Abstract
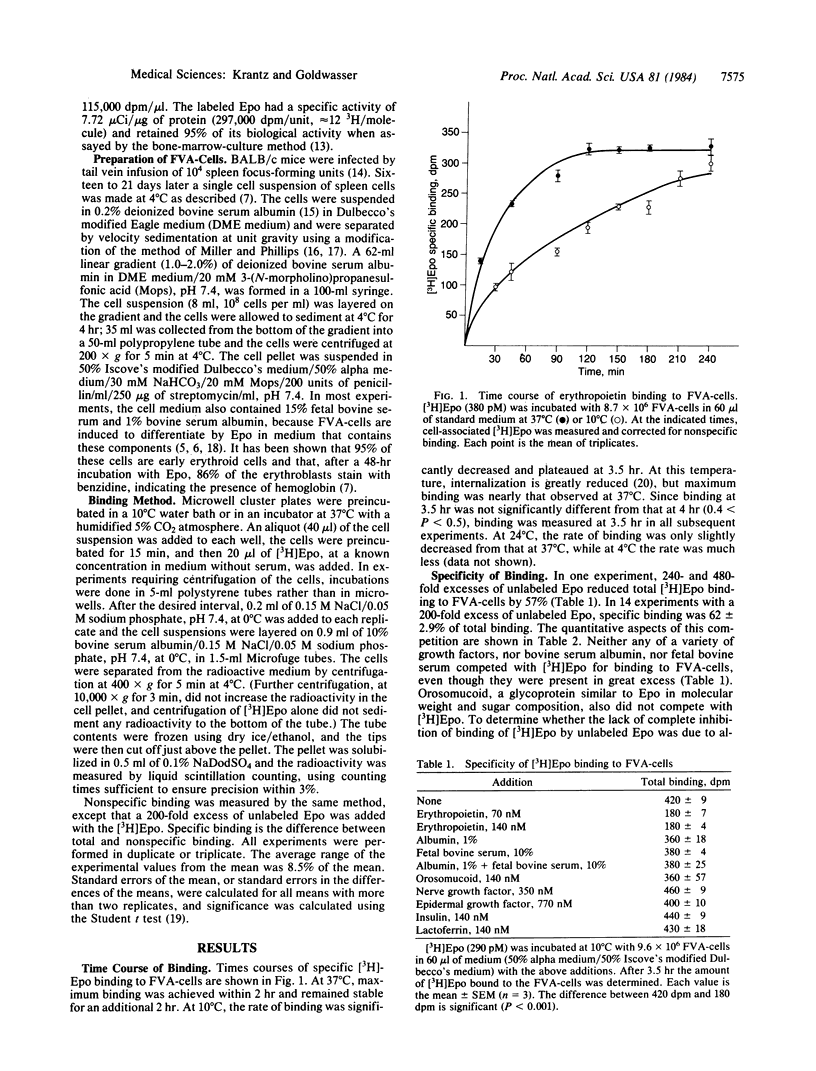
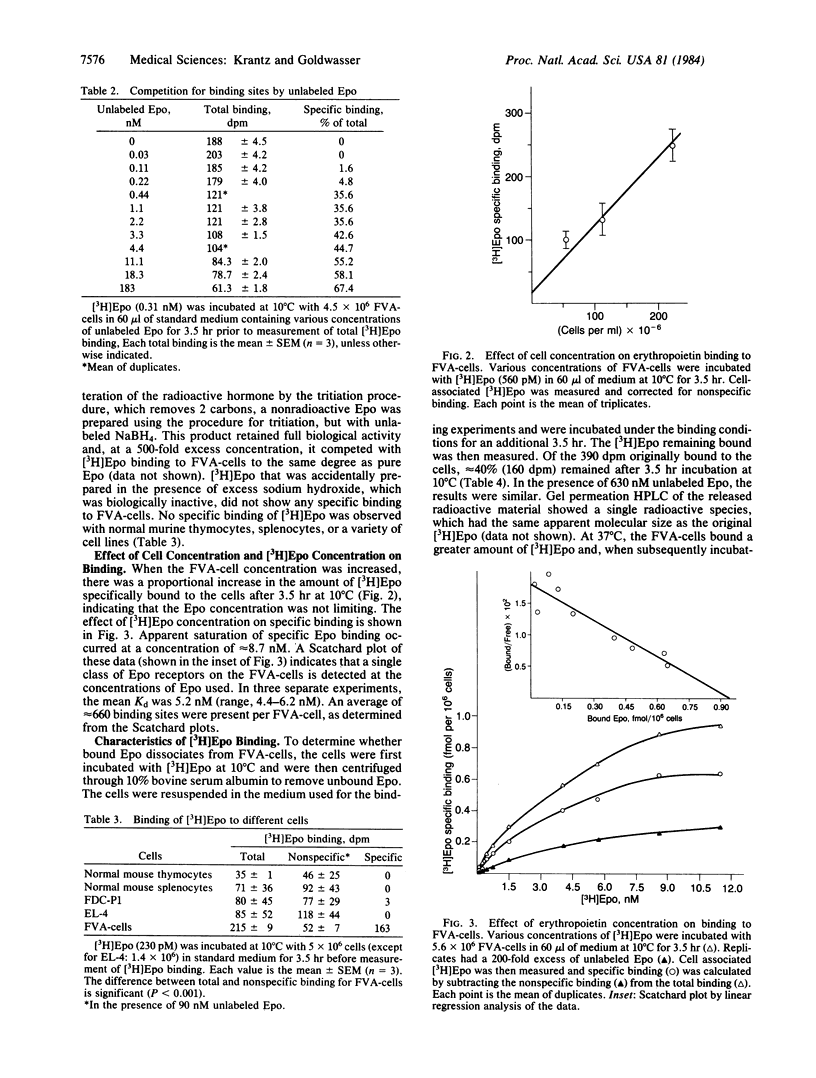
Tritiated erythropoietin with full biological activity has been prepared, and a relatively homogeneous population of enriched progenitor cells that respond to the hormone has been generated by infection of mice with the Friend virus that produces anemia. These cells, obtained from the spleens of infected mice, develop into mature erythroblasts and erythrocytes in the presence of erythropoietin. We have measured the binding of erythropoietin to these target cells; 62% of the binding was inhibited by excess unlabeled erythropoietin, but no inhibition occurred with albumin, serum, or a variety of growth factors and glycoproteins. Apparent equilibrium was reached by 2 hr at 37 degrees C and by 3.5-4 hr at 10 degrees C. The extent of specific binding increased linearly with cell concentration. In binding experiments at 10 degrees C, apparent saturation of specific binding occurred at approximately equal to 8.7 nM. Scatchard analysis showed a single class of binding sites. The dissociation constant is 5.2 nM with an average of 660 binding sites per cell. At 0.06 nM, where most of the cells are induced to terminally differentiate in vitro, an average of only 8 erythropoietin molecules bound per cell. These studies indicate that erythropoietin attaches to specific binding sites, which are most likely receptors since they manifest high affinity and specificity, and that the biologic effect of the hormone may be produced by attachment of a very small number of erythropoietin molecules.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AXELRAD A. A., STEEVES R. A. ASSAY FOR FRIEND LEUKEMIA VIRUS: RAPID QUANTITATIVE METHOD BASED ON ENUMERATION OF MACROSCOPIC SPLEEN FOCI IN MICE. Virology. 1964 Nov;24:513–518. doi: 10.1016/0042-6822(64)90199-0. [DOI] [PubMed] [Google Scholar]
- Bondurant M., Koury M., Krantz S. B., Blevins T., Duncan D. T. Isolation of erythropoietin-sensitive cells from Friend virus-infected marrow cultures: characteristics of the erythropoietin response. Blood. 1983 Apr;61(4):751–758. [PubMed] [Google Scholar]
- Chang S. C., Sikkema D., Goldwasser E. Evidence for an erythropoietin receptor protein on rat bone marrow cells. Biochem Biophys Res Commun. 1974 Mar 25;57(2):399–405. doi: 10.1016/0006-291x(74)90944-9. [DOI] [PubMed] [Google Scholar]
- FILMANOWICZ E., GURNEY C. W. Studies on erythropoiesis. XVI. Response to a single dose of erythropoietin in polycythemic mouse. J Lab Clin Med. 1961 Jan;57:65–72. [PubMed] [Google Scholar]
- Gregory C. J. Erythropoietin sensitivity as a differentiation marker in the hemopoietic system: studies of three erythropoietic colony responses in culture. J Cell Physiol. 1976 Oct;89(2):289–301. doi: 10.1002/jcp.1040890212. [DOI] [PubMed] [Google Scholar]
- Hankins W. D., Troxler D. Polycythemia- and anemia-inducing erythroleukemia viruses exhibit differential erythroid transforming effects in vitro. Cell. 1980 Dec;22(3):693–699. doi: 10.1016/0092-8674(80)90545-0. [DOI] [PubMed] [Google Scholar]
- Hilfiker M. L., Moore R. N., Farrar J. J. Biologic properties of chromatographically separated murine thymoma-derived Interleukin 2 and colony-stimulating factor. J Immunol. 1981 Nov;127(5):1983–1987. [PubMed] [Google Scholar]
- Ihle J. N., Keller J., Greenberger J. S., Henderson L., Yetter R. A., Morse H. C., 3rd Phenotypic characteristics of cell lines requiring interleukin 3 for growth. J Immunol. 1982 Oct;129(4):1377–1383. [PubMed] [Google Scholar]
- Iscove N. N. The role of erythropoietin in regulation of population size and cell cycling of early and late erythroid precursors in mouse bone marrow. Cell Tissue Kinet. 1977 Jul;10(4):323–334. doi: 10.1111/j.1365-2184.1977.tb00300.x. [DOI] [PubMed] [Google Scholar]
- Kost T. A., Hankins W. D., Krantz S. B. Combined effect of friend polycythemia virus and erythropoietin on erythroid burst formation in vitro. Exp Hematol. 1980;8 (Suppl 8):248–258. [PubMed] [Google Scholar]
- Kost T. A., Koury M. J., Krantz S. B. Mature erythroid burst-forming units are target cells for Friend virus-induced erythroid bursts. Virology. 1981 Jan 30;108(2):309–317. doi: 10.1016/0042-6822(81)90439-6. [DOI] [PubMed] [Google Scholar]
- Koury M. J., Bondurant M. C., Duncan D. T., Krantz S. B., Hankins W. D. Specific differentiation events induced by erythropoietin in cells infected in vitro with the anemia strain of Friend virus. Proc Natl Acad Sci U S A. 1982 Jan;79(2):635–639. doi: 10.1073/pnas.79.2.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koury M. J., Krantz S. B. Velocity sedimentation profiles of normal and regenerating primitive erythroid burst-forming units: relation to phase of cell cycle and growth in vitro. Cell Tissue Kinet. 1982 Jan;15(1):59–67. doi: 10.1111/j.1365-2184.1982.tb01024.x. [DOI] [PubMed] [Google Scholar]
- Krantz S. B., Goldwasser E. On the mechanism of erythropoietin-induced differentiation. IV. Some characteristics of erythropoietin action on hemoglobin synthesis in marrow cell culture. Biochim Biophys Acta. 1965 Nov 8;108(3):455–462. [PubMed] [Google Scholar]
- McLeod D. L., Shreeve M. M., Axelrad A. A. Improved plasma culture system for production of erythrocytic colonies in vitro: quantitative assay method for CFU-E. Blood. 1974 Oct;44(4):517–534. [PubMed] [Google Scholar]
- Miller R. G., Phillips R. A. Separation of cells by velocity sedimentation. J Cell Physiol. 1969 Jun;73(3):191–201. doi: 10.1002/jcp.1040730305. [DOI] [PubMed] [Google Scholar]
- Miyake T., Kung C. K., Goldwasser E. Purification of human erythropoietin. J Biol Chem. 1977 Aug 10;252(15):5558–5564. [PubMed] [Google Scholar]
- Nijhof W., Wierenga P. K. Isolation and characterization of the erythroid progenitor cell: CFU-E. J Cell Biol. 1983 Feb;96(2):386–392. doi: 10.1083/jcb.96.2.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouellette P. L., Monette F. C. Erythroid progenitors forming clusters in vitro demonstrate high erythropoietin sensitivity. J Cell Physiol. 1980 Oct;105(1):181–184. doi: 10.1002/jcp.1041050119. [DOI] [PubMed] [Google Scholar]
- Roodman G. D., Spivak J. L., Zanjani E. D. Stimulation of erythroid colony formation in vitro by erythropoietin immobilized on agarose-bound lectins. J Lab Clin Med. 1981 Nov;98(5):684–690. [PubMed] [Google Scholar]
- Stanley E. R., Guilbert L. J., Tushinski R. J., Bartelmez S. H. CSF-1--a mononuclear phagocyte lineage-specific hemopoietic growth factor. J Cell Biochem. 1983;21(2):151–159. doi: 10.1002/jcb.240210206. [DOI] [PubMed] [Google Scholar]
- Steinman R. M., Mellman I. S., Muller W. A., Cohn Z. A. Endocytosis and the recycling of plasma membrane. J Cell Biol. 1983 Jan;96(1):1–27. doi: 10.1083/jcb.96.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troxler D. H., Ruscetti S. K., Scolnick E. M. The molecular biology of Friend virus. Biochim Biophys Acta. 1980 Sep 22;605(3):305–324. doi: 10.1016/0304-419x(80)90014-1. [DOI] [PubMed] [Google Scholar]
- Van Lenten L., Ashwell G. Studies on the chemical and enzymatic modification of glycoproteins. A general method for the tritiation of sialic acid-containing glycoproteins. J Biol Chem. 1971 Mar 25;246(6):1889–1894. [PubMed] [Google Scholar]
- Whitehead P. H., Sammons H. G. A simple technique for the isolation of orosomucoid from normal and pathological sera. Biochim Biophys Acta. 1966 Jul 27;124(1):209–211. doi: 10.1016/0304-4165(66)90336-9. [DOI] [PubMed] [Google Scholar]
- Worton R. G., McCulloch E. A., Till J. E. Physical separation of hemopoietic stem cells from cells forming colonies in culture. J Cell Physiol. 1969 Oct;74(2):171–182. doi: 10.1002/jcp.1040740209. [DOI] [PubMed] [Google Scholar]



