Summary
Objective
To identify prediction factors for the development of leptospirosis-associated pulmonary hemorrhage syndrome (LPHS).
Methods
The study comprised of 203 patients, aged ≥14 years, admitted with complications of the severe form of leptospirosis at the Emílio Ribas Institute of Infectology (Sao Paulo, Brazil) between 1998 to 2004. Laboratory and demographic data were obtained and the severity of illness score and involvement of the lungs and others organs were determined. Logistic regression was performed to identify independent predictors of LPHS. A validation cohort of 97 subjects with severe form of leptospirosis admitted at the same hospital between 2004-2006 was used to independently evaluate the predictive value of the model.
Results
The overall mortality rate was 7.9%. Multivariate logistic regression revealed that five factors were independently associated with the development of LPHS: serum potassium (mmol/L) (OR=2.6; 95%CI=1.1-5.9); serum creatinine (μmol/L) (OR=1.2; 95%CI=1.1-1.4); respiratory rate (breaths/min) (OR=1.1; 95%CI=1.1-1.2); presenting shock (OR=69.9; 95%CI=20.1-236.4), and Glasgow Coma Scale Score (GCS)<15 (OR=7.7; 95%CI = 1.3-23.0). We used these findings to calculate the risk of LPHS by the use of a spreadsheet. In the validation cohort, the equation classified correctly 92% of patients (Kappa statistic = 0.80).
Conclusions
We developed and validated a multivariate model for predicting LPHS. This tool should prove useful in identifying LPHS patients, allowing earlier management and thereby reducing mortality.
Keywords: Leptospirosis, Leptospirosis-associated pulmonary hemorrhage syndrome, ROC curve, Predictive model, Brazil
Introduction
Leptospirosis, a spirochetal zoonosis, has been increasingly recognized as an important cause of pulmonary haemorrhage syndrome.1-4 Five to fifteen percent of the clinical infections progresses to develop severe disease manifestations.1-3,5 The Nicaragua outbreak in 1995 raised awareness of leptospirosis as the cause of severe pulmonary haemorrhage.4 Whereas case fatality is 5-15% for Weil’s disease, it is more than 50% in patients who develop leptospirosis-associated pulmonary haemorrhage syndrome (LPHS).5-8
Early identification and triage of patients at risk for developing LPHS is essential to reducing the high case fatality rate. LPHS patients require intensive care unit (ICU) monitoring and aggressive supportive care for concomitant acute respiratory distress syndrome (ARDS), acute kidney injury and hypotension.9-14 Protective mechanical ventilation modalities14 and daily hemodialysis15 has been shown to provide beneficial outcomes in clinical trials which included leptospirosis patients as subjects. However, identification of patients at risk for developing LPHS is difficult10-14, especially during initial hospital evaluation. Therefore predictive markers (e.g.: hematology-test and biochemistry-test) need to be identified such that patients at risk for developing LPHS can be effectively identified and triaged.
We herein report the findings of a study which aimed to develop and validate a predictive model which can be used to identify patients at risk for developing LPHS at the time of hospital admission.
Patients And Methods
Patient cohorts
The study was performed at Emílio Ribas Institute of Infectology, the state infectious disease hospital in Sao Paulo, Brazil. This 200 bed hospital serves as the reference center for leptospirosis in the city which has a population of 10 million inhabitants. From October 1998 through December 2006, the study team of clinicians reviewed hospital admission records to consecutively identify suspected cases of leptospirosis during the first 24 hours of hospitalization. Patients were prospectively enrolled into the cohort who had suspected leptospirosis based on clinical and laboratory criteria established by the Brazilian Ministry of Health16, were hospitalized at the study site, had age greater or equal to 14 years and provided written informed consent to participate in the study, according to protocols approved by Committee of Emílio Ribas Institute of Infectology and the National Ethics Committee in Research for the Brazilian Ministry of Health. Patients were excluded from the study if they had laboratory or radiologic evidence for a disease other than leptospirosis during hospitalization and did not have laboratory-confirmed diagnosis of leptospirosis.
Data Collection
Information was obtained on potential predictive factors for LPHS at the time of initial emergency room evaluation. A standardized data form was used to extract information on demographic characteristics, clinical signs and symptoms and laboratory findings from the medical records. A complete history, physical examination, complete blood count, serum electrolytes and coagulation parameters (prothrombin and prothromboplastin time) were obtained as part of routine emergency room evaluation for suspected leptospirosis. Haemoptysis was encoded as expectoration of blood-tinged sputum. The Glasgow Coma scale (GCS) was used to assess the mental status of subjects. Arterial blood gas evaluation was performed on a subset of patients who had signs of dyspnea and hypotension. Diuresis was assessed by evaluating urinary volume during the first 24 hours of hospital admission. Oliguria was defined as a 24 hour urinary volume of less than 400 mL. Shock was defined as the use of vasoactive drugs (one or more) to maintain a systolic blood pressure >90 mmHg after fluid resuscitation in the first 24 hours of hospitalization. The study team determined the Acute Physiology and Chronic Health Evaluation (APACHE) II score for patients based on findings obtained during emergency room evaluation.
During hospitalization all patients received antimicrobial therapy with penicillin, ampicillin or ceftriaxone. When indicated, patients received intravenous fluid resuscitation, vasoactive drugs, supplemental oxygen, blood component transfusions and ICU monitoring. Intravenous fluid overload was avoided in patients with ARDS and pulmonary edema. Renal replacement therapy was initiated for indications of oliguria, hyperkalemia, severe acidosis, fluid overload and uremic syndrome. The study team followed patients daily during hospitalization to evaluate outcomes of LPHS, defined as massive pulmonary bleeding (hemoptysis >300ml or aspiration of fresh blood after endotracheal intubation which did not clear with suctioning) and respiratory failure requiring mechanical ventilation, and mortality.
Laboratory confirmation
Serum samples were collected at the time of hospital admission and a period of 10 to 14 days afterwards during convalescence. The IgM ELISA assay, microscopic agglutination test (MAT) and culture isolation was performed as previously reported. Immunohistochemical and immunofluorescence studies of autopsy specimens was routinely performed with anti-Leptospira antibodies1,3,7,8,10. A laboratory-confirmed diagnosis of leptospirosis was defined as a positive IgM ELISA result, seroconversion or four-fold rise in the MAT titer between acute- and convalescent-phase samples, a single MAT titre ≥1:800, a positive Leptospira culture or identification of leptospires in autopsy specimens, detected through culture or immunohistochemical staining, in clinical specimens. The presumptive infecting serogroup was determined based on the serovar against which highest agglutination titres were directed.
Statistical analysis
The cohort was divided into two groups, a derivation cohort that was enrolled from January 1998 through October 2004 and a validation cohort that was enrolled from November 2004 through December 2006. Bivariate analyses were performed with information from the derivation cohort to identify clinical and laboratory findings at the time of hospital admission which were associated with the risk of developing LPHS during hospitalization was compared to those of patients who did not develop LPHS. Continuous variables were evaluated for their distribution. The Mann-Whitney and chi-square test was used to compare continuous and categorical variables, respectively, for patients who did and did not develop LPHS. Significant differences were defined as a P-value <0.05. Odds ratios (OR) and 95% confidence intervals (95% CI) were calculated to estimate the risk of developing LPHS associated with baseline findings.
Logistic regression was performed to identify independent predictors of LPHS. Variables which were found to be significant in the bivariate analyses were included in the regression analysis. Continuous variables were maintained as such in the model, unless preliminary analyses indicated that categorization of the variable improved the predictive value. Due to co-variation among clinical and laboratory findings, variables were grouped into categories according to pulmonary, renal, hepatic and hemodynamic complications. A forward stepwise approach was used to identify significant independent predictors of LPHS within each group. To develop a prediction rule, a scoring system was developed based on multiplying by different values the natural logarithm of the OR for each risk factor identified in the logistic regression analysis and rounding the value to the nearest integer. However we did not identify a point scoring system that had sufficient predictive value in comparison to the regression model. Therefore the predictive model used in the study was based on the exponential equation derived from the logistic regression analysis.
Cutoff points were determined by constructing a receiver operating characteristic (ROC) curves. Based on the evaluation of a range of threshold criteria for the predictive model, a final criteria of Pr(LPHS) of ≥0.5 was used. An Excel spreadsheet to calculate risk of LPHS is available as supplementary material.
Information from the validation cohort was used to independently evaluate the predictive value of the model. The discriminatory ability of the predictive model was used to assess by calculating the area under und the ROC curve. Sensitivity, specificity and positive and negative predictive values and their 95% CI for the predictive model were calculated for the derivation, validation and combined cohorts, using standard definitions and methods. All analyses were performed using STATA version 8.0 (Stata Corporation, College Station, TX, USA).
Results
Derivation cohort
From January 1998 through October 2004, 281 patients with suspected leptospirosis were hospitalized at the study site. Of these 27 patients were excluded because they had evidence of disease other than leptospirosis or did not have a laboratory-confirmed diagnosis of leptospirosis and 51 were excluded for being under 14 years of age. Of the 203 subjects enrolled in the derivation cohort, 172, 29 and 2 had a confirmed diagnosis on the basis of MAT, IgM ELISA and culture isolation criteria, respectively. The presumptive infecting serogroup was L. interrogans serogroup Icterohaemorrhagiae in 71% (144) of the patients.
The most common infecting serovars (%) in the LPHS group was Leptospira interrogans serovar Icterohaemorrhagiae (38%), followed by L. interrogans serovar Copenhageni (33.3%), L. kirschneri serovar Cynopteri (23.8%), and L. interrogans serovar Canicola (4.8%), and L. kirschneri serovar Grippotyphosa (4.7%). Among the non-LPHS group infecting serovars was Leptospira interrogans serovar Icterohaemorrhagiae (32%), followed by L. interrogans serovar copenhageni (31%), L. kirschneri serovar Gripppotyphosa (7.8%), L. kirschneri serovar Cinopteri (7.8%), L. interrogans serovar Canicola (4.4%), and L. interrogans serovar hebdomadis (1.1%), L. interrogans serovar Pomona (1.1%), and L. interrogans serovar Pyogenes (1.1%).
Subjects in the derivation cohort had clinical manifestations of Weil’s disease. Acute kidney injury, defined as a serum creatinine level ≥176.8 μmol/L, oliguria and jaundice were observed in 70.9% (144), 10% (21) and 86.7% (176), respectively, of the patients, while 26.1% (53) had signs of gastrointestinal bleeding, hemoptysis or others hemorrhagic manifestations. Of note, significant differences were observed between LPHS and non-LPHS patients with respect to hemorrhagic manifestations at hospital admission 39% vs. 22%, respectively, P = 0.01. No statistically difference was seen in LPHS and non-LHPS in chest x-rays at hospital admission 23.5% vs. 17.5%, respectively, P = 0.41. During the first 24 hours of hospitalization, 21% (43) developed shock.
During hospitalization, 132 (65%) and 28% of the patients required ICU monitoring and dialysis. Among the 203 patients, 51 (25%) developed LPHS based on the findings of massive pulmonary haemorrhage and respiratory failure requiring mechanical ventilation. The overall case fatality for the derivation cohort was 8% (16 deaths). All of the deaths occurred among patients who had developed LPHS (16 of 51 patients; case fatality rate, 31%).
Risk factors for developing LPHS during hospitalization
Table 1 shows the clinical and laboratory findings at the time of hospital admission for patients who did and did not develop LPHS. Of the 203 subjects, 167 (82.3%) presented serologic evidence of leptospirosis on the MAT and 71% (100) infected with the serogroup Icterohaemorrhagiae. Significant differences were not found between the two groups with respect to symptoms of fever, headache, myalgia or gastrointestinal manifestations (results not shown), and between the infecting serogroup in terms of organ involvement, pulmonary hemorrhage or death. LPHS patients had significantly increased frequency of having renal, hepatic, respiratory and cardiovascular complications and altered mental status at the time of hospital admission (Table 1).
Table 1.
Age, clinical signs and laboratory values obtained at admission in 203 patients with severe leptospirosis
| Factor | Normal values | LPHS |
P value | |||
|---|---|---|---|---|---|---|
| Absent |
Present |
|||||
| n | Mean (SD) | n | Mean (SD) | |||
| Demographic | ||||||
| Age (years) | 152 | 33.5(1.0) | 51 | 39.3(2.0) | 0.015 | |
| Sex (male/female)(n/%) | 152 | 142/10 | 51 | 46/5 | 0.44 | |
| Renal involvement | ||||||
| Blood urea nitrogen (mmol/L) | 2.1–7.1 | 152 | 41.0(2.35) | 51 | 54.8(3.8) | <0.001 |
| Creatinine (μmol/L) | 70–120 | 152 | 309.4(17.7) | 51 | 433.2(35.4) | <0.001 |
| Potassium (mmol/L) | 3.5–4.5 | 152 | 3.6(0.04) | 51 | 3.9(0.08) | 0.014 |
| Hepatic involvement | ||||||
| Aspartate aminotransferase (μkat/L) | 0–0.58 | 151 | 1.97(0.15) | 51 | 3.2(0.45) | 0.002 |
| Total bilirubin (mmol/L) | 2–21 | 149 | 217.2(15.4) | 51 | 307.8(29.1) | 0.002 |
| Albumin (g/L) | 34–47 | 100 | 28(0.4) | 43 | 24(0.6) | <0.001 |
| Muscle involvement | ||||||
| Creatine kinase (μkat/L) | 0.53–4.45 | 124 | 15.8(29.2) | 45 | 38.5(41.2) | <0.001 |
| Hematologic involvement | ||||||
| WBC count (×109/L) | 4.8–10.8 | 152 | 11.0(4.8) | 51 | 13.7(7.7) | 0.02 |
| Haematocrit | 39–49 | 151 | 37.0(5.9) | 51 | 33.8(6.4) | 0.001 |
| Platelet count | (×109/L) | 150–450 | 152 | 103.0(7.7) | 51 63.0(4.9) | <0.001 |
| Prothrombin time (sec) | 11–15 | 120 | 14.0(5.0) | 47 | 13.2(2.4) | 0.47 |
| Respiratory manifestations | ||||||
| Respiratory rate (n/min) | 12–20 | 152 | 23.6(7.5) | 51 | 31.4(11.8) | <0.001 |
| Arterial oxygen (kPa) | 11.04–14.36 | 118 | 10.9(3.3) | 51 | 10.1(4.1) | 0.18 |
| PaO2/FiO2 (ratio) | >350 | 120 | 383.7(76.9) | 51 | 290.4(123.4) | <0.001 |
| Arterial pH (units) | 7.35–7.45 | 118 | 7.42(0.05) | 51 | 7.34(0.1) | <0.001 |
| Arterial bicarbonate (mmol/L) | 22–29 | 118 | 18.1(3.8) | 51 | 16.0(4.3) | 0.002 |
| Cardiac manifestations | ||||||
| Heart rate (n/min) | 60–100 | 152 | 90.2(19.0) | 51 | 106.4(16.6) | <0.001 |
| MAP (mmHg) | 80–90 | 152 | 88.8(16.1) | 51 | 84.2(21.5) | 0.10 |
| Monitoring | ||||||
| Fever (n/%) | No | 152 | 123(80.9) | 51 | 37(74) | 0.29 |
| Pulse oximetry (SaO2) | 0.95–1.0 | 118 | 0.94(0.4) | 51 | 0.88(0.1) | <0.001 |
| APACHE II (score) | 0 | 146 | 9.0(4.5) | 51 | 15.4(4.9) | <0.001 |
| Shock | No | 162 | 145 | 41 | 34 | <0.001 |
| GCS < 15 | 15 | 178 | 143 | 23 | 15 | <0.001 |
| Diuresis (mL/24 h) | 800–2000 | 121 | 2732(168) | 44 | 1816(293) | 0.001 |
LPHS = leptospirosis-associated pulmonary hemorrhage syndrome; WBC = white blood cells; PaO2 = arterial oxygen tension; FiO2 = fraction of inspired oxygen; MAP = mean arterial pressure; SaO2 = arterial oxygen saturation; APACHE = acute physiological and chronic health evaluation; shock = hemodynamic instability in the first 24 h; GCS = Glasgow Coma Scale.
Logistic regression analysis identified five independent risk factors for developing LPHS based on clinical and laboratory parameters at the time of admission (Table 2). The presence of shock (OR=41.4; 95% CI 20.1-236.4) and GCS <15 (OR=5.5; 95% CI 1.3-23.0) were the strongest predictors of LPHS. The model correctly classified 88% of the subjects in the derivation cohort, with a sensitivity of 73% and a specificity of 93%. A point scoring system was not identified which had sufficient performance as compared with the predictive model.
Table 2.
Crude and adjusted odds ratios and 95% confidence intervals for risk factors associated with leptospirosis-associated pulmonary hemorrhage syndrome.
| Factor | Crude OR |
Adjusted ORb (95% CI) |
P value |
|---|---|---|---|
| Potassium (mmol/L)a | 1.9 | 2.6 (1.1–5.9) | 0.02 |
| Serum creatinine (μmol/L)a |
1.2 | 1.2 (1.1–1.4) | 0.003 |
| Respiratory rate (n/min)a |
1.1 | 1.1 (1.1–1.2) | <0.0001 |
| Shock | |||
| No | 1.0c | – | |
| Yes | 41.4 | 69.2 (20.1–236.4) | <0.0001 |
| Glasgow Coma Scale | |||
| 15 | 1.0c | – | |
| <15 | 7.7 | 5.5 (1.3–23.0) | 0.02 |
Shock = hemodynamic instability in the first 24 h.
Used as continuous variables in the prediction model.
Adjusted for the other factors shown in the table.
Reference group.
Validation cohort
A second independent cohort of 97 patients who fulfilled the study criteria were enrolled from November 2004 to December 2006. Acute kidney injury, defined as a serum creatinine ≥ 176.8 μmol/L, oliguria, jaundice and shock were observed in 72.6% (70), 6% (6), 70.1% (67) and 49% (48), respectively, of the patients. During hospitalization, 29 (29.9%) developed LPHS. The overall case fatality for the validation cohort was 6.8% (6 deaths). All of the deaths occurred among patients who had developed LPHS.
Accuracy of prediction model in identifying patients who developed LPHS
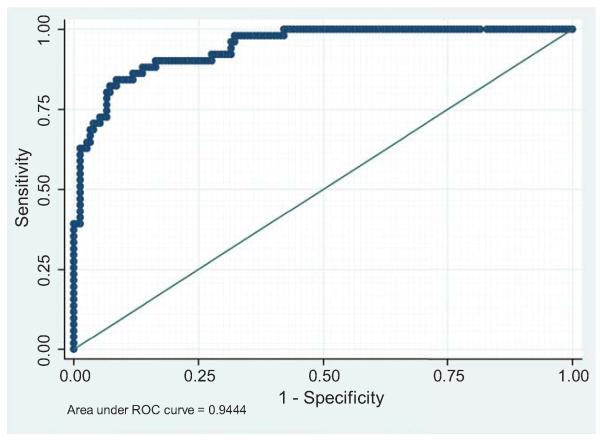
We assessed the performance of the logistic regression model in predicting pulmonary hemorrhage by constructing the ROC curve (Figure 1). The area under the curve was 0.94 (sensitivity: 72.5%; specificity: 93.4%; positive predictive value: 78.7%; negative predictive value: 91%).
Fig. 1.
ROC curve assessing the performance of the logistic regression model in predicting pulmonary hemorrhage
We compared the performance of the predictive model in identifying LPHS during hospitalization with the APACHE II scoring system. The area under the ROC curve was significantly larger for the predictive model than the APACHE II scoring system (0.94; 95% CI 0.91-0.98 vs. 0.83; 95% CI 0.76-0.90; P<0.05). The sensitivity, specificity, positive predictive value and negative predictive values for the APACHE II scoring system were 57%, 91%, 67% and 86%, respectively, and were overall lower than those for the predictive model.
The prediction equation for LPHS is as follows:
The equation was applied to the validation cohort, and 89 (92%) patients were correctly classified. In the non-LPHS group, 6 cases were judged to have LPHS, whereas 2 cases in the LPHS group were judged to not have LPHS (Kappa statistic = 0.80).
Discussion
Leptospirosis presents a wide variety of clinical manifestations and prognoses. Leptospirosis presenting as LPHS is associated with a high mortality rate. However, the clinical course of LPHS can be improved by early diagnosis and prompt treatment.
In the present study, we developed and validated a multivariate prediction model for LPHS based on five readily available variables. Assigning patient values to each variable, the equation we developed can be used to estimate individual risk. This prediction model proved to be excellent tool for distinguishing between patients with LPHS and those with other severe forms of leptospirosis (area under the ROC curve = 0.94). This constitutes a new tool that, using data routinely available at hospital admission, provides physicians with a simple method of accurately assessing LPHS risk. Patients in the high-risk group would benefit the most from rapid triage to a reference center in order to receive aggressive therapies, including monitoring, hemodynamic resuscitation, invasive mechanical ventilation and early dialysis.
To our knowledge, this is the first study using multivariate analysis to assess clinical factors associated with LPHS and proposing a tool to predict patient risk at hospital admission. However, previous studies included similar factors in prognostic models for leptospirosis. Panaphut et al.5 found that oliguria, hyperkalemia, pulmonary rales or hypotension on admission in patients with leptospirosis were predictors for mortality. Niwattayakul et al. and Doudier et al.9,17 found that hypotension, oliguria and abnormal chest auscultation were related to prognosis. As with our results, Esen et al.18 demonstrated that altered mental status and potassium levels at the time of hospital admission were seen to be independent risk factors for motality.5,7,8,18 The higher serum potassium levels may have been provoked by more severe renal dysfunction, metabolic derangement, or rhabdomyolisis.8,18 Altered mental status has previously been reported as a powerful independent risk factor for mortality.7,18 The laboratory findings in LPHS patients in the present study are consistent with the observations made by Craig et al.19 When leptospirosis is suspected, patients presenting with higher levels of blood urea, serum creatinine, and leukocytosis and lowers levels of serum albunin, haematocrit and platelets may need to be closely monitored and have aggressive treatment regimes directed toward them.
It is no coincidence that our findings and those of such previous studies point to similar factors, LPHS being the main cause of death among patients with leptospirosis.6-9,11-15
Another strength of our study is the higher number of consecutive patients included in our cohort – more than 200 patients, 25% of them with LPHS. In addition, all of them had been admitted to the same hospital, which resulted in homogenous criteria at admission and during follow-up evaluations. Finally, our model was validated in an independent sample of 97 consecutive patients, 29% of them with LPHS, hospitalized with severe leptospirosis, in which the use of our equation resulted in the correct classification of 92% of the patients.
Certain limitations of this model should be considered. The model includes only variables available from our dataset, although there could be other factors affecting the outcome which are not routinely collected in our center. In addition, although the data used to develop the model were subjected to local validation, the performance of the model in predicting LPHS should be tested in primary care hospitals and in areas of lower incidence. Furthermore, the validation in another settings would be desirable.
In conclusion, we have developed a multivariate prediction model, based on three clinical factors and two laboratory testing factors, to predict LPHS risk in an urban area with a high incidence of leptospirosis. These variables are, in descending order of statistical significance, as follows: hemodynamic instability (shock within the first 24 h), altered mental status (GCS score < 15), serum potassium, serum creatinine and respiratory rate. The model can be used at hospital admission to calculate patient-specific risk based on the logistic equation. This tool could also be useful to clinicians in the identification of patients with LPHS and should help reduce mortality by allowing earlier management of these cases.
Supplementary Material
Acknowledgements
We would like to thank Mr. Carlos C. F. Marotto, Ms. Kesia Santos and Ms. Adriana Sanudo for the technical assistance. This work was supported, in part, by the National Institutes of Health (grants R01 AI052473 and D43 TW00919) and the Secretary of Health, Sao Paulo State, Brazil.
Footnotes
Conflict of interest
The authors state that there is no conflict of interest.
References
- 1.Faine S, Adler B, Bolin C, Perolat P. Leptospira and Leptospirosis. 2nd edition MediSci; Melbourne, Australia: 1999. [Google Scholar]
- 2.Yersin V, Bovet P, Mérien F, Clément J, Laille M, VanRanst M, et al. Pulmonary haemorrhage as a predominant cause of death in leptospirosis in Seychelles. Trans R Soc Trop Med Hyg. 2000;94:71–6. doi: 10.1016/s0035-9203(00)90445-0. [DOI] [PubMed] [Google Scholar]
- 3.Levett PN. Leptospirosis. Clin Microbiol Rev. 2001;14:296–326. doi: 10.1128/CMR.14.2.296-326.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trevejo RT, Rigau-Pérez JG, Ashford DA, Zaki SR, Shieh WJ, Peters CJ, et al. Epidemic leptospirosis associated with pulmonary hemorrhage – Nicaragua, 1995. J Infect Dis. 1998;178:1457–63. doi: 10.1086/314424. [DOI] [PubMed] [Google Scholar]
- 5.Panaphut T, Domrongkitchaiporn S, Thinkamrop B. Prognostic factors of death in leptospirosis: a prospective cohort study in Khon Kaen, Thailand. Int J Infect Dis. 2002;6:52–9. doi: 10.1016/s1201-9712(02)90137-2. [DOI] [PubMed] [Google Scholar]
- 6.Dupont H, Dupont-Perdrizet D, Perie JL, Zehner-Hansen S, Jarrige B, Daijardin JB. Leptospirosis: prognostic factors associated with mortality. Clin Infect Dis. 1997;25:720–4. doi: 10.1086/513767. [DOI] [PubMed] [Google Scholar]
- 7.Ko AI, Galvão Reis M, Ribeiro Dourado CM, Johnson WD, Jr., Riley LW, Salvador Leptospirosis Study Group Urban epidemic of severe leptospirosis in Brazil. Lancet. 1999;354:820–5. doi: 10.1016/s0140-6736(99)80012-9. [DOI] [PubMed] [Google Scholar]
- 8.Marotto PC, Nascimento CM, Eluf-Neto J, Marotto MS, Andrade L, Sztajnbok J, et al. Acute lung injury in leptospirosis: clinical and laboratory features, outcome, and factors associated with mortality. Clin Infect Dis. 1999;29:1561–3. doi: 10.1086/313501. [DOI] [PubMed] [Google Scholar]
- 9.Niwattayakul K, Homvijitkul J, Niwattayakul S, Khow O, Sitprija V. Hypotension, renal failure, and pulmonary complications in leptospirosis. Ren Fail. 2002;24:297–305. doi: 10.1081/jdi-120005363. [DOI] [PubMed] [Google Scholar]
- 10.Bharti AR, Nally JE, Ricaldi JN, Matthias MA, Diaz MM, Lovett MA, et al. Leptospirosis: a zoonotic disease of global importance. Lancet Infect Dis. 2003;2003;3:757–71. doi: 10.1016/s1473-3099(03)00830-2. [DOI] [PubMed] [Google Scholar]
- 11.Tattevin P, Léveiller G, Flicoteaux R, Jauréguiberry S, Le Tulzo Y, Dupont M, et al. Respiratory manifestations of leptospirosis: a retrospective study. Lung. 2005;183:283–9. doi: 10.1007/s00408-004-2541-0. [DOI] [PubMed] [Google Scholar]
- 12.Paganin F, Bourdin A, Dalban C, Courtin JP, Poubeau P, Borgherini G, et al. Leptospirosis in Reunion Island (Indian Ocean): analysis of factors associated with severity in 147 confirmed cases. Intensive Care Med. 2007;33:1959–66. doi: 10.1007/s00134-007-0776-y. [DOI] [PubMed] [Google Scholar]
- 13.Segura ER, Ganoza CA, Campos K, Ricaldi JN, Torres S, Silva H, et al. Clinical spectrum of pulmonary involvement in leptospirosis in a region of endemicity, with quantification of leptospiral burden. Clin Infect Dis. 2005;2005;40:343–51. doi: 10.1086/427110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dolhnikoff M, Mauad T, Bethlem E, Carvalho CRR. Leptospiral pneumonias. Curr Opin Pulm Med. 2007;2007;13:230–5. doi: 10.1097/MCP.0b013e3280f9df74. [DOI] [PubMed] [Google Scholar]
- 15.Andrade L, de Francesco Daher E, Seguro AC. Leptospiral nephropathy. Semin Nephrol. 2008;28(4):383–94. doi: 10.1016/j.semnephrol.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 16.Brasil. Ministério da Saúde. Secretaria de Vigilância em Saúde. Guia de vigilância epidemiológica/Ministério da Saúde, Secretaria de Vigilância em Saúde. – 6. ed. – Brasília: Ministério da Saúde. http://bvsms.saude.gov.br/bvs/publicacoes/Guia_Vig_Epid_novo2.pdf http://dtr2001.saude.gov.br/svs/epi/situacao_doencas/planilhas_doencas.htm
- 17.Doudier B, Garcia S, Quennee V, Jarno P, Brouqui P. Prognostic factors associated with severe leptospirosis. Clin Microbiol Infect. 2006;12:299–300. doi: 10.1111/j.1469-0691.2005.01335.x. [DOI] [PubMed] [Google Scholar]
- 18.Esen S, Sunbul M, Leblebicioglu H, Eroglu C, Turan D. Impacto f clinical and laboratory findings on prognosis in leptospirosis. Swiss Med Wkly. 2004;134:347–52. doi: 10.4414/smw.2004.10436. [DOI] [PubMed] [Google Scholar]
- 19.Craig SB, Graham GC, Burns M-A, Dohnt MF, Smithe LD, McKay DB. Ann Trop Med Parasitol. 2009;103(4):333–41. doi: 10.1179/136485909X435058. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.