Abstract
Electron paramagnetic resonance (EPR) spectroscopy has been well established as a viable technique for measurement of free radicals and oxygen in biological systems, from in vitro cellular systems to in vivo small animal models of disease. However, the use of EPR in human subjects in the clinical setting, although attractive for a variety of important applications such as oxygen measurement, is challenged with several factors including the need for instrumentation customized for human subjects, probe and regulatory constraints. This paper describes the rationale and development of the first clinical EPR systems for two important clinical applications, namely, measurement of tissue oxygen (oximetry), and radiation dose (dosimetry) in humans. The clinical spectrometers operate at 1.2 GHz frequency and use surface loop resonators capable of providing topical measurements up to 1 cm depth in tissues. Tissue pO2 measurements can be carried out noninvasively and repeatedly after placement of an oxygen-sensitive paramagnetic material (currently India ink) at the site of interest. Our EPR dosimetry system is capable of measuring radiation-induced free radicals in the tooth of irradiated human subjects to determine the exposure dose. These developments offer potential opportunities for clinical dosimetry and oximetry, which include guiding therapy for individual patients with tumors or vascular disease, by monitoring of tissue oxygenation. Further work is in progress to translate this unique technology to routine clinical practice.
Keywords: Electron Paramagnetic Resonance, free radical, oxygen, hyperoxygenation, tumor therapy, radiation therapy, dosimetry
Introduction
Electron paramagnetic resonance (EPR) (also called electron spin resonance, (ESR)) spectroscopy shares many of the features of nuclear magnetic resonance (NMR), spectroscopy and magnetic resonance imaging (MRI) including underlying principles, discovery period, and their evolution to become an indispensable tool for in vivo biological applications. Particularly, proton MRI has rapidly emerged to become a unique device for noninvasive measurement (imaging) of tissue pathophysiology in the clinic. On the other hand, EPR, which relies on paramagnetic species with unpaired electrons, despite its superiority to NMR in terms of detection sensitivity, has not advanced to use for pertinent clinical applications for a variety of reasons. The most important impediments are the lack of adequate levels of paramagnetic species in biological systems, shorter relaxation times of unpaired electrons when compared to protons, and the need to use microwave radiation as source of excitation. In spite of the limitations, the last three decades have seen some innovative and concerted successful progress that has developed this unique technology for making very useful measurements in living systems [1–3]. The most pertinent advances include the development of low-frequency instrumentation including lumped-circuit resonators, time-domain detection, imaging capabilities, and molecular probes for extracting specific information of interest from tissues of both animal models and human [4]. The technological advances have now reached a stage where useful clinical measurements such as tissue oxygenation (oximetry) or radiation exposure (dosimetry) in human subjects have become a reality. This article presents an overview of the unique opportunities of the EPR technology for measurements in humans and challenges that need to be addressed before it can achieve widespread acceptance as a useful clinical device. Two potential areas of clinical application, namely oximetry and dosimetry, for which substantial progresses have been made are highlighted.
Measurement of tissue oxygenation (Oximetry)
Although the discovery of oxygen was made in the 18th century, measurements of oxygen concentration (oximetry) in living systems (in vivo) are only a recent phenomenon. Some measurements were made in the 1960s, but it was in the late 1980s when the computerized polarographic needle electrode system became available that it was used extensively to assess the oxygenation in tumors clinically. The use of this technique helped to establish the role of hypoxia in the efficacy of radiation therapy [5]. Now, there are several potentially clinically useful methods[6] that are based on other principles, including fluorescence-quenching, phosphorescence, optical detection, immunohistochemical, and magnetic resonance techniques. Some of the important criteria for improvements in the ability to make successful clinical measurements of oxygenation include: minimal or no invasiveness; capability to make repeated measurements; accessibility to the region of interest; appropriate spatial resolution; adequate depth of measurement; accuracy and robustness of measurements; usefulness of the parameter reported for clinical purposes; measurement time consistent with use in patients; ease of use, in the clinical setting; and potential for the instrumentation to be commercially available. It is especially important that the method should enable repeated measurements from the region of interest in order to follow the changes in oxygenation over a period of time, preferably for up to several weeks, months, or even years [7, 8]. The technique should also provide appropriate spatial and temporal resolution. The depth of measurement (penetration) and accessibility to the region of interest are some of the important factors for establishing the scope of applicability of the technique.
Existing methods for tissue oximetry in vivo
While a few techniques are available that can provide direct measurements of tissue pO2 (especially oxygen polarographic electrodes, the OxyLite fluorescence-quenching technique and direct injection of oxygen-sensitive NMR probes based on fluorine [9–11]), these techniques have the disadvantage of not being able to be used repeatedly. The inability to make repeated measurements is a disadvantage because the most valuable clinical usefulness depends on obtaining repeated measurements so that changes due to the treatment and the disease process can be followed. There are also some concerns about the potential for artifacts because the invasiveness of these techniques can cause perturbations precisely at the time that the measurements are being made [12–16]. To date, only the polarographic method has been systematically used in humans [17].
Some widely available non-invasive techniques to assess oxygenation provide data on parameters that, while related to tissue pO2, do not measure pO2 directly. This especially includes techniques such as blood oxygen level dependent (BOLD) MRI, NMR proton spectroscopy, diffusion-weighted imaging (DWI) MRI, duplex Doppler ultrasound, 18F-Miso positron emission tomography (PET), and near-infrared (NIR) measurements of hemoglobin. These techniques can provide information on the saturation of hemoglobin within the vascular system or hemodynamics or redox-related metabolites or reactions, but, while this can be valuable to know, it does not provide direct quantification of the pO2 in the site of interest and surrounding tissues. The clinical importance of direct measurement of pO2 is especially high in tumors, where typically the microvasculature is complex and irregular and therefore is very difficult to extrapolate these indirect measurements to the pO2 in the tumor [18, 19]. EPR oximetry could provide the data to facilitate relating these indirect methods to the actual tumor or tissue pO2, thereby clarifying the conditions under which these other methods can provide the desired clinically relevant information on pO2.
EPR Oximetry for biological systems including viable tissues
EPR oximetry refers to the measurement of oxygen concentration by EPR spectroscopy [20]. The principle of EPR oximetry is based on the paramagnetic characteristics of molecular oxygen, which in its ground state has two unpaired electrons, and undergoes spin-exchange interaction with a paramagnetic EPR spin-probe. This process is sensitive to the amount of oxygen present in the local environment. In most cases the relaxation rate of the spin-probe increases proportionately as a function of oxygen content (concentration or pressure) [20, 21], but other mechanisms also can occur. The increased spin-spin relaxation rate results in increased line-broadening. The fact that the linewidths of EPR resonance lines correlate with oxygen concentration has been used in a variety of biological settings [22–35]. The development of low-frequency EPR instrumentation at L-band (1–2 GHz) and even lower frequencies (600 MHz and 300 MHz) has made it possible to perform EPR oximetry measurements on complex biological systems such as intact animals and isolated functioning organs [23, 36, 37]. Unfortunately, clinical EPR must confront the fact that the depth of penetration will continue to be a limiting factor, unless further advances in hardware design and their applications are successful. Generally speaking, noninvasive EPR measurements are limited to a penetration depth on the order of 1 cm at L-band (~1.2 GHz). Penetration depth can be increased to 8 cm or more by lowering the frequency to the 300–600 MHz range. The tradeoff, however, is that the signal-to-noise ratio significantly decreases at these lower frequencies, causing a decrease in spatial resolution and accuracy of pO2 measurements. Implantable resonators are one potential way to overcome this restriction for the successful clinical application of EPR for oximetry measurements at deeper sites [38].
Paramagnetic probes for possible use in clinical EPR oximetry
Measurement of oxygen-induced EPR line-broadening requires the presence of a suitable paramagnetic probe in the tissue region of interest. Two types of probes are used: (i) soluble probes that report the concentration of dissolved oxygen and (ii) particulate probes that measure partial pressure of oxygen (pO2) in the milieu. Considerable progress has been made in the development and use of both types of probes. The soluble probes provide the potential for imaging, but have the potential limitations of being limited in their sensitivity and the ability to repeat the measurements. Nevertheless they have been very successful in a number of applications in preclinical models. Because they need to be administered directly their translation to clinical applications hinges on the success of having a sponsor who will be willing and able to invest the many hundreds of millions of dollars required for achieving regulatory approval for their use in human subjects.
There are certain advantages in using particulate probes including that they can more readily be approved for use in human subjects as described below. The other attractive features of particulate probes include (i) high resolution - resolutions in the range of 0.1 torr (mmHg) can be obtained; (ii) suitability for repeated measurements in vivo without reintroduction of the probe into the tissue; (iii) non-invasive measurement - may require one-time introduction of the probe; however, subsequent measurements are performed under noninvasive conditions (iv) accuracy - highly reproducible and correlate closely with measurements by other methods; (v) localized measurements - the measurement is made from a single voxel/region containing the particulate – thus the spatial resolution is the size of the particulate deposit; (vi) insolubility in aqueous solvents; (vii) no effect of the various biological oxidoreductants, pH, temperature etc.; (viii) Nontoxic - the probes are very inert in biological systems; (ix) temporal response is very good, usually less than a seconds (x) respond to partial pressure of oxygen (pO2), rather than concentration of oxygen, the latter may be quite heterogeneous in cellular/tissue environments because of the solubility of oxygen and hence calibration can be difficult . The use of both the naturally occurring (coals, fusinite), semisynthetic (India ink, sugar chars, carbon blacks) and purely synthetic crystalline materials, e.g., lithium phthalocyanine (LiPc), lithium naphthalocyanine (LiNc), and lithium octabutoxynaphthalocyanine (LiNc-BuO) have been reported for EPR oximetry [33, 39–47].
The particulate probes typically are introduced into tissues using a small gauge needle (21 – 25 gauge), often several days prior to measurement in order to minimize trauma and allow for healing and incorporation of the material into the tissue prior to pO2 measurement. An advantage of the particulate approach is that once the probes have been introduced, they may be used to make repeated measurements at the same location without the need to introduce additional material or wait for introduced material to be cleared. This characteristic is particularly useful for the monitoring of tissue oxygenation during the development of disease or in response to therapy, as needed for the optimal treatment of solid tumor cancers and peripheral vascular disease.
India ink has been the first probe used for oxygen measurements in human [40]. India ink has a long history of clinical use as an anatomic marker for surgery and radiotherapy and, fortuitously, the suspended carbon black particles contain stable radical species at sufficient concentrations with EPR spectra that are highly sensitive to the presence of oxygen [43, 48]. Charcoal, a naturally occurring carbonaceous material, has been extensively characterized for EPR oximetry in animal models [49–51]. Charcoal has been approved for clinical use (as a tissue marker) in Europe. Despite their availability for clinical use, the carbon-based oxygen sensors have some limitations insensitivity to oxygen and if used as suspensions may diffuse. Alternatively, purely synthetic materials such as lithium phthalocyanine [33], or naphthalocyanine [52] and derivatives [45, 47], are ideally-suited for clinical EPR oximetry. However, these materials need regulatory approvals for use in human. The most notable drawback and potential barrier to the use of these materials in clinical EPR applications is the need to leave these particulate probe materials permanently in the tissue, which may present practical barriers for obtaining approval for use in human subjects. Therefore, we are developing an alternative approach for their clinical use: to embed them in biocompatible materials that have high oxygen permeability [49, 53–55]. The probes are effectively shielded from interaction with the biological milieu that could result in biochemical degradation and breakdown, as well as local and/or systemic toxicity effects that may occur due to these reactions. The implants could be left in the tissue or removed when no longer needed.
Although India ink has been the only sensor currently in use for measurement in humans, the crystalline materials such as LiPc and LiNc-BuO have some potential advantages. The crystalline sensors have significantly higher EPR detection sensitivity, largely due to higher paramagnetic spin content [33, 43, 47] and narrower lineshape when compared to India ink. The narrower lineshape of the crystalline sensors also contribute to their higher oxygen resolutions, typically in the range of 0.2–0.5 mmHg. Our laboratory is keenly focused on developing the crystalline sensors for use in clinical oximetry.
Multisite oximetry
Traditional EPR oximetry provides average pO2 estimates based on the summed signals detected from spins within the sensitive volume of the resonator. In order to provide additional spatial resolution of pO2 within the tissues, we have developed procedures for simultaneous measurements from multiple implants, as well as implantable resonators with multiple sensing tips that are spatially distributed within the tissue [27, 38, 56]. The multi-site measurement technique uses magnetic field gradients to spectrally distinguish their respective signals. A number of analytic methods have been developed [57–59] which exploit this sparseness in spatial spin distribution to limit the number of degrees of freedom involved in pO2 estimation and significantly reduce the data acquisition time as compared to conventional spectral-spatial imaging. Our laboratory has pioneered an approach coined ‘‘multi-site oximetry” where sparseness of the spin distribution is exploited [57, 58].
Clinical potential of EPR oximetry
Although several existing other methods are utilized to try to measure oxygen concentrations in vivo in human subjects, a suitable technique for noninvasive and repeated direct measurements of oxygen in the same tissue or cells on a temporal scale is not available currently. While electrode techniques have evolved as the standard methods for measurement of oxygen, they are complex to use and can generate analytical artifacts during assay procedures at the freshly probed sites and they are not suitable for repeated measurements [17]. Near-infrared and magnetic resonance techniques such as nuclear magnetic resonance, blood oxygen level-dependent (BOLD) magnetic resonance imaging, Overhauser-enhanced magnetic resonance imaging (OMRI) are noninvasive methods. However, they do not report the absolute values of oxygen concentration in the tissues [10, 60–64]. EPR oximetry enables reliable and accurate measurements of the pO2 [20]. EPR oximetry can measure directly in the tissue and can be done at the actual site(s) of interest. The placement of the paramagnetic material directly at the site of interest, where it remains and can be interrogated after any temporary perturbation has resolved, also avoids potential concerns about damaging the tissue at the time of measurement [8]. The ability of EPR oximetry to make repeated measurements from localized sites provides a very important capability that can enable critical aspects of a number of biomedical applications.
Constraints for Clinical EPR development
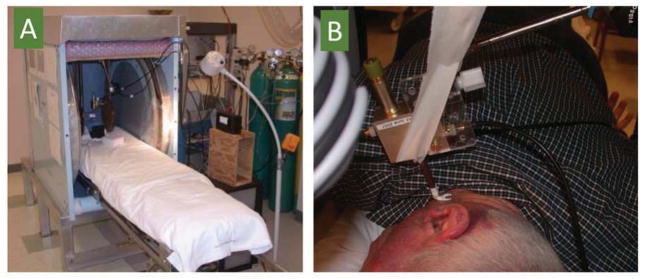
At present the EPR systems for use in laboratory animals have been fairly well developed using low-frequency (1.2 GHz or below) source and lumped circuit resonators capable of providing whole-body measurements including imaging [65]. However, clinical EPR is faced with several constraints and challenges with respect to instrumentation, probe administration, and regulatory issues [2, 3, 8]. Magnets with large gap and sufficient magnetic-field homogeneity are needed to accommodate and enable measurements in human subject. Our laboratory has succeeded in using large permanent magnets (gap of 50 cm) with field homogeneity suitable for even the very narrow linewidth of anoxic lithium phthalocyanine (20 milligauss) for whole-body oximetry measurements (Figure 1). We have also developed magnet designs customized for head, and for dosimetry measurements in human tooth in vivo. The applicability of these clinical EPR systems have been demonstrated, to date, in a large number of normal volunteers and more than 30 patients. Another major constraint for clinical EPR oximetry is the need to use an exogenous probe, which is placed in the desired location of the tissue, using a minimally invasive surgical procedure. The probe, once implanted, will stay in the tissue for a desired length of period or permanently enabling repeated measurements of pO2. This raises safety and ethical concerns for use in human and thus necessitates elaborative studies, which are both expensive and time-consuming before getting FDA clearance. Of the oximetry probes characterized in animal models, India ink has previously been approved for clinical use, and hence our laboratory has started to use India ink for clinical measurements. Although we have alternative probes, based on lithium phthalocyanine (LiPc) derivatives [33, 47, 66] that are much superior to India ink in terms on oxygen sensitivity, and other desirable properties their use in human awaits regulatory approval. One important consideration towards increasing the safety of the implanted probe material, which is usually in the form of microcrystalline powder, is to encapsulate it in a biocompatible, oxygen-permeable polymer [54, 55, 67–69]. The encapsulation will not only hold the probe particulates from smearing or migration over a period of time but also avoid direct contact of the probe with the cells. We have used polydimethylsiloxane (PDMS), a silicone polymer with properties desirable for encapsulation of oximetry probes. It is a biocompatible, highly flexible, highly oxygen permeable [70] and has been used in a wide range of medical device and health care applications [71]. PDMS has been approved for use in human subjects and is one of the reference materials provided by the National Heart Lung and Blood Institute for standardized biocompatibility testing [72]. We used PDMS to encapsulate LiNc-BuO and developed the implants in the form of thin films (OxyChip). The efficacy and safety of OxyChip have been well characterized in animal models [55, 69]. We have taken steps to obtain investigational device exemption status from FDA for testing the OxyChips in human subjects. The OxyChip implants will be used for subcutaneous and tissue measurements up to a depth of 10 mm. For measurements deeper than 10 mm we will use implantable resonators, which we have developed and demonstrated in animal models [27, 38, 56]. The implantable resonators (Figure 2) are capable of deep tissue pO2 measurements anywhere in the body, and at multiple sites, simultaneously. FDA clearance for the use of implantable resonator in human is expected be less problematic.
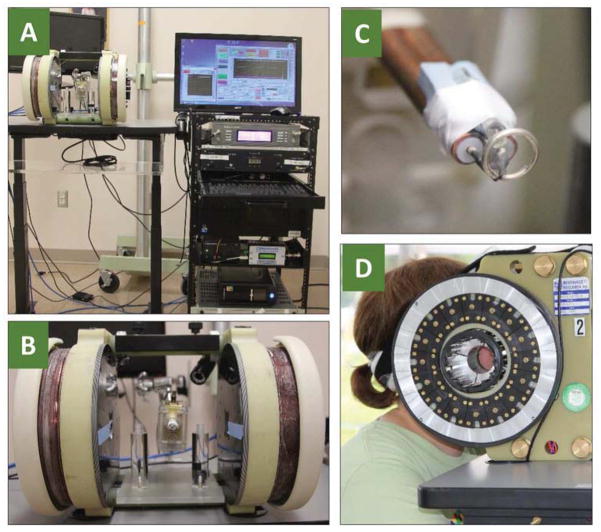
Figure 1. Clinical EPR system.
(A) Our 1.2 GHz EPR spectrometer using a large-gap permanent magnet and surface loop resonator. Also shown is a moveable bed for patient positioning. (B) Measurement of tumor pO2 in a head & neck cancer patient suing surface loop resonator.
Figure 2. Implantable resonator design.
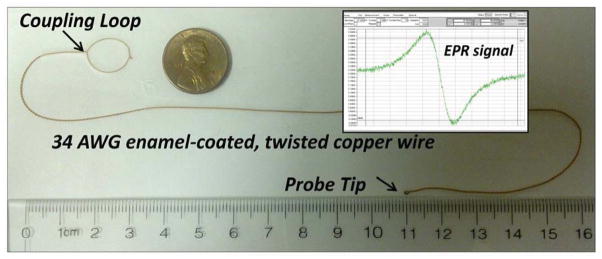
Photograph of a 25-cm-long implantable resonator made of 34-AWG enamel-coated copper wire containing PDMS-encapsulated LiNc-BuO sensor at one end (Probe tip) and coupling loop at the other end. The inset shows an EPR spectrum obtained using the resonator.
Other than instrumentation and probe, there are also additional challenges in the use of clinical EPR. Translation of the new technology for clinical practice involves a variety of factors, most importantly, the human factors including operators (technicians), physicians, and patients and the need to fit the measurements into the established clinical routines or emergency response. The EPR Center at Dartmouth is also working on the translational aspects of this technology for clinical acceptance and practice.
Clinical oximetry measurements
We have demonstrated that oxygen measurements can be made in human subjects in superficial tumors using India ink, the only currently approved material for use in human subjects. Extensive pre-clinical and clinical development has shown the feasibility and safety of repeated oxygen measurements for easily-accessible, subcutaneous sites. The development of India ink as a clinically-applicable oxygen reporter was carried out jointly by the Dartmouth laboratory and Dr. Gallez’ research group in Belgium [3, 40, 41, 43, 48, 73–75]. At the Dartmouth EPR Center, EPR oximetry measurements in human subjects are performed using the clinical whole-body L-band EPR spectrometer [3, 8, 48, 76]. This spectrometer consists of a 420-G permanent magnet, magnetic field modulation and sweep coils, RF detection system including surface-loop resonators, and computer control [1, 8]. The spectrometer operates at an L-band frequency (~1.2 GHz), where a practical compromise between RF penetration depth and EPR sensitivity is achieved. At this frequency RF penetration depths of approximately 1 cm are achieved. Data on oxygen measurements in 11 superficial tumors at different sites in 10 patients is summarized in Table 2
Table 2.
pO2 measurements in human tumors measured in vivo with challenge by breathing enriched oxygen.
| Number | Cancer | Site | Response to Oxygen | With Air (mmHg) | With Oxygen (mmHg) |
|---|---|---|---|---|---|
| 1 | Melanoma | Knee | Yes | <10 | >50 |
| 2 | Lymphoma | Post. Thigh | Yes | <5 | >100 |
| 3 | Melanoma | Foot | No | Anoxic | Anoxic |
| 4 | Melanoma | Scalp | No | Anoxic | Anoxic |
| 5 | Sarcoma | Nose | No | ~10 | ~10 |
| 6 | Melanoma | Scalp & Neck | Yes | ~6 | ~12 |
| 7 | Melanoma | Scapula | Yes | <1 | <5 |
| 8 | Melanoma | Chest | No | ~10 | ~10 |
| 9 | Melanoma | Calf | Yes | Anoxic | ~3 |
| 10 | Sarcoma | Post. Thigh | Yes | <3 | >10 |
| 11 | Sarcoma | Post. Thigh | Yes | ~1 | >5 |
The experimental procedure was relatively simple in order to make the measurements under conditions that could readily be incorporated into normal clinical processes. The India ink was inserted into the tumors in the outpatient clinical, with local anesthesia and sterile procedures. A 21 to 23-gauge needle was used to insert 10 – 25 microliters of a sterilized slurry of India Ink, whose response to oxygen had been well calibrated in vitro and in vivo in animals. One - five days later a baseline measurement was made and then the patient was given an enriched oxygen mixture to breathe through a regular simple face mask. EPR measurements were made continuously while breathing the enriched oxygen mixture and then when the patient returned to breathing room air. The procedure took less than an hour in total and was very well tolerated by all subjects. The data as summarized in Table 2 lead to several tentative conclusions: (i) The limited amount of data and small number of tumor types permit only qualitative implications to be drawn; (ii) The tumors varied considerably in their baseline pO2, which ranged from 0 to 10 torr; (iii) The subjects varied considerably as to whether their tumors responded to increased oxygen in the breathing gas (four out of 11 did not respond); (iv) The amount of the increase in tumor oxygen varied widely among the responders (3 to 100 torr).
Even with such a limited amount of data, these results indicate that the ability to make repeated measurements of oxygen in tumors could be quite useful clinically. The measurements could be made under conditions compatible with usual clinical practice. The observation that a number of tumors did not respond at all to increases in the amount of oxygen in the breathing gas illustrates how misleading it could be to try to evaluate the ability of hyperoxygenation strategies to improve outcomes without being able to detect whether the treatment did actually change pO2 in the tumor. The potential for individualizing therapy also is illustrated because the time patterns of increased oxygen and return to normal varied among the subjects (data not shown).
The other systematic clinical oximetry study that has been initiated is directed towards the use of EPR oximetry to follow oxygen levels in critical tissues in patients with peripheral vascular disease. Our initial studies have been limited to normal subjects with injections into the subcutaneous tissues. We have been able to make repeated measurements of tissue oxygen in the foot at the sites where the major pathophysiology usually occurs, under the first metatarsal [3]. Measurements have been made successfully for more than five years from an initial single injection, including continuing responses to temporary interruption of the blood flow and to hyperoxygenation from breathing enriched normobaric oxygen.
We also have related the measurements with EPR with in vivo NIR and also with polarographic transcutaneous oxygen. There have been no reported or observed side effects from the injections of Indian Ink. We have, however found that in some subjects there is up to several weeks after initial injection before the ink becomes responsive to oxygen. We are investigating the basis for this effect; this lack of responsiveness has not been observed in animals or in tumors in human subjects.
While still at an early stage, the oximetry measurements in human subjects have demonstrated the feasibility of the technique, including the capability of carrying out the measurements within the constraints of usual clinical practice. Extensive studies are now being planned for a systematic development and evaluation of EPR oximetry in cancer patients.
Dosimetry
Another major use of clinical EPR is for the purposes of dosimetry [3, 77, 78]. The importance of this area is reflected in the high level of funding that has been made available for its development and the widespread recognition of the unique contributions that can be made by in vivo EPR to solve some of the critical problems in this area. As a consequence of the high level of activity that has been supported for this application development of instrumentation and techniques, the technical capabilities for all types of clinical EPR have been significantly advanced, while extensive experience has been gained in the practical aspects of making measurements in human subjects with in vivo EPR. The technical/practical advances that will be applicable to all types of clinical applications of EPR include: (i) Development of transportable EPR instruments, which will facilitate the adoption of clinical EPR to bedside and intra-operative applications; (ii) Development of operating characteristics so that it can be used by non-expert operators in any environment; (iii) Features that accommodate routine, minimally perturbing, and rapid measurements with an instrument that is appropriate for use with compromised human subjects; and (iv) Extensive demonstration of the safety and efficacy of EPR measurements in human subjects.
The potential value of the use of EPR dosimetry arises from an unfortunate fact of our current world: the potential for widespread exposures of large numbers of individuals to levels of ionizing radiation that are acutely life-threatening. The exposures could come from accidents (although these are unlikely to result in acutely life-threatening levels of exposures to many people), terrorism, or war. There are treatments available that reduce the severity of the accurate radiation syndrome but these are too complex and too potentially toxic to allow them to be used except if there is a reasonable probability that the subject has received potentially lethal doses of radiation. With a capability to screen large numbers of individuals rapidly, responders could direct into the health care system those who have received potentially life-threatening exposures and allow the rest of the population to know that they do not need acute treatment. This problem could be ameliorated if everyone would wear badges to detect significant radiation exposures, but the consensus is that this is impractical because of failure to comply and potential malfunction or misuse of passive radiation badges such as ones intrinsic to credit cards or driver’s licenses. Fortunately, however, it turns out that most of us are wearing a dosimeter, our teeth and finger/toe nails [3, 78]. These have sufficiently stable radiation-induced free radicals to act as passive radiation dosimeters. These potentially can be assessed directly by EPR spectroscopy in vivo.
The basis of EPR dosimetry resides in its unique ability to directly measure free radicals. Ionizing radiation generates large numbers of free radicals and while most of these react quickly and disappear in microseconds or less, they can persist if they are generated in materials that have little liquid water and have some systemic organization. The hydroxyapatite matrix in tooth enamel is such a tissue and it has been established that linearly dose-dependent radiation-induced changes in teeth last millions of years and there serve as excellent dosimeters. The EPR signal arises from radical ions generated from carbonate randomly distributed in the hydroxyapatite. The keratin–rich regions of nails also provide a site where radiation-induced radicals are stabilized. These persist for weeks but are more complex to analyze because mechanical forces also can generate radicals in keratin [79–82].
Currently almost all of the measurements in human subjects for EPR radiation dosimetry have been done using teeth as the dosimetric site (Figure 3). The focus is on the use of the two upper central incisor teeth because of their favorable anatomy (they are relatively flat), the higher probability that they will be present and without major restorations, and the convenience of making measurements in teeth at this position. With the support of BARDA (Biomedical Advanced Research and Development Authority) we are moving forward with the goal of producing an FDA-approved manufacturable dosimeter that can be employed in the field as part of the planned responses to a large radiation event. The characteristics for this instrument include features that will be very useful for all clinical applications including simplified operation, rapid measurement time (less than five minutes), attention to the needs for comfort and accessibility of potentially compromised subjects, rugged and reproducible construction, and full safety features compatible with clinical use. The current status of the developments are indicated by the initial data on dose response of human subjects measured in vivo, using patients undergoing total body irradiation (TBI) as the model for radiation effects in teeth of human subjects irradiated in the field. The current resolution of dose in the mouth model using human teeth is about 0.5 Gray standard error of inverse prediction (SEIP). In irradiated subjects, preliminary results indicate that it may be feasible to achieve the same dose resolution, but more data are needed to confirm this. This level of accuracy is more than sufficient for the purpose of initial triage.
Figure 3. In vivo tooth dosimetry using deployable EPR dosimeter.
(A) 1.15 GHz EPR dosimeter setup. (B) Magnet, modulation coil, and resonator assembly. (C) Surface-loop resonator (~9-mm diameter loop). (D) Side view of the dosimeter with the person undergoing tooth measurement.
The status of in vivo nail dosimetry is less advanced but quite promising. Because the radiation-induced signals in nails are weaker than those in teeth, we are assuming that the measurements will need to be made with higher frequency EPR (9 GHz vs. the 1.1 GHz) used for other clinical measurements. Satisfactory measurements of radiation-induced signals in fingernail models have been made at 9 GHz [78]. The challenge for this application is to design and build resonators that limit the microwave field to the nail, avoiding the soft tissue below because the latter would result in non-resonant absorption of the microwave. Considerable progress has been made on such resonators so that useful spectra have been obtained from living fingers (Figure 4). Developments are proceeding to enable the measurements to be made of the radiation-induced signals in the nails of patients undergoing radiation therapy that results in significant doses to their nails.
Figure 4. Photographs illustrating the position of fingers in resonators for in vivo nail dosimetry.
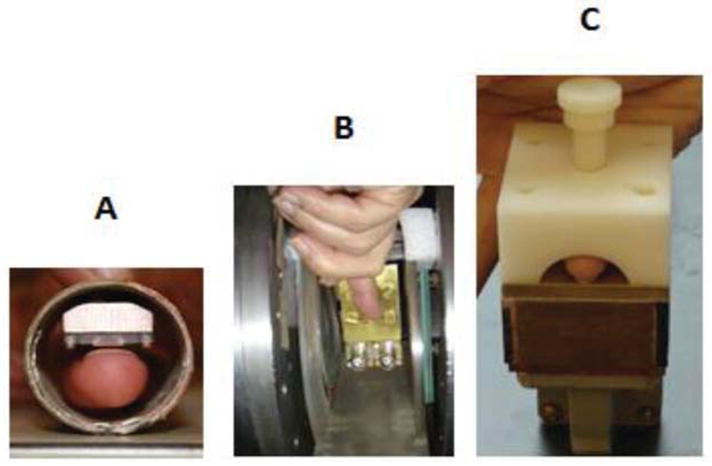
(A) SRA resonator, (B) rectangular TE102 cavity aperture resonator (AR), and (C) hemispherical TE121 AR.
Conclusion
The recent developments in EPR instrumentation, probes, and methods offer potential opportunities for clinical oximetry and dosimetry. The EPR oximetry is valuable for guiding therapy for individual patients with tumors or vascular disease, by monitoring of tissue oxygenation. Further work is in progress to translate this unique technology to routine clinical practice. The use of in vivo EPR dosimetry appears to be sufficient for the initial stage of triage in a large-scale radiation event and further improvements are underway, consistent with the goal of becoming an intrinsic part of the response system within 2–3 years.
Table 1.
Clinical EPR at Dartmouth
| Clinical problem | Parameter to be measured | Status of measurements in human subjects | Rationale for using in vivo EPR measurements |
|---|---|---|---|
| Dosimetry | Radiation-induced free radicals | Extensive measurements underway | Fills unique niche for emergency dosimetry based on physical parameter |
| Peripheral vascular disease | Oxygen at sites of likely pathologies | Measurements underway in normal volunteers; patients to be studied in near future | The pO2 in the tissues is the most significant pathophysiological variable; no other method available to make such direct measurements. The pO2 measurements will facilitate evaluating progression of disease and success of therapeutic intervention |
| Cancer | pO2 in tumors | Measurements underway in patients with superficial tumors; IDEs submitted for permission to start studies with embedded materials | The response of tumors to cytotoxic therapy, especially ionizing radiation, is critically dependent on pO2. Antitumor therapies are given repeatedly and often change pO2. Knowledge of the changes in individual patients would significantly optimize the timing of the therapy |
| Wound healing | pO2 at various sites in wounds, Perhaps reactive oxygen species | Existing clinical protocols use an apparatus compatible with EPR measurements. | The pO2 is a critical variable for successful healing of wounds. Direct measurements would identify patients likely to have poor healing and follow responses to therapy |
| Radiation Induced Fibrosis | pO2 in irradiated tumor beds and peripheral normal tissue | Initial studies have been started | Radiation-induced hypoxia may play a critical role in the signaling of pro-inflammatory, pro-fibrotic, and pro-angiogenic growth factors and cytokines that lead to tissue fibrosis. |
Acknowledgments
Funding acknowledgements: US Department of Health and Human Services – Biomedical Advanced Research and Development Authority (HHSO100201100024C); NIH NIAID (U19-AI091173); NIH NIBIB (R01 EB004031)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Salikhov I, Walczak T, Lesniewski P, et al. EPR spectrometer for clinical applications. Magn Reson Med. 2005;54(5):1317–20. doi: 10.1002/mrm.20689. [DOI] [PubMed] [Google Scholar]
- 2.Swartz HM, Walczak T. Developing in vivo EPR oximetry for clinical use. Adv Exp Med Biol. 1998;454:243–52. doi: 10.1007/978-1-4615-4863-8_29. [DOI] [PubMed] [Google Scholar]
- 3.Williams BB, Khan N, Zaki B, Hartford A, Ernstoff MS, Swartz HM. Clinical electron paramagnetic resonance (EPR) oximetry using India ink. Adv Exp Med Biol. 2010;662:149–56. doi: 10.1007/978-1-4419-1241-1_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Swartz HM, Berliner LJ. Biological magnetic resonance. New York: Plenum Press; 2003. [Google Scholar]
- 5.Rockwell S, Moulder JE. Hypoxic fractions of human tumors xenografted into mice: a review. Int J Radiat Oncol Biol Phys. 1990;19(1):197–202. doi: 10.1016/0360-3016(90)90154-c. [DOI] [PubMed] [Google Scholar]
- 6.Vikram DS, Zweier JL, Kuppusamy P. Methods for noninvasive imaging of tissue hypoxia. Antioxid Redox Signal. 2007;9(10):1745–56. doi: 10.1089/ars.2007.1717. [DOI] [PubMed] [Google Scholar]
- 7.Swartz HM. Using EPR to measure a critical but often unmeasured component of oxidative damage: oxygen. Antioxid Redox Signal. 2004;6(3):677–86. doi: 10.1089/152308604773934440. [DOI] [PubMed] [Google Scholar]
- 8.Swartz HM, Khan N, Buckey J, et al. Clinical applications of EPR: overview and perspectives. NMR Biomed. 2004;17(5):335–51. doi: 10.1002/nbm.911. [DOI] [PubMed] [Google Scholar]
- 9.Springett R, Swartz HM. Measurements of oxygen in vivo: overview and perspectives on methods to measure oxygen within cells and tissues. Antioxid Redox Signal. 2007;9(8):1295–301. doi: 10.1089/ars.2007.1620. [DOI] [PubMed] [Google Scholar]
- 10.Mason RP, Hunjan S, Le D, et al. Regional tumor oxygen tension: fluorine echo planar imaging of hexafluorobenzene reveals heterogeneity of dynamics. Int J Radiat Oncol Biol Phys. 1998;42(4):747–50. doi: 10.1016/s0360-3016(98)00306-x. [DOI] [PubMed] [Google Scholar]
- 11.Griffiths JR, Robinson SP. The OxyLite: a fibre-optic oxygen sensor. Br J Radiol. 1999;72(859):627–30. doi: 10.1259/bjr.72.859.10624317. [DOI] [PubMed] [Google Scholar]
- 12.Braun RD, Lanzen JL, Snyder SA, Dewhirst MW. Comparison of tumor and normal tissue oxygen tension measurements using OxyLite or microelectrodes in rodents. Am J Physiol Heart Circ Physiol. 2001;280(6):H2533–44. doi: 10.1152/ajpheart.2001.280.6.H2533. [DOI] [PubMed] [Google Scholar]
- 13.Nozue M, Lee I, Yuan F, et al. Interlaboratory variation in oxygen tension measurement by Eppendorf “Histograph” and comparison with hypoxic marker. J Surg Oncol. 1997;66(1):30–8. doi: 10.1002/(sici)1096-9098(199709)66:1<30::aid-jso7>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 14.O'Hara JA, Hou H, Demidenko E, Springett RJ, Khan N, Swartz HM. Simultaneous measurement of rat brain cortex PtO2 using EPR oximetry and a fluorescence fiber-optic sensor during normoxia and hyperoxia. Physiol Meas. 2005;26(3):203–13. doi: 10.1088/0967-3334/26/3/006. [DOI] [PubMed] [Google Scholar]
- 15.O'Hara JA, Khan N, Hou H, et al. Comparison of EPR oximetry and Eppendorf polarographic electrode assessments of rat brain PtO2. Physiol Meas. 2004;25(6):1413–23. doi: 10.1088/0967-3334/25/6/007. [DOI] [PubMed] [Google Scholar]
- 16.Vikram DS, Bratasz A, Ahmad R, Kuppusamy P. A comparative evaluation of EPR and OxyLite oximetry using a random sampling of pO(2) in a murine tumor. Radiat Res. 2007;168(3):308–15. doi: 10.1667/RR0854.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vaupel P, Schlenger K, Knoop C, Hockel M. Oxygenation of human tumors: evaluation of tissue oxygen distribution in breast cancers by computerized O2 tension measurements. Cancer Res. 1991;51(12):3316–22. [PubMed] [Google Scholar]
- 18.Baudelet C, Gallez B. How does blood oxygen level-dependent (BOLD) contrast correlate with oxygen partial pressure (pO2) inside tumors? Magn Reson Med. 2002;48(6):980–6. doi: 10.1002/mrm.10318. [DOI] [PubMed] [Google Scholar]
- 19.Jordan BF, Crokart N, Baudelet C, Cron GO, Ansiaux R, Gallez B. Complex relationship between changes in oxygenation status and changes in R*2: the case of insulin and NS-398, two inhibitors of oxygen consumption. Magn Reson Med. 2006;56(3):637–43. doi: 10.1002/mrm.20963. [DOI] [PubMed] [Google Scholar]
- 20.Ahmad R, Kuppusamy P. Theory, instrumentation, and applications of electron paramagnetic resonance oximetry. Chemical reviews. 2010;110(5):3212–36. doi: 10.1021/cr900396q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smirnov AI, Norby SW, Walczak T, Liu KJ, Swartz HM. Physical and instrumental considerations in the use of lithium phthalocyanine for measurements of the concentration of the oxygen. Journal of magnetic resonance Series B. 1994;103(2):95–102. doi: 10.1006/jmrb.1994.1016. [DOI] [PubMed] [Google Scholar]
- 22.Elas M, Ahn KH, Parasca A, et al. Electron paramagnetic resonance oxygen images correlate spatially and quantitatively with Oxylite oxygen measurements. Clin Cancer Res. 2006;12(14 Pt 1):4209–17. doi: 10.1158/1078-0432.CCR-05-0446. [DOI] [PubMed] [Google Scholar]
- 23.Froncisz W, Lai CS, Hyde JS. Spin-label oximetry: kinetic study of cell respiration using a rapidpassage T1-sensitive electron spin resonance display. Proc Natl Acad Sci U S A. 1985;82(2):411–5. doi: 10.1073/pnas.82.2.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gallez B, Bacic G, Goda F, et al. Use of nitroxides for assessing perfusion, oxygenation, and viability of tissues: in vivo EPR and MRI studies. Magn Reson Med. 1996;35(1):97–106. doi: 10.1002/mrm.1910350113. [DOI] [PubMed] [Google Scholar]
- 25.Grinberg OY, Friedman BJ, Swartz HM. Intramyocardial pO2 measured by EPR. Adv Exp Med Biol. 1997;428:261–8. doi: 10.1007/978-1-4615-5399-1_36. [DOI] [PubMed] [Google Scholar]
- 26.Halpern HJ, Yu C, Peric M, et al. Measurement of differences in pO2 in response to perfluorocarbon/carbogen in FSa and NFSa murine fibrosarcomas with low-frequency electron paramagnetic resonance oximetry. Radiat Res. 1996;145(5):610–8. [PubMed] [Google Scholar]
- 27.Hou H, Dong R, Li H, et al. Dynamic changes in oxygenation of intracranial tumor and contralateral brain during tumor growth and carbogen breathing: a multisite EPR oximetry with implantable resonators. J Magn Reson. 2012;214(1):22–8. doi: 10.1016/j.jmr.2011.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ilangovan G, Li H, Zweier JL, Kuppusamy P. Effect of carbogen-breathing on redox status of the RIF-1 tumor. Adv Exp Med Biol. 2003;510:13–7. doi: 10.1007/978-1-4615-0205-0_3. [DOI] [PubMed] [Google Scholar]
- 29.Ilangovan G, Liebgott T, Kutala VK, Petryakov S, Zweier JL, Kuppusamy P. EPR oximetry in the beating heart: myocardial oxygen consumption rate as an index of postischemic recovery. Magn Reson Med. 2004;51(4):835–42. doi: 10.1002/mrm.20000. [DOI] [PubMed] [Google Scholar]
- 30.Khan M, Kutala VK, Wisel S, et al. Measurement of oxygenation at the site of stem cell therapy in a murine model of myocardial infarction. Adv Exp Med Biol. 2008;614:45–52. doi: 10.1007/978-0-387-74911-2_6. [DOI] [PubMed] [Google Scholar]
- 31.Khan M, Mohan IK, Kutala VK, et al. Sulfaphenazole protects heart against ischemia-reperfusion injury and cardiac dysfunction by overexpression of iNOS, leading to enhancement of nitric oxide bioavailability and tissue oxygenation. Antioxid Redox Signal. 2009;11(4):725–38. doi: 10.1089/ars.2008.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuppusamy P, Afeworki M, Shankar RA, et al. In vivo electron paramagnetic resonance imaging of tumor heterogeneity and oxygenation in a murine model. Cancer Res. 1998;58(7):1562–8. [PubMed] [Google Scholar]
- 33.Liu KJ, Gast P, Moussavi M, et al. Lithium phthalocyanine: a probe for electron paramagnetic resonance oximetry in viable biological systems. Proc Natl Acad Sci U S A. 1993;90(12):5438–42. doi: 10.1073/pnas.90.12.5438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krishna MC, English S, Yamada K, et al. Overhauser enhanced magnetic resonance imaging for tumor oximetry: coregistration of tumor anatomy and tissue oxygen concentration. Proc Natl Acad Sci U S A. 2002;99(4):2216–21. doi: 10.1073/pnas.042671399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matsumoto A, Matsumoto K, Matsumoto S, et al. Intracellular hypoxia of tumor tissue estimated by noninvasive electron paramagnetic resonance oximetry technique using paramagnetic probes. Biological & pharmaceutical bulletin. 2011;34(1):142–5. doi: 10.1248/bpb.34.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosen GM, Halpern HJ, Brunsting LA, et al. Direct measurement of nitroxide pharmacokinetics in isolated hearts situated in a low-frequency electron spin resonance spectrometer: implications for spin trapping and in vivo oxymetry. Proc Natl Acad Sci U S A. 1988;85(20):7772–6. doi: 10.1073/pnas.85.20.7772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zweier JL, Kuppusamy P. Electron paramagnetic resonance measurements of free radicals in the intact beating heart: a technique for detection and characterization of free radicals in whole biological tissues. Proc Natl Acad Sci U S A. 1988;85(15):5703–7. doi: 10.1073/pnas.85.15.5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li H, Hou H, Sucheta A, et al. Implantable resonators--a technique for repeated measurement of oxygen at multiple deep sites with in vivo EPR. Adv Exp Med Biol. 2010;662:265–72. doi: 10.1007/978-1-4419-1241-1_38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vahidi N, Clarkson RB, Liu KJ, Norby SW, Wu M, Swartz HM. In vivo and in vitro EPR oximetry with fusinite: a new coal-derived, particulate EPR probe. Magn Reson Med. 1994;31(2):139–46. doi: 10.1002/mrm.1910310207. [DOI] [PubMed] [Google Scholar]
- 40.Swartz HM, Liu KJ, Goda F, Walczak T. India ink: a potential clinically applicable EPR oximetry probe. Magn Reson Med. 1994;31(2):229–32. doi: 10.1002/mrm.1910310218. [DOI] [PubMed] [Google Scholar]
- 41.Nakashima T, Goda F, Jiang J, Shima T, Swartz HM. Use of EPR oximetry with India ink to measure the pO2 in the liver in vivo in mice. Magn Reson Med. 1995;34(6):888–92. doi: 10.1002/mrm.1910340614. [DOI] [PubMed] [Google Scholar]
- 42.James PE, Goda F, Grinberg OY, Szybinski KG, Swartz HM. Intrarenal pO2 measured by EPR oximetry and the effects of bacterial endotoxin. Adv Exp Med Biol. 1997;411:557–68. doi: 10.1007/978-1-4615-5865-1_69. [DOI] [PubMed] [Google Scholar]
- 43.Goda F, Liu KJ, Walczak T, O'Hara JA, Jiang J, Swartz HM. In vivo oximetry using EPR and India ink. Magn Reson Med. 1995;33(2):237–45. doi: 10.1002/mrm.1910330214. [DOI] [PubMed] [Google Scholar]
- 44.Pandian RP, Dolgos M, Dang V, Sostaric JZ, Woodward PM, Kuppusamy P. Structure and Oxygen-Sensing Paramagnetic Properties of a New Lithium 1,8,15,22-Tetraphenoxyphthalocyanine Radical Probe for Biological Oximetry. Chem Mater. 2007;19:3545–52. [Google Scholar]
- 45.Pandian RP, Dolgos M, Marginean C, et al. Molecular packing and magnetic properties of lithium naphthalocyanine crystals: hollow channels enabling permeability and paramagnetic sensitivity to molecular oxygen. Journal of materials chemistry. 2009;19(24):4138–47. doi: 10.1039/b901886g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pandian RP, Kim YI, Woodward PM, Zweier JL, Manoharan PT, Kuppusamy P. Open molecular framework in lithium octabutoxy-naphthalocyanine paramagnetic crystal: Implications for the detection of oxygen and nitric oxide by EPR spectroscopy. J Mater Chem. 2006;16:3609–18. [Google Scholar]
- 47.Pandian RP, Parinandi NL, Ilangovan G, Zweier JL, Kuppusamy P. Novel particulate spin probe for targeted determination of oxygen in cells and tissues. Free Radic Biol Med. 2003;35(9):1138–48. doi: 10.1016/s0891-5849(03)00496-9. [DOI] [PubMed] [Google Scholar]
- 48.Khan N, Hou H, Hein P, et al. Black magic and EPR oximetry: from lab to initial clinical trials. Adv Exp Med Biol. 2005;566:119–25. doi: 10.1007/0-387-26206-7_17. [DOI] [PubMed] [Google Scholar]
- 49.Gallez B, Debuyst R, Dejehet F, et al. Small particles of fusinite and carbohydrate chars coated with aqueous soluble polymers: preparation and applications for in vivo EPR oximetry. Magn Reson Med. 1998;40(1):152–9. doi: 10.1002/mrm.1910400120. [DOI] [PubMed] [Google Scholar]
- 50.Jordan BF, Baudelet C, Gallez B. Carbon-centered radicals as oxygen sensors for in vivo electron paramagnetic resonance: screening for an optimal probe among commercially available charcoals. Magma. 1998;7(2):121–9. doi: 10.1007/BF02592236. [DOI] [PubMed] [Google Scholar]
- 51.Mahy P, De Bast M, Gallez B, et al. In vivo colocalization of 2-nitroimidazole EF5 fluorescence intensity and electron paramagnetic resonance oximetry in mouse tumors. Radiother Oncol. 2003;67(1):53–61. doi: 10.1016/s0167-8140(03)00028-8. [DOI] [PubMed] [Google Scholar]
- 52.Ilangovan G, Manivannan A, Li H, Yanagi H, Zweier JL, Kuppusamy P. A naphthalocyanine-based EPR probe for localized measurements of tissue oxygenation. Free Radic Biol Med. 2002;32(2):139–47. doi: 10.1016/s0891-5849(01)00784-5. [DOI] [PubMed] [Google Scholar]
- 53.He J, Beghein N, Ceroke P, Clarkson RB, Swartz HM, Gallez B. Development of biocompatible oxygen-permeable films holding paramagnetic carbon particles: evaluation of their performance and stability in EPR oximetry. Magn Reson Med. 2001;46(3):610–4. doi: 10.1002/mrm.1234. [DOI] [PubMed] [Google Scholar]
- 54.Meenakshisundaram G, Eteshola E, Pandian RP, Bratasz A, Lee SC, Kuppusamy P. Fabrication and physical evaluation of a polymer-encapsulated paramagnetic probe for biomedical oximetry. Biomed Microdevices. 2009;11(4):773–82. doi: 10.1007/s10544-009-9292-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Meenakshisundaram G, Pandian RP, Eteshola E, Lee SC, Kuppusamy P. A paramagnetic implant containing lithium naphthalocyanine microcrystals for high-resolution biological oximetry. J Magn Reson. 2010;203(1):185–9. doi: 10.1016/j.jmr.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hou H, Li H, Dong R, Mupparaju S, Khan N, Swartz H. Cerebral oxygenation of the cortex and striatum following normobaric hyperoxia and mild hypoxia in rats by EPR oximetry using multiprobe implantable resonators. Adv Exp Med Biol. 2011;701:61–7. doi: 10.1007/978-1-4419-7756-4_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Smirnov AI, Norby SW, Clarkson RB, Walczak T, Swartz HM. Simultaneous multi-site EPR spectroscopy in vivo. Magn Reson Med. 1993;30(2):213–20. doi: 10.1002/mrm.1910300210. [DOI] [PubMed] [Google Scholar]
- 58.Grinberg OY, Smirnov AI, Swartz HM. High spatial resolution multi-site EPR oximetry. The use of convolution-based fitting method. J Magn Reson. 2001;152(2):247–58. doi: 10.1006/jmre.2001.2408. [DOI] [PubMed] [Google Scholar]
- 59.Som S, Potter LC, Ahmad R, Vikram DS, Kuppusamy P. EPR oximetry in three spatial dimensions using sparse spin distribution. J Magn Reson. 2008;193(2):210–7. doi: 10.1016/j.jmr.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Aboagye EO, Maxwell RJ, Horsman MR, et al. The relationship between tumour oxygenation determined by oxygen electrode measurements and magnetic resonance spectroscopy of the fluorinated 2-nitroimidazole SR-4554. Br J Cancer. 1998;77(1):65–70. doi: 10.1038/bjc.1998.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim JG, Zhao D, Song Y, Constantinescu A, Mason RP, Liu H. Interplay of tumor vascular oxygenation and tumor pO2 observed using near-infrared spectroscopy, an oxygen needle electrode, and 19F MR pO2 mapping. J Biomed Opt. 2003;8(1):53–62. doi: 10.1117/1.1527049. [DOI] [PubMed] [Google Scholar]
- 62.Laukemper-Ostendorf S, Scholz A, Burger K, et al. 19F-MRI of perflubron for measurement of oxygen partial pressure in porcine lungs during partial liquid ventilation. Magn Reson Med. 2002;47(1):82–9. doi: 10.1002/mrm.10008. [DOI] [PubMed] [Google Scholar]
- 63.Mason RP, Rodbumrung W, Antich PP. Hexafluorobenzene: a sensitive 19F NMR indicator of tumor oxygenation. NMR Biomed. 1996;9(3):125–34. doi: 10.1002/(SICI)1099-1492(199605)9:3<125::AID-NBM405>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 64.Prasad PV, Edelman RR, Epstein FH. Noninvasive evaluation of intrarenal oxygenation with BOLD MRI. Circulation. 1996;94(12):3271–5. doi: 10.1161/01.cir.94.12.3271. [DOI] [PubMed] [Google Scholar]
- 65.Berliner LJ. In vivo EPR(ESR): Theory and Applications. In: Berliner LJ, editor. Biological magnetic Resonance. New York: Plenum; 2002. [Google Scholar]
- 66.Pandian RP, Chacko SM, Kuppusamy ML, Rivera BK, Kuppusamy P. Evaluation of lithium naphthalocyanine (LiNc) microcrystals for biological EPR oximetry. Adv Exp Med Biol. 2011;701:29–36. doi: 10.1007/978-1-4419-7756-4_5. [DOI] [PubMed] [Google Scholar]
- 67.Dinguizli M, Beghein N, Gallez B. Retrievable micro-inserts containing oxygen sensors for monitoring tissue oxygenation using EPR oximetry. Physiol Meas. 2008;29(11):1247–54. doi: 10.1088/0967-3334/29/11/001. [DOI] [PubMed] [Google Scholar]
- 68.Dinguizli M, Jeumont S, Beghein N, et al. Development and evaluation of biocompatible films of polytetrafluoroethylene polymers holding lithium phthalocyanine crystals for their use in EPR oximetry. Biosens Bioelectron. 2006;21(7):1015–22. doi: 10.1016/j.bios.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 69.Meenakshisundaram G, Eteshola E, Pandian RP, et al. Oxygen sensitivity and biocompatibility of an implantable paramagnetic probe for repeated measurements of tissue oxygenation. Biomed Microdevices. 2009;11(4):817–26. doi: 10.1007/s10544-009-9298-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mata A, Fleischman AJ, Roy S. Characterization of polydimethylsiloxane (PDMS) properties for biomedical micro/nanosystems. Biomed Microdevices. 2005;7(4):281–93. doi: 10.1007/s10544-005-6070-2. [DOI] [PubMed] [Google Scholar]
- 71.Abbasi F, Mirzadeh H, Katbab AA. Modification of polysiloxane polymers for biomedical applications: a review. Polymer International. 2001;50(12):1279–87. [Google Scholar]
- 72.Belanger MC, Marois Y. Hemocompatibility, biocompatibility, inflammatory and in vivo studies of primary reference materials low-density polyethylene and polydimethylsiloxane: a review. Journal of biomedical materials research. 2001;58(5):467–77. doi: 10.1002/jbm.1043. [DOI] [PubMed] [Google Scholar]
- 73.Jiang J, Nakashima T, Liu KJ, Goda F, Shima T, Swartz HM. Measurement of PO2 in liver using EPR oximetry. J Appl Physiol. 1996;80(2):552–8. doi: 10.1152/jappl.1996.80.2.552. [DOI] [PubMed] [Google Scholar]
- 74.Liu KJ, Miyake M, James PE, Swartz HM. Separation and enrichment of the active component of carbon based paramagnetic materials for use in EPR oximetry. J Magn Reson. 1998;133(2):291–8. doi: 10.1006/jmre.1998.1480. [DOI] [PubMed] [Google Scholar]
- 75.O'Hara JA, Goda F, Demidenko E, Swartz HM. Effect on regrowth delay in a murine tumor of scheduling split-dose irradiation based on direct pO2 measurements by electron paramagnetic resonance oximetry. Radiat Res. 1998;150(5):549–56. [PubMed] [Google Scholar]
- 76.Khan N, Williams BB, Hou H, Li H, Swartz HM. Repetitive tissue pO2 measurements by electron paramagnetic resonance oximetry: current status and future potential for experimental and clinical studies. Antioxid Redox Signal. 2007;9(8):1169–82. doi: 10.1089/ars.2007.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Swartz HM, Burke G, Coey M, et al. In Vivo EPR For Dosimetry. Radiat Meas. 2007;42(6–7):1075–84. doi: 10.1016/j.radmeas.2007.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Swartz HM, Flood AB, Williams BB, et al. Electron paramagnetic resonance dosimetry for a largescale radiation incident. Health Phys. 2012;103(3):255–67. doi: 10.1097/HP.0b013e3182588d92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Black PJ, Swarts SG. Ex vivo analysis of irradiated fingernails: chemical yields and properties of radiation-induced and mechanically-induced radicals. Health Phys. 2010;98(2):301–8. doi: 10.1097/HP.0b013e3181b0c045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.He X, Gui J, Matthews TP, et al. Advances towards using finger/toenail dosimetry to triage a large population after potential exposure to ionizing radiation. Radiat Meas. 2011;46(9):882–7. doi: 10.1016/j.radmeas.2011.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Romanyukha A, Reyes RA, Trompier F, Benevides LA. Fingernail dosimetry: current status and perspectives. Health Phys. 2010;98(2):296–300. doi: 10.1097/01.HP.0000347999.01948.74. [DOI] [PubMed] [Google Scholar]
- 82.Wilcox DE, He X, Gui J, et al. Dosimetry based on EPR spectral analysis of fingernail clippings. Health Phys. 2010;98(2):309–17. doi: 10.1097/HP.0b013e3181b27502. [DOI] [PMC free article] [PubMed] [Google Scholar]