Abstract
This study was performed to assess the in vivo ocular toxicity of benzalkonium chloride (BAK) homologs compared with commercially available BAK (BAK mixture) and to assess the ocular toxicity of BAK homolog after repeated ocular application. Rabbit eyes were examined by ophthalmology and scanning electron microscopy (SEM) after 10 applications of BAK homologs with C12 (C12-BAK) and C14 (C14-BAK) alkyl chain lengths and a BAK mixture at concentrations of 0.001% (w/v), 0.003% (w/v), 0.005% (w/v), 0.01% (w/v) and 0.03% (w/v). The ocular toxicity of C12-BAK to rabbit eyes was examined by ophthalmology and histopathology after repeated ocular application for 39 weeks. In addition, the antimicrobial activities of C12-BAK and C14-BAK against A. niger, S. aureus and P. aeruginosa were assessed. Ocular toxicity of C12-BAK was less than those of the BAK mixture and C14-BAK. No ocular toxicity was noted after ocular application of 0.01% C12-BAK to rabbits for 39 weeks. C12-BAK showed antimicrobial activities at a concentration of 0.003%. These results suggest that the use of C12-BAK to replace BAK mixture as a preservative in ophthalmic solutions should be considered in order to reduce the incidence of the corneal epithelial cell injury induced clinically by BAK.
Keywords: benzalkonium chloride, homolog, ocular toxicity, rabbit
Introduction
Many ophthalmic solutions contain preservatives to prevent contamination by microorganisms. Benzalkonium chloride (BAK) is a widely used preservative in ophthalmic solutions. The ocular toxicity of BAK has been investigated in vitro using cell culture systems, such as human corneal cell lines1,2,3,4,5, human conjunctival cell lines1,2,6,7,8,9,10,11, a three-dimensional model of human corneal epithelium12,13, rabbit corneal cell lines6,14 and bovine corneal cell lines6. The ocular toxicity of BAK has also been investigated by several in vivo experiments following application on the ocular surface of rats15, rabbits16,17,18, monkeys17 and cats18. In these studies, BAK was deleterious to corneas and cultured cells at concentrations used clinically. Based on in vitro and in vivo toxicity studies, BAK has undergone considerable criticism in recent years. In an attempt to reduce the adverse effects of BAK, several ophthalmic solutions have been marketed during the last several years as BAK-free formulations. In addition, new preservatives with a wide range of activity and good safety profiles have been introduced. Commercially available BAK is composed of a mixture of different homologs with alkyl chain lengths ranging from 8−18 carbon atoms. Ocular toxicity of BAK homologs is reported to be dependent on the alkyl chain length of the molecule. The studies were done using various models including in vitro studies of bovine corneas19, transepithelial electrical resistance (TER) of live rabbit corneas20, cytotoxicity assays of rabbit corneal epithelial (NRCE) cells20 and cytotoxicity assays of human corneal epithelial (HCE) and conjunctival epithelial (IOBA-NHC) cells21. These studies suggest that the use of BAK homologs instead of the commercially available BAK mixture ophthalmic solution may lessen the adverse events for patients. However, the relative order of cytotoxicity of BAK homologs and BAK mixture varied in these in vitro studies. To date, the ocular toxicities of BAK homologs and a BAK mixture have not been compared in vivo, which is a first step in comparing the possible toxicities of these homologs with the mixture for clinical use.
In the present study, we compared the ocular toxicity of BAK homologs (C12- and C14-BAK) and a BAK mixture by ophthalmology and scanning electron microscopy (SEM) after 10 ocular applications to rabbits and assessed the ocular toxicity of a BAK homolog (C12-BAK) by ophthalmology and histopathology after repeated ocular application for 39 weeks in rabbits. In addition, the antimicrobial activities of C12-BAK and C14-BAK against A. niger, S. aureus, and P. aeruginosa were assessed.
Materials and Methods
Experiments 1 and 3 were conducted in the Nara Research & Development Center of Santen Pharmaceutical Co., Ltd. (Osaka, Japan), and experiment 2 was conducted in the Drug Safety Research Laboratories of Shin Nippon Biomedical Laboratories, Ltd. (SNBL DSR; Kagoshima, Japan). Animal care and experiments involving animals in experiment 1 were performed in accordance with the Law for the Humane Treatment and Management of Animals (Law No. 105, 1973) and Standards Relating to the Care and Management of Experimental Animals (Notice No. 6 of the Prime Minister’s Office, 1980) in Japan. Experiment 2 was approved by the Institutional Animal Care and Use Committee and was performed in accordance with the animal welfare bylaws of SNBL DSR. Experiment 2 was performed in compliance with Good Laboratory Practice Regulations for Nonclinical Safety Studies on Drugs (Ministry of Health and Welfare, Japan, Ordinance No. 21, March 26, 1997).
Experiment 1
Animals: Eight- to nine-week-old male Japanese white rabbits (Kbs:JW) were supplied by Kitayama Labes Co., Ltd. (Nagano, Japan). They were quarantined and acclimatized to laboratory conditions for 7 days before experiments. Animals were individually housed in a room with a temperature of 22–24°C (acceptable range), relative humidity of 45–65% (acceptable range), 12-h light/dark cycles and air ventilation at 14 times per h. They were provided with water ad libitum and were given approximately 130 g/day of solid food (RC-4; Oriental Yeast Co., Ltd., Tokyo, Japan).
Administration: Benzyldimethyldodecylammonium chloride (C12-BAK) was obtained as a powder from Okami Chemical Industry Co., Ltd. (Kyoto, Japan), benzyldimethyltetradecylammonium chloride (C14-BAK) was purchased as a powder from Sigma-Aldrich Co., Ltd. (St. Louis, MO, USA), and an industrial batch of BAK mixture powder (60.1% (w/w) C12, 37.1% (w/w) C14, 2.8% (w/w) C16) was obtained from FeF Chemicals A/S (Køge, Denmark). C12-BAK, C14-BAK and BAK mixture were stored at room temperature. The results of assays (calculated on anhydrous basis) of C12-BAK and BAK mixture were 104.1% and 101.3%, respectively.
Solutions containing C12-BAK and BAK mixture were prepared in concentrations of 0.001% (w/v), 0.003% (w/v), 0.005% (w/v) and 0.01% (w/v) because BAK is generally used at concentrations ranging from 0.001% to 0.01% in ophthalmic solutions. In addition, 0.03% (w/v) was selected as the highest concentration to assess the toxicity of an excess concentration. Solutions containing C14-BAK were prepared in concentrations of 0.005% (w/v) and 0.03% (w/v). BAK was dissolved in 0.9% saline (Otsuka Pharmaceutical Factory, Inc., Tokyo, Japan). Saline alone was used as a control.
Fifty microliters of saline or BAK solution, which is equal to one drop from an eye drop bottle, was bilaterally applied 10 times (every 30 minutes) to the ocular surface of eyes of 19 rabbits (38 eyes). Saline was applied to 2 eyes, and each BAK solution was applied to 3 eyes.
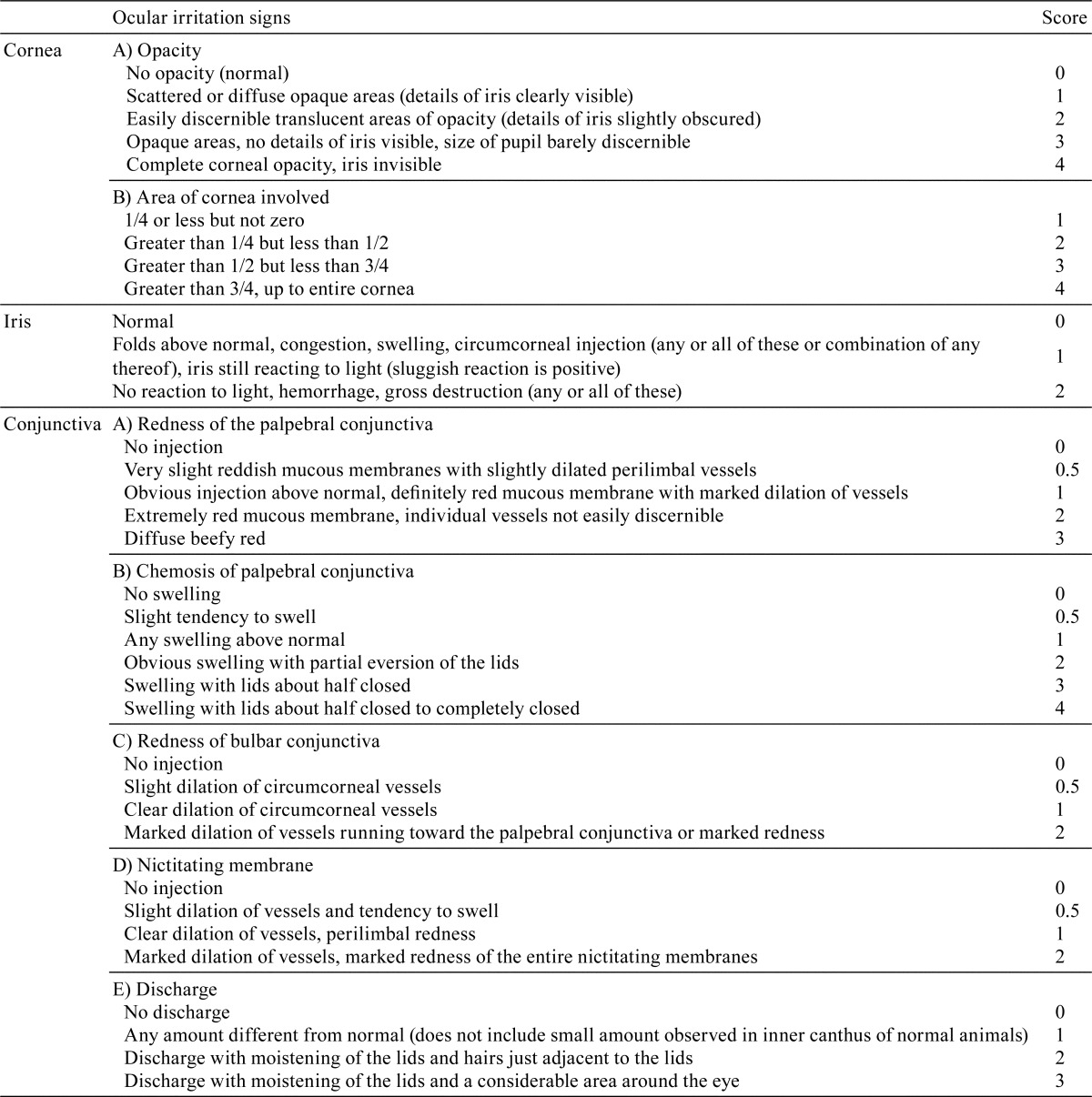
Observations and examinations: The cornea and conjunctiva were macroscopically observed at approximately 30 minutes after the last application, and ocular irritation scores were recorded according to the modified Draize scoring system22 (Table 1). One to two hours after the last instillation of test solutions, rabbits were anesthetized by an intravenous injection of sodium pentobarbital solution (50 mg/mL, 0.5 mL/kg; Dainippon Sumitomo, Pharma Co., Ltd., Osaka, Japan) into the auricular vein and euthanized by exsanguination. Eyeballs from each animal were removed and fixed by submersion in 2.5% glutaraldehyde solution in 0.1 M phosphate buffer (pH 7.4). Corneas were excised from fixed eyeballs, rinsed and postfixed in 1% buffered osmium tetroxide solution. After dehydration in ethanol at increasing concentrations, they were transferred to amyl acetate, dried with liquid carbon dioxide using a critical point dryer (HCP-2; Hitachi Koki Co., Ltd., Tokyo, Japan) and sputter-coated with gold in an ion coater (IB-3; Eiko Engineering Co., Ltd., Tokyo, Japan). The corneal epithelium and endothelium were viewed using SEM (JSM840A; JEOL, Tokyo, Japan), their levels of damage were estimated semiquantitatively.
Table 1. Modified Draize Scoring System for Ocular Irritation.
Experiment 2
Animals: Eight-week-old male and female rabbits (Kbl:JW) were supplied by Kitayama Labes Co., Ltd. Rabbits were quarantined and acclimatized to laboratory conditions for approximately 2 weeks before experiments and individually housed in a room with a temperature of 20–24°C (acceptable range), relative humidity of 40–60% (acceptable range), 12-h light/dark cycle and air ventilation at 15 times per hour. They were provided with water ad libitum and were given approximately 120 g/day of solid food (CR-1; CLEA Japan, Inc., Tokyo, Japan) once daily.
Administration: C12-BAK was obtained as a powder from Okami Chemical Industry Co., Ltd. Solutions containing C12-BAK were prepared in concentrations of 0.01% and were supplied by Santen Pharmaceutical Co., Ltd. C12-BAK was dissolved in phosphate buffer containing 0.75% NaCl and 0.15% KCl (pH 7.5).
Fifty microliters of the solution containing 0.01% C12-BAK was applied on the ocular surface of the left eye in 6 males and 6 females 7 times/day for 39 weeks. The right eye was not treated.
Ophthalmology: Once in weeks 4, 6, 8, 13, 26 and 39, the cornea and conjunctiva were macroscopically observed at approximately 1 h after the last application of the day, and ocular irritation scores were recorded according to the modified Draize scoring system (Table 1). After macroscopic examination, corneal epithelia were stained with fluorescein paper (Wyeth-Ayerst Laboratories Inc., Wayne, PA, USA), observed using a slit lamp (SL-14; Kouda Denshi Co., Ltd., Kahoku, Japan) and evaluated according to the scoring system for fluorescein staining.
Histopathology of the eyeball and its accessory organs: On the day following the end of applications, rabbits were anesthetized by an intravenous injection of sodium pentobarbital solution (64.8 mg/mL, 0.5 mL/kg; Tokyo Chemical Industry Co., Ltd., Tokyo, Japan) into the auricular vein and euthanized by exsanguination. Eyeballs (including the optic nerve and bulbar conjunctiva) from each animal were removed and fixed in a solution of 2.5% formaldehyde and 1% glutaraldehyde. Eyelids (including the palpebral conjunctiva) and lacrimal glands were fixed in 10% neutral buffered formalin. Organs and tissues were trimmed, embedded in paraffin, sectioned, stained with hematoxylin and eosin and examined microscopically.
Observation of corneal epithelium and endothelium by SEM: Corneas were excised from eyeballs of two males and two females that were fixed by 2.5% formaldehyde and 1% glutaraldehyde, rinsed and postfixed in 1% buffered osmium tetroxide solution. After dehydration in ethanol at increasing concentrations, they were transferred to amyl acetate, dried with liquid carbon dioxide using a critical point dryer and sputter-coated with gold in an ion coater. The corneal epithelium and endothelium were viewed using SEM (JSM-5200; JEOL Co., Ltd.).
Experiment 3
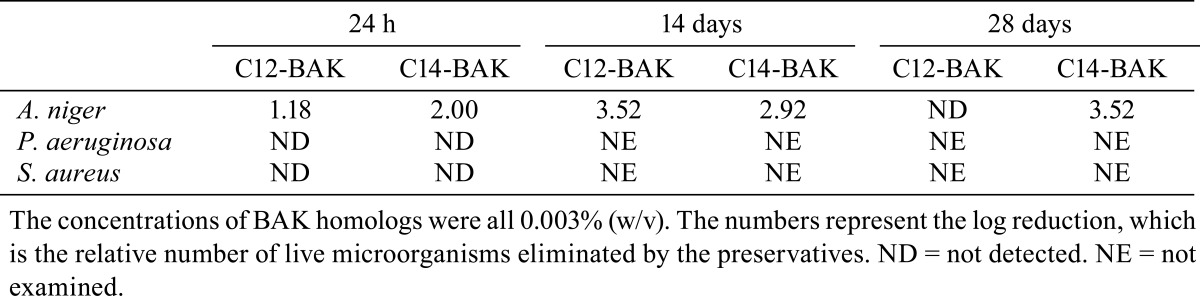
The antimicrobial activity of BAK homologs: The preservative effectiveness tests assessed the antimicrobial activities of 0.003% C12-BAK and C14-BAK in phosphate buffer containing 0.9% NaCl (pH 7.0), according to the preservative effectiveness tests described in the Japanese Pharmacopeia (fifteenth edition). Test strains were incubated on the surfaces of agar slants, using Soybean-Casein Digest Agar Medium for bacteria and Sabouraud Dextrose Agar for A.niger. The saline suspensions of microorganisms were prepared to harvest the cells aseptically from agar slants after incubation. The cell suspensions were inoculated into samples and adjusted so that the final cell numbers per milliliter were between 105 and 106. Samples were incubated at 22.5 ± 2.5°C, and the viable organism numbers of A. niger at 24 h, 14 days and 28 days and those of P. aeruginosa and S. aureus at 24 h were determined by the Pour-Plate method.
Results
Experiment 1
In eyes treated with 0.03% C12-BAK, C14-BAK and BAK mixture, macroscopic ocular signs such as redness of the palpebral conjunctiva, bulbar conjunctiva and nictitating membrane were observed (Table 2). In addition, conjunctiva discharge with the 0.03% BAK mixture and conjunctiva discharge and chemosis of the palpebral conjunctiva with 0.03% C14-BAK were noted. Therefore, the relative order of BAK homologs and BAK mixture with regard to ocular insult was C12-BAK < BAK mixture < C14-BAK. In eyes treated with 0.01% or less of C12-BAK, C14-BAK and BAK mixture, macroscopic ocular insults were not observed.
Table 2. Ocular Irritation Scores after 10 Applications of BAK Homologs and BAK Mixture.
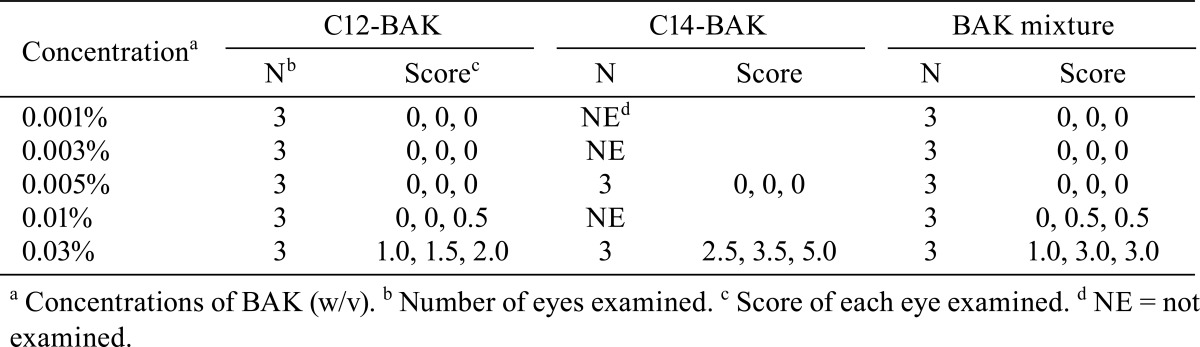
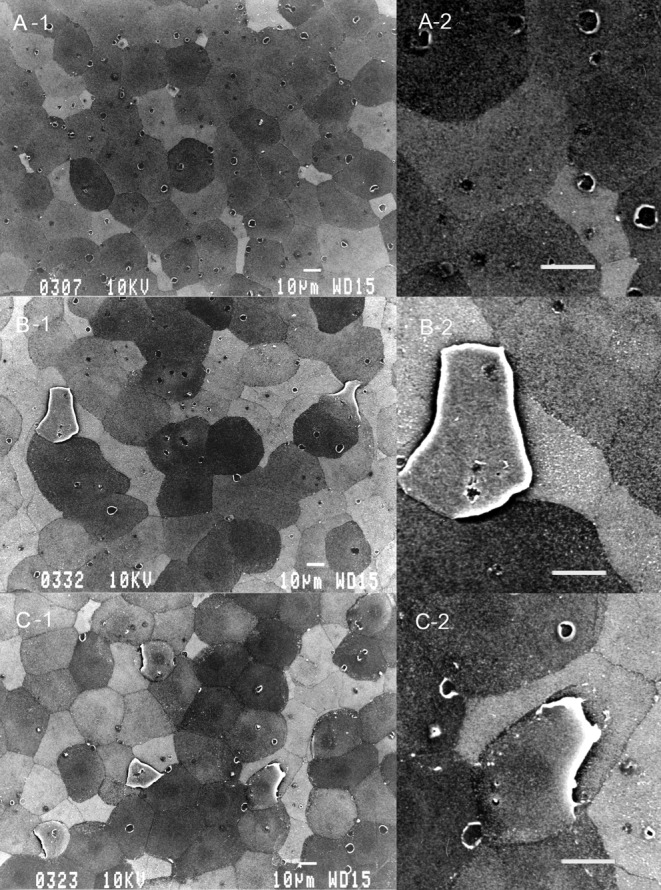
Table 3 summarizes SEM results of the corneal epithelium following 10 applications of the drugs. The corneal epithelium surface treated with 0.9% saline consisted of irregularly-shaped polygonal cells. Epithelial cells could be separated into light, medium and dark cells on the basis of their brightness. Light cells were smaller than dark or medium cells. There were a large number of microvilli and crater-like depressions, which are called epithelial holes23, on the surface of light, medium and dark cells. No damage was detected on corneas treated with concentrations of ≤ 0.005% C12-BAK or treated with the 0.001% BAK mixture. Application of 0.01% C12-BAK, 0.005% C14-BAK and 0.003%-0.01% BAK mixture caused exfoliation of a small number of cells (Fig. 1). Treatments with 0.03% C12-BAK, C14-BAK and BAK mixture resulted in significant cell exfoliation (Fig. 2), with decreases in epithelial holes and microvilli of corneal epithelial cells. Corneal epithelial changes produced by C12-BAK were weaker than with BAK mixture and C14-BAK. No abnormalities of the corneal endothelium were noted at any concentrations of C12-BAK, C14-BAK, and BAK mixture following 10 applications.
Table 3. SEM Analysis of the Cornea Epithelium after 10 Applications of BAK Homologs and BAK Mixture.
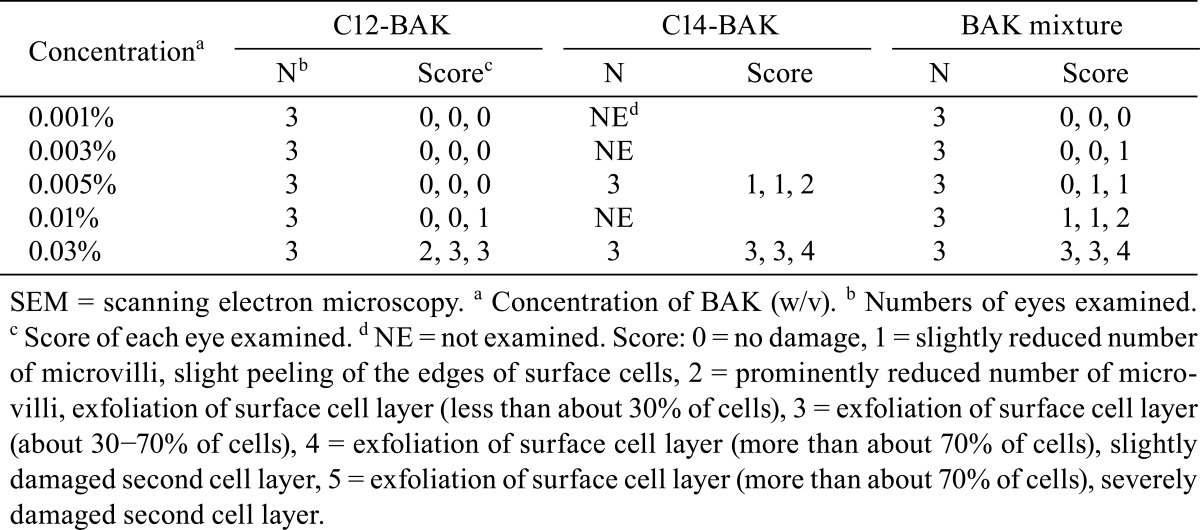
Fig. 1.
Scanning electron microscopy of the corneal epithelium after 10 applications of 0.005% BAK homologs and BAK mixture. C12-BAK (A-1) induced no damage. The BAK mixture (B-1) and C14-BAK (C-1) induced exfoliation of a small number of cells. Original magnification ×500. Photos A-2, B-2 and C-2 are high-magnification versions of photos A-1, B-1 and C-1, respectively. Bar = 10 μm.
Fig. 2.
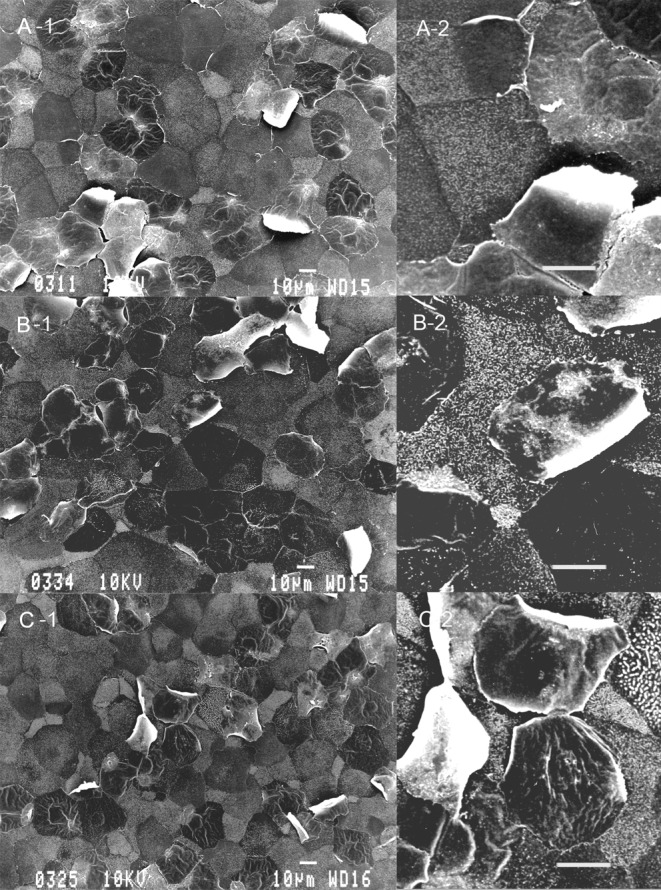
Scanning electron microscopy of the corneal epithelium after 10 applications of 0.03% BAK homologs and BAK mixture. C12-BAK (A-1), BAK mixture (B-1) and C14-BAK (C-1) caused exfoliation of a large number of cells. Microvilli and epithelial holes on the corneal surface are decreased. Original magnification ×500. Photos A-2, B-2 and C-2 are high-magnification versions of photos A-1, B-1 and C-1, respectively. Bar = 10 μm.
Experiment 2
During application of 0.01% C12-BAK for 39 weeks in rabbits, no BAK-related ocular irritation on the cornea, iris or conjunctiva or fluorescein staining on the cornea was found in ophthalmologic examinations. No histopathological lesions related to C12-BAK application were noted in the eyeballs, eyelids and lacrimal glands. There were no changes in the epithelium and endothelium of the corneas when observed by SEM examination.
Experiment 3
The antimicrobial activity of C14-BAK against A. niger at 24 h was slightly greater than C12-BAK in this formulation (Table 4). At 14 days and 28 days, the antimicrobial activity of C12-BAK was slightly greater than C14-BAK. In contrast, the antimicrobial activities of C12-BAK and C14-BAK against S. aureus and P. aeruginosa could not be detected at 24 h.
Table 4. Antimicrobial Activity of BAK Homologs.
Discussion
A recent large-scale European study that enrolled 9658 patients compared the prevalence of side effects between eye drops with or without preservatives. A significant decrease in all ocular symptoms and signs was observed in patients using lesser amounts of preserved eye drops or using preservative-free drops24. This study suggested that the use of formulations with a lower preservative concentration or less toxic preservatives may lessen the adverse events. In addition, in vitro and in vivo studies showed that corneal toxicity of BAK homologs was dependent on the alkyl chain length of the molecule19,20,21. The cytotoxicity of BAK homologs and BAK mixture has been compared by in vitro studies20,21. The corneal toxicity of BAK homologs has also been assessed by an in vivo study20. However, the corneal toxicity of BAK homologs has not been compared with that of a BAK mixture by an in vivo study. In the present study, we compared the corneal toxicities of BAK homologs and a BAK mixture by SEM after application to rabbits to determine whether the use of BAK homologs lessens the corneal toxicity compared with a BAK mixture. When treated with 0.9% saline, the corneal surface consisted of polygonal cells with epithelial holes and a large number of microvilli, in which light, medium and dark cells were distinguishable. This has also been described in the studies of normal surfaces of rabbit corneal epithelium23,25. Application of 0.01−0.03% C12-BAK, 0.005−0.03% C14-BAK and 0.003−0.03% BAK mixture caused cell exfoliation together with decreased epithelial holes and loss of microvilli. The decrease of epithelial holes from the corneal epithelium has also been observed on the cornea of rabbits receiving several surfactants and several local anesthetics (our unpublished data). Therefore, we suggest that this change may be a common result of corneal injury by toxicants and not specific to BAK. The present study shows that the ocular toxicity of BAK homologs and BAK mixture is ranked as C12-BAK < BAK mixture < C14-BAK by the ocular irritation scores and SEM analyses of the corneas. The results were as expected considering that the BAK mixture used in the present study was mainly composed of C12-BAK and C14-BAK. The nontoxic dose of 10 applications (every 30 minutes), which is excessive compared with 1−7 applications per day in clinical use of ophthalmic solutions, was 0.005% for C12-BAK and 0.001% for the BAK mixture. This indicates that the ocular toxicity of C12-BAK is five times weaker than than that of the BAK mixture. The results of the present study are consistent with a previous in vitro study using transepithelial electrical resistance (TER) of live rabbit corneas and a cytotoxicity assay20. In this in vitro study, the ocular toxicity was ranked C12-BAK < BAK mixture < C14-BAK. The in vitro study using the bovine corneas also showed that the toxicity of C12-BAK was weaker than than that of C14-BAK and that the corneal toxicity of the BAK mixture decreased when the relative proportion of C12-BAK increased19. It is known that the longer the hydrocarbon chain of BAK homologs become, the greater the BAK homologs have the tendency to initiate the increase in cell permeability that precedes cell death26. Therefore, it is important to adjust the composition of the BAK mixture in ophthalmic solutions to decrease the ocular toxicity. Furthermore, the use of C12-BAK instead of a BAK mixture as a preservative in ophthalmic solutions could be significantly safer for patients. In contrast, it was reported that the relative order of cytotoxicity was BAK mixture < C12-BAK < C14-BAK in HCE cells, and BAK mixture < C14-BAK < C12-BAK in IOBA-NHC cells21. The reasons for the differences in results among in vitro experiments using different alkyl chain lengths are unknown, but the differences may be due to differences in experimental conditions such as the test system, cell lines, duration of drug exposure and culture medium.
The repeated toxicity of C12-BAK by ocular application has not been previously reported. For this reason, in the present study we assessed the repeated toxicity of 0.01% C12-BAK after ocular application 7 times per day for 39 weeks because BAK is generally used at concentrations up to 0.01% in ophthalmic solutions and ophthalmic solutions are generally applied up to 7 times per day in clinical use. The results of this investigation demonstrated that 0.01% C12-BAK did not cause ophthalmological changes suggestive of irritation or corneal damage, histopathological changes in the eyeball and its accessory organs and changes in the corneas as demonstrated by SEM. Therefore, it is possible that C12-BAK is nontoxic after repeated ocular application up to at least a concentration of 0.01% under an application frequency generally used in clinical practice. In previous studies using in vitro methods, C12-BAK showed cytotoxicity at concentrations below 0.01%19,20,21. The most significant reason why the toxicity of C12-BAK in the present study was weaker than that observed from in vitro studies is the pharmacokinetics of the drug in the tear film following ocular application. The physiological tear turnover rate is about 16% per minute in humans27 and is increased due to stimulation resulting from drop instillation28. The instilled dose leaves the precorneal area within 2 minutes of application29. It was also shown that most of an applied eye drop is lost to drainage in the first 15 to 30 seconds after application in humans28. Therefore, the contact time of the BAK with the cornea is possibly shorter than that in in vitro studies19,20,21.
We investigated the antimicrobial activities of C12-BAK and C14-BAK against A. niger, S. aureus, and P. aeruginosa. Both C12-BAK and C14-BAK showed antimicrobial activities at a concentration of 0.003%, similar to that used in ophthalmic solutions. The concentration of 0.003% is also nontoxic for C12-BAK, as observed by examination after repeated applications.
In conclusion, the present study demonstrates that C12-BAK is less toxic than C14-BAK and a BAK mixture and that ocular toxicity of 0.01% C12-BAK was not noted after repeated ocular application. The use of C12-BAK instead of a BAK mixture as a preservative in ophthalmic solutions should be considered in order to reduce the incidence of the corneal epithelial cell injury induced clinically by BAK.
Acknowledgments
We would like to thank Ms. Kiyoko Yamashita, Mr. Takashi Yamanouchi, and Mr. Tadao Fujita for their technical assistance. We also would like to thank Mr. Yoshihiro Takahashi and the staff of SNBL DSR for all their help during experiment 2 (administration, ophthalmology, histopathology, SEM observation, etc.).
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives (by-nc-nd) License <http://creativecommons.org/licenses/by-nc-nd/3.0/>.
References
- 1.Ammar DA, Noecker RJ, and Kahook MY. Effects of benzalkonium chloride- and polyquad-preserved combination glaucoma medications on cultured human ocular surface cells. Adv Ther. 28: 501–510 2011. [DOI] [PubMed] [Google Scholar]
- 2.tiftein SP, Ahdoot M, Marcus E, and Asbell PA. Comparative toxicity of preservatives on immortalized corneal and conjunctival epithelial cells. J Ocul Pharmacol Ther. 25: 113–119 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li J, and Yan X. Toxicity of benzalkonium chloride on human corneal epithelial cells in vitro. Chin Ophthal Res. 26: 814–817 2008 [Google Scholar]
- 4.Tripathi BJ, Tripathi RC, and Kolli SP. Cytotoxicity of ophthalmic preservatives on human corneal epithelium. Lens Eye Toxic Res. 9: 361–375 1992. [PubMed] [Google Scholar]
- 5.Tripathi BJ, and Tripathi RC. Cytotoxic effects of benzalkonium chloride and chlorobutanol on human corneal epithelial cells in vitro. Lens Eye Toxic Res. 6: 395–403 1989. [PubMed] [Google Scholar]
- 6.Ayaki M, and Iwasawa A. Cell viability of four corneoconjunctival cell lines exposed to five preservatives and a surfactant used for infection control in eye drops. Biocontrol Sci. 16: 117–121 2011. [DOI] [PubMed] [Google Scholar]
- 7.Brasnu E, Brignole-Baudouin F, Riancho L, Guenoun JM, Warnet JM, and Baudouin C. In vitro effects of preservative-free tafluprost and preserved latanoprost, travoprost, and bimatoprost in a conjunctival epithelial cell line. Curr Eye Res. 33: 303–312 2008. [DOI] [PubMed] [Google Scholar]
- 8.Brasnu E, Brignole-Baudouin F, Riancho L, Warnet JM, and Baudouin C. Comparative study on the cytotoxic effects of benzalkonium chloride on the Wong-Kilbourne derivative of Chang conjunctival and IOBA-NHC cell lines. Mol Vis. 14: 394–402 2008. [PMC free article] [PubMed] [Google Scholar]
- 9.Debbasch C, Rat P, Warnet JM, De Saint Jean M, Baudouin C, and Pisella PJ. Evaluation of the toxicity of benzalkonium chloride on the ocular surface. J Toxicol Cut Ocular Toxicol. 19: 105–115 2000 [Google Scholar]
- 10.Debbasch C, De Saint Jean M, Pisella PJ, Rat P, Warnet JM, and Baudouin C. Influence of preservatives on conjunctival cells in vitro. J Toxicol Cut Ocular Toxicol. 19: 79–88 2000 [Google Scholar]
- 11.De Saint Jean M, Brignole F, Bringuier AF, Bauchet A, Feldmann G, and Baudouin C. Effects of benzalkonium chloride on growth and survival of Chang conjunctival cells. Invest Ophthalmol Vis Sci. 40: 619–630 1999. [PubMed] [Google Scholar]
- 12.Liang H, Pauly A, Riancho L, Baudouin C, and Brignole-Baudouin F. Toxicological evaluation of preservative-containing and preservative-free topical prostaglandin analogues on a three-dimensional- reconstituted corneal epithelium system. Br J Ophthalmol. 95: 869–875 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pauly A, Meloni M, Brignole-Baudouin F, Warnet JM, and Baudouin C. Multiple endpoint analysis of the 3D-reconstituted corneal epithelium after treatment with benzalkonium chloride: Early detection of toxic damage. Invest Ophthalmol Vis Sci. 50: 1644–1652 2009. [DOI] [PubMed] [Google Scholar]
- 14.Cha SH, Lee JS, Oum BS, and Kim CD. Corneal epithelial cellular dysfunction from benzalkonium chloride (BAC) in vitro. Clin Experiment Ophthalmol. 32: 180–184 2004. [DOI] [PubMed] [Google Scholar]
- 15.Becquet F, Goldschild M, Moldovan MS, Ettaiche M, Gastaud P, and Baudouin C. Histopathological effects of topical ophthalmic preservatives on rat corneoconjunctival surface. Curr Eye Res. 17: 419–425 1998. [DOI] [PubMed] [Google Scholar]
- 16.Pfister RR, and Burstein N. The effects of ophthalmic drugs, vehicles, and preservatives on corneal epithelium: a scanning electron microscope study. Invest Ophthalmol. 15: 246–259 1976. [PubMed] [Google Scholar]
- 17.Tønjum AM. Effects of benzalkonium chloride upon the corneal epithelium studied with scanning electron microscopy. Acta Ophthalmol (Copenh). 53: 358–366 1975. [DOI] [PubMed] [Google Scholar]
- 18.Burstein NL. Preservative cytotoxic threshold for benzalkonium chloride and chlorhexidine digluconate in cat and rabbit corneas. Invest Ophthalmol Vis Sci. 19: 308–313 1980. [PubMed] [Google Scholar]
- 19.Erhart M, Zilliox P, De Burlet GL, and Andermann G. Differences in ocular toxicity and antimicrobial activity of benzalkonium chlorides. Concepts Toxicol. 4: 145–151 1987 [Google Scholar]
- 20.Uematsu M, Kumagami T, Shimoda K, Kusano M, Teshima M, Sasaki H, and Kitaoka T. Influence of alkyl chain length of benzalkonium chloride on acute corneal epithelial toxicity. Cornea. 29: 1296–1301 2010. [DOI] [PubMed] [Google Scholar]
- 21.Pellinen P, Huhtala A, Tolonen A, Lokkila J, Mäenpää J, and Uusitalo H. The cytotoxic effects of preserved and preservative-free prostaglandin analogs on human corneal and conjunctival epithelium in vitro and the distribution of benzalkonium chloride homologs in ocular surface tissues in vivo. Curr Eye Res. 37: 145–154 2012. [DOI] [PubMed] [Google Scholar]
- 22.Draize JH, Woodard G, and Calvery HO. Methods for the study of irritation and toxicity of substances applied topically to the skin and mucous membranes. J Pharmacol Exp Ther. 82: 377–390 1944 [Google Scholar]
- 23.Pfister RR. The normal surface of corneal epithelium: a scanning electron microscopic study. Invest Ophthalmol. 12: 654–668 1973. [PubMed] [Google Scholar]
- 24.Jaenen N, Baudouin C, Pouliquen P, Manni G, Figueiredo A, and Zeyen T. Ocular symptoms and signs with preserved and preservative-free glaucoma medications. Eur J Ophthalmol. 17: 341–349 2007. [DOI] [PubMed] [Google Scholar]
- 25.Doughty MJ. Analyses of areas and shapes of cells on the corneal surface of the albino rabbit by scanning electron microscopy. Curr Eye Res. 9: 295–306 1990. [DOI] [PubMed] [Google Scholar]
- 26.Blois DW, and Swarbrick J. Interaction of quaternary ammonium bactericides with biological materials. I. Conductometric studies with cephalin. J Pharm Sci. 61: 390–392 1972. [DOI] [PubMed] [Google Scholar]
- 27.Mishima S, Gasset A, Klyce SD, and Baum JL. Determination of tear volume and tear flow. Invest Ophthalmol. 5: 264–276 1966. [PubMed] [Google Scholar]
- 28.Shell JW. Pharmacokinetics of topically applied ophthalmic drugs. Surv Ophthalmol. 26: 207–218 1982. [DOI] [PubMed] [Google Scholar]
- 29.Eaga CM, Kandukuri JM, Allenki V, and Yamsani MR. In-situ gels –a novel approach for ocular drug delivery. Der Pharmacia Lettre. 1: 21–33 2009 [Google Scholar]


