Abstract
Schizophrenia is considered a neurodevelopmental disorder, but whether the adolescent period, proximal to onset, is associated with aberrant development in individuals at clinical high risk (CHR) for psychosis is incompletely understood. While abnormal gray and white matter development has been observed, alterations in functional neuroimaging (fMRI) parameters during adolescence as related to conversion to psychosis have not yet been investigated. Twenty CHR individuals and 19 typically developing controls (TDC), (ages 14-21), were recruited from the Center for Assessment and Prevention of Prodromal States (CAPPS) at UCLA. Participants performed a Sternberg-style verbal working memory (WMem) task during fMRI and data were analyzed using a cross-sectional design to test the hypothesis that there is a deviant developmental trajectory in WMem associated neural circuitry in those at risk for psychosis. Eight of the CHR adolescents converted to psychosis within 2 years of initial assessment. A voxel-wise regression examining the relationship between age and activation revealed a significant group-by-age interaction. TDC showed a negative association between age and functional activation in the WMem circuitry while CHR adolescents showed a positive association. Moreover, CHR patients who later converted to overt psychosis showed a distinct pattern of abnormal age-associated activation in the frontal cortex relative to controls, while non-converters showed a more diffuse posterior pattern. Finding that age related variation in baseline patterns of neural activity differentiate individuals who subsequently convert to psychosis from healthy subjects suggests that these differences are likely to be clinically relevant.
Keywords: schizophrenia, prodrome, fMRI, working memory, development, psychosis
1. Introduction
Schizophrenia is a neurodevelopmental disorder, with cognitive, motor and social abnormalities observable many years before disease onset (Bearden et al., 2000), although overt illness onset typically does not occur until adolescence. This pattern suggests that some risk-factors that contribute to disease onset may occur very early with others occurring more proximally to disease onset. Specifying how cognitive and clinical features observed in adult patients arise over the course of development may have critical implications for intervention and ultimately, prevention.
Given that schizophrenia onset or its treatment may alter brain function, it is necessary to assess developmental trajectories before onset occurs (Karlsgodt et al., 2008), for instance by assessing clinically defined high-risk subjects (CHR) identified based on expression of sub-psychotic symptoms and changes in function. CHR adolescents who converted to psychosis have previously shown frontal lobe gray matter reductions compared with non-converters (Dazzan et al., 2012; Sun et al., 2009a) and smaller volumes in temporal regions (Mechelli et al., 2011). Further, CHR youth failed to show the typical age-associated increase in DTI measures of white matter integrity, and lower baseline integrity predicted later functioning (Karlsgodt et al., 2009a). Given these patterns, we hypothesized that, compared with healthy adolescents, CHR youth would show an altered association between age and brain physiology as assessed with functional magnetic resonance imaging (fMRI).
Patients with psychotic and schizophrenia spectrum disorders and those at genetic and clinical high-risk have deficits in a number of cognitive domains, including working memory (WMem) (Kuperberg and Heckers, 2000), and WMem has been proposed as an endophentype for this disorder (Glahn et al., 2003). Consistent with findings that the frontal lobe is relatively late to fully mature, in healthy individuals WMem processes continue to develop into adulthood. The basic components of the WMem circuitry are functional by the end of childhood; even young children engage a fronto-parietal network similar to adults during WMem (Geier et al., 2009; Nelson et al., 2000). However, with increased task difficulty or complexity, children tend to activate a more diffuse, less focused and efficient network than adults (Geier et al., 2009; O’Hare et al., 2008). WMem-associated activation continues to change across adolescence (Schweinsburg et al., 2005), which suggests maturational changes that are likely to reflect refinement of the dorsolateral prefrontal cortical (DLPFC) contribution to WMem across adolescence (Geier et al., 2009). CHR individuals have shown abnormalities in fMRI activation during WMem (Broome et al., 2009; Choi et al., 2011; Fusar-Poli et al., 2010; Morey et al., 2005) but it is not clear how these differences may vary with age during adolescence.
The goals of this study were to determine whether CHR youth show a differential association between age and functional activation during adolescence compared with typically developing controls (TDC), and to clarify whether such a putative differential association is limited to adolescents who convert to psychosis. A differential association between age and functional activation between CHR and TD adolescents would suggest that an age-related developmental process may contribute to observed differences in activation between adult psychotic patients and controls. Alternatively, if the groups show similar differences in functional activation across age and differ only in mean activation, effects of an earlier risk factor provide a more likely explanation for activation differences between psychotic patients and controls.
2. Methods
2.1 Participants
Twenty CHR youth and 19 TDCs (Table 1) participated in an ongoing study at the University of California, Los Angeles. TDCs age matched to the CHR sample were recruited from the community via advertising. While the intent was also to match gender, exclusion for below chance performance, motion, and imaging artifacts resulted in proportionately more TDC females; thus all analyses were co-varied for sex. fMRI data was obtained at baseline and analyzed cross-sectionally, while clinical follow-ups over a subsequent 2 year period allowed for longitudinal determination of conversion status. CHR participants met criteria for one of three prodromal syndrome categories, as assessed by the Structured Interview for Prodromal Syndromes (SIPS(McGlashan et al., 2001)): (1) attenuated psychotic symptoms; (2) transient, recent-onset psychotic symptoms; or (3) a substantial drop in functioning in conjunction with schizotypal personality disorder or a first-degree relative with psychosis. TDC youth did not meet DSM-IV criteria for a psychiatric disorder based on SCID-I/P or K-SADS interviews, have a first-degree family history of psychosis, or meet the prodromal state criteria. Additional exclusions for all participants included: neurological disorder, drug/alcohol abuse or dependence within the past 6 months, insufficient English fluency, and/or IQ below 70. Details regarding SIPS criteria, reliability and consensus procedures are described elsewhere (Meyer et al., 2005). All participants completed informed consent or assent (parental consent was obtained for minors), approved by the UCLA Institutional Review Board, and were compensated for participation.
Table 1.
Subject Demographics
| CHR (n=20) | TDC (n=19) | |
|---|---|---|
|
|
||
| Age (mean ± std) | 16.85 (2.06) | 17.84 (2.11) |
| (range) | 14-20 | 14-21 |
| Gender: % female (n) | 15% (3)* | 47.36% (9)* |
| Race: percent (n) | ||
| % Caucasian | 50% (10) | 42.11% (8) |
| % African American | 10% (2) | 5.2% (1) |
| % Asian American/Pacific Islander | 15 %(3) | 10.5% (2) |
| % Latino/Hispanic | 20% (4) | 10.5% (2) |
| % Other | 5% (1) | 44.4% (4) |
| Primary SIPS diagnosis: percent (n) | ||
| Brief Intermittent Psychotic (BIPS) | 20% (4) | |
| Attenuated Positive Symptoms (APS) | 75% (15) | |
| Genetic Risk & Deterioration (GRD) | 5% (1) | |
| Medication Status (n) | ||
| Current atypical antipsychotic | 4 | |
| Current SSRI/SNRI | 7 | |
| Current mood stabilizer | 2 | |
| Current psychostimulant | 2 | |
| Current Other (cogentin, abilify, klonopin) | 3 | |
| No current medication | 10 | |
| One current medication | 5 | |
| Two current medications | 4 | |
| Three current medications | 0 | |
| Four current medications | 1 | |
2.2 Procedures
2.2.1 Cognitive Paradigm
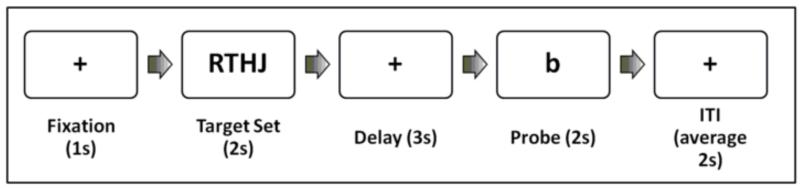
Participants performed a Sternberg-style item recognition task during fMRI at the baseline assessment (Sternberg, 1966) as described in (Karlsgodt et al., 2007; Karlsgodt et al., 2009b) (Figure 1) The experiment was run using E-Prime Software (Psychology Software Tools), images were displayed using goggles (Resonance Technologies, Inc), and responses were collected via a button box (Current Designs).
Figure 1.
Task Design
2.2.2 Clinical Assessments
After the baseline scan the SIPS and SCID were re-administered at 6-month intervals, for up to 24 months with additional assessment if clinical deterioration was observed. Conversion to psychosis was defined according to SIPS criteria (Cannon et al., 2008). Briefly, the patient must exhibit psychotic symptoms of certain intensity (e.g., delusional conviction) and frequency or duration (one hour/day for four days/week during the last month) or having a severe impact (seriously disorganizing/dangerous). Psychosis is the primary defining feature of schizophrenia but may occur in other DSM-IV categories. Eight subjects converted to a psychotic disorder with diagnoses of: schizophrenia (n=3), schizoaffective disorder (n=1), schizophreniform disorder with a secondary diagnosis of depressive disorder NOS (n=1), mood disorder with psychotic features (n=1), and psychosis not otherwise specified (NOS; n=2). One individual with psychosis NOS also had a secondary diagnosis of depressive disorder NOS. Individuals who did not convert had diagnoses of: major depressive disorder in full or partial remission (n=7), pervasive developmental disorder (n=1), mood disorder NOS (n=1), ADHD (n=1), with one diagnosis remaining unclear at last follow-up.
2.2.3 Imaging Parameters
Scans were acquired on a 3T Siemens Allegra scanner at UCLA. A T2 weighted image with 1.5mm in-plane resolution was taken using a set of high-resolution EPI localizers (TR/TE 5000/33ms, 33 3-mm slices with 1mm gap, 128x128 matrix, 200mm FOV). To match any B0-related distortions, the T2 images had the same readout bandwidth along the phase encoding direction as functional scans. Functional slices matched the AC-PC aligned slices in the T2 image, and utilized an echo planar (EPI) sequence (TR/TE 3000/45ms, 90° flip angle, 33 3mm slices, 1mm gap, interleaved acquisition). The voxel size was 3.125 × 3.125 × 3.99. The task consisted of 180 time points with a total duration of 9 minutes.
2.2.4 Image Processing
A study-specific group averaged T2-weighted brain was created using Automated Image Registration (AIR) (Woods, 1998). It was used as the common space to which all subjects were registered and in which group statistics were performed. This approach minimizes misregistration of the functional data during spatial normalization and ensures similar levels of spatial transformation from individual to template space between comparison groups. To generate coordinates comparable with other data sets, statistical maps were registered to MNI-152 space.
Functional analysis was performed using FSL (FMRIB’s Software Library v4.1; Smith et al, 2004). Data were motion corrected then co-registered, first the EPI to the subject’s individual T2, then the T2 to the study specific common brain (Jenkinson et al., 2002; Jenkinson and Smith, 2001). Individual subject analyses employed FEAT (FMRI Expert Analysis Tool) using a 5mm (FWHM) Gaussian smoothing kernel and 72s high-pass filter, with slice timing correction. Time-series statistical analysis was carried out using FILM (FMRIB’s Improved Linear Model) with local autocorrelation correction (Woolrich et al., 2001). Each load was modeled separately, and each trial was modeled in a block design fashion. Motion parameters were entered as covariates.
2.2.5 Statistical Analyses
We have previously demonstrated that behavioral performance on this verbal WMem task may influence functional activation, and that capacity may vary even within diagnostic group (Karlsgodt et al., 2007; Karlsgodt et al., 2009b). Therefore, for this analysis we took a conservative approach in which we calculated WMem capacity at each load using the following formula, where k=capacity, n=# items in the display, H=hit rate, and CR=correct rejection rate (Cowan, 2001):
| (1) |
The subject’s overall capacity was set to the highest capacity among those calculated and subsequent analyses used only data from the load that most closely represented the subjects’ capacity. This was achieved by loading the lower level cope images for the load defined as being at capacity for each subject individually into the group model.
To model the interaction of the age-activation relationship between groups, age was demeaned over all subjects and two separate age regressors, one for each group, were added to the model as well as regressors modeling the mean of each group, for a total of four regressors. We then tested for voxels in which the relationship between age and neural activity was greater in CHR compared with TDC and vice versa. In a subsequent analysis, the CHR group was divided into individuals who did or did not convert to psychosis during follow up and each subset was again compared to TDC. We tested for voxels in which the age relationship was greater in converters compared with controls, and non-converters compared with controls, and vice versa. Group analyses were carried out using FLAME Stage 1 (FMRIB’s Local Analysis of Mixed Effects) (Behrens et al., 2003; Smith et al., 2004). Subjects with average translational motion greater than 3mm were excluded. To correct for multiple comparisons, cluster correction was performed in Feat (Z>2.3, P=0.05) (Forman et al.; Friston et al.; Worsley et al.). (Jenkinson and Smith, 2001). To confirm the direction of the voxel-wise effects, the omnibus of all significant voxels were graphed for each group using a robust multiple regression in Stata v8, for each analysis we first checked for outliers (studentized residuals greater than 2.5 and excluded those people from the regression).
2.2.6 Post-Hoc Analyses
Because our samples with useable neuroimaging data were not matched for sex, we conducted additional analyses to test for potentially confounding sex effects. Specifically, we examined the differences between males and females in the TDC group using an exploratory region of interest analysis (using functional regions defined in a previous study employing the same task in healthy controls and first episode patients, to avoid circularity (Karlsgodt et al., 2009b)). In addition, as approximately half of the CHR group was on medication, we performed additional analyses to test for medication related effects.
3. Results
3.1 Behavioral Results
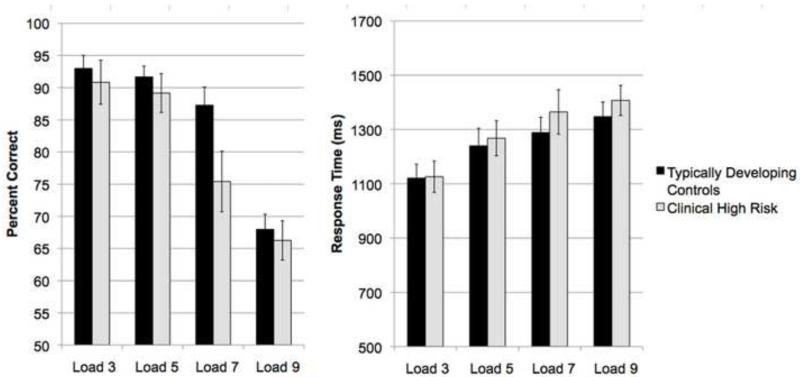
In a repeated measures ANOVA assessing performance across loads by group (CHR and TDC), there was a significant main effect of load (F(3)=50.12, p<.001), but no significant effect of group (F(1)=1.93, p=0.17) and a non-significant trend toward a load by group interaction (F(3)=2.3, p=.08). For response time, there was a significant main effect of load (F(3)=26.14, p<.001) but no significant effect of group (F(1)=0.29, p=0.59) or load by group interaction (F(3)=0.54, p=0.66) (Figure 2). CHR and TDC groups did not differ in overall WMem capacity, as calculated by Formula 1 (t(37)=0.28, p=0.78). Within the CHR group, a one-way ANOVA revealed no difference in WMem capacity between converters and non-converters (F(1)<.001, p>.99) or overall average performance (F(1)=.001, p=.972).
Figure 2.
Task performance and response time in CHR and TDC groups (mean ± standard error)
3.2 Imaging Results
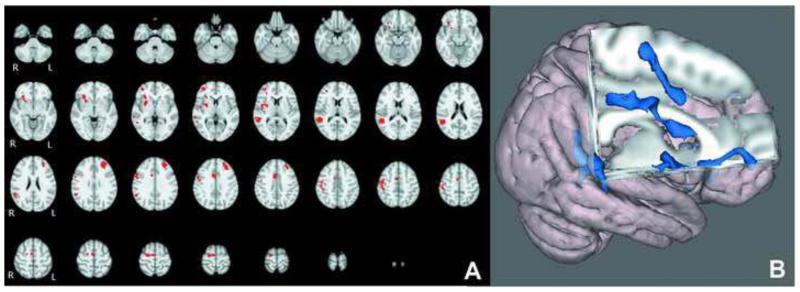
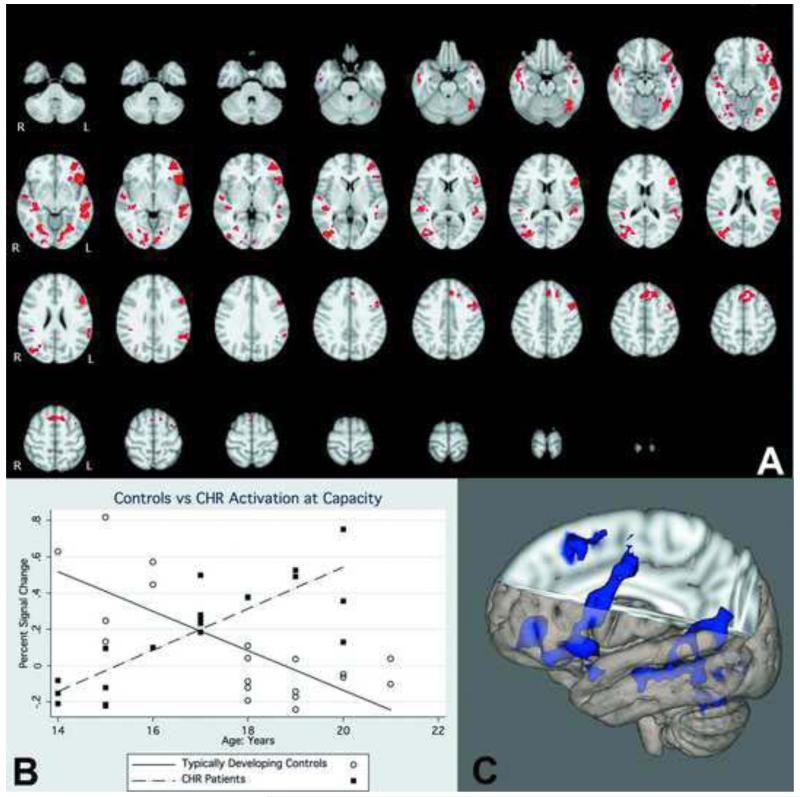
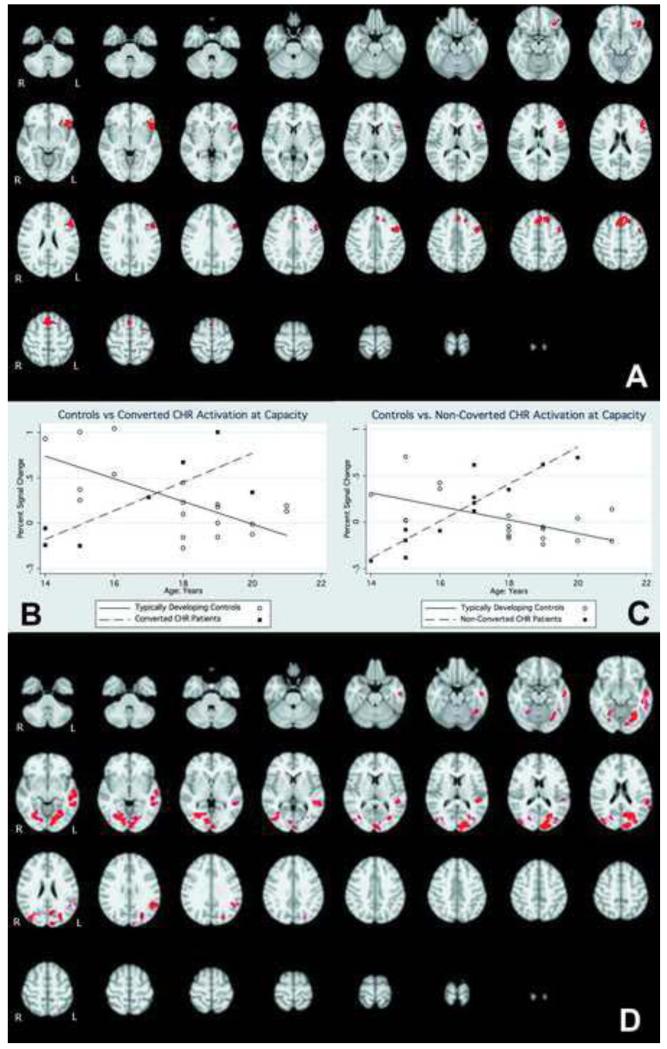
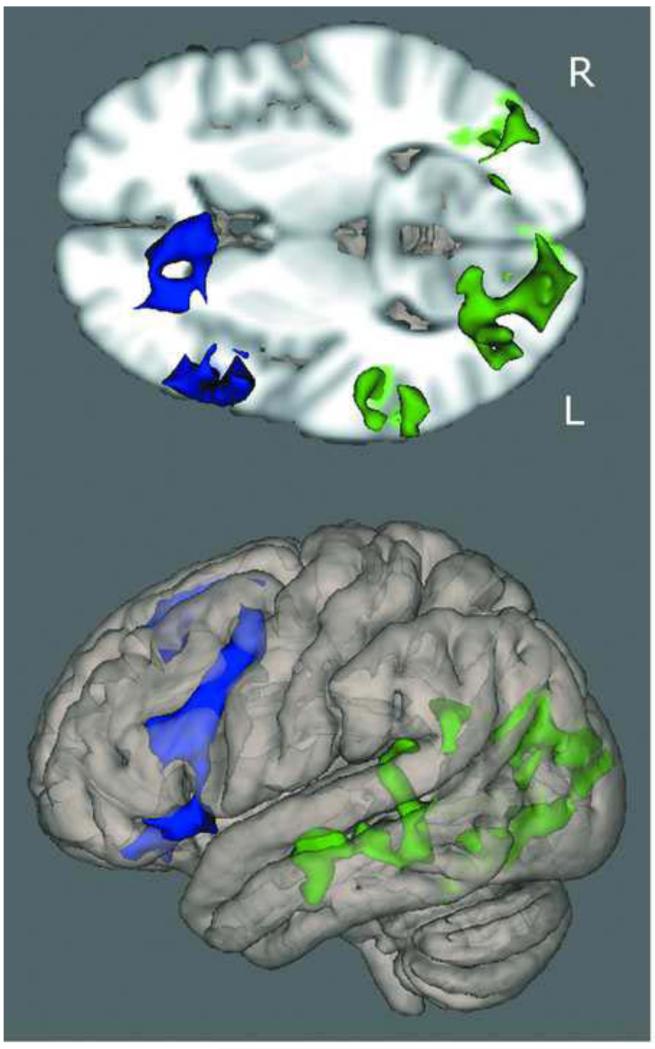
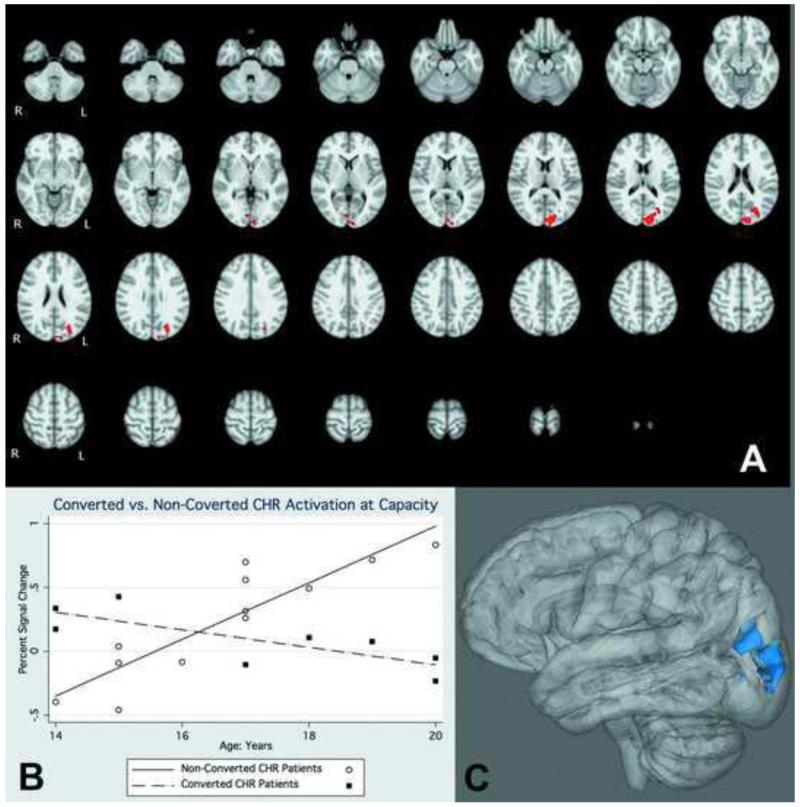
Overall, the TDC-CHR comparison at capacity revealed regions where CHR individuals showed greater activation than controls, and no regions where controls were greater. Significant regions included the left middle frontal gyrus, and a number of regions on the right including frontal eye fields, cingulate gyrus, putamen, and frontal pole, potentially indicating a greater reliance on right hemisphere regions to complete the task (Figure 3; Table 2). The cross-sectional voxel-wise analysis showed several regions with a significantly greater positive association between age and activation in CHR than TDC adolescents (Figure 4). These regions included the frontal pole, inferior frontal gyrus, dorsolateral prefrontal cortex, middle temporal gyrus, superior temporal gyrus, and parietal cortex (Table 3). There were no regions in which TDC adolescents showed a significantly greater positive association between age and activation than CHR adolescents. In the subsequent analysis we compared the age-activation association between CHR adolescents who later converted to psychosis (N=8) and those who did not (N=12) with TDC adolescents. In the comparison of CHR converters to TDC, the regions in which age showed a significantly greater positive association with functional activation were all localized to the frontal lobe and included DLPFC, inferior frontal gyrus, frontal eye fields, and a superior frontal gyrus region (Figure 5; Table 4). In contrast, in the comparison of CHR non-converters to TDC, the regions in which age showed a significantly greater positive associations with functional activation were more diffuse, and localized to the temporal, parietal, and occipital lobes, with no significant between group differences in the frontal lobe (Figure 6, Table 5). CHR patients who converted did not show any regions with a greater age-activation slope than non-converters. However, CHR non-converters had a steeper age-associated increase in the occipital lobe than converters (Figure 7, Table 6).
Figure 3.
A). Voxel-wise difference between TDC and CHR groups. B). 3-dimensional rendering of group difference. Significant voxels represent regions where patients showed greater activation than controls.
Table 2.
Active regions in comparison of CHR and TDC overall activation
| Region | Hemisphere | Zstat | X | Y | Z |
|---|---|---|---|---|---|
|
| |||||
| frontal pole | R | 4.26 | 38 | 50 | 2 |
| Insula | R | 3.74 | 30 | 26 | −2 |
| Orbitofrontal cortex | R | 3.68 | 34 | 30 | −12 |
| putamen | R | 3.36 | 28 | 0 | 6 |
| angular gyrus | R | 4.47 | 52 | −40 | 12 |
| Tempero-occipital region | R | 2.82 | 52 | −50 | 0 |
| Supplementary motor area | R | 3.57 | 8 | −10 | 66 |
| Cingulate | R | 3.53 | 6 | 6 | 38 |
| Middle frontal gyrus | L | 4.23 | −30 | 46 | 28 |
| Frontal eye field | R | 4.19 | 56 | 4 | 34 |
X, Y, Z reflect MNI coordinates at max Z statistic in region.
Figure 4.
A). Interaction of functional activation and age between CHR patients and TDC. Red colors indicate voxels in which the age-activation slope differs in patients and controls. B). Graphical rendering of activation difference with age in CHR and TDC participants, across an omnibus ROI of all significant voxels. C). 3-dimensional rendering of CHR-TDC age difference.
Table 3.
Active regions in comparison of CHR and TDC age-activation association
| Region | Hemisphere | Zstat | X | Y | Z |
|---|---|---|---|---|---|
|
| |||||
| frontal pole | L | 4.54 | −40 | 52 | 2 |
| inferior frontal gyrus | L | 4.03 | −56 | 18 | −4 |
| DLPFC | L | 4 | −54 | 18 | 26 |
| middle temporal gyrus | L | 4 | −52 | −30 | −6 |
| middle temporal gyrus | L | 3.79 | −56 | −12 | −12 |
| inferior temporal gyrus | L | 3.55 | −54 | −44 | −10 |
| parietal operculum cortex | L | 3.52 | −60 | −36 | 20 |
| lateral occipital cortex | R | 4.43 | 46 | −80 | −6 |
| fusiform | R | 3.53 | 32 | −64 | −8 |
| superior temporal gyrus | R | 3.68 | 46 | −28 | 4 |
| posterior hippocampus | R | 3.35 | 38 | −24 | −8 |
| fusiform | L | 3.71 | −28 | −64 | −10 |
| lingual gyrus | L | 3.2 | −4 | −88 | −4 |
| medial aspect of superior frontal gyrus |
L | 3.62 | −2 | 32 | 48 |
| superior frontal gyrus | L | 3.32 | −18 | 28 | 48 |
X, Y, Z reflect MNI coordinates at max Z statistic in region.
Figure 5.
A) Interaction of functional activation and age between CHR subjects who converted to psychosis within 2 years of the baseline scan, and TDC. Red colors indicate voxels in which the age-activation slope differs in patients and controls. B). Graphical rendering of activation difference with age in converted CHR and TDC participants, across an omnibus ROI of all significant voxels. (One outlier has been left out of this graph). C). Interaction of functional activation and age in CHR patients who did not convert to psychosis within 2 years of this baseline scan, compared to TDC. Red colors indicate voxels in which the age-activation slope differs in patients and controls. D). Graphical rendering of activation difference with age in non-converted CHR and TDC participants, across an omnibus ROI of all significant voxels.
Table 4.
Active regions in comparison of converted CHR subjects and TDC age-activation association
| Region | Hemisphere | Zstat | X | Y | Z |
|---|---|---|---|---|---|
|
| |||||
| frontal eye field | L | 3.62 | −42 | 4 | 54 |
| DLPFC | L | 4.93 | −54 | 24 | 22 |
| inferior frontal gyrus | L | 4.22 | −56 | 18 | −4 |
| medial aspect of superior frontal gyrus | L | 3.77 | −2 | 32 | 48 |
X, Y, Z reflect MNI coordinates at max Z statistic in region.
Figure 6.
Three-dimensional rendering of age-activation slope differences between TDC and converted and unconverted groups. Blue regions represent voxels that showed a difference in slope between converters and controls; Green regions represent voxels that showed a difference in slope between non-converters and controls.
Table 5.
Active regions in comparisons of unconverted CHR subjects and TDC age-activation association
| Region | Hemisphere | Zstat | X | Y | Z |
|---|---|---|---|---|---|
|
| |||||
| occipital lobe | L | 4.92 | −10 | −92 | 16 |
| fusiform gyrus | L | 4.17 | −30 | −64 | −12 |
| fusiform gyrus | R | 3.92 | 28 | −72 | −4 |
| lingual gyrus | L | 3.87 | −12 | −74 | −4 |
| middle temporal | |||||
| gyrus | L | 3.96 | −58 | −14 | −8 |
| temporo-occipital | L | 3.6 | −54 | −46 | −12 |
| planum temporale | L | 3.54 | −50 | −32 | 12 |
X, Y, Z reflect MNI coordinates at max Z statistic in region.
Figure 7.
A). Interaction of functional activation and age in CHR patients who did not convert to psychosis within 2 years of this baseline scan, compared to those who did convert to psychosis. Red colors indicate voxels in which the age-activation slope differs in patients and controls. B). Graphical rendering of activation difference with age in non-converted CHR and converted CHR participants, across an omnibus ROI of all significant voxels. C). 3-dimensional rendering of converter-non converter differences.
Table 6.
Active regions in comparisons of unconverted and converted CHR subjects age-activation association
| Region | Hemisphere | Zstat | X | Y | Z |
|---|---|---|---|---|---|
|
| |||||
| occipital pole | L | 4.46 | −10 | −92 | 16 |
| Superior lateral | |||||
| occipital lobe | L | 4.03 | −28 | −76 | 26 |
| Intracalcarine | |||||
| cortex | R | 2.76 | 4 | −82 | 2 |
X, Y, Z reflect MNI coordinates at max Z statistic in region.
Post-hoc tests for sex effects did not reveal an age difference between sexes in either the TDC group (t(17)=.9582, p=.3514) or the CHR group (t(18)=-1.052, p=.3066). In the exploratory ROI analysis we found no significant difference in average activation between males and females [left DLPFC (t(17)=.3620, p=.7218), right DLPFC (t(17)=0043, p=.9966), left parietal (t(17)=-.1599, p=..8748, and right parietal lobes (t(17)=.7403, p=.4692)]. Finally, there was no interaction of sex by age in the TDC group, indicating that in this sample there was not a difference in the association of age with functional activation between males and females [right DLPFC (F(3,15)=3.08, p=.0595, p=.314, left DLPFC (F(3,15)=.87, p=.4786, right parietal (F(3,15)=1.19, p=.3470, and left parietal lobes (F(3,15)=.89, p=.4699)].
As a subset of the CHR subjects were medicated, we also performed post-hoc tests of medication effects. When the four subjects treated with antipsychotics were removed, the regression equations remained significant (CHR patients vs controls: F(3,31)=12.74, p<.001) and they also remained significant when all currently medicated subjects were removed [(F(3,25)=9.95, p<.001)]. Furthermore, the distribution of converted subjects did not differ between medicated and unmedicated groups (Chi-sq=.833, p=.361).
4. Discussion
The principal finding of this cross-sectional study is that TDC controls showed a negative association between age and functional activation during verbal WMem when controlled for capacity while CHR youth showed the opposite. The negative age-activation association in TDC adolescents may represent maturation and increased efficiency of the WMem circuitry. In contrast, the positive age-activation association in CHR youth may represent an emerging hyperactivity. One possible hypothesis is that perhaps this change is an early indicator of compensatory increases in physiologic activity in response to underlying structural changes such as accelerated pruning. The differences in age-activation relationships between TDC and CHR is consistent with the hypothesis that the period leading up to psychosis onset may be characterized by an aberrant developmental trajectory. Additionally, the CHR converter-TDC difference in age-activation association was localized to the frontal lobe, in areas commonly disrupted in psychosis and schizophrenia, while the CHR non-converter-TDC difference showed a more diffuse pattern. Further, CHR patients who did not convert showed a significantly different age-activation relationship in posterior regions relative to CHR patients who subsequently converted. Taken together, these findings suggest that it is not simply a general disruption in development, but a specific pattern of disruption, affecting the frontal lobe, that is associated with psychosis onset (and is detectable prior to the onset of overt illness). In this respect the findings, and the hypothesis that they may reflect compensatory activity, are consistent with observations of a steeper rate of surface contraction in the prefrontal cortex among CHR youth (Sun et al., 2009a).
Previous work has investigated functional changes in individuals at risk for psychosis during a variety of tasks (for example(Allen et al., 2012; Broome et al., 2009; Choi et al., 2011; Fusar-Poli et al., 2010; Gee et al., 2012; Juckel et al., 2012; Morey et al., 2005)), with a recent meta-analysis showing consistent frontal lobe abnormalities(Fusar-Poli, 2012). However, to our knowledge this is the first study to compare associations of age with functional activation during WMem performance between CHR adolescents that convert, or do not convert to psychosis and TDC adolescents. One prior fMRI study investigated age trajectories during emotion processing in CHR youth, and showed similar differential relationships between age and activation patterns in CHR compared to TDC groups in a number of regions as well as an emerging fronto-amygdala disconnection in CHR individuals (Gee et al., 2012). However, the study had a smaller sample and was not able to assess the differences between those who did and did not convert to psychosis. The interpretation of the non-conversion group is complicated by the heterogeneity of those subjects. For instance, Schlosser et al (Schlosser et al., 2012) found that this group contains individuals who improve both symptoms and function, those who only improve symptom levels and those who only improve function levels after 2 years. Given the continuation of functional and/or symptomatic differences in these individuals even without conversion, it would be expected that they would not show a normal developmental trajectory. However, more studies in larger groups of non-converters are needed to understand the within-group differences in neural function.
The relationship of behavioral performance to functional activation is an important consideration (Callicott et al., 2003; Karlsgodt et al., 2009b; Manoach, 2003). As CHR and TDC groups did not differ behaviorally, the observed differences are not likely to be explained by differences in performance or task engagement. The lack of a case-control difference in behavioral accuracy in this study may reflect that prior to onset, individuals who will later convert to psychosis are able to compensate for emerging brain pathology in the WMem circuitry (e.g., by increasing the activation of frontal regions), and/or that WMem declines from pre- to post-onset phases of psychosis (Kumra et al., 2005).
The strengths of this study are the very well-characterized CHR and TDC samples, the use of a well-established verbal WMem task (Karlsgodt et al., 2007; Karlsgodt et al., 2009b), individualized analysis of data at capacity to avoid performance confounds, and the application of rigorous whole-brain corrected voxelwise analysis to ensure the most stringent statistical testing, which resulted in significant findings despite the relatively small sample of converters in the study. A weakness is the small sample size and cross-sectional design of the study; a prospective longitudinal fMRI study is needed to test within-subject change over time. In addition, while we did not find evidence for a sex-specific pattern of age-activation associations in TDC, it is unknown whether such differences exist in CHR adolescents. Our sample was limited in that it was not evenly matched for gender. A larger study with more female CHR adolescents is needed to test any hypotheses related to sex differences. A further limitation is that 10 of the 20 CHR subjects were currently medicated, however we did not find evidence that medication affected the pattern of the results. Future studies with unmedicated subjects, or with standardized medication regimes will be important for determining the role of medication effects. Finally, it is possible that some of the non-converted subjects did in fact convert after the follow up period was completed, which adds potential heterogeneity to that group.
Overall, the differential age-activation relationships between CHR converters, CHR non-converters, and controls observed in this study suggest that there is a difference in the pattern of of WMem-associated functional activation across adolescence in CHR individuals compared with controls and that changes in frontal lobe physiology may be specific to CHR patients who convert to psychosis. These findings suggest that that specific abnormalities in the development of frontal lobe activity may be associated with the onset of psychosis.
Acknowledgements
We would like to thank the participants in the study and to acknowledge the contributions of Jeanette Mumford to the statistical design and analysis, Tara Niendam to subject ascertainment and diagnosis, Molly Hardt to functional data acquisition, Anna Xu to functional imaging data management, and Aron Jacobson, to behavioral and demographic data management.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen P, Chaddock CA, Howes OD, Egerton A, Seal ML, Fusar-Poli P, Valli I, Day F, McGuire PK. Abnormal relationship between medial temporal lobe and subcortical dopamine function in people with an ultra high risk for psychosis. Schizophrenia Bulletin. 2012;38:1040–1049. doi: 10.1093/schbul/sbr017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearden CE, Rosso IM, Hollister JM, Sanchez LE, Hadley T, Cannon TD. A prospective cohort study of childhood behavioral deviance and language abnormalities as predictors of adult schizophrenia. Schizophrenia Bulletin. 2000;26:395–410. doi: 10.1093/oxfordjournals.schbul.a033461. [DOI] [PubMed] [Google Scholar]
- Behrens TE, Johansen-Berg H, Woolrich MW, Smith SM, Wheeler-Kingshott CA, Boulby PA, Barker GJ, Sillery EL, Sheehan K, Ciccarelli O, Thompson AJ, Brady JM, Matthews PM. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nature Neuroscience. 2003;6:750–757. doi: 10.1038/nn1075. [DOI] [PubMed] [Google Scholar]
- Broome MR, Matthiasson P, Fusar-Poli P, Woolley JB, Johns LC, Tabraham P, Bramon E, Valmaggia L, Williams SC, Brammer MJ, Chitnis X, McGuire PK. Neural correlates of executive function and working memory in the ‘at-risk mental state’. The British Journal of Psychiatry: The Journal of Mental Science. 2009;194:25–33. doi: 10.1192/bjp.bp.107.046789. [DOI] [PubMed] [Google Scholar]
- Callicott JH, Egan MF, Mattay VS, Bertolino A, Bone AD, Verchinksi B, Weinberger DR. Abnormal fMRI response of the dorsolateral prefrontal cortex in cognitively intact siblings of patients with schizophrenia. The American Journal of Psychiatry. 2003;160:709–719. doi: 10.1176/appi.ajp.160.4.709. [DOI] [PubMed] [Google Scholar]
- Cannon TD, Cadenhead K, Cornblatt B, Woods SW, Addington J, Walker E, Seidman LJ, Perkins D, Tsuang M, McGlashan T, Heinssen R. Prediction of psychosis in youth at high clinical risk: a multisite longitudinal study in North America. Archives of General Psychiatry. 2008;65:28–37. doi: 10.1001/archgenpsychiatry.2007.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JS, Park JY, Jung MH, Jang JH, Kang DH, Jung WH, Han JY, Choi CH, Hong KS, Kwon JS. Phase-specific brain change of spatial working memory processing in genetic and ultra-high risk groups of schizophrenia. Schizophrenia Bulletin. 2011 doi: 10.1093/schbul/sbr038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan N. The magical number 4 in short-term memory: a reconsideration of mental storage capacity. Behavioral Brain Science. 2001;24:87–114. doi: 10.1017/s0140525x01003922. discussion 114-185. [DOI] [PubMed] [Google Scholar]
- Dazzan P, Soulsby B, Mechelli A, Wood SJ, Velakoulis D, Phillips LJ, Yung AR, Chitnis X, Lin A, Murray RM, McGorry PD, McGuire PK, Pantelis C. Volumetric abnormalities predating the onset of schizophrenia and affective psychoses: an MRI study in subjects at ultrahigh risk of psychosis. Schizophrenia Bulletin. 2012;38:1083–1091. doi: 10.1093/schbul/sbr035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magnetic Resonance Medicine. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Worsley K, Frackowiak R, Mazziotta JC, Evans AC. Assessing the significance of focal activations using their spatial extent. Human Brain Mapping. 1994;1:214–220. doi: 10.1002/hbm.460010306. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P. Voxel-wise meta-analysis of fMRI studies in patients at clinical high risk for psychosis. Journal of Psychiatry & Neuroscience. 2012;37:106–112. doi: 10.1503/jpn.110021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P, Broome MR, Matthiasson P, Woolley JB, Johns LC, Tabraham P, Bramon E, Valmaggia L, Williams SC, McGuire P. Spatial working memory in individuals at high risk for psychosis: longitudinal fMRI study. Schizophrenia Research. 2010;123:45–52. doi: 10.1016/j.schres.2010.06.008. [DOI] [PubMed] [Google Scholar]
- Gee DG, Karlsgodt KH, van Erp TG, Bearden CE, Lieberman MD, Belger A, Perkins DO, Olvet DM, Cornblatt BA, Constable T, Woods SW, Addington J, Cadenhead KS, McGlashan TH, Seidman LJ, Tsuang MT, Walker EF, Cannon TD. Altered age-related trajectories of amygdala-prefrontal circuitry in adolescents at clinical high risk for psychosis: a preliminary study. Schizophrenia Research. 2012;134:1–9. doi: 10.1016/j.schres.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier CF, Garver K, Terwilliger R, Luna B. Development of working memory maintenance. J Neurophysiology. 2009;101:84–99. doi: 10.1152/jn.90562.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glahn DC, Therman S, Manninen M, Huttunen M, Kaprio J, Lonnqvist J, Cannon TD. Spatial working memory as an endophenotype for schizophrenia. Biological Psychiatry. 2003;53:624–626. doi: 10.1016/s0006-3223(02)01641-4. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Medical Image Analysis. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Juckel G, Friedel E, Koslowski M, Witthaus H, Ozgurdal S, Gudlowski Y, Knutson B, Wrase J, Brune M, Heinz A, Schlagenhauf F. Ventral striatal activation during reward processing in subjects with ultra-high risk for schizophrenia. Neuropsychobiology. 2012;66:50–56. doi: 10.1159/000337130. [DOI] [PubMed] [Google Scholar]
- Karlsgodt K, Glahn DC, van Erp TG, Therman S, Huttunen M, Manninen M, Kaprio J, Cohen MS, Lönnqvist J, Cannon TD. The relationship between performance and fMRI signal during working memory in patients with schizophrenia, unaffected co-twins, and control subjects. Schizophrenia Research. 2007;89:191–197. doi: 10.1016/j.schres.2006.08.016. [DOI] [PubMed] [Google Scholar]
- Karlsgodt K, Niendam TA, Bearden CE, Cannon TD. White matter integrity and prediction of social and role functioning in subjects at ultra-high risk for psychosis. Biological Psychiatry. 2009a;66:562–569. doi: 10.1016/j.biopsych.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsgodt K, Sanz J, van Erp TG, Bearden CE, Nuechterlein KH, Cannon TD. Re-evaluating dorsolateral prefrontal cortex activation during working memory in schizophrenia. Schizophrenia Research. 2009b;108:143–150. doi: 10.1016/j.schres.2008.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsgodt K, Sun D, Jimenez AM, Lutkenhoff ES, Willhite R, van Erp TG, Cannon TD. Developmental disruptions in neural connectivity in the pathophysiology of schizophrenia. Development and Psychopathology. 2008;20:1297–1327. doi: 10.1017/S095457940800062X. [DOI] [PubMed] [Google Scholar]
- Kumra S, Ashtari M, Cervellione KL, Henderson I, Kester H, Roofeh D, Wu J, Clarke T, Thaden E, Kane JM, Rhinewine J, Lencz T, Diamond A, Ardekani BA, Szeszko PR. White matter abnormalities in early-onset schizophrenia: a voxel-based diffusion tensor imaging study. Journal of the American Academy of Child and Adolescent Psychiatry. 2005;44:934–941. doi: 10.1097/01.chi.0000170553.15798.94. [DOI] [PubMed] [Google Scholar]
- Kuperberg G, Heckers S. Schizophrenia and cognitive function. Current Opinions in Neurobiology. 2000;10:205–210. doi: 10.1016/s0959-4388(00)00068-4. [DOI] [PubMed] [Google Scholar]
- Manoach DS. Prefrontal cortex dysfunction during working memory performance in schizophrenia: reconciling discrepant findings. Schizophrenia Research. 2003;60:285–298. doi: 10.1016/s0920-9964(02)00294-3. [DOI] [PubMed] [Google Scholar]
- McGlashan TH, Miller TJ, Woods SW, Rosen JL, Hoffman RE, Davidson L. Structured Clinical Interview for Prodromal Syndromes v3.1. PRIME Research Clinic; Yale School of Medicine: 2001. [Google Scholar]
- Mechelli A, Riecher-Rossler A, Meisenzahl EM, Tognin S, Wood SJ, Borgwardt SJ, Koutsouleris N, Yung AR, Stone JM, Phillips LJ, McGorry PD, Valli I, Velakoulis D, Woolley J, Pantelis C, McGuire P. Neuroanatomical abnormalities that predate the onset of psychosis: a multicenter study. Archives of General Psychiatry. 2011;68:489–495. doi: 10.1001/archgenpsychiatry.2011.42. [DOI] [PubMed] [Google Scholar]
- Meyer SE, Bearden CE, Lux SR, Gordon JL, Johnson JK, O’Brien MP, Niendam TA, Loewy RL, Ventura J, Cannon TD. The psychosis prodrome in adolescent patients viewed through the lens of DSM-IV. Journal of Child and Adolescent Psychopharmacology. 2005;15:434–451. doi: 10.1089/cap.2005.15.434. [DOI] [PubMed] [Google Scholar]
- Morey RA, Inan S, Mitchell TV, Perkins DO, Lieberman JA, Belger A. Imaging frontostriatal function in ultra-high-risk, early, and chronic schizophrenia during executive processing. Archives of General Psychiatry. 2005;62:254–262. doi: 10.1001/archpsyc.62.3.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CA, Monk CS, Lin J, Carver LJ, Thomas KM, Truwit CL. Functional neuroanatomy of spatial working memory in children. Developmental Psychology. 2000;36:109–116. doi: 10.1037//0012-1649.36.1.109. [DOI] [PubMed] [Google Scholar]
- O’Hare ED, Lu LH, Houston SM, Bookheimer SY, Sowell ER. Neurodevelopmental changes in verbal working memory load-dependency: an fMRI investigation. NeuroImage. 2008;42:1678–1685. doi: 10.1016/j.neuroimage.2008.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlosser DA, Jacobson S, Chen Q, Sugar CA, Niendam TA, Li G, Bearden CE, Cannon TD. Recovery from an at-risk state: clinical and functional outcomes of putatively prodromal youth who do not develop psychosis. Schizophrenia Bulletin. 2012;38:1225–1233. doi: 10.1093/schbul/sbr098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg AD, Nagel BJ, Tapert SF. fMRI reveals alteration of spatial working memory networks across adolescence. Journal of the International Neuropsychological Society. 2005;11:631–644. doi: 10.1017/S1355617705050757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23(Suppl 1):S208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Sternberg S. High-speed scanning in human memory. Science. 1966;153:652–654. doi: 10.1126/science.153.3736.652. [DOI] [PubMed] [Google Scholar]
- Sun D, Stuart GW, Phillips L, Velakoulis D, Jenkinson M, Yung A, McGorry PD, Wood SJ, van Erp TGM, Thompson PM, Toga AW, Cannon TD, Pantelis C. Progressive brain structural changes mapped as psychosis develops in ‘at risk’ individuals. Schizophrenia Research. 2009a;108:85–92. doi: 10.1016/j.schres.2008.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of FMRI data. NeuroImage. 2001;14:1370–1386. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Evans AC, Marrett S, Neelin P. A three-dimensional statistical analysis for CBF activation studies in human brain. Journal of Cerebral Blood Flow and Metabolism. 1992;12:900–918. doi: 10.1038/jcbfm.1992.127. [DOI] [PubMed] [Google Scholar]