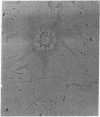
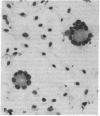
Abstract
We studied the effects of human T-lymphotropic virus type I (HTLV-I) on human endothelial cells in vitro. During cocultivation with an HTLV-I producer cell line (C91/PL), endothelial cells formed characteristic multinucleated syncytial giant cells. Inoculation with concentrated cell-free supernatant fluid from C91/PL cultures produced similar cytopathic effects, which were neutralized by pretreatment with HTLV-I specific human serum. HTLV-I antigens were detected in the cytoplasm of the multinucleated cells by indirect immunofluorescence. When endothelial cells showed maximal cytopathic changes, reverse transcriptase activity was demonstrated in the supernatant fluid and HTLV-I was isolated by cocultivation with peripheral blood mononuclear cells. This study demonstrates that HTLV-I tropism is not limited to lymphoid cells but extends to human endothelial cells as well.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashida E. R., Johnson A. R., Lipsky P. E. Human endothelial cell-lymphocyte interaction. Endothelial cells function as accessory cells necessary for mitogen-induced human T lymphocyte activation in vitro. J Clin Invest. 1981 May;67(5):1490–1499. doi: 10.1172/JCI110179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barré-Sinoussi F., Chermann J. C., Rey F., Nugeyre M. T., Chamaret S., Gruest J., Dauguet C., Axler-Blin C., Vézinet-Brun F., Rouzioux C. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science. 1983 May 20;220(4599):868–871. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- Blayney D. W., Blattner W. A., Robert-Guroff M., Jaffe E. S., Fisher R. I., Bunn P. A., Jr, Patton M. G., Rarick H. R., Gallo R. C. The human T-cell leukemia-lymphoma virus in the southeastern United States. JAMA. 1983 Aug 26;250(8):1048–1052. [PubMed] [Google Scholar]
- Bunn P. A., Jr, Schechter G. P., Jaffe E., Blayney D., Young R. C., Matthews M. J., Blattner W., Broder S., Robert-Guroff M., Gallo R. C. Clinical course of retrovirus-associated adult T-cell lymphoma in the United States. N Engl J Med. 1983 Aug 4;309(5):257–264. doi: 10.1056/NEJM198308043090501. [DOI] [PubMed] [Google Scholar]
- Catovsky D., Greaves M. F., Rose M., Galton D. A., Goolden A. W., McCluskey D. R., White J. M., Lampert I., Bourikas G., Ireland R. Adult T-cell lymphoma-leukaemia in Blacks from the West Indies. Lancet. 1982 Mar 20;1(8273):639–643. doi: 10.1016/s0140-6736(82)92200-0. [DOI] [PubMed] [Google Scholar]
- Chin Y. H., Carey G. D., Woodruff J. J. Lymphocyte recognition of lymph node high endothelium. V. Isolation of adhesion molecules from lysates of rat lymphocytes. J Immunol. 1983 Sep;131(3):1368–1374. [PubMed] [Google Scholar]
- Clapham P., Nagy K., Cheingsong-Popov R., Exley M., Weiss R. A. Productive infection and cell-free transmission of human T-cell leukemia virus in a nonlymphoid cell line. Science. 1983 Dec 9;222(4628):1125–1127. doi: 10.1126/science.6316502. [DOI] [PubMed] [Google Scholar]
- Diglio C. A., Ferrer J. F. Induction of syncytia by the bovine C-type leukemia virus. Cancer Res. 1976 Mar;36(3):1056–1067. [PubMed] [Google Scholar]
- Essex M., McLane M. F., Lee T. H., Falk L., Howe C. W., Mullins J. I., Cabradilla C., Francis D. P. Antibodies to cell membrane antigens associated with human T-cell leukemia virus in patients with AIDS. Science. 1983 May 20;220(4599):859–862. doi: 10.1126/science.6342136. [DOI] [PubMed] [Google Scholar]
- Flotte T. J., Hatcher V. A., Friedman-Kien A. E. Factor VIII-related antigen in Kaposi's sarcoma in young homosexual men. Arch Dermatol. 1984 Feb;120(2):180–182. [PubMed] [Google Scholar]
- Friedman H. M., Macarak E. J., MacGregor R. R., Wolfe J., Kefalides N. A. Virus infection of endothelial cells. J Infect Dis. 1981 Feb;143(2):266–273. doi: 10.1093/infdis/143.2.266. [DOI] [PubMed] [Google Scholar]
- Gallo R. C., Salahuddin S. Z., Popovic M., Shearer G. M., Kaplan M., Haynes B. F., Palker T. J., Redfield R., Oleske J., Safai B. Frequent detection and isolation of cytopathic retroviruses (HTLV-III) from patients with AIDS and at risk for AIDS. Science. 1984 May 4;224(4648):500–503. doi: 10.1126/science.6200936. [DOI] [PubMed] [Google Scholar]
- Gallo R. C., Sarin P. S., Gelmann E. P., Robert-Guroff M., Richardson E., Kalyanaraman V. S., Mann D., Sidhu G. D., Stahl R. E., Zolla-Pazner S. Isolation of human T-cell leukemia virus in acquired immune deficiency syndrome (AIDS). Science. 1983 May 20;220(4599):865–867. doi: 10.1126/science.6601823. [DOI] [PubMed] [Google Scholar]
- Gelmann E. P., Popovic M., Blayney D., Masur H., Sidhu G., Stahl R. E., Gallo R. C. Proviral DNA of a retrovirus, human T-cell leukemia virus, in two patients with AIDS. Science. 1983 May 20;220(4599):862–865. doi: 10.1126/science.6601822. [DOI] [PubMed] [Google Scholar]
- Gimbrone M. A., Jr, Cotran R. S., Folkman J. Human vascular endothelial cells in culture. Growth and DNA synthesis. J Cell Biol. 1974 Mar;60(3):673–684. doi: 10.1083/jcb.60.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb M. S., Groopman J. E., Weinstein W. M., Fahey J. L., Detels R. The acquired immunodeficiency syndrome. Ann Intern Med. 1983 Aug;99(2):208–220. doi: 10.7326/0003-4819-99-2-208. [DOI] [PubMed] [Google Scholar]
- Guarda L. G., Silva E. G., Ordóez N. G., Smith J. L., Jr Factor VIII in Kaposi's sarcoma. Am J Clin Pathol. 1981 Aug;76(2):197–200. doi: 10.1093/ajcp/76.2.197. [DOI] [PubMed] [Google Scholar]
- Hayami M., Tsujimoto H., Komuro A., Hinuma Y., Fujiwara K. Transmission of adult T-cell leukemia virus from lymphoid cells to non-lymphoid cells associated with cell membrane fusion. Gan. 1984 Feb;75(2):99–102. [PubMed] [Google Scholar]
- Hinuma Y., Nagata K., Hanaoka M., Nakai M., Matsumoto T., Kinoshita K. I., Shirakawa S., Miyoshi I. Adult T-cell leukemia: antigen in an ATL cell line and detection of antibodies to the antigen in human sera. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6476–6480. doi: 10.1073/pnas.78.10.6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunsmann G., Schneider J., Schmitt J., Yamamoto N. Detection of serum antibodies to adult T-cell leukemia virus in non-human primates and in people from Africa. Int J Cancer. 1983 Sep 15;32(3):329–332. doi: 10.1002/ijc.2910320311. [DOI] [PubMed] [Google Scholar]
- Kaplan J., Mastrangelo R., Peterson W. D., Jr Childhood lymphoblastic lymphoma, a cancer of thymus-derived lymphocytes. Cancer Res. 1974 Mar;34(3):521–525. [PubMed] [Google Scholar]
- Klement V., Rowe W. P., Hartley J. W., Pugh W. E. Mixed culture cytopathogenicity: a new test for growth of murine leukemia viruses in tissue culture. Proc Natl Acad Sci U S A. 1969 Jul;63(3):753–758. doi: 10.1073/pnas.63.3.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciag T., Hoover G. A., Stemerman M. B., Weinstein R. Serial propagation of human endothelial cells in vitro. J Cell Biol. 1981 Nov;91(2 Pt 1):420–426. doi: 10.1083/jcb.91.2.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy K., Clapham P., Cheingsong-Popov R., Weiss R. A. Human T-cell leukemia virus type I: induction of syncytia and inhibition by patients' sera. Int J Cancer. 1983 Sep 15;32(3):321–328. doi: 10.1002/ijc.2910320310. [DOI] [PubMed] [Google Scholar]
- Poiesz B. J., Ruscetti F. W., Gazdar A. F., Bunn P. A., Minna J. D., Gallo R. C. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovic M., Lange-Wantzin G., Sarin P. S., Mann D., Gallo R. C. Transformation of human umbilical cord blood T cells by human T-cell leukemia/lymphoma virus. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5402–5406. doi: 10.1073/pnas.80.17.5402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovic M., Sarin P. S., Robert-Gurroff M., Kalyanaraman V. S., Mann D., Minowada J., Gallo R. C. Isolation and transmission of human retrovirus (human t-cell leukemia virus). Science. 1983 Feb 18;219(4586):856–859. doi: 10.1126/science.6600519. [DOI] [PubMed] [Google Scholar]
- Popovic M., Sarngadharan M. G., Read E., Gallo R. C. Detection, isolation, and continuous production of cytopathic retroviruses (HTLV-III) from patients with AIDS and pre-AIDS. Science. 1984 May 4;224(4648):497–500. doi: 10.1126/science.6200935. [DOI] [PubMed] [Google Scholar]
- Rand K. H., Long C. W., Wei T. T., Gilden R. V. Plaque assay for the Mason-Pfizer monkey virus. J Natl Cancer Inst. 1974 Aug;53(2):449–452. doi: 10.1093/jnci/53.2.449. [DOI] [PubMed] [Google Scholar]
- Rangan S. R., Ueberhorst P. J., Wong M. C. Syncytial giant cell focus assay for viruses derived from feline leukemia and a simian sarcoma. Proc Soc Exp Biol Med. 1973 Apr;142(4):1077–1082. doi: 10.3181/00379727-142-37180. [DOI] [PubMed] [Google Scholar]
- Sarngadharan M. G., Popovic M., Bruch L., Schüpbach J., Gallo R. C. Antibodies reactive with human T-lymphotropic retroviruses (HTLV-III) in the serum of patients with AIDS. Science. 1984 May 4;224(4648):506–508. doi: 10.1126/science.6324345. [DOI] [PubMed] [Google Scholar]
- Schüpbach J., Popovic M., Gilden R. V., Gonda M. A., Sarngadharan M. G., Gallo R. C. Serological analysis of a subgroup of human T-lymphotropic retroviruses (HTLV-III) associated with AIDS. Science. 1984 May 4;224(4648):503–505. doi: 10.1126/science.6200937. [DOI] [PubMed] [Google Scholar]
- Stevens S. K., Weissman I. L., Butcher E. C. Differences in the migration of B and T lymphocytes: organ-selective localization in vivo and the role of lymphocyte-endothelial cell recognition. J Immunol. 1982 Feb;128(2):844–851. [PubMed] [Google Scholar]