 11% | 196 | 19% | 332 | 14% |
| Total: | 966 | 323 | 1044 | 2333 | ||||
Neural Circuits
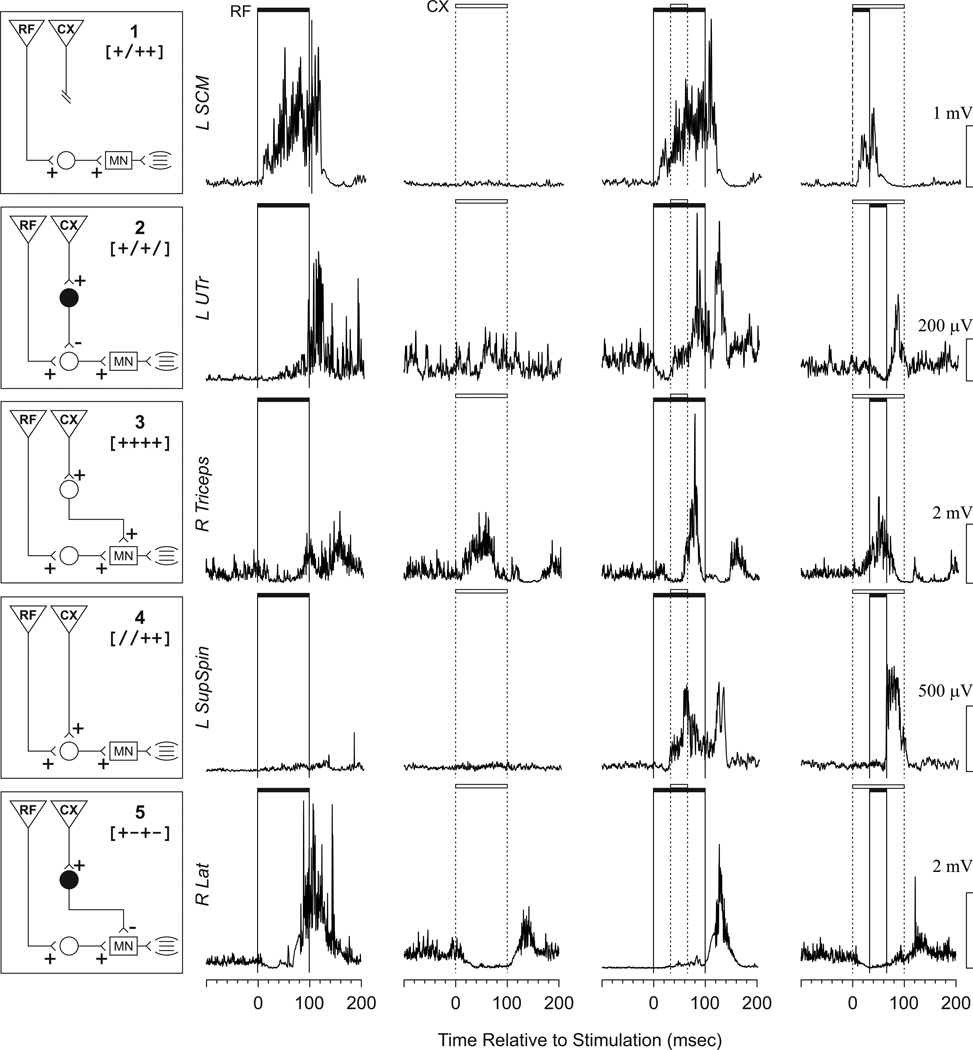
Five simple theoretical neural circuits were sufficient to explain the predictable, commonly observed patterns. For each circuit, an example of a set of EMG responses that would be explained by that circuit is provided (Fig. 3). Some of the circuits were useful to explain the patterns more often than others (Table 2). Circuit 1 (Total = 856, 37%) was the most observed circuit. Responses explained by this circuit were cases where, for the muscle in question, only one of the stimulation sites had an effect (either CX or RF). Circuit 2 (Total = 751, 32%) was the second most predominant circuit observed, which could be explained by an indirect inhibitory effect from the CX onto a premotor interneuron blocking disynaptic excitation from RF. Circuit 3 (Total = 296, 13%) was the third most frequent, and in this case the muscle responded with facilitation from RF and from CX, with the excitatory activities combined when both were stimulated. The last two circuits (4 and 5) each represent <5% of the observations. Circuit 4 represented a joint pathway through a common interneuron, such that nether site alone produced a visible response, but the two stimulated together did. Circuit 5 portrayed disynaptic excitation from one system and disynaptic inhibition from the other. In this case, if the site producing inhibition was activated first, the site producing facilitation had little or no effect. But if the site producing facilitation was activated first, activation of the site producing inhibition had little or no effect. The patterns that were labeled as unexpected would have required complex circuits that were considered too speculative for this report.
Figure 3.
Diagrams displaying the neural circuits used to explain the observed patterns, along with examples of a pattern for each. In the boxes on the left, inverted triangles represent projections from the descending systems (RF and CX), while circles represent interneurons (white excitatory, black inhibitory). The rectangles represent motoneuron pools in the spinal cord. An example of a set of EMG responses that would fit each of the theoretical neural circuits is shown in each row to the right of each circuit. The first column of EMG shows the response to reticular formation stimulation only (RF). The second column shows the response to cortical stimulation only (CX). The third and fourth columns represent joint stimulation. The filled horizontal bar indicates the duration of RF stimulation, and the open bar indicates CX stimulation. Scales for each EMG are shown to the right. The names of the muscles used as the examples are shown to the left. Further explanation of the circuits is provided in the discussion.
Table 2.
Categorization of circuits per subject and in total.
| Category | H | % | O | % | N | % | Total | % |
|---|---|---|---|---|---|---|---|---|
| Circuit 1 | 354 | 34% | 151 | 47% | 351 | 36% | 856 | 37% |
| Circuit 2 | 350 | 34% | 84 | 26% | 317 | 33% | 751 | 32% |
| Circuit 3 | 105 | 10% | 50 | 15% | 141 | 15% | 296 | 13% |
| Circuit 4 | 20 | 2% | 0 | 0% | 48 | 5% | 68 | 3% |
| Circuit 5 | 19 | 2% | 3 | 1% | 8 | 1% | 30 | 1% |
| Unpredictable | 194 | 19% | 35 | 11% | 101 | 10% | 330 | 14% |
| Total | 1042 | 323 | 966 | 2331 |
Under each subject’s ID (H, N, and O) the number of response patterns that matched each circuit is shown. The percentages are relative to the total observations per subject. Circuit 1 was the most predominant in all subjects, followed by circuit 2. Circuit 3 was observed frequently, whereas circuits 4 and 5 were relatively rare. On average, 14% of the patterns were unexplained by the above circuits.
DISCUSSION
To the authors’ knowledge, this experiment is the first attempt to understand the interactions from the reticulospinal and corticospinal systems for the control of upper limb muscles in the awake, behaving primate. Five theoretical circuits based on simple disynaptic projections to the motor pools were able to explain 86% of the responses. The three patterns observed most frequently (the first three circuits) were consistent in each subject. The first circuit, portraying effects from only one system, was expected as it is known that both systems have direct effects on muscle recruitment, and it would be unrealistic to expect that every pair of electrode sites would always affect any one given muscle, even when both were within armrelated areas. The second circuit was more interesting. This indicates that corticospinal outputs, while not producing overt inhibition on their own, might serve to suppress output from the reticulospinal system. This has also been proposed as an explanation for changes in brain organization after stroke [22], where this lack of cortical control could explain the bilateral movement responses observed when patients recovering from stroke attempt unilateral movements. The theoretical circuit drawn here proposes that the interaction occurs at the segmental level in the spinal cord, but could also involve corticoreticular connections or presynaptic inhibition [23;24]. This is also consistent with the concept of gating of reticulospinal outputs observed in the cat [25]. The third circuit displayed a simple combination of facilitation that was fully expected [15].
The two remaining circuits explained a relatively small portion of the responses. For circuit 4, having both systems produce weak, unobservable effects alone, but clear effects in combination, could also be explained by a variety of circuits. A shared interneuron as shown would be the classic explanation, but this pattern could also be explained by both systems having parallel and independent connections that were too weak alone but effective through spatial summation (e.g., circuit 3). Circuit 5 postulated independent, disynaptic pathways, one excitatory and the other inhibitory. Again, there might be other ways to explain the observations that seemed to fit this circuit, but this seemed the simplest explanation. Having these five circuits capture 86% of the observations raises the question on what caused the remaining 14% of the patterns. While we could presumably have devised a circuit for each, the complexity was such that this seemed too speculative.
Although beyond the scope of this short paper, there are further questions to be addressed in this dataset. Are certain patterns more common in the limb contralateral to the cortex v. the ipsilateral limb? Do the patterns observed depend on which side of the brainstem or which of the cortical motor areas was stimulated? And, do the patterns observed differ for proximal v. distal muscles? Analyses to address these questions are currently underway. Technical improvements in the detection and classification of responses would also strengthen the analysis. Using signal processing techniques such as Wavelets [26;27] and classification algorithms such as Enhanced Probabalistic Neural Networks (EPNN) [28] could enhance the detection and classification of responses [29]. A similar approach has been demonstrated in detecting and classifying EMG signals from Parkinson’s patients [30].
CONCLUSIONS
In conclusion, this report presents a first approach to the analysis and understanding of interactions between corticospinal and reticulospinal systems for muscle recruitment. It presents five neural circuits that explain 86% of the observed EMG patterns during stimulation. This preliminary approach shows evidence for a variety of interactions, some simple, and some complex. Overall, a strength of the present study is that the conclusions are based on responses in awake behaving subjects, not subject to limitations of a reduced preparation. A potential limitation is that with the relatively long stimulus trains required to elicit responses, there is the potential for some of the responses to have been elicited through indirect interactions with pathways not considered here. However, we would argue that the consistency of the responses among subjects suggests that the responses observed were most likely due to relatively direct pathways, which would also be consistent among subjects. The results presented demonstrate the value of this experimental approach and warrant further study.
HIGHLIGHTS.
The motor cortex and reticulospinal system were stimulated conjointly, or one at a time, in the awake behaving monkey
EMG activity was recorded from proximal and distal muscles, flexors and extensors, in both upper limbs
Patterns of coordinated motor output from these two major parts of the motor system were analyzed
Simple neural circuits explained the most commonly observed patterns of responses.
ACKNOWLEDGEMENTS
The authors thank Stephanie Moran, Wendy Herbert, and Lynnette Montgomery for assistance in collecting these data. This work was supported in part by NIH NINDS R01 NS37822 and the Summer Research Opportunity Program (SROP) at the Ohio State University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Drew T, Prentice S, Schepens B. Cortical and brainstem control of locomotion. Prog. Brain Res. 2004;143:251–261. doi: 10.1016/S0079-6123(03)43025-2. [DOI] [PubMed] [Google Scholar]
- 2.Matsuyama K, Mori F, Nakajima K, Drew T, Aoki M, Mori S. Locomotor role of the corticoreticularreticulospinal-spinal interneuronal system. Prog. Brain Res. 2004;143:239–249. doi: 10.1016/S0079-6123(03)43024-0. [DOI] [PubMed] [Google Scholar]
- 3.Peterson BW, Pitts NG, Fukushima K. Reticulospinal connections with limb and axial motoneurons. Exp. Brain Res. 1979;36:1–20. doi: 10.1007/BF00238464. [DOI] [PubMed] [Google Scholar]
- 4.Stapley PJ, Drew T. The pontomedullary reticular formation contributes to the compensatory postural responses observed following removal of the support surface in the standing cat. J. Neurophysiol. 2009;101:1334–1350. doi: 10.1152/jn.91013.2008. [DOI] [PubMed] [Google Scholar]
- 5.Davidson AG, Buford JA. Bilateral actions of the reticulospinal tract on arm and shoulder muscles in the monkey: stimulus triggered averaging. 2006:25–39. doi: 10.1007/s00221-006-0374-1. [DOI] [PubMed] [Google Scholar]
- 6.Davidson AG, Schieber MH, Buford JA. Bilateral spike-triggered average effects in arm and shoulder muscles from the monkey pontomedullary reticular formation. J. Neurosci. 2007;27:8053–8058. doi: 10.1523/JNEUROSCI.0040-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schepens B, Drew T. Independent and convergent signals from the pontomedullary reticular formation contribute to the control of posture and movement during reaching in the cat. J. Neurophysiol. 2004;92:2217–2238. doi: 10.1152/jn.01189.2003. [DOI] [PubMed] [Google Scholar]
- 8.Baker SN. The primate reticulospinal tract, hand function and functional recovery. J. Physiol. 2011;589:5603–5612. doi: 10.1113/jphysiol.2011.215160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Riddle CN, Edgley SA, Baker SN. Direct and indirect connections with upper limb motoneurons from the primate reticulospinal tract. J Neurosci. 2009;29:4993–4999. doi: 10.1523/JNEUROSCI.3720-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buford JA. Reticulospinal System. In: Squire LR, editor. Encyclopedia of Neuroscience. Vol. 8. Oxford: Academic Press; 2008. pp. 151–158. [Google Scholar]
- 11.Buford JA, Davidson AG. Movement-related and preparatory activity in the reticulospinal system of the monkey. Exp. Brain Res. 2004;159:284–300. doi: 10.1007/s00221-004-1956-4. [DOI] [PubMed] [Google Scholar]
- 12.Davidson AG, Buford JA. Bilateral actions of the reticulospinal tract on arm and shoulder muscles in the monkey: stimulus triggered averaging. Exp. Brain Res. 2006;173:25–39. doi: 10.1007/s00221-006-0374-1. [DOI] [PubMed] [Google Scholar]
- 13.Martin JH. The corticospinal system: from development to motor control. Neuroscientist. 2005;11:161–173. doi: 10.1177/1073858404270843. [DOI] [PubMed] [Google Scholar]
- 14.Lemon RN, Griffiths J. Comparing the function of the corticospinal system in different species: organizational differences for motor specialization? Muscle Nerve. 2005;32:261–279. doi: 10.1002/mus.20333. [DOI] [PubMed] [Google Scholar]
- 15.Galea MP, Hammar I, Nilsson E, Jankowska E. Bilateral postsynaptic actions of pyramidal tract and reticulospinal neurons on feline erector spinae motoneurons. J. Neurosci. 2010;30:858–869. doi: 10.1523/JNEUROSCI.4859-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jankowska E, Edgley SA. How can corticospinal tract neurons contribute to ipsilateral movements? A question with implications for recovery of motor functions. Neuroscientist. 2006;12:67–79. doi: 10.1177/1073858405283392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Montgomery LR, Herbert WJ, Buford JA. Recruitment of ipsilateral and contralateral upper limb muscles following stimulation of the cortical motor areas in the monkey. Exp. Brain Res. 2013 doi: 10.1007/s00221-013-3639-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roland PE, Larsen B, Lassen NA, Skinhoj E. Supplementary motor area and other cortical areas in organization of voluntary movements in man. J. Neurophysiol. 1980;43:118–136. doi: 10.1152/jn.1980.43.1.118. [DOI] [PubMed] [Google Scholar]
- 19.Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. Cereb. Cortex. 1991;1:1–47. doi: 10.1093/cercor/1.1.1-a. [DOI] [PubMed] [Google Scholar]
- 20.Davidson AG, Buford JA. Bilateral actions of the reticulospinal tract on arm and shoulder muscles in the monkey: stimulus triggered averaging. Exp. Brain Res. 2006;173:25–39. doi: 10.1007/s00221-006-0374-1. [DOI] [PubMed] [Google Scholar]
- 21.Riddle CN, Edgley SA, Baker SN. Direct and indirect connections with upper limb motoneurons from the primate reticulospinal tract. J. Neurosci. 2009;29:4993–4999. doi: 10.1523/JNEUROSCI.3720-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bradnam LV, Stinear CM, Byblow WD. Ipsilateral motor pathways after stroke: implications for non-invasive brain stimulation. Front Hum. Neurosci. 2013;7:184. doi: 10.3389/fnhum.2013.00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fisher KM, Zaaimi B, Baker SN. Reticular formation responses to magnetic brain stimulation of primary motor cortex. J. Physiol. 2012;590:4045–4060. doi: 10.1113/jphysiol.2011.226209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jankowska E, Edgley SA. How can corticospinal tract neurons contribute to ipsilateral movements? A question with implications for recovery of motor functions. Neuroscientist. 2006;12:67–79. doi: 10.1177/1073858405283392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schepens B, Drew T. Descending signals from the pontomedullary reticular formation are bilateral, asymmetric, and gated during reaching movements in the cat. J. Neurophysiol. 2006;96:2229–2252. doi: 10.1152/jn.00342.2006. [DOI] [PubMed] [Google Scholar]
- 26.Adeli H, Ghosh-Dastidar S, Dadmehr N. A spatio-temporal wavelet-chaos methodology for EEGbased diagnosis of Alzheimer's disease. Neurosci. Lett. 2008;444:190–194. doi: 10.1016/j.neulet.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 27.Adeli H, Ghosh-Dastidar S. Automated EEG-based Diagnosis of Neurological Disorders - Inventing the Future of Neurology. Boca Raton: CRC Press Taylor & Francis; 2010. [Google Scholar]
- 28.Ahmadlou M, Adeli H. Enhanced Probabilistic Neural Network with Local Decision Circles: A Robust Classifier. Integrated Computer-Aided Engineering. 2010;17:197–210. [Google Scholar]
- 29.Ortiz-Rosario A, Adeli H. Brain-computer interface technologies: from signal to action. Rev. Neurosci. 2013;24:537–552. doi: 10.1515/revneuro-2013-0032. [DOI] [PubMed] [Google Scholar]
- 30.Hossen A. A neural network approach for feature extraction and discrimination between Parkinsonian tremor and essential tremor. Technol. Health Care. 2013;21:345–356. doi: 10.3233/THC-130735. [DOI] [PubMed] [Google Scholar]