Abstract
It is well known that human papillomavirus (HPV) is the causative agent of cervical cancer. The integration of HPV genes into the host genome causes the upregulation of E6 and E7 oncogenes. E6 and E7 proteins inactivate and degrade tumor suppressors p53 and retinoblastoma, respectively, leading to malignant progression. HPV E6 and E7 antigens are ideal targets for the development of therapies for cervical cancer and precursor lesions because they are constitutively expressed in infected cells and malignant tumors but not in normal cells and they are essential for cell immortalization and transformation. Immunotherapies are being developed to target E6/E7 by eliciting antigen-specific immune responses. siRNA technologies target E6/E7 by modulating the expression of the oncoproteins. Proteasome inhibitors and histone deacetylase inhibitors are being developed to indirectly target E6/E7 by interfering with their oncogenic activities. The ultimate goal for HPV-targeted therapies is the progression through clinical trials to commercialization.
Keywords: cervical cancer, targeted therapy, human papillomavirus, vaccine, proteasome inhibitor, histone deacetylase inhibitor, siRNA, gynecological cancer, HPV-targeted therapies
1. Introduction
Cervical cancer is one of the leading causes of cancer death in women worldwide [1,2]. A recent estimate indicates that there are approximately 529,800 cases and 275,100 deaths due to cervical cancer annually [3]. The current standard of care for advanced cervical cancer includes the use of a chemotherapeutic drug, cisplatin, in conjunction with local radiation therapy [4]. In many cases, these treatments are responsible for significant adverse effects [5]. Despite improvements noted with combination therapy, five-year survival in most patients affected by advanced cervical cancer is approximately 30% [6]. Furthermore, analyses of conservative surgical treatments for cervical dysplasia also found that conization and LEEP had pregnancy-related morbidities such as pre-term delivery, low birth weight, vaginal hemorrhage, and post-birth infections [5]. Additionally, these treatments are associated with significant recurrence rates of up to 10% of cases. Hence, the efficacy of existing surgical treatments may drop due to the risk of relapse, possibly insufficient clearance of affected tissue and potentially undesirable side effects. Furthermore, although the commercialization of preventive HPV vaccines, Gardasil and Cervarix, are effective in preventing cervical cancer, they do not have therapeutic effects against preexisting precancer or cancer lesions. Thus, there is an urgent need for innovative therapies that can reduce the number of cervical cancer cases as well as improve patients’ lives and outcomes.
It is well known that human papillomavirus (HPV) is the causative agent of cervical cancer (for review, see [7,8]). More than 100 types of HPV have been identified [9] and among those, 12 are considered ‘definite carcinogens’ due to their oncogenic potential [10-12]. These high-risk HPV types are associated with the development of high-grade lesions and malignant tumors. Persistent infection with a high-risk HPV type has been proven to be causative and necessary for the development of squamous intraepithelial lesions (SIL) (also known as cervical intraepithelial neoplasia or CIN), and malignant cervical cancer [13]. HPV-16 and HPV-18 are the most common high-risk HPV types associated with cervical cancer and are responsible for about 62.6% and 15.7% of cervical cancers, respectively [14]. Therefore, HPV-16 and HPV-18 have been the primary focus of targeted therapies for cervical cancer.
HPV is a non-enveloped, circular, double-stranded DNA virus that belongs to the Papillomaviridae family. HPV is comprised of an icosahedral capsid enclosing an approximately 8 kilobase pair genome that encodes early proteins, including the oncoproteins E6 and E7, and late proteins that form the capsid of the virion (for review, see [15]). HPV infects basal cells of the cervical epithelium and other epithelial tissues upon tissue microtrauma [16] (Figure 1). While most HPV infections are self-limiting and transient (for review, see [1]), in a productive infection of HPV, expression of the HPV genome correlates with the maturation of the infected cell. Immature epithelial cells in the basal layer of the epithelium allow expression of the HPV early genes while the late genes are expressed in terminally differentiated cells, allowing encapsidated virions to be released from the superficial epithelial layers. This localization of HPV results in pathological low-grade squamous intraepithelial lesions (LSIL, also known as CIN 1). In LSIL, the HPV genome is typically in the episomal form. High-grade squamous intraepithelial lesions (HSIL, also known as CIN 2/3) are of greater concern because they can progress to malignancies. In some HSILs, high-risk HPV types may integrate into the host genome. This integration of HPV genes causes the upregulation of E6 and E7 oncogenes because E2, a transcriptional repressor of E6 and E7, is deleted by viral integration into the host genome. These E6 and E7 proteins inactivate and degrade tumor suppressors p53 and retinoblastoma (Rb), respectively, leading to cell cycle deregulation, genomic instability, and uncontrolled proliferation of the host cell (for review, see [13] [17,18]).
Figure 1. Cervical squamous intraepithelial lesions (SILs) and HPV-associated pathogenesis.
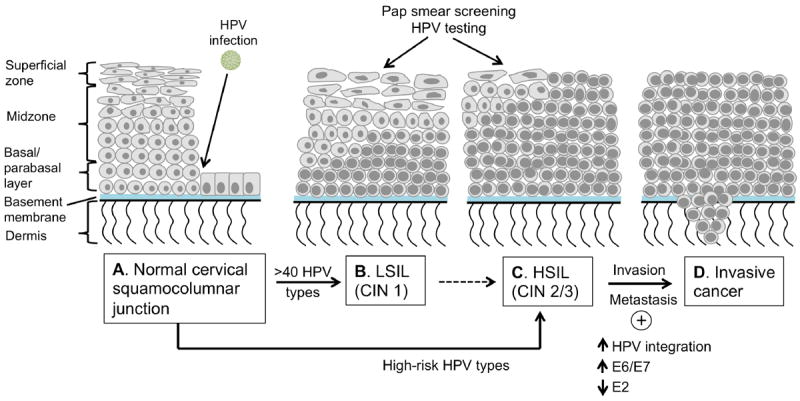
A. The normal cervical squamocolumnar junction. The layer of basal cells rests on the basement membrane is the normal barrier between the epithelium and the underlying stromal tissue. The parabasal cells form layers of one to two cells thick just above the basal cell layer. Normal squamous epithelium differentiates as shown, with the nuclear/cytoplasmic ratio decreasing closer to the surface. The squamocolumnar junction is the most common site for cervical cancer to develop. B. Productive infections produce low-grade squamous intraepithelial lesions (LSILs), in which the basaloid cells occupy the lower third of the epithelium. C. The cancerous precursor pathway is usually initiated by high-risk HPV infections and produces high-grade squamous intraepithelial lesions (HSILs). HSILs show less cellular differentiation and the basaloid cells occupy at least the lower two thirds and up to the full thickness of the epithelium. Pap smears and HPV tests can be used to detect SILs. D. If untreated, premalignant lesions can progress into microinvasive or invasive cancer, in which tumor cells breach the basement membrane. This process is associated with integration of the HPV genome into the host chromosomes, loss of E2 and upregulation of viral oncogene expression and genomic instability.
HPV E6 and E7 antigens are ideal targets for the development of targeted therapies against cervical cancer and precursor lesion because they 1) are constitutively expressed in infected cells and malignant tumors but not in normal cells [19], 2) are essential for cell immortalization and transformation. Therefore, many cervical cancer targeted therapeutic strategies have mainly focused on HPV E6 and E7 oncogenic proteins (Figure 2). Therapies targeting HPV E6 and E7 are being explored by various approaches in preclinical studies as well as different phases of clinical trials. Immunotherapies are being developed to target E6 and E7 by eliciting antigen-specific immune responses. siRNA technologies also target E6 and E7 by modulating the expression of the oncoproteins. Proteasome inhibitors and histone deacetylase inhibitors are also being developed to indirectly target E6 and E7 by interfering with their oncogenic activities. The ultimate goal for HPV-targeted therapies is the progression through clinical trials to commercialization.
Figure 2. Summary of targeted therapies.
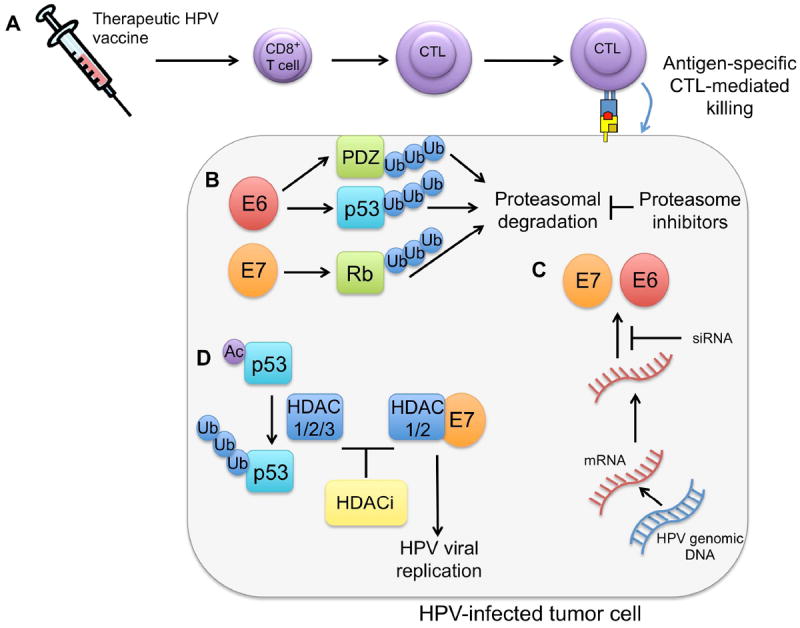
A. Therapeutic HPV vaccines: Vector, peptide, protein, DNA and dendritic cell vaccines generate E6/E7-specific cytotoxic T lymphocyte (CTL) immune responses, which result in the killing of tumor cells presenting antigen on MHC class I molecules. B. Proteasome inhibitors: HPV E6 induces ubiquitination of p53 and PDZ family proteins by the E6-AP ubiquitin ligase. HPV E7 induces ubiquitination of Rb by the CUL2 ubiquitin ligase complex. Proteasome inhibitors, such as bortezomib, impede proteasome-mediated degradation of p53 and Rb. C. siRNA targeting E6/E7: siRNA specific to E6 and/or E7 lead to the degradiation of E6/E7 mRNA, thereby inhibiting E6/E7 expression. D. HDACi: HDACi alleviate HDAC-mediated repression of p53-dependent transcriptional activation, apoptosis, and growth arrest. Additionally, HDACi prevent E7/HDAC1/2-mediated upregulation of HPV viral replication.
2. Targeted therapeutic strategies
2.1 Immunotherapies
HPV E6 and E7 oncogenic proteins are ideal targets for the development of immunotherapy against HPV-associated lesions. As mentioned above, they are constitutively expressed in infected cells and malignant tumors but not in normal cells. Furthermore, because they are foreign antigens, they do not have problem of central immune tolerance, which is commonly seen when endogenous antigens are used for vaccine development. In addition, because they are essential for cell immortalization and malignant progression, they do not have issues of antigenic loss. Therefore, many cervical cancer immunotherapeutic strategies have mainly focused on eliciting HPV E6 and E7-specific T cell immune responses to control and inhibit the progression of HPV-associated lesions. Therapeutic HPV vaccines have been evaluated in a variety of preclinical models and clinical trials using live vector, peptide, protein, DNA, RNA replicon, and dendritic cell (DC)-based vaccines targeting HPV E6 and E7 oncoproteins (for review, see [20,21]). Table 1 provides a summary of therapeutic HPV vaccines in clinical development.
Table 1.
Therapeutic HPV vaccines in clinical development
| Vaccine | Antigen(s) | Construct | Company | Phase | Ref |
|---|---|---|---|---|---|
| ADXS11-001 (Lm-LLO-E7) | HPV-16 E7 | Lm secreting fusion LLO-HPV-16 E7 protein | Advaxis Inc. | Phase I for cervical cancer patients | [26] |
| Phase I in patients with HPV-associated oropharyngeal cancer | NCT01598792 [28] | ||||
| Phase II in CIN 2/3 patients | NCT01116245 [30] | ||||
| Phase II in women with recurrent cervical carcinoma | NCT01266460 [29] | ||||
| TA-HPV | HPV-16 E6 and E7 And HPV-18 E6 and E7 | Recombinant vaccinia virus expressing E6 and E7 from both HPV-16 and HPV-18 | Celtic Pharma | Phase I for stage Ib and IIa cervical cancer patients | [41] |
| Phase I/II for late stage cervical cancer patients | [40] | ||||
| Phase II for VIN 3 and VAIN2 patients | [43] | ||||
| Phase II for VIN 3 patients | [42] | ||||
| European Organization for Research and Treatment of Cancer | Phase II for stage Ib and IIa cervical cancer patients | NCT00002916 [45] | |||
| MVA E2 | HPV-16 E2 | Recombinant Modified Vaccinia Ankara encoding E2 from BPV | Instituto Mexicano del Seguro Social | Phase I/II for CIN1-3 patients | [121] |
| Phase I/II for patients with flat condyloma lesions | [122] | ||||
| Phase II for high-grade CIN patients | [123] | ||||
| TG4001/R3484 | HPV-16 E6/E7 | Recombinant Modified Vaccinia Ankara expressing HPV-16 E6, E7, and IL-2 | Transgene/Roche | Phase IIa for CIN2/3 patients | [124] |
| Phase IIb for CIN2/3 patients | [47] | ||||
| Lipopeptide | HPV-16 E7 | Lipidated E7 linked to PADRE peptide | Cytel | Phase I for cervical cancer patients | [51] |
| Peptide & Montanide ISA-51 adjuvant | HPV-16 E7 | HPV-16 E7 epitopes emulsified in Montanide ISA-51 adjuvant | Cytel | Phase I/II for cervical cancer patients | [52] |
| Phase I/II for cervical cancer patients | [53] | ||||
| Peptide & Montanide ISA-51 adjuvant | HPV-16 E7 | HPV E7 (aa 12-20) peptide coadministered with 24 amino acid lipopeptide consisting of linker peptide, PADRE helper peptide, and E7 peptide (aa 86-93) | National Cancer Institute | Phase I for high-grade CIN and VIN patients | [54] |
| Overlapping long peptide and Montanide ISA-51 adjuvant | HPV-16 E6 and E7 | 13 peptides representing the entire sequence of HPV-16 E6 and E7 formulated in Montanide ISA-51 adjuvant | ISA Pharmaceuticals | Phase I for end-stage cervical cancer patients | [55] |
| Phase II for Ib1 cervical cancer patients | [56] | ||||
| Phase II for VIN 3 patients | [57] | ||||
| Phase II for HSIL patients | [59] | ||||
| HPV-16 immunotherapeutic | HPV-16 E6 and E7 | Recombinant HPV-16 E6/E7 fusion protein with ISCOMATRIX adjuvant | CSL Limited | Phase I for CIN 1-3 patients | [125] |
| Phase I for HIV-positive male patients with HPV-associated AIN | [126] | ||||
| PD-E7 | HPV-16 E7 | Modified HPV-16 E7/Hib protein D fusion protein formulated in AS02B adjuvant | GlaxoSmithKline | PhaseI/II for CIN 1 or CIN 3 patients | [127] |
| SGN-00101 | HPV-16 E7 | HPV-16 E7/M. bovis Hsp65 fusion protein | Nventa Biopharmaceuticals/Akela Pharma | Phase I for anal HSIL patients | [128] |
| Phase II for anal HSIL patients | [128] | ||||
| Phase I/II for high-grade AIN patients | [129] | ||||
| Phase II for ASCUS and LSIL patients | NCT00091130 | ||||
| Phase II for recurrent respiratory papillomatosis patients | [130] | ||||
| Phase II for high-grade CIN patients | [60] | ||||
| Phase II for CIN 3 patients | NCT00054041 | ||||
| Phase II for CIN 3 patients (followed by biopsy) | [60-62] | ||||
| SGN-00101 in poly ICLC adjuvant | HPV-16 E7 | HPV-16 E7/M. bovis Hsp65 fusion protein in poly ICLC adjuvant | Nventa Biopharmaceuticals/Akela Pharma | Phase I for CIN 1-3 patients | NCT00493545 |
| ZYC101 | HPV-16 E7 | Plasmid DNA encoding HPV-16 E7, encapsulated in poly microparticles | Eisai | Phase I for males patients with high-grade AIN | [66] |
| Phase I for CIN 2/3 patients | [67] | ||||
| ZYC101a | HPV-16 and HPV-18 E6 and E7 | Plasmid DNA encoding fragments of HPV-16 and HPV-18 E6 and E7, encapsulated in poly microparticles | Eisai | Phase II for high grade CIN | [68] |
| Phase II for CIN 2/3 patients | NCT00264732 [70] | ||||
| pNGVL4a-Sig/E7(detox)/Hsp7 0 | HPV-16 E7 | Plasmid DNA expressing mutated HPV-16 E7 fused to Sig and Hsp70 | National Cancer Institute | Phase I for CIN 2/3 patients | NCT00121173 [131] |
| Phase I for advanced HNSCC patients | *Gillison | ||||
| pNGVL4a-CRT/E7(detox) | HPV-16 E7 | Plasmid DNA expressing mutated HPV-16 E7 fused to calreticulin | National Cancer Institute | Ongoing phase I for CIN 2/3 patients | NCT00988559 [76] |
| VGX-3100 | HPV-16 and HPV-18 E6 and E7 | Plasmid DNA expressing HPV-16 and HPV-18 E6 and E7 proteins | VGX Pharma | Ongoing phase I for CIN2/3 patients who already underwent surgical or ablative treatment | NCT00685412 [132] |
| Ongoing Phase I for CIN 2/3 patients for fourth dose of VGX-3100 | NCT01188850 [133] | ||||
| Phase II for CIN 2/3 patients | NCT01304524 [73] | ||||
| DC + KLH | HPV-16 and HPV-18 E7 | Dendritic Cells pulsed with HPV-16 and HPV-18 E7 and keyhole limpet hemocyanin | National Institute of Health | Phase I for stage Ib and IIa cervical cancer patients | [80] |
| DC | HPV-16 E7 | Dendritic Cells pulsed with HPV-16 E7 peptide | National Taiwan University Hospital | Phase I for recurrent cervical cancer patients | NCT00155766 [134] |
| TA-CIN/TA-HPV prime/boost | HPV-16 and HPV-18 E6 and E7 and HPV-16 L2 | Prime with TA-CIN, boost with TA-HPV | Celtic Pharma | Phase II for AGIN 3 patients | [49] |
| Phase II for high-grade AGIN patients | [48] | ||||
| TA-HPV/TA-CIN prime/boost | HPV-16 and HPV-18 E6 and E7 and HPV-16 L2 | Prime with TA-HPV, boost with TA-CIN | Celtic Pharma | Phase I/II for high-grade AGIN patients | [135] |
| pNGVL4a-Sig/E7(detox)/Hsp7 0 and TA-HPV prime/boost + imiquimod | HPV-16 and HPV-18 E6 and E7 | Prime with pNGVL4a-Sig/E7(detox)/Hsp70, boost with TA-HPV and add imiquimod | National Cancer Institute | Phase I for patients with HPV infection and CIN 3 | NCT00788164 [50] |
2.1.1. Live vector-based Therapeutic HPV vaccines
The concept of live vector vaccines encompasses the use of bacterial and viral vectors, which replicate within the body and facilitate the spread of the encoded antigens. Live vector-based therapeutic HPV vaccines can deliver E6 and E7 antigens to antigen-presenting cells in order to stimulate antigen presentation through MHC class I to CD8+ cytotoxic T cells and MHC class II to CD4+ T helper cells. However, live vectors inherently pose a safety risk, particularly to immunocompromised individuals. Live vectors may also face limited capacity for repeated administration due to the stimulation of vector-specific neutralizing antibodies and/or pre-existing vector-specific immunity.
Bacterial vector-based vaccines
Several bacterial vectors have been explored for therapeutic HPV vaccines including Listeria monocytogenes [22,23], Lactobacillus casei [24], and Lactococcus lactis [25]. Among these, L. monocytogenes has generated significant interest. L. monocytogenes is a gram-positive intracellular bacterium that invades macrophages and evades phagocytosis within the phagosome by using listeriolysin O (LLO), a pore-forming toxin. Upon its escape into the cytoplasm of antigen presenting cells (APCs), antigens are delivered and processed in both MHC class I and class II pathways that activate CD4+ and CD8+ T cells
L. monocytogenes bacterial vector-based vaccines have been used in clinical arena. ADXS11-001 is a live, attenuated L. monocytogenes bacterial vector secreting HPV-16 E7 fused to LLO. In Phase I trials in patients with advanced cervical cancer, ADXS11-001 was found to be safe and well tolerated with dose limiting toxicities of hypotension and flu-like symptoms [26,27]. Antigen-specific T cell responses and some clinical responses were observed, which will be further assessed in phase II trials. ADXS11-001 is currently being studied in three clinical trials with active enrollment. An open-label, Phase I dose escalation trial will assess the safety of ADXS11-001 in patients with HPV-associated oropharyngeal cancer (NCT01598792)[28]. An ongoing Phase II trial will assess the safety and efficacy of ADXS11-001 in treating women with persistent or recurrent cervical carcinoma (NCT01266460)[29]. A randomized, single-blind, placebo-controlled Phase II study is testing whether three doses of ADXS11-001 in women with CIN2/3, for whom surgery is indicated, can safely reverse disease (NCT01116245)[30,31].
Viral vector-based
The high immunogenicity of viral vectors makes them attractive for use in therapeutic HPV vaccines. There have been several viral vectors used to deliver HPV E6 and E7 antigens, including adenoviruses [32,33], alphaviruses [34-36] and the vaccinia virus [37-39]. The vaccinia virus, an enveloped, double-stranded DNA virus within the Poxviridae family, is a promising viral vector because of its large genome and high infectivity.
Vaccinia vector-based therapeutic HPV vaccines have translated to clinical trials. A recombinant vaccinia virus expressing HPV-16/18 E6/E7 antigens (TA-HPV) has been evaluated in Phase I/II clinical trials in patients with early-stage cervical cancer [40], late-stage cervical cancer [41] vulvar intraepithelial neoplasia [42] and vaginal intraepithelial neoplasia [43]. TA-HPV was found to be safe, well tolerated and potent in stimulating vaccinia-specific antibody responses and HPV antigen-specific CTL responses [40,41,44]. A Phase II trial in patients with early stage cervical cancer has been conducted to study the safety and immunological effects of TA-HPV in combination with surgery [40,45]. The results of this trial indicated that TA-HPV is safe, causing only mild to moderate toxicities, and was able to generate HPV-specific CTLs as well as serological responses in some patients [40].
Another vaccinia-based therapeutic HPV vaccine in clinical trials is the Ankara vector, a modified vaccinia vector expressing HPV-16 E6 and E7 antigens and the adjuvant IL-2 (MVA-HPV-IL2, also known as TG4001/R3484). TG4001/R3484 was designed to: (1) alert the immune system specifically to HPV-16-infected cells presenting HPV-16 E6 and E7 antigens and (2) further stimulate the immune system in clearing the infection using IL-2. In Phase II clinical trials, TG4001/R3484 was found to be both safe and effective in producing clinical responses in women with HPV-16-positive CIN 2/3 [46]. TG4001/R3484 was tested in a placebo controlled Phase IIb trial on patients with HPV-related CIN 2/3 lesions. Interim results demonstrated proof of concept for the therapeutic vaccine in HPV-16 monotherapy, but the trial did not reach its primary endpoint of six month resolution in the CIN 2/3 indication and is not progressing to a Phase III trial [47].
Vaccinia vector-based vaccines have also been assessed in clinical trials using heterologous prime boost regimens. In a Phase I clinical trial, 29 female patients with HPV-associated high-grade anogenital intraepithelial neoplasia received three doses of TA-CIN, a fusion protein-based HPV vaccine, followed by one dose of TA-HPV [48] [49]. The vaccine was safe and well tolerated in patients without any significant adverse side effects. Moreover, full and partial clinical responses were seen in 17% of patients while 62% had symptomatic improvement. A separate Phase I trial of a therapeutic HPV DNA vaccine with TA-HPV prime boost in combination with topical imiquimod in CIN3 patients is recruiting patients (NCT00788164)[50].
2.1.2. Peptide-based Therapeutic HPV vaccines
Direct administration of peptides derived from HPV antigens can lead to the uptake of peptides by dendritic cells for antigen processing and presentation, thus activating antigen-specific T cell immunity. Peptide-based vaccines are stable, easy to produce, and have a good safety profile. Their creation involves the identification of CTL epitopes and CD4+ T helper epitopes that stimulate potent antigen-specific CD8+ and CD4+ T cell immune responses, respectively. Research has focused on addressing the main limitations of peptide-based vaccines, namely their low immunogenicity and the obstacle of MHC restriction.
Adjuvants can be used to enhance the immunogenicity of peptide-based vaccines and have been explored in clinical studies. Because HLA-A*0201 is the most common human MHC class I molecule carried by over 50% of the general population, peptide-based therapeutic HPV vaccine studies have focused on HPV-16 E7 CTL epitopes that are presented by HLA-A*0201. Vaccination with CTL epitope peptide, strong adjuvant, and nonspecific help might prime CTLs against the weakly immunogenic CTL epitope and result in clearance of HPV in preclinical models. The combination of CTL epitope peptide with nonspecific T cell help has been explored in the form of a lipopeptide construct of HPV-16 E7 aa86-93 peptide linked to Pan HLA-DR epitope (PADRE) peptide in women with late stage cervical cancer [51]. Further similar studies have focused on combining two E7-derived epitopes (aa11-20 and aa 86-93) with Montanide ISA 51 as adjuvant in combination with PADRE universal T helper peptide [52] [53]. However, among these trials, only weak immune responses were detected without evidence of antitumor benefits [52] [53]. A Phase I clinical trial found that a vaccine consisting of HPV-16 E7 peptide (aa 12-20) administered along with a construct of Incomplete Freund’s adjuvant, HPV E7 lipopeptide (aa 86-93) and PADRE, was able to stimulate an immune response in a significant proportion of eighteen HLA-A2-positive patients with CIN/VIN II/III [54]. The vaccine was well tolerated and led to complete regression of CIN lesions in 3 of 17 evaluable patients. However, as these epitopes are HLA-A2*0201-restricted, these peptide vaccines would only have clinical benefit for the population of patients who are positive for this MHC class I.
The limitation of MHC restriction associated with peptide-based vaccines necessitates the identification of immunogenic epitopes corresponding to the polymorphic MHC molecules within the population. Long overlapping peptides circumvent MHC restriction by including a range of antigenic epitopes of HPV E6 and E7 proteins and have renewed interest in therapeutic HPV E6/E7 peptide-based vaccines. These larger peptides have been tested in recent clinical trials in end-stage cervical cancer patients. A vaccine comprised of 13 overlapping peptides representing HPV-16 E6 and E7 antigens mixed with Montanide ISA 51 adjuvant was safe and able to elicit broad T cell responses in end-stage cervical cancer patients [55]. A second clinical trial using a broad array of epitopes in early-stage cervical cancer patients generated increased HPV-16-specific CD4+ and CD8+ T cell responses compared to unvaccinated patients [56]. Phase II clinical trials of this vaccine demonstrated great efficacy in HPV-16-positive high-grade vulvar intraepithelial neoplasia (VIN) patients. Half of the patients with histologically confirmed HPV-16-positive VIN3 displayed complete regression of their lesion after 3 or 4 vaccinations with HPV-16 E6/E7 overlapping peptide [57]. Further investigation into vaccine-induced immune responses showed that a high ratio of the number of HPV16-specific effector T cells to the number of HPV16-specific CD4+CD25+Foxp3+ T reg cells was predictive of clinical success [58]. Recently, a placebo-controlled randomized Phase II study demonstrated that this HPV-16 E6/E7 synthetic overlapping long-peptide vaccine increased numbers of circulating IFN-γ-producing HPV-16 antigen-specific T cells in patients with HPV-16+ HSIL [59]. The vaccination had few side effects but no HPV clearance was observed at the time of lesion excision, nor could conclusions be drawn regarding vaccine-induced T cell lesional infiltration due to patient accrual problems. The focus remains on developing a well-tolerated vaccine capable of generating strong immune responses in patients with precancer lesions.
2.1.3. Protein-based vaccines
Protein-based vaccines are promising forms of therapeutic HPV vaccines. They are safer than live-vector based vaccines and avoid MHC restriction since they include epitopes that bind to all haplotypes of MHC class I and II molecules. However, protein-based vaccines have some disadvantages, including relatively poor immunogenicity. Additionally, protein-based vaccines are processed through the endocytic pathway and are presented via the MHC class II pathway, generating predominantly antibody responses rather than CTL responses. Thus, protein-based vaccines require strategies that increase their immunogenicity as well as the induction of CD8+ T cell-mediated immune responses to promote antitumor immunity.
In clinical trials, several protein-based therapeutic HPV candidates have been explored. Namely, HspE7, a chimeric protein composed of bacille Calmette-Guerin heat shock protein (Hsp65) and HPV-16 E7, has generated considerable interest. HspE7 was found to be well-tolerated as a single-agent therapy in both Phase I and Phase II clinical trials, promoting lesion regression in several HPV-associated diseases, including CIN 2/3 [60-62].
TA-CIN, a fusion protein-based vaccine expressing HPV-16 L2-E6-E7 conjugated proteins, has shown tremendous potential in clinical studies. TA-CIN has been shown to boost E6- and E7-specific CD8+ T cell immune responses in healthy volunteers [63]. TA-CIN has also been tested in conjunction with imiquimod in a Phase II clinical trial in patients with high-grade vulvar intraepithelial neoplasia (VIN). Intramuscular administration of TA-CIN and topical application of imiquimod was well tolerated without adverse effects. Antigen-specific antibody titers were generated; however, titers were not significantly different after imiquimod application or vaccination in “responders” versus “non-responders.” “Responders” to the therapy demonstrated high levels of CD4+ and CD8+ T cells locally as well as within HPV-associated lesions. Imiquimod was shown to increase T cell infiltration, leading to the complete regression of VIN lesions in 63% of patients one year after treatment. Additionally, 36% of patients with VIN lesions showed complete HPV clearance and 79% of women remained symptom free. Phase III clinical trials will be needed to assess the comparative efficacy of this combinatorial approach. Additional clinical trials have tested TA-CIN with TA-HPV as mentioned in section 2.1.1 [48,49]. TA-CIN has been tested preclinically with GPI-0100, a semi-synthetic saponin adjuvant, and shown to generate significant HPV antigen-specific CD8+ T cell immune responses and therapeutic antitumor effects against HPV-16 E6/E7-expressing tumors [64]. The encouraging results have led to the preparation of several clinical trials (Roden, personal communication).
2.1.4. DNA Vaccines
Among the different forms of therapeutic HPV vaccines, DNA vaccines have become an attractive approach due to their stability, simplicity and safety. DNA vaccination involves direct injection of plasmid DNA encoding antigen of interest into host cells, promoting expression and presentation of the encoded antigen by transfected cells and stimulating cell-mediated and/or humoral immune responses against the encoded antigen. DNA vaccines do not pose the intrinsic safety risks associated with introducing a tumor cell, bacteria, or virus into a patient. Naked DNA is easy to manufacture and can sustain antigen expression by target cells for longer durations than RNA vaccines. Additionally, DNA vaccines do not elicit neutralizing antibodies in vivo as do live vector-based vaccines, therefore allowing repeated administration. However, in general DNA vaccines have poor immunogenicity and require additional strategies to increase vaccine potency. Many strategies to enhance DNA vaccine potency focus on targeting DNA vaccines to dendritic cells (DCs), the potent activators of antigen-specific immune responses. In general, these strategies to improve the potency of therapeutic HPV DNA vaccines through DC modification can be classified by: 1) increasing the number of antigen-expressing/antigen-loaded DCs, (2) improving HPV antigen expression, processing and presentation in DCs, and 3) enhancing DC and T cell interaction (for review, see [21]). Encouraging data from preclinical studies have led to testing several therapeutic HPV DNA vaccines in clinical trials. Strategies to increase the number of antigen-expressing/antigen-loaded DCs include enhanced vaccine delivery methods such as gene gun, microencapsulation, and electroporation (for review see [65]).
Another delivery technique that has been evaluated in several clinical trials is microencapsulation of DNA vaccines [66-68] (for review, see [69]). Of considerable interest is ZYC101, a plasmid encoding several HPV-16 E7-specific HLA-A2 restricted CTL epitopes encapsulated in 1-2 μm biopolymer microparticles composed of poly-lactide co-glycolide (PLG). A Phase 1 trial examined the effects of ZYC101 on patients with high-grade CIN and found that five of 15 patients experienced complete histological regression and 11 showed substantial HPV-specific T cell responses with no serious adverse effects [67]. A newer version of the plasmid, amolimogene bepiplasmid (ZYC101a), encoding HPV-16 and -18 E6 and E7 protein fragments was tested in a Phase II clinical trial of 127 subjects with high-grade CIN. The vaccine was well tolerated and promoted resolution of CIN 2/3 in patients under 25 years of age (70% versus 23% in the placebo group) [68]. Amolimogene was recently explored in a Phase II/III double-blinded, randomized, placebo-controlled clinical trial examining its efficacy and safety in the treatment of patients with CIN 2/3. Eligible subjects were randomized to either drug or placebo groups and during the six-month study period and were monitored by colposcopic, cytologic, and HPV testing. Persistence or resolution of disease was determined by a loop electrosurgical excision procedure (LEEP) performed at study exit [70]. Results showed that 11 of 21 patients receiving amolimogene generated enhanced T cell responses to HLA-A2 restricted HPV 16/18 peptides compared to baseline and 6 of those subjects experienced resolution of CIN 2/3 lesions [70].
Electroporation following intramuscular injection has also been assessed as a potential vaccine delivery method [71]. For example, VGX-3100, a DNA vaccine incorporating plasmids targeting HPV-16/18 E6 and E7 proteins, is delivered via intramuscular injection followed by electroporation using a CELLECTRA constant current device to deliver a small electrical charge. In a Phase I clinical trial, subjects with a history of high-grade CIN were vaccinated with VGX-3100, which was well tolerated. VGX-3100 immunization elicited a T cell response in 14 out of 18 subjects and all subjects had antibody positivity to at least two antigens [72]. Currently, VGX-3100 is being tested in individuals with histologically confirmed HPV-16/18 associated high-grade CIN in a double-blind, randomized, placebo-controlled Phase II clinical trial (NCT01304524) [73].
Several clinical trials have been conducted to examine the effects of vaccines designed to improve MHC class I processing. A Phase I clinical trial showed that 8 of 15 patients with high-grade CIN had increased E7-specific T-cell responses after intramuscular injection with a therapeutic HPV DNA vaccine, pNGVL4a-Sig/E7(detox)/HSP70. The vaccine pNGVL4a-Sig/E7(detox)/HSP70 consists of DNA encoding a signal peptide (Sig), linked to an attenuated form of HPV-16 E7 (E7(detox)) fused to Mycobacterium tuberculosis heat shock protein 70 (HSP70). Although the DNA vaccine was well tolerated, subjects treated with the vaccine did not experience significantly improved therapeutic effects compared to unvaccinated subjects [74]. Despite poor immunogenicity, complete histologic regression did occur in 33% of patients vaccinated with the highest dose of pNGVL4a-Sig/E7(detox)/HSP70 (3 mg per vaccination) [74]. The same vaccine has also been used in patients with HPV-16+ head and neck cancer (Gillison and Wu, personal communication). It is now clear that approximately 20% of head and neck cancers are associated with HPV, particularly HPV-16 (for review, see [75]). These early phase clinical trials with pNGVLA4a-Sig/E7(detox)/HSP70 demonstrate great safety without significant side effects. The results of the early phase clinical trials suggest that the DNA, although safe, is not sufficient to generate impressive therapeutic effects on its own. Therefore, prime-boost regimens with different expression vectors or the adoption of different delivery methods, such as electroporation or intradermal administration via gene gun, may be necessary to increase DNA vaccine potency. For example, an ongoing Phase I clinical trial is examining three routes of administration (intradermal administration via gene gun, intramuscular administration, and intralesional delivery) of a DNA vaccine encoding calreticulin (CRT) linked to HPV-16 E7 protein, pNGVL4a-CRT/E7(detox), to treat CIN 2/3 lesions in HPV-16 positive patients (NCT00988559) [76].
Other methods have also been explored for improving the immunogenicity of therapeutic HPV DNA vaccines. Of particular interest are toll-like receptor (TLR) agonists, which are immunomodulators that generate robust immune responses and increase the potency of therapeutic HPV vaccines. For example, imiquimod, a TLR7 agonist, has been shown to promote the activation of APCs leading to the production of the cytokines IFN-α, IL-6, and TNF-α [77]. Cytokines induce a robust and potent immune response by facilitating adaptive immune cell activation and differentiation. Thus, imiquimod is a promising adjuvant for therapeutic HPV DNA vaccines. An ongoing Phase I clinical trial investigates treatment of CIN3 with a DNA prime-vaccinia boost regimen combined with topical imiquimod. The subjects were intramuscularly primed with pNGVL4a-Sig/E7(detox)/HSP70 DNA vaccine and then boosted with a recombinant vaccinia virus encoding HPV-16 and 18 E6 and E7 (TA-HPV) with local application of imiquimod on the CIN lesion [50].
2.1.5. Dendritic cell based-vaccines
Dendritic cells are professional APCs and are thus able to induce the adaptive immune response, T cell-mediated immune responses in particular, by processing antigen and priming T cells in both the MHC class I and class II pathways. The process of pulsing DCs with HPV antigenic peptides, proteins, or DNA encoding antigens ex vivo enables loading of MHC class I and class II molecules with HPV epitopes and subsequently allows DCs to differentiate and mature. Upon re-introduction of the DCs into the body, T cells become primed and elicit a cell-mediated immune response. Understanding DC biology, such as differentiation and maturation, as well as antigen processing and presentation has been helpful in providing a rationale for improving DC-based vaccines. For example, siRNAs used to target pro-apoptotic proteins have been used to enhance the potency of therapeutic HPV DC-based vaccines. Specifically, a therapeutic HPV vaccine consisting of E7-loaded DCs transfected with BAK/BAX siRNA elicited potent E7-specific CD8+ T cell immune responses and antitumor effects in TC-1 tumor-bearing mice by prolonging the life of DCs [78,79].
A Phase I clinical trial assessed the safety and immunogenicity of a DC-based vaccine using HPV-16 E7 and/or HPV-18 E7 in 10 patients diagnosed with early-stage cervical cancer [80]. All patients developed antibody and CD4+ T cell responses to the HPV E7-loaded DC vaccination and 8 of the 10 patients had increased E7-specific CD8+ T cell counts compared to prevaccination levels. Overall, the vaccine was determined to be safe and immunogenic. Although DC-based vaccines may be used in advanced cases of cervical cancer, it is unlikely that they will be used to treat CIN lesions because the procedures involved in this treatment are relatively labor-intensive and costly.
2.1.6 Combinatorial Approaches
Although strategies to enhance different types of therapeutic HPV vaccines have been developed, the combination of strategies may further increase the immunogenicity and efficacy of therapeutic HPV vaccines. Hence, the focus of therapeutic HPV vaccine strategies has shifted in the direction of combinatorial approaches to work toward commercialization. One combinatorial approach uses fusion protein antigens concurrently with low-dose radiation treatment of tumors in preclinical models [81]. Vaccination of mice with DNA vaccine encoding calreticulin (CRT) fused with HPV-16 E7 (CRT/E7) combined with radiation therapy showed an increase in therapeutic efficacy as compared to DNA vaccinated mice alone [81]. The combination of therapies elicited the highest frequency E7-specific CD8+ T cell response and increased tumor susceptibility to E7-specific CTL activity in the tumor-microenvironment [81]. This response slowed and stabilized tumor growth, which ultimately led to an increase in mouse long-term survival rates [81]. Additionally, radiation therapy was successful in causing apoptosis of tumor cells, indicating that radiation is a useful method in stabilizing tumor cell growth when applied with immunotherapy. The combination of chemotherapy, radiation therapy, and vaccination suggests effective antitumor effects.
The combination of chemotherapeutic agents with DNA-based vaccines is another emerging strategy that may be an effective HPV therapy as shown in preclinical models [82-86]. For example, the chemotherapeutic agent, apigenin, was used concurrently with a DNA encoding heat shock protein 70 (HSP70) and HPV-16 E7 [87]. Vaccination with E7-HSP70 DNA in conjunction with apigenin chemotherapy demonstrated the highest frequency of effector CD8+ T cells and memory CD8+ T cells. Vaccination and chemotherapy caused tumor susceptibility to E7-specific cytotoxic immune responses that led to a reduction in tumor size and an increase in survival rates. Apigenin treatment also proved to increase tumor cell apoptosis in a dose-dependent manner. Overall, the combination of chemotherapy and DNA-based HPV vaccination generated the greatest antitumor effect.
2.2 Proteasome inhibitors
A major oncogenic activity of HPV E6 is to bind to the E3 ubiquitin ligase E6-AP and redirect its activity to promote the rapid proteasomal degradation of p53 and PDZ family proteins (ex. hDlg, hScribble, and hMAGI) [88-90], thereby abolishing cell cycle regulation. This creates the opportunity to target ubiquitin-dependent protein degradation for the treatment of cervical cancer. Thus, treatment with proteasome inhibitors may restore near normal levels of p53 and in so doing, promote cell death of abnormally growing HPV-associated cervical cancer cells.
Bortezomib (marketed by Millennium Pharmaceuticals as Velcade) is a widely used proteasome inhibitor that has been recognized as a potent chemotherapeutic agent (for review, see [91]). It is currently approved for use in humans to treat relapsed multiple myeloma and mantle cell lymphoma (for review see [92]). Bortezomib-induced tumor cell apoptosis may enhance the immunogenicity of tumor cells and provide an opportunity for generating tumor-specific immunity [93]. The therapeutic effects of bortezomib have been examined in preclinical models of cervical cancer. Bortezomib has been found to dramatically decrease p53 degradation in HeLa cells and to a lesser extent in CaSki cells [94] as well as increase the expression of pRb in both cell lines [95]. Furthermore, bortezomib enhances E7-specific CD8+ T cell-mediated immune responses generated by therapeutic HPV DNA vaccine (CRT/E7(detox)) in mice bearing E7-expressing tumors [84]. Notably, bortezomib has been tested in a clinical trial in combination with another chemotherapeutic drug. A Phase I/II trial is testing bortezomib in combination with vandetanib, a chemotherapeutic drug for used for thyroid cancer treatment, in patients with a variety of cancers, including cervical cancer, for cancer reduction and duration (NCT00923247). The results of this study are pending.
Several different proteasome inhibitors have been explored for their potential as targeted therapeutics against HPV-expressing cervical cancer in cell culture systems. A collection of chalcone-derivatives lacking aminoacidic components (termed RAMBs), ubiquitin-proteasome system stressors that inhibit ubiquitin-mediated protein degradation upstream of the 20S proteasomal catalytic activities, were found to selectively kill cervical cancer cells in vitro [96]. Furthermore, MG132 is a protein aldehyde that blocks the function of the 26S proteasome complex. MG132 was found to be capable of inhibiting the growth of HeLa cells by inducing cell cycle arrest as well as triggering apoptosis [97]. Thus, these proteasome inhibitors offer an opportunity to treat cervical cancer by indirectly targeting the oncogenic pathway contributed by HPV oncoprotein E6.
2.3 Histone deacetylase inhibitors
Histone deacetylases (HDACs) have been examined as targets for cervical cancer therapies. Class I HDACs deacetylate p53 and thereby repress p53-dependent transcriptional activation, apoptosis, and growth arrest [98]. This indicates that inhibition of p53 deacetylation may improve p53 levels, and consequently promote cell cycle arrest and apoptosis of tumor cells. Additionally, E7 oncoprotein binds indirectly to HDAC1 and HDAC2, leading to upregulation of the E2F2 promoter, and consequently HPV viral replication [99,100]. HDAC inhibitors (HDACi) have been shown to induce intrinsic apoptosis in HPV E7-expressing cells [101,102]. Furthermore, HDACi represent a great opportunity for boosting the potency of DNA vaccines. Previous studies have found that HDACi enhance the antitumor effects of therapeutic DNA vaccines in preclinical models by enhancing the expression of the protein/antigen encoded by the DNA vaccine [103,104]. HDACi can also lead to the upregulation of MHC class I and II molecules in treated tumor cells [105]. The upregulation of MHC class I and/or II molecules on tumor cells may render them more susceptible to T cell-mediated killing.
Suberoylanilide hydroxamic acid (SAHA), also known as Vorinostat, is an FDA approved HDACi used in the treatment of cutaneous T-cell lymphoma [106]. SAHA has been tested in human cervical cancer cell lines in combination with bortezomib and the combination of drugs was found to synergistically promote cancer cell apoptosis [94,107]. SAHA has also been tested in combination with cisplatin found similar synergistic killing effects on HeLa cells [108]. Although SAHA has not been clinically tested on cervical cancer patients, it is being tested in another HPV-associated malignancy. A Phase I study examining the effects of SAHA on patients with advanced stage oropharyngeal squamous cell carcinoma is currently recruiting participants and will include analysis of HPV-specific T cell immune responses in patients with HPV+ tumors (NCT01064921).
AR-42 is a novel HDACi that is similar in structure to SAHA and has recently received attention for its potential as an anticancer drug [109]. AR-42 has an optimized structure, which causes it to be a potent inhibitor of HDACs [109]. Indeed, AR-42 was shown to have greater potency and antitumor effects against various cancers in cell culture systems [110-112] as well as in hepatocellular carcinoma [113] in vivo compared to clinically available SAHA. Importantly, it has recently been shown that AR-42, but not other clinically available HDACi, can generate potent antigen-specific C8+ T cell-mediated immune responses and antitumor effects against a murine HPV-16 E6/E7-expressing tumor model when combined with a therapeutic HPV DNA vaccine [114]. While the effects of AR-42 on cervical cancer has only been tested in preclinical models to date, patients with acute myeloid leukemia (NCT01798901) and with advanced or relapsed multiple myeloma, chronic lymphocytic leukemia or lymphoma (NCT01129193) are being recruited for testing AR-42 in clinical trials. If these clinical trials demonstrate a high safety profile for AR-42, it potentially can be used in conjunction with a therapeutic HPV vaccine to further improve HPV therapeutic vaccine potency.
HDACi, such as Trichostatin A, have been used in combination with proteasome inhibitors, such as bortezomib, in HeLa xenografts and were found to have more potent antitumor effects than either drug alone [94]. Thus, it will potentially be rewarding to identify the ideal HDACi and proteasome inhibitors for their combined usage for the control of HPV-associated malignancies.
2.4 siRNA technologies targeting E6/E7
Small interfering RNA (siRNA) technologies have been widely employed in cancer gene therapy to modulate the expression of targeted proteins (for review, see [115]). Notably, siRNA has been used to induce selective silencing of E6 and E7 in mammalian cells [116]. Although siRNA therapeutic strategies have been tested quite successfully in cell culture systems, data is limited on the effects of siRNA on HPV-16 E6/E7-expressing tumors in animal models. Nevertheless, a few studies have generated interesting results. HPV16 E6-targeting siRNA was found to significantly reduce tumor growth in CaSki tumor-bearing mice compared to non-specific siRNA [117]. Additionally, Chang et al developed potent siRNAs targeting HPV18 E6 and E7 and found that they substantially suppressed tumor growth when intratumorally injected in nude mice bearing HeLa xenografts [118]. Fuji et al also tested HPV18 E6 and E7-targeting siRNA in SKC-II tumor-bearing nude mice in combination with atelocollagen, which served a carrier [119]. They found that HPV18 E6/E7 siRNA decreased tumor volume and tumor cell proliferation. A novel method of intravenous delivery of siRNA to cervical tumors within lipid particles protects siRNA from nuclease degradation and was shown to reduce target gene expression by 50% in TC-1 tumor-bearing mice [120].
There are not yet clinical trials testing the efficacy of siRNA against cervical cancer. Currently, siRNA technology remains limited by specific delivery and efficient biofunctionality and repeated administration is often necessary. As such, it is uncertain that clinical translation of this targeted treatment will occur soon.
3. Conclusion
HPV oncoproteins E6 and E7 are the most promising targets for the development of targeted therapy against HPV-associated cervical cancer. Numerous immunotherapeutic and other targeted therapeutic strategies are in development. Furthermore, indirect targeting of E6 and E7 by obstructing and/or circumventing their oncogenic functions is also a promising strategy. Indeed, multiple proteasome inhibitors and HDACi are progressing toward clinical translation for the treatment of cervical cancer.
As some of the targeted therapeutic strategies such as immunotherapy targeting E6/E7 are translating to the clinic at different rates, it is worth noting that therapeutic efficacy of these strategies might improve by addressing the immunosuppressive tumor microenvironment in order to improve the antitumor immune response and clinical outcomes. Furthermore, these strategies could be used in conjunction with the current standard of care for cervical cancer, chemotherapy and/or radiation, to more effectively control cervical tumors. As research in the development of targeted therapies for cervical cancer continues, optimal strategies will be created that will drive cervical cancer closer to eradication.
Acknowledgments
This review is not intended to be an encyclopedic one, and the authors apologize to those not cited. This work was supported by the NCI SPORE in Cervical Cancer P50 CA098252 and NCI 1 RO1 CA114425.
Chien-Fu Hung is pending the receipt of NIH grants.
T.-C. Wu is a consultant for and has received royalties from Papivax Biotech Inc; is a counsultant for Papivax, LLC; has received NIH grants; has received honoraria from National Health Research Institute, Taiwan; and has receieved travel/acommodations expenses covered by NCI.
Footnotes
Conflict of Interest
Jayne Knoff and Benjamin Yang declare they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Einstein MH, Schiller JT, Viscidi RP, Strickler HD, Coursaget P, et al. Clinician’s guide to human papillomavirus immunology: knowns and unknowns. Lancet Infect Dis. 2009;9:347–356. doi: 10.1016/S1473-3099(09)70108-2. [DOI] [PubMed] [Google Scholar]
- 2.Parkin DM, Bray F. Chapter 2: The burden of HPV-related cancers. Vaccine. 2006;24(Suppl 3):S3/11–25. doi: 10.1016/j.vaccine.2006.05.111. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Bray F, Center MM, Ferlay J, Ward E, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 4.Reducing uncertainties about the effects of chemoradiotherapy for cervical cancer: a systematic review and meta-analysis of individual patient data from 18 randomized trials. J Clin Oncol. 2008;26:5802–5812. doi: 10.1200/JCO.2008.16.4368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kyrgiou M, Koliopoulos G, Martin-Hirsch P, Arbyn M, Prendiville W, et al. Obstetric outcomes after conservative treatment for intraepithelial or early invasive cervical lesions: systematic review and meta-analysis. Lancet. 2006;367:489–498. doi: 10.1016/S0140-6736(06)68181-6. [DOI] [PubMed] [Google Scholar]
- 6.Society AC Cervical Cancer. American Cancer Society; Jun 29, 2012. http://www.cancer.org/Cancer/CervicalCancer/DetailedGuide/cervical-cancer-survival. [Google Scholar]
- 7.Hoory T, Monie A, Gravitt P, Wu TC. Molecular epidemiology of human papillomavirus. J Formos Med Assoc. 2008;107:198–217. doi: 10.1016/S0929-6646(08)60138-2. [DOI] [PubMed] [Google Scholar]
- 8.de Martel C, Ferlay J, Franceschi S, Vignat J, Bray F, et al. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol. 2012;13:607–615. doi: 10.1016/S1470-2045(12)70137-7. [DOI] [PubMed] [Google Scholar]
- 9.Bernard HU, Burk RD, Chen Z, van Doorslaer K, Hausen H, et al. Classification of papillomaviruses (PVs) based on 189 PV types and proposal of taxonomic amendments. Virology. 2010;401:70–79. doi: 10.1016/j.virol.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Munoz N, Bosch FX, de Sanjose S, Herrero R, Castellsague X, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348:518–527. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- 11.Cogliano V, Baan R, Straif K, Grosse Y, Secretan B, et al. Carcinogenicity of human papillomaviruses. Lancet Oncol. 2005;6:204. doi: 10.1016/s1470-2045(05)70086-3. [DOI] [PubMed] [Google Scholar]
- 12.Schiffman M, Clifford G, Buonaguro FM. Classification of weakly carcinogenic human papillomavirus types: addressing the limits of epidemiology at the borderline. Infect Agent Cancer. 2009;4:8. doi: 10.1186/1750-9378-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer. 2002;2:342–350. doi: 10.1038/nrc798. [DOI] [PubMed] [Google Scholar]
- 14.Guan P, Howell-Jones R, Li N, Bruni L, de Sanjose S, et al. Human papillomavirus types in 115,789 HPV-positive women: A meta-analysis from cervical infection to cancer. Int J Cancer. 2012;131:2349–2359. doi: 10.1002/ijc.27485. [DOI] [PubMed] [Google Scholar]
- 15.Doorbar J. Molecular biology of human papillomavirus infection and cervical cancer. Clin Sci (Lond) 2006;110:525–541. doi: 10.1042/CS20050369. [DOI] [PubMed] [Google Scholar]
- 16.Kines RC, Thompson CD, Lowy DR, Schiller JT, Day PM. The initial steps leading to papillomavirus infection occur on the basement membrane prior to cell surface binding. Proc Natl Acad Sci U S A. 2009;106:20458–20463. doi: 10.1073/pnas.0908502106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lehoux M, D’Abramo CM, Archambault J. Molecular mechanisms of human papillomavirus-induced carcinogenesis. Public Health Genomics. 2009;12:268–280. doi: 10.1159/000214918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klingelhutz AJ, Roman A. Cellular transformation by human papillomaviruses: lessons learned by comparing high- and low-risk viruses. Virology. 2012;424:77–98. doi: 10.1016/j.virol.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yugawa T, Kiyono T. Molecular mechanisms of cervical carcinogenesis by high-risk human papillomaviruses: novel functions of E6 and E7 oncoproteins. Rev Med Virol. 2009;19:97–113. doi: 10.1002/rmv.605. [DOI] [PubMed] [Google Scholar]
- 20.Lin J, Xu J, Albers AE, Kaufmann AM. New Developments inTherapeutic HPV Vaccines. Curr Obstet Gynecol Rep. 2012;1:106–115. [Google Scholar]
- 21.Lin K, Roosinovich E, Ma B, Hung CF, Wu TC. Therapeutic HPV DNA vaccines. Immunol Res. 2010;47:86–112. doi: 10.1007/s12026-009-8141-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Souders NC, Sewell DA, Pan ZK, Hussain SF, Rodriguez A, et al. Listeria-based vaccines can overcome tolerance by expanding low avidity CD8+ T cells capable of eradicating a solid tumor in a transgenic mouse model of cancer. Cancer Immun. 2007;7:2. [PMC free article] [PubMed] [Google Scholar]
- 23.Sewell DA, Pan ZK, Paterson Y. Listeria-based HPV-16 E7 vaccines limit autochthonous tumor growth in a transgenic mouse model for HPV-16 transformed tumors. Vaccine. 2008;26:5315–5320. doi: 10.1016/j.vaccine.2008.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adachi K, Kawana K, Yokoyama T, Fujii T, Tomio A, et al. Oral immunization with a Lactobacillus casei vaccine expressing human papillomavirus (HPV) type 16 E7 is an effective strategy to induce mucosal cytotoxic lymphocytes against HPV16 E7. Vaccine. 2010;28:2810–2817. doi: 10.1016/j.vaccine.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 25.Quistian-Martinez D, Villatoro-Hernandez J, Loera-Arias MJ, Rangel-Colmenero BR, Zavala-Flores LM, et al. Efficient secretion of a modified E7 protein from human papilloma virus type-16 by Lactococcus lactis. Lett Appl Microbiol. 2010;51:383–387. doi: 10.1111/j.1472-765X.2010.02905.x. [DOI] [PubMed] [Google Scholar]
- 26.Maciag PC, Radulovic S, Rothman J. The first clinical use of a live-attenuated Listeria monocytogenes vaccine: a Phase I safety study of Lm-LLO-E7 in patients with advanced carcinoma of the cervix. Vaccine. 2009;27:3975–3983. doi: 10.1016/j.vaccine.2009.04.041. [DOI] [PubMed] [Google Scholar]
- 27.Radulovic S, Brankovic-Magic M, Malisic E, Jankovic R, Dobricic J, et al. Therapeutic cancer vaccines in cervical cancer: phase I study of Lovaxin-C. J BUON. 2009;14(Suppl 1):S165–168. [PubMed] [Google Scholar]
- 28.Advaxis. REALISTIC: A Phase I, Dose Escalation Trial Of Recombinant Listeria Monocytogenes (Lm)-Based Vaccine Encoding Human Papilloma Virus Genotype 16 Target Antigens (ADXS11-001) In Patients With HPV-16 +ve Oropharyngeal Carcinoma 2012 [Google Scholar]
- 29.NCI. A Phase II Evaluation of ADXS11-001 (NSC 752718, BB-IND#13,712) in the Treatment of Persistent or Recurrent Squamous or Non-Squamous Cell Carcinoma of the Cervix 2012 [Google Scholar]
- 30.Advaxis. A Randomized, Single Blind, Placebo Controlled Phase 2 Study to Assess the Safety of ADXS11-001 for the Treatment of Cervical Intraepithelial Neoplasia Grade 2/3 2012 [Google Scholar]
- 31.Wallecha A, French C, Petit R, Singh R, Amin A, et al. Lm-LLO-Based Immunotherapies and HPV-Associated Disease. J Oncol. 2012;2012:542851. doi: 10.1155/2012/542851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee DW, Anderson ME, Wu S, Lee JH. Development of an adenoviral vaccine against E6 and E7 oncoproteins to prevent growth of human papillomavirus-positive cancer. Arch Otolaryngol Head Neck Surg. 2008;134:1316–1323. doi: 10.1001/archoto.2008.507. [DOI] [PubMed] [Google Scholar]
- 33.Gomez-Gutierrez JG, Elpek KG, Montes de Oca-Luna R, Shirwan H, Sam Zhou H, et al. Vaccination with an adenoviral vector expressing calreticulin-human papillomavirus 16 E7 fusion protein eradicates E7 expressing established tumors in mice. Cancer Immunol Immunother. 2007;56:997–1007. doi: 10.1007/s00262-006-0247-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Daemen T, Riezebos-Brilman A, Regts J, Dontje B, van der Zee A, et al. Superior therapeutic efficacy of alphavirus-mediated immunization against human papilloma virus type 16 antigens in a murine tumour model: effects of the route of immunization. Antivir Ther. 2004;9:733–742. [PubMed] [Google Scholar]
- 35.Cassetti MC, McElhiney SP, Shahabi V, Pullen JK, Le Poole IC, et al. Antitumor efficacy of Venezuelan equine encephalitis virus replicon particles encoding mutated HPV16 E6 and E7 genes. Vaccine. 2004;22:520–527. doi: 10.1016/j.vaccine.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 36.Velders MP, McElhiney S, Cassetti MC, Eiben GL, Higgins T, et al. Eradication of established tumors by vaccination with Venezuelan equine encephalitis virus replicon particles delivering human papillomavirus 16 E7 RNA. Cancer Res. 2001;61:7861–7867. [PubMed] [Google Scholar]
- 37.Hibbitts S. TA-CIN, a vaccine incorporating a recombinant HPV fusion protein (HPV16 L2E6E7) for the potential treatment of HPV16-associated genital diseases. Curr Opin Mol Ther. 2010;12:598–606. [PubMed] [Google Scholar]
- 38.Zurkova K, Babiarova K, Hainz P, Krystofova J, Kutinova L, et al. The expression of the soluble isoform of hFlt3 ligand by recombinant vaccinia virus enhances immunogenicity of the vector. Oncol Rep. 2009;21:1335–1343. doi: 10.3892/or_00000359. [DOI] [PubMed] [Google Scholar]
- 39.Hsieh CJ, Kim TW, Hung CF, Juang J, Moniz M, et al. Enhancement of vaccinia vaccine potency by linkage of tumor antigen gene to gene encoding calreticulin. Vaccine. 2004;22:3993–4001. doi: 10.1016/j.vaccine.2004.03.057. [DOI] [PubMed] [Google Scholar]
- 40.Kaufmann AM, Stern PL, Rankin EM, Sommer H, Nuessler V, et al. Safety and immunogenicity of TA-HPV, a recombinant vaccinia virus expressing modified human papillomavirus (HPV)-16 and HPV-18 E6 and E7 genes, in women with progressive cervical cancer. Clin Cancer Res. 2002;8:3676–3685. [PubMed] [Google Scholar]
- 41.Borysiewicz LK, Fiander A, Nimako M, Man S, Wilkinson GW, et al. A recombinant vaccinia virus encoding human papillomavirus types 16 and 18, E6 and E7 proteins as immunotherapy for cervical cancer. Lancet. 1996;347:1523–1527. doi: 10.1016/s0140-6736(96)90674-1. [DOI] [PubMed] [Google Scholar]
- 42.Davidson EJ, Boswell CM, Sehr P, Pawlita M, Tomlinson AE, et al. Immunological and clinical responses in women with vulval intraepithelial neoplasia vaccinated with a vaccinia virus encoding human papillomavirus 16/18 oncoproteins. Cancer Res. 2003;63:6032–6041. [PubMed] [Google Scholar]
- 43.Baldwin PJ, van der Burg SH, Boswell CM, Offringa R, Hickling JK, et al. Vaccinia-expressed human papillomavirus 16 and 18 e6 and e7 as a therapeutic vaccination for vulval and vaginal intraepithelial neoplasia. Clin Cancer Res. 2003;9:5205–5213. [PubMed] [Google Scholar]
- 44.Adams M, Borysiewicz L, Fiander A, Man S, Jasani B, et al. Clinical studies of human papilloma vaccines in pre-invasive and invasive cancer. Vaccine. 2001;19:2549–2556. doi: 10.1016/s0264-410x(00)00488-6. [DOI] [PubMed] [Google Scholar]
- 45.Cancer EOfRaTo. A Phase II Trial in Patients With Early Cervical Cancer to Study The Safety and The Immunological Effects of Vaccination With TA-HPV, A Live Recombinant Vaccinia Virus Expressing The Human Papilloma Virus 16 and 18 E6 and E7 Proteins. Bethesda (MD): 1999. ClinicalTrialsgov [Internet] [Google Scholar]
- 46.Brun JL, Dalstein V, Leveque J, Mathevet P, Raulic P, et al. Regression of high-grade cervical intraepithelial neoplasia with TG4001 targeted immunotherapy. Am J Obstet Gynecol. 2011;204:169 e161–168. doi: 10.1016/j.ajog.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 47.Transgene. Transgene Reports Randomized Phase 2b Data with its Therapeutic HPV Vaccine TG4001 in Women with CIN2/3 Intraepithelial Cervical Neoplasia. 2012 Jul 20; http://www.transgene.fr/index.php?option=com_press_release&task=download&id=208&l=en.
- 48.Fiander AN, Tristram AJ, Davidson EJ, Tomlinson AE, Man S, et al. Prime-boost vaccination strategy in women with high-grade, noncervical anogenital intraepithelial neoplasia: clinical results from a multicenter phase II trial. Int J Gynecol Cancer. 2006;16:1075–1081. doi: 10.1111/j.1525-1438.2006.00598.x. [DOI] [PubMed] [Google Scholar]
- 49.Smyth LJ, Van Poelgeest MI, Davidson EJ, Kwappenberg KM, Burt D, et al. Immunological responses in women with human papillomavirus type 16 (HPV-16)-associated anogenital intraepithelial neoplasia induced by heterologous prime-boost HPV-16 oncogene vaccination. Clin Cancer Res. 2004;10:2954–2961. doi: 10.1158/1078-0432.ccr-03-0703. [DOI] [PubMed] [Google Scholar]
- 50.University JH. Vaccine Therapy With or Without Imiquimod in Treating Patients With Grade 3 Cervical Intraepithelial Neoplasia. Bethesda (MD): National Library of Medicine (US); 2008. ClinicalTrials.gov [Internet] [Google Scholar]
- 51.Steller MA, Gurski KJ, Murakami M, Daniel RW, Shah KV, et al. Cell-mediated immunological responses in cervical and vaginal cancer patients immunized with a lipidated epitope of human papillomavirus type 16 E7. Clin Cancer Res. 1998;4:2103–2109. [PubMed] [Google Scholar]
- 52.van Driel WJ, Ressing ME, Kenter GG, Brandt RM, Krul EJ, et al. Vaccination with HPV16 peptides of patients with advanced cervical carcinoma: clinical evaluation of a phase I-II trial. Eur J Cancer. 1999;35:946–952. doi: 10.1016/s0959-8049(99)00048-9. [DOI] [PubMed] [Google Scholar]
- 53.Ressing ME, van Driel WJ, Brandt RM, Kenter GG, de Jong JH, et al. Detection of T helper responses, but not of human papillomavirus-specific cytotoxic T lymphocyte responses, after peptide vaccination of patients with cervical carcinoma. J Immunother. 2000;23:255–266. doi: 10.1097/00002371-200003000-00010. [DOI] [PubMed] [Google Scholar]
- 54.Muderspach L, Wilczynski S, Roman L, Bade L, Felix J, et al. A phase I trial of a human papillomavirus (HPV) peptide vaccine for women with high-grade cervical and vulvar intraepithelial neoplasia who are HPV 16 positive. Clin Cancer Res. 2000;6:3406–3416. [PubMed] [Google Scholar]
- 55.Kenter GG, Welters MJ, Valentijn AR, Lowik MJ, Berends-van der Meer DM, et al. Phase I immunotherapeutic trial with long peptides spanning the E6 and E7 sequences of high-risk human papillomavirus 16 in end-stage cervical cancer patients shows low toxicity and robust immunogenicity. Clin Cancer Res. 2008;14:169–177. doi: 10.1158/1078-0432.CCR-07-1881. [DOI] [PubMed] [Google Scholar]
- 56.Welters MJ, Kenter GG, Piersma SJ, Vloon AP, Lowik MJ, et al. Induction of tumor-specific CD4+ and CD8+ T-cell immunity in cervical cancer patients by a human papillomavirus type 16 E6 and E7 long peptides vaccine. Clin Cancer Res. 2008;14:178–187. doi: 10.1158/1078-0432.CCR-07-1880. [DOI] [PubMed] [Google Scholar]
- 57.Kenter GG, Welters MJ, Valentijn AR, Lowik MJ, Berends-van der Meer DM, et al. Vaccination against HPV-16 oncoproteins for vulvar intraepithelial neoplasia. N Engl J Med. 2009;361:1838–1847. doi: 10.1056/NEJMoa0810097. [DOI] [PubMed] [Google Scholar]
- 58.Welters MJ, Kenter GG, de Vos van Steenwijk PJ, Lowik MJ, Berends-van der Meer DM, et al. Success or failure of vaccination for HPV16-positive vulvar lesions correlates with kinetics and phenotype of induced T-cell responses. Proc Natl Acad Sci U S A. 2010;107:11895–11899. doi: 10.1073/pnas.1006500107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.de Vos van Steenwijk PJ, Ramwadhdoebe TH, Lowik MJ, van der Minne CE, Berends-van der Meer DM, et al. A placebo-controlled randomized HPV16 synthetic long-peptide vaccination study in women with high-grade cervical squamous intraepithelial lesions. Cancer Immunol Immunother. 2012 doi: 10.1007/s00262-012-1292-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Roman LD, Wilczynski S, Muderspach LI, Burnett AF, O’Meara A, et al. A phase II study of Hsp-7 (SGN-00101) in women with high-grade cervical intraepithelial neoplasia. Gynecol Oncol. 2007;106:558–566. doi: 10.1016/j.ygyno.2007.05.038. [DOI] [PubMed] [Google Scholar]
- 61.Einstein MH, Kadish AS, Burk RD, Kim MY, Wadler S, et al. Heat shock fusion protein-based immunotherapy for treatment of cervical intraepithelial neoplasia III. Gynecol Oncol. 2007;106:453–460. doi: 10.1016/j.ygyno.2007.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Van Doorslaer K, Reimers LL, Studentsov YY, Einstein MH, Burk RD. Serological response to an HPV16 E7 based therapeutic vaccine in women with high-grade cervical dysplasia. Gynecol Oncol. 2010;116:208–212. doi: 10.1016/j.ygyno.2009.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.de Jong A, O’Neill T, Khan AY, Kwappenberg KM, Chisholm SE, et al. Enhancement of human papillomavirus (HPV) type 16 E6 and E7-specific T-cell immunity in healthy volunteers through vaccination with TA-CIN, an HPV16 L2E7E6 fusion protein vaccine. Vaccine. 2002;20:3456–3464. doi: 10.1016/s0264-410x(02)00350-x. [DOI] [PubMed] [Google Scholar]
- 64.Karanam B, Gambhira R, Peng S, Jagu S, Kim DJ, et al. Vaccination with HPV16 L2E6E7 fusion protein in GPI-0100 adjuvant elicits protective humoral and cell-mediated immunity. Vaccine. 2009;27:1040–1049. doi: 10.1016/j.vaccine.2008.11.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hung CF, Monie A, Weng WH, Wu T. DNA vaccines for cervical cancer. Am J Transl Res. 2010;2:75–87. [PMC free article] [PubMed] [Google Scholar]
- 66.Klencke B, Matijevic M, Urban RG, Lathey JL, Hedley ML, et al. Encapsulated plasmid DNA treatment for human papillomavirus 16-associated anal dysplasia: a Phase I study of ZYC101. Clin Cancer Res. 2002;8:1028–1037. [PubMed] [Google Scholar]
- 67.Sheets EE, Urban RG, Crum CP, Hedley ML, Politch JA, et al. Immunotherapy of human cervical high-grade cervical intraepithelial neoplasia with microparticle-delivered human papillomavirus 16 E7 plasmid DNA. Am J Obstet Gynecol. 2003;188:916–926. doi: 10.1067/mob.2003.256. [DOI] [PubMed] [Google Scholar]
- 68.Garcia F, Petry KU, Muderspach L, Gold MA, Braly P, et al. ZYC101a for treatment of high-grade cervical intraepithelial neoplasia: a randomized controlled trial. Obstet Gynecol. 2004;103:317–326. doi: 10.1097/01.AOG.0000110246.93627.17. [DOI] [PubMed] [Google Scholar]
- 69.Alvarez-Salas LM. Amolimogene bepiplasmid, a DNA-based therapeutic encoding the E6 and E7 epitopes from HPV, for cervical and anal dysplasia. Curr Opin Mol Ther. 2008;10:622–628. [PubMed] [Google Scholar]
- 70.Matijevic M, Hedley ML, Urban RG, Chicz RM, Lajoie C, et al. Immunization with a poly (lactide co-glycolide) encapsulated plasmid DNA expressing antigenic regions of HPV 16 and 18 results in an increase in the precursor frequency of T cells that respond to epitopes from HPV 16, 18, 6 and 11. Cell Immunol. 2011;270:62–69. doi: 10.1016/j.cellimm.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bodles-Brakhop AM, Heller R, Draghia-Akli R. Electroporation for the delivery of DNA-based vaccines and immunotherapeutics: current clinical developments. Mol Ther. 2009;17:585–592. doi: 10.1038/mt.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bagarazzi ML, Yan J, Morrow MP, Shen X, Parker RL, et al. Immunotherapy Against HPV16/18 Generates Potent TH1 and Cytotoxic Cellular Immune Responses. Sci Transl Med. 2012;4:155ra138. doi: 10.1126/scitranslmed.3004414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pharmaceuticals I. Phase II Placebo Controlled Study of VGX-3100, (HPV16 E6/E7, HPV18 E6/E7 DNA Vaccine) Delivered IM Followed by Electroporation With Cellectra-5P for the Treatment of Biopsy-proven CIN 2/3 or CIN 3 With Documented HPV 16 or 18 2012 [Google Scholar]
- 74.Trimble CL, Peng S, Kos F, Gravitt P, Viscidi R, et al. A phase I trial of a human papillomavirus DNA vaccine for HPV16+ cervical intraepithelial neoplasia 2/3. Clin Cancer Res. 2009;15:361–367. doi: 10.1158/1078-0432.CCR-08-1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chung CH, Gillison ML. Human papillomavirus in head and neck cancer: its role in pathogenesis and clinical implications. Clin Cancer Res. 2009;15:6758–6762. doi: 10.1158/1078-0432.CCR-09-0784. [DOI] [PubMed] [Google Scholar]
- 76.Center SKCC. A Pilot Study of pnGVL4a-CRT/E7 (Detox) for the Treatment of Patients With HPV16+ Cervical Intraepithelial Neoplasia 2/3 (CIN2/3) Bethesda (MD): National Library of Medicine (US); 2009. ClinicalTrialsgov [Internet] [Google Scholar]
- 77.Bilu D, Sauder DN. Imiquimod: modes of action. Br J Dermatol. 2003;149(Suppl 66):5–8. doi: 10.1046/j.0366-077x.2003.05628.x. [DOI] [PubMed] [Google Scholar]
- 78.Peng S, Kim TW, Lee JH, Yang M, He L, et al. Vaccination with dendritic cells transfected with BAK and BAX siRNA enhances antigen-specific immune responses by prolonging dendritic cell life. Hum Gene Ther. 2005;16:584–593. doi: 10.1089/hum.2005.16.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kang TH, Lee JH, Noh KH, Han HD, Shin BC, et al. Enhancing dendritic cell vaccine potency by combining a BAK/BAX siRNA-mediated antiapoptotic strategy to prolong dendritic cell life with an intracellular strategy to target antigen to lysosomal compartments. Int J Cancer. 2007;120:1696–1703. doi: 10.1002/ijc.22377. [DOI] [PubMed] [Google Scholar]
- 80.Santin AD, Bellone S, Palmieri M, Zanolini A, Ravaggi A, et al. Human papillomavirus type 16 and 18 E7-pulsed dendritic cell vaccination of stage IB or IIA cervical cancer patients: a phase I escalating-dose trial. J Virol. 2008;82:1968–1979. doi: 10.1128/JVI.02343-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tseng CW, Trimble C, Zeng Q, Monie A, Alvarez RD, et al. Low-dose radiation enhances therapeutic HPV DNA vaccination in tumor-bearing hosts. Cancer Immunol Immunother. 2009;58:737–748. doi: 10.1007/s00262-008-0596-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kang TH, Lee JH, Song CK, Han HD, Shin BC, et al. Epigallocatechin-3-gallate enhances CD8+ T cell-mediated antitumor immunity induced by DNA vaccination. Cancer Res. 2007;67:802–811. doi: 10.1158/0008-5472.CAN-06-2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tseng CW, Hung CF, Alvarez RD, Trimble C, Huh WK, et al. Pretreatment with cisplatin enhances E7-specific CD8+ T-Cell-mediated antitumor immunity induced by DNA vaccination. Clin Cancer Res. 2008;14:3185–3192. doi: 10.1158/1078-0432.CCR-08-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tseng CW, Monie A, Wu CY, Huang B, Wang MC, et al. Treatment with proteasome inhibitor bortezomib enhances antigen-specific CD8+ T-cell-mediated antitumor immunity induced by DNA vaccination. J Mol Med (Berl) 2008;86:899–908. doi: 10.1007/s00109-008-0370-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zeng Q, Peng S, Monie A, Yang M, Pang X, et al. Control of cervicovaginal HPV-16 E7-expressing tumors by the combination of therapeutic HPV vaccination and vascular disrupting agents. Hum Gene Ther. 2011;22:809–819. doi: 10.1089/hum.2010.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Peng S, Monie A, Pang X, Hung CF, Wu TC. Vascular disrupting agent DMXAA enhances the antitumor effects generated by therapeutic HPV DNA vaccines. J Biomed Sci. 2011;18:21. doi: 10.1186/1423-0127-18-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chuang CM, Monie A, Wu A, Hung CF. Combination of apigenin treatment with therapeutic HPV DNA vaccination generates enhanced therapeutic antitumor effects. J Biomed Sci. 2009;16:49. doi: 10.1186/1423-0127-16-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Werness BA, Levine AJ, Howley PM. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science. 1990;248:76–79. doi: 10.1126/science.2157286. [DOI] [PubMed] [Google Scholar]
- 89.Scheffner M, Huibregtse JM, Vierstra RD, Howley PM. The HPV-16 E6 and E6-AP complex functions as a ubiquitin-protein ligase in the ubiquitination of p53. Cell. 1993;75:495–505. doi: 10.1016/0092-8674(93)90384-3. [DOI] [PubMed] [Google Scholar]
- 90.Yi JW, Jang M, Kim SJ, Kim SS, Rhee JE. Degradation of p53 by natural variants of the E6 protein of human papillomavirus type 16. Oncol Rep. 2013;29:1617–1622. doi: 10.3892/or.2013.2281. [DOI] [PubMed] [Google Scholar]
- 91.Chen D, Frezza M, Schmitt S, Kanwar J, Dou QP. Bortezomib as the first proteasome inhibitor anticancer drug: current status and future perspectives. Curr Cancer Drug Targets. 2011;11:239–253. doi: 10.2174/156800911794519752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pellom ST, Jr, Shanker A. Development of Proteasome Inhibitors as Therapeutic Drugs. J Clin Cell Immunol. 2012;S5:5. doi: 10.4172/2155-9899.s5-005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Spisek R, Charalambous A, Mazumder A, Vesole DH, Jagannath S, et al. Bortezomib enhances dendritic cell (DC)-mediated induction of immunity to human myeloma via exposure of cell surface heat shock protein 90 on dying tumor cells: therapeutic implications. Blood. 2007;109:4839–4845. doi: 10.1182/blood-2006-10-054221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lin Z, Bazzaro M, Wang MC, Chan KC, Peng S, et al. Combination of proteasome and HDAC inhibitors for uterine cervical cancer treatment. Clin Cancer Res. 2009;15:570–577. doi: 10.1158/1078-0432.CCR-08-1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Miyamoto Y, Nakagawa S, Wada-Hiraike O, Seiki T, Tanikawa M, et al. Sequential effects of the proteasome inhibitor bortezomib and chemotherapeutic agents in uterine cervical cancer cell lines. Oncol Rep. 2013;29:51–57. doi: 10.3892/or.2012.2072. [DOI] [PubMed] [Google Scholar]
- 96.Anchoori RK, Khan SR, Sueblinvong T, Felthauser A, Iizuka Y, et al. Stressing the ubiquitin-proteasome system without 20S proteolytic inhibition selectively kills cervical cancer cells. PLoS One. 2011;6:e23888. doi: 10.1371/journal.pone.0023888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Han YH, Moon HJ, You BR, Park WH. The effect of MG132, a proteasome inhibitor on HeLa cells in relation to cell growth, reactive oxygen species and GSH. Oncol Rep. 2009;22:215–221. [PubMed] [Google Scholar]
- 98.Juan LJ, Shia WJ, Chen MH, Yang WM, Seto E, et al. Histone deacetylases specifically down-regulate p53-dependent gene activation. J Biol Chem. 2000;275:20436–20443. doi: 10.1074/jbc.M000202200. [DOI] [PubMed] [Google Scholar]
- 99.Longworth MS, Wilson R, Laimins LA. HPV31 E7 facilitates replication by activating E2F2 transcription through its interaction with HDACs. EMBO J. 2005;24:1821–1830. doi: 10.1038/sj.emboj.7600651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Brehm A, Nielsen SJ, Miska EA, McCance DJ, Reid JL, et al. The E7 oncoprotein associates with Mi2 and histone deacetylase activity to promote cell growth. EMBO J. 1999;18:2449–2458. doi: 10.1093/emboj/18.9.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Finzer P, Krueger A, Stohr M, Brenner D, Soto U, et al. HDAC inhibitors trigger apoptosis in HPV-positive cells by inducing the E2F-p73 pathway. Oncogene. 2004;23:4807–4817. doi: 10.1038/sj.onc.1207620. [DOI] [PubMed] [Google Scholar]
- 102.Finzer P, Kuntzen C, Soto U, zur Hausen H, Rosl F. Inhibitors of histone deacetylase arrest cell cycle and induce apoptosis in cervical carcinoma cells circumventing human papillomavirus oncogene expression. Oncogene. 2001;20:4768–4776. doi: 10.1038/sj.onc.1204652. [DOI] [PubMed] [Google Scholar]
- 103.Vanniasinkam T, Ertl H, Tang Q. Trichostatin-A enhances adaptive immune responses to DNA vaccination. J Clin Virol. 2006;36:292–297. doi: 10.1016/j.jcv.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 104.Lai MD, Chen CS, Yang CR, Yuan SY, Tsai JJ, et al. An HDAC inhibitor enhances the antitumor activity of a CMV promoter-driven DNA vaccine. Cancer Gene Ther. 2010;17:203–211. doi: 10.1038/cgt.2009.65. [DOI] [PubMed] [Google Scholar]
- 105.Magner WJ, Kazim AL, Stewart C, Romano MA, Catalano G, et al. Activation of MHC class I, II, and CD40 gene expression by histone deacetylase inhibitors. J Immunol. 2000;165:7017–7024. doi: 10.4049/jimmunol.165.12.7017. [DOI] [PubMed] [Google Scholar]
- 106.Mann BS, Johnson JR, He K, Sridhara R, Abraham S, et al. Vorinostat for treatment of cutaneous manifestations of advanced primary cutaneous T-cell lymphoma. Clin Cancer Res. 2007;13:2318–2322. doi: 10.1158/1078-0432.CCR-06-2672. [DOI] [PubMed] [Google Scholar]
- 107.Jiang Y, Wang Y, Su Z, Yang L, Guo W, et al. Synergistic induction of apoptosis in HeLa cells by the proteasome inhibitor bortezomib and histone deacetylase inhibitor SAHA. Mol Med Rep. 2010;3:613–619. doi: 10.3892/mmr_00000305. [DOI] [PubMed] [Google Scholar]
- 108.Jin KL, Park JY, Noh EJ, Hoe KL, Lee JH, et al. The effect of combined treatment with cisplatin and histone deacetylase inhibitors on HeLa cells. J Gynecol Oncol. 2010;21:262–268. doi: 10.3802/jgo.2010.21.4.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lu Q, Yang YT, Chen CS, Davis M, Byrd JC, et al. Zn2+-chelating motif-tethered short-chain fatty acids as a novel class of histone deacetylase inhibitors. J Med Chem. 2004;47:467–474. doi: 10.1021/jm0303655. [DOI] [PubMed] [Google Scholar]
- 110.Bai LY, Omar HA, Chiu CF, Chi ZP, Hu JL, et al. Antitumor effects of (S)-HDAC42, a phenylbutyrate-derived histone deacetylase inhibitor, in multiple myeloma cells. Cancer Chemother Pharmacol. 2011;68:489–496. doi: 10.1007/s00280-010-1501-z. [DOI] [PubMed] [Google Scholar]
- 111.Tang YA, Wen WL, Chang JW, Wei TT, Tan YH, et al. A novel histone deacetylase inhibitor exhibits antitumor activity via apoptosis induction, F-actin disruption and gene acetylation in lung cancer. PLoS One. 2010;5:e12417. doi: 10.1371/journal.pone.0012417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kulp SK, Chen CS, Wang DS, Chen CY. Antitumor effects of a novel phenylbutyrate-based histone deacetylase inhibitor, (S)-HDAC-42, in prostate cancer. Clin Cancer Res. 2006;12:5199–5206. doi: 10.1158/1078-0432.CCR-06-0429. [DOI] [PubMed] [Google Scholar]
- 113.Lu YS, Kashida Y, Kulp SK, Wang YC, Wang D, et al. Efficacy of a novel histone deacetylase inhibitor in murine models of hepatocellular carcinoma. Hepatology. 2007;46:1119–1130. doi: 10.1002/hep.21804. [DOI] [PubMed] [Google Scholar]
- 114.Lee SY, Huang Z, Kang TH, Soong RS, Knoff J, et al. Histone deacetylase inhibitor AR-42 enhances E7-specific CD8(+) T cell-mediated antitumor immunity induced by therapeutic HPV DNA vaccination. J Mol Med (Berl) 2013;91:1221–1231. doi: 10.1007/s00109-013-1054-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Resnier P, Montier T, Mathieu V, Benoit JP, Passirani C. A review of the current status of siRNA nanomedicines in the treatment of cancer. Biomaterials. 2013;34:6429–6443. doi: 10.1016/j.biomaterials.2013.04.060. [DOI] [PubMed] [Google Scholar]
- 116.Jiang M, Milner J. Selective silencing of viral gene expression in HPV-positive human cervical carcinoma cells treated with siRNA, a primer of RNA interference. Oncogene. 2002;21:6041–6048. doi: 10.1038/sj.onc.1205878. [DOI] [PubMed] [Google Scholar]
- 117.Niu XY, Peng ZL, Duan WQ, Wang H, Wang P. Inhibition of HPV 16 E6 oncogene expression by RNA interference in vitro and in vivo. Int J Gynecol Cancer. 2006;16:743–751. doi: 10.1111/j.1525-1438.2006.00384.x. [DOI] [PubMed] [Google Scholar]
- 118.Chang JT, Kuo TF, Chen YJ, Chiu CC, Lu YC, et al. Highly potent and specific siRNAs against E6 or E7 genes of HPV16- or HPV18-infected cervical cancers. Cancer Gene Ther. 2010;17:827–836. doi: 10.1038/cgt.2010.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fujii T, Saito M, Iwasaki E, Ochiya T, Takei Y, et al. Intratumor injection of small interfering RNA-targeting human papillomavirus 18 E6 and E7 successfully inhibits the growth of cervical cancer. Int J Oncol. 2006;29:541–548. [PubMed] [Google Scholar]
- 120.Wu SY, Singhania A, Burgess M, Putral LN, Kirkpatrick C, et al. Systemic delivery of E6/7 siRNA using novel lipidic particles and its application with cisplatin in cervical cancer mouse models. Gene Ther. 2011;18:14–22. doi: 10.1038/gt.2010.113. [DOI] [PubMed] [Google Scholar]
- 121.Corona Gutierrez CM, Tinoco A, Navarro T, Contreras ML, Cortes RR, et al. Therapeutic vaccination with MVA E2 can eliminate precancerous lesions (CIN 1, CIN 2, and CIN 3) associated with infection by oncogenic human papillomavirus. Hum Gene Ther. 2004;15:421–431. doi: 10.1089/10430340460745757. [DOI] [PubMed] [Google Scholar]
- 122.Garcia-Hernandez E, Gonzalez-Sanchez JL, Andrade-Manzano A, Contreras ML, Padilla S, et al. Regression of papilloma high-grade lesions (CIN 2 and CIN 3) is stimulated by therapeutic vaccination with MVA E2 recombinant vaccine. Cancer Gene Ther. 2006;13:592–597. doi: 10.1038/sj.cgt.7700937. [DOI] [PubMed] [Google Scholar]
- 123.Albarran YCA, de la Garza A, Cruz Quiroz BJ, Vazquez Zea E, Diaz Estrada I, et al. MVA E2 recombinant vaccine in the treatment of human papillomavirus infection in men presenting intraurethral flat condyloma: a phase I/II study. BioDrugs. 2007;21:47–59. doi: 10.2165/00063030-200721010-00006. [DOI] [PubMed] [Google Scholar]
- 124.Transgene. TRANSGENE AND ROCHE MODIFY THE CLINICAL DEVELOPMENT PROGRAMME FOR THEIR HPV TARGETED IMMUNOTHERAPY TG4001/R3484. 2008 Jul 24; http://www.transgene.fr/index.php?option=com_press_release&task=download&id=22&l=en 2012.
- 125.Frazer IH, Quinn M, Nicklin JL, Tan J, Perrin LC, et al. Phase 1 study of HPV16-specific immunotherapy with E6E7 fusion protein and ISCOMATRIX adjuvant in women with cervical intraepithelial neoplasia. Vaccine. 2004;23:172–181. doi: 10.1016/j.vaccine.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 126.Anderson JS, Hoy J, Hillman R, Barnden M, Eu B, et al. A randomized, placebo-controlled, dose-escalation study to determine the safety, tolerability, and immunogenicity of an HPV-16 therapeutic vaccine in HIV-positive participants with oncogenic HPV infection of the anus. J Acquir Immune Defic Syndr. 2009;52:371–381. doi: 10.1097/QAI.0b013e3181b7354c. [DOI] [PubMed] [Google Scholar]
- 127.Hallez S, Simon P, Maudoux F, Doyen J, Noel JC, et al. Phase I/II trial of immunogenicity of a human papillomavirus (HPV) type 16 E7 protein-based vaccine in women with oncogenic HPV-positive cervical intraepithelial neoplasia. Cancer Immunol Immunother. 2004;53:642–650. doi: 10.1007/s00262-004-0501-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Goldstone SE, Palefsky JM, Winnett MT, Neefe JR. Activity of HspE7, a novel immunotherapy, in patients with anogenital warts. Dis Colon Rectum. 2002;45:502–507. doi: 10.1007/s10350-004-6229-6. [DOI] [PubMed] [Google Scholar]
- 129.Palefsky JM, Berry JM, Jay N, Krogstad M, Da Costa M, et al. A trial of SGN-00101 (HspE7) to treat high-grade anal intraepithelial neoplasia in HIV-positive individuals. AIDS. 2006;20:1151–1155. doi: 10.1097/01.aids.0000226955.02719.26. [DOI] [PubMed] [Google Scholar]
- 130.Derkay CS, Smith RJ, McClay J, van Burik JA, Wiatrak BJ, et al. HspE7 treatment of pediatric recurrent respiratory papillomatosis: final results of an open-label trial. Ann Otol Rhinol Laryngol. 2005;114:730–737. doi: 10.1177/000348940511400913. [DOI] [PubMed] [Google Scholar]
- 131.Center SKCC. A Phase I/II Clinical Trial of pNGVL4a-Sig/E7 (Detox)/HSP70 for the Treatment of Patients With HPV 16+ Cervical Intraepithelial Neoplasia 2/3 (CIN2/3) Bethesda (MD): National Library of Medicine (US); 2005. Jul 19, ClinicalTrials.gov [Internet] [Google Scholar]
- 132.Pharmaceuticals I. Phase I Open Label, Dose Escalation Study to Evaluate the Safety, Tolerability and Immunogenicity of Human Papillomavirus (HPV) DNA Plasmid (VGX-3100) + Electroporation (EP) in Adult Females Post Surgical or Ablative Treatment of Grade 2 or 3 Cervical Intraepithelial Neoplasia (CIN) Bethesda (MD): National Library of Medicine (US); 2008. May 22, ClinicalTrials.gov [Internet] [Google Scholar]
- 133.Pharmaceuticals I. Phase I, Open-label Study to Evaluate the Safety, Tolerability and Immunogenicity of a Fourth Dose of Human Papillomavirus (HPV) DNA Plasmid (VGX-3100) + Electroporation (EP) in Adult Females Previously Immunized With VGX-3100. Bethesda (MD): National Library of Medicine (US); 2010. ClinicalTrialsgov [Internet] [Google Scholar]
- 134.Hospital NTU. A Pilot Study for the Immunotherapy of Recurrent Cervical Cancers Using Dendritic Cells (DCs) Pulsed With Human Papillomavirus Type 16 E7 Antigen. Bethesda (MD): National Library of Medicine (US); 2005. Sep 9, ClinicalTrials.gov [Internet] [Google Scholar]
- 135.Davidson EJ, Faulkner RL, Sehr P, Pawlita M, Smyth LJ, et al. Effect of TA-CIN (HPV 16 L2E6E7) booster immunisation in vulval intraepithelial neoplasia patients previously vaccinated with TA-HPV (vaccinia virus encoding HPV 16/18 E6E7) Vaccine. 2004;22:2722–2729. doi: 10.1016/j.vaccine.2004.01.049. [DOI] [PubMed] [Google Scholar]


