Abstract
The antithyroid drugs (ATDs) methimazole (MMI) and propylthiouracil (PTU) have been used for treatment of hyperthyroidism for more than several decades, despite the fact that they are associated with adverse drug reactions that are thought to be autoimmune mediated. We therefore examined histopathologic responses in the immune system in male and female rats given MMI (2, 20 and 200 mg/kg/day, p.o., in experiment 1; 200 mg/kg/day, p.o., in experiment 3) or PTU (25 and 250 mg/kg/day, p.o., in experiment 2; 200 mg/kg/day, p.o., in experiment 3) for two weeks. In experiments 1 and 2, highest doses of MMI and PTU induced histopathologic changes in the spleen consistent with those in experiment 3 without any changes in the other peripheral lymphoid organs and tissues. In experiment 3, histopathological evaluation of the spleen along with hematological and bone marrow examinations were performed. In both male and female rats, MMI or PTU induced histopathological changes in the spleen characterized by development of germinal centers and an increase in the number of IgG-positive plasma cells in the red pulp; these changes were most prevalent in the MMI-treated female rats. Total red and white blood cell counts were decreased in the MMI-treated male and female rats; lymphocytes and monocytes were lower in male and female rats, respectively. Bone marrow nucleated cells were significantly lower in the MMI-treated males. This is the first study to demonstrate that ATDs induce spleen specific B-cell reactions in rats
Keywords: antithyroid drug, methimazole, propylthiouracil, spleen, germinal center, autoantibody
Introduction
Methimazole (MMI) and propylthiouracil (PTU) are antithyroid drugs (ATDs) that have been used to treat hyperthyroidism for more than several decades. However, ATDs are associated with potentially life-threatening adverse drug reactions (ADRs), including agranulocytosis, hepatotoxicity and vasculitis that is often associated with acute renal and respiratory symptoms; minor ADRs include skin reactions and arthralgia1. The incidence of agranulocytosis and vasculitis is rare; both are thought to be autoimmune mediated since anti-granulocyte autoantibodies, such as anti-neutrophil cytoplasmic antibodies (ANCAs), and myeloperoxidase (MPO), one of the major antigens of ANCA, are often detected in hyperthyroid patients taking ATDs1,2. Typically, drug-induced autoimmunity is idiosyncratic; because the incidence is very low and highly species specific, it is difficult to predict in preclinical studies3,4 and is therefore excluded from the scope of the ICH S8 guideline on immunotoxicity studies for human pharmaceuticals5.
Germinal centers are main sites in which high affinity antibody-secreting plasma cells and memory B cells are generated6. The development of germinal centers is therefore reflective of enhanced antibody responses to antigens draining the regional lymphoid tissues. To date, there are no reports that ATDs induce germinal center development in lymphoid tissues in rodent preclinical studies, although only a few compounds have been reported to cause such lesions7. Thus, the aim of this study was to examine the effect of ATDs on histopathology in peripheral lymphoid organs in rats. We also examined the effects of the drugs on hematologic parameters.
Materials and Methods
Test substances
MMI and PTU were purchased from Sigma-Aldrich Japan (Tokyo, Japan), dissolved in propylene glycol at the dosing volume of 5 mL/kg body weight, stored at 4°C in the dark and used within a week after preparation.
Animals
Male and female Crl:CD(SD) rats were purchased from Charles River Laboratories Japan Inc. (Kanagawa, Japan) at five weeks of age. The rats were housed individually in plastic cages (265 × 425 × 200 mm) embedded with ALPHA-dri (Shepherd Specialty Papers Inc., Kalamazoo, MI, USA), fed CE-2 solid chow (Oriental Yeast Co., Ltd., Chiba, Japan) and given tap water ad libitum. The animal room conditions were as follows: 1) temperature, 20–26°C; 2) relative humidity, 40–75%; 3) ventilation, 15 to 25 air changes per hour; and 4) lighting, 07:00 to 19:00. Serological, bacteriological and microscopic examinations of sentinel rats kept simultaneously in the same animal room for health monitoring revealed no signs of major viral, bacterial or parasitic infections.
Treatment
After one week of acclimatization, rats were divided into the vehicle- (propylene glycol; control) and ATD-treated groups (n=5/sex/group) for each of the following three experiments.
In experiments 1 and 2, MMI (2, 20 and 200 mg/kg/day) and PTU (25 and 250 mg/kg/day), respectively, were administered by gavage once daily for 14 days.
In experiment 3, according to the results of experiments 1 and 2, MMI and PTU were administered to rats of both sexes at the dose levels of 200 and 250 mg/kg/day, respectively, once daily for 14 days.
Clinical observation
Clinical observations were recorded daily, and body weights were measured twice a week during the dosing period.
Postmortem examination
One day after the final dosing, blood samples for hematology were collected under pentobarbital anesthesia from the jugular vein into EDTA-2K-coated vacutainers. The rats were then killed by exsanguination for organ weight measurement and histopathological/immunohistochemical examinations.
Hematology
The parameters determined were red (RBC) and white blood cell (WBC) counts, hemoglobin (HGB), hematocrit (HCT), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC) and platelet (PTL) count, which were determined using an automated hematology analyzer (K-4500, Sysmex Corp., Hyogo, Japan). Blood smears were prepared from the blood samples and stained with Wright-Giemsa stain; differential leukocyte counts were obtained for 100 leukocytes per rat under light microscopy.
Bone marrow examination
In experiment 3, femurs were taken at necropsy, and bone marrow cells were individually obtained by flushing the femurs with 2 mL of Minimal Essential Medium, Alpha Modification (Nacalai Tesque Inc., Kyoto, Japan), and bone marrow nucleated cell counts were measured using the previously mentioned hematology analyzer. Differential cell counts were thereafter determined by counting 500 cells per animal on bone marrow smears stained with Wright-Giemsa stain.
Organ weight measurement
In experiment 3, spleens, thymi and thyroid glands were excised and weighed.
Histopathology and immunohistochemistry of the spleen
In experiments 1 and 2, thymi, spleens, submaxillary lymph nodes and mesenteric lymph nodes were excised and fixed in 10% phosphate-buffered formalin for histopathological evaluations.
In experiment 3, excised spleens were fixed in 10% phosphate-buffered formalin for histopathological and immunohistochemical evaluations. The formalin-fixed spleens were embedded in paraffin, cut into thin sections, stained with hematoxylin and eosin (HE) and examined by light microscopy for histopathological examination including determination of the number of germinal centers per transverse section of the spleen.
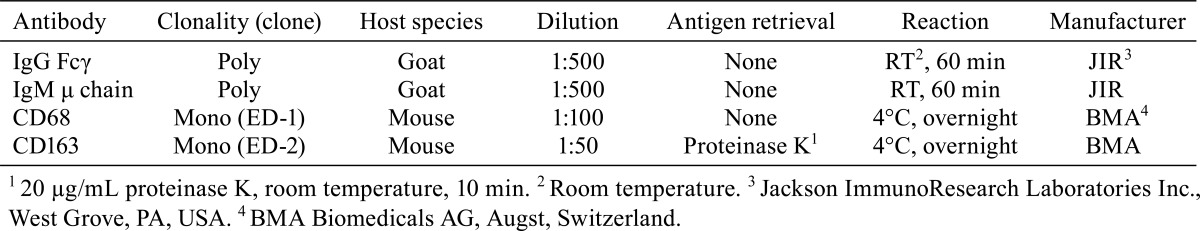
Immunohistochemistry of the spleen with antibodies against IgG Fcγ, IgM μ chain, CD68 (ED-1) and CD163 (ED-2) was also performed. The deparaffinized sections were incubated in methanol supplemented with 0.3% hydrogen peroxide for 30 min at room temperature to inactivate endogenous peroxidases. After washing with tris-buffered saline, the sections were incubated for 10 min with Blocking One Histo (Nacalai Tesque Inc.) to inhibit nonspecific protein binding. Subsequently, the sections were incubated with primary antibodies (see Table 1). Color development was then performed using commercially available kits, Histofine Simple Stain MAX PO (G) or (MULTI) (Nichirei Biosciences, Inc., Tokyo, Japan) and a Vector DAB Substrate Kit (Vector Laboratories Ltd, Peterborough, UK), and the sections were counterstained with Carrazzi’s hematoxylin.
Table 1. Primary Antibodies Used for Immunohistochemistry.
Statistics
For comparison between the control and each of the drug treatment groups, in experiment 1 and 2, Bartlett’s test was performed followed by the nonparametric Dunnett’s test when the difference was significant or the parametric Dunnett’s test when the difference was not significant. In experiment 3, the F-test was performed followed by the Aspin-Welch test when the difference was significant or the Student’s t-test when the difference was not significant. The level of significance was set at P<0.05.
Animal welfare
The experimental protocol was approved by the Animal Research Committee of ASKA Pharmaceutical Co., Ltd., prior to the study initiation. All studies were performed in compliance with the Rules for the Care and Use of Laboratory Animals at ASKA Pharmaceutical Co., Ltd., and the Basic Guidelines for Proper Conduct of Animal Testing and Related Activities in the Research Institutions under the Jurisdiction of the Ministry of Health, Labour and Welfare of Japan.
Results
Experiments 1 and 2
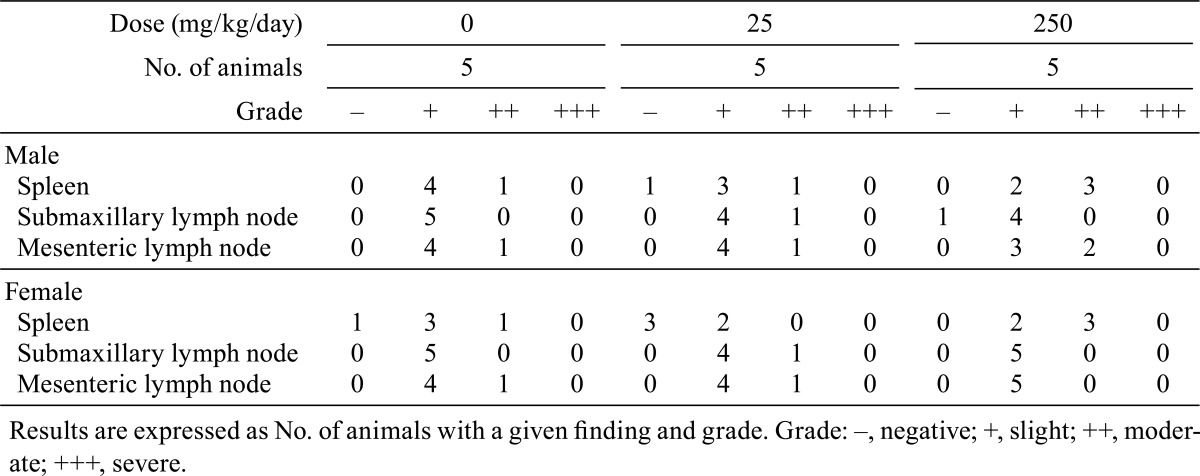
Rats given the highest doses of MMI and PTU showed the same changes in body weight gain and hematological parameters as those in experiment 3 (data not shown). Goiter was noted in the rats treated with MMI at a dose of 20 or 200 mg/kg/day and in the rats treated with either dose of PTU. Histopathological examination showed germinal center development in spleens from the rats treated with ATDs at their respective highest doses, and no histopathological changes, including germinal center development, were noted in other peripheral lymphoid organs and tissues in any animals (Tables 2 and 3).
Table 2. Germinal Center Development in the Peripheral Lymphoid Tissues in Rats Treated with Methimazole (Experiment 1).
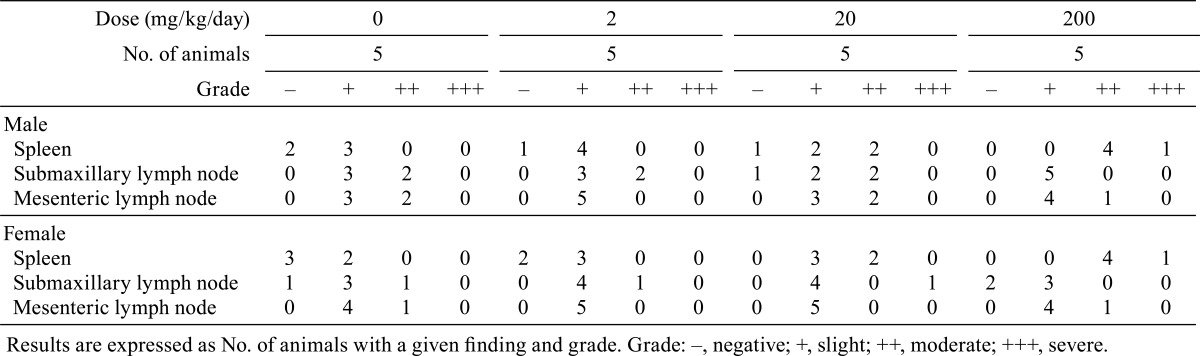
Table 3. Germinal Center Development in the Peripheral Lymphoid Tissues in Rats Treated with Propylthiouracil (Experiment 2).
Experiment 3
Clinical observations and body weights: There were no treatment-related changes in clinical observations or deaths in any group. Body weights in both MMI- and PTU-treated male rats were significantly lower than in controls due to the decreased body weight gains but not decreased body weights by themselves, and those of the ATD-treated females tended to be lower; however, these changes were not statistically significant (Fig. 1).
Fig. 1.
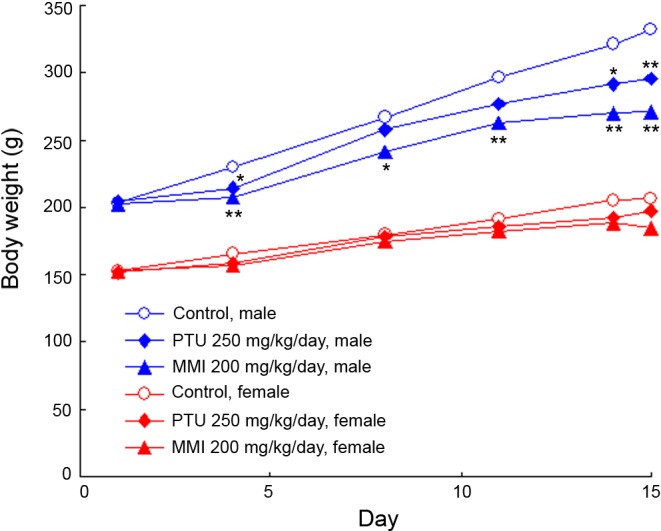
Changes in body weights in the rats treated with antithyroid drugs during the 14-day treatment period.
Organ weights
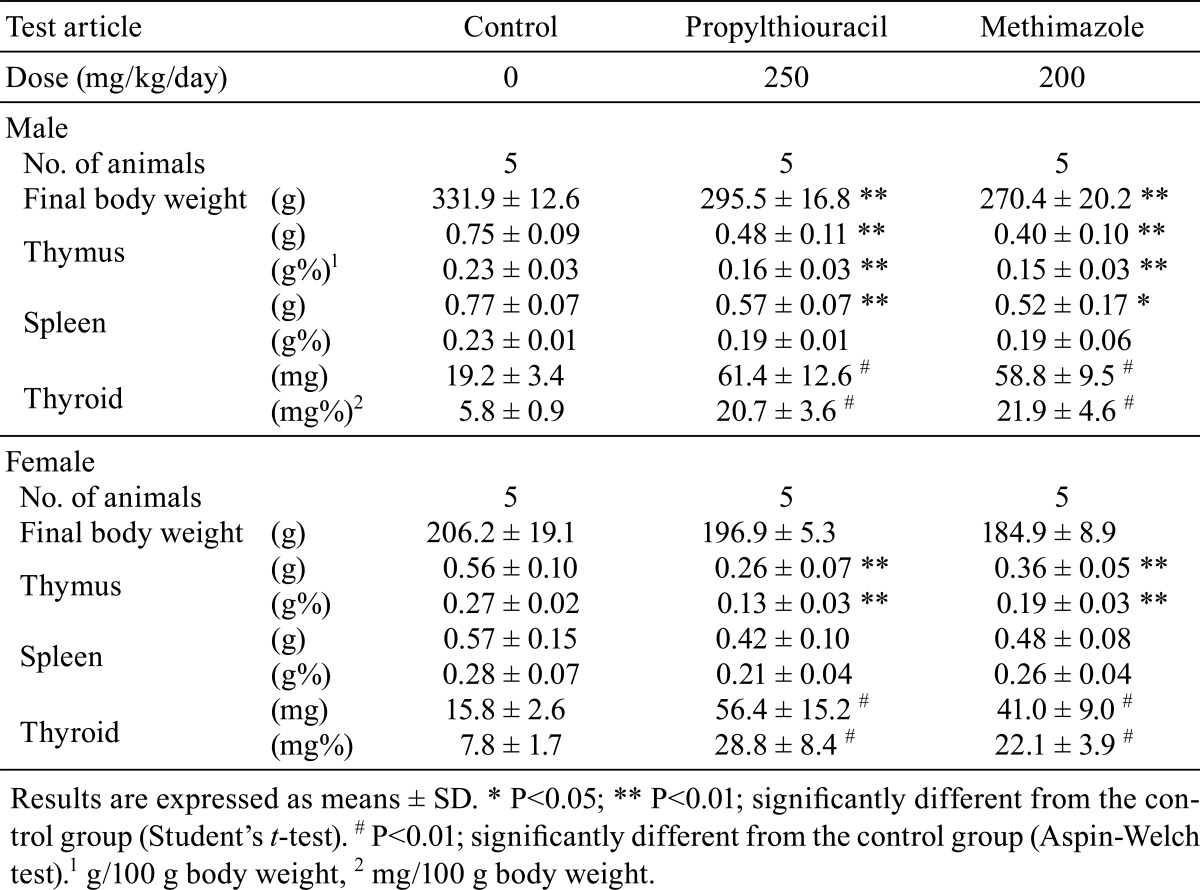
The results of organ weight measurement are shown in Table 4. Absolute and relative thymus weights were significantly decreased in both male and female rats treated with either of the ATDs; conversely, absolute and relative thyroid gland weights were significantly higher in all the ATD-treated rats. Absolute spleen weights were significantly lower in the ATD-treated males, and those of females were slightly lower but statistically nonsignificant.
Table 4. Final Body and Organ Weights in Rats Treated with Antithyroid Drugs.
Hematology
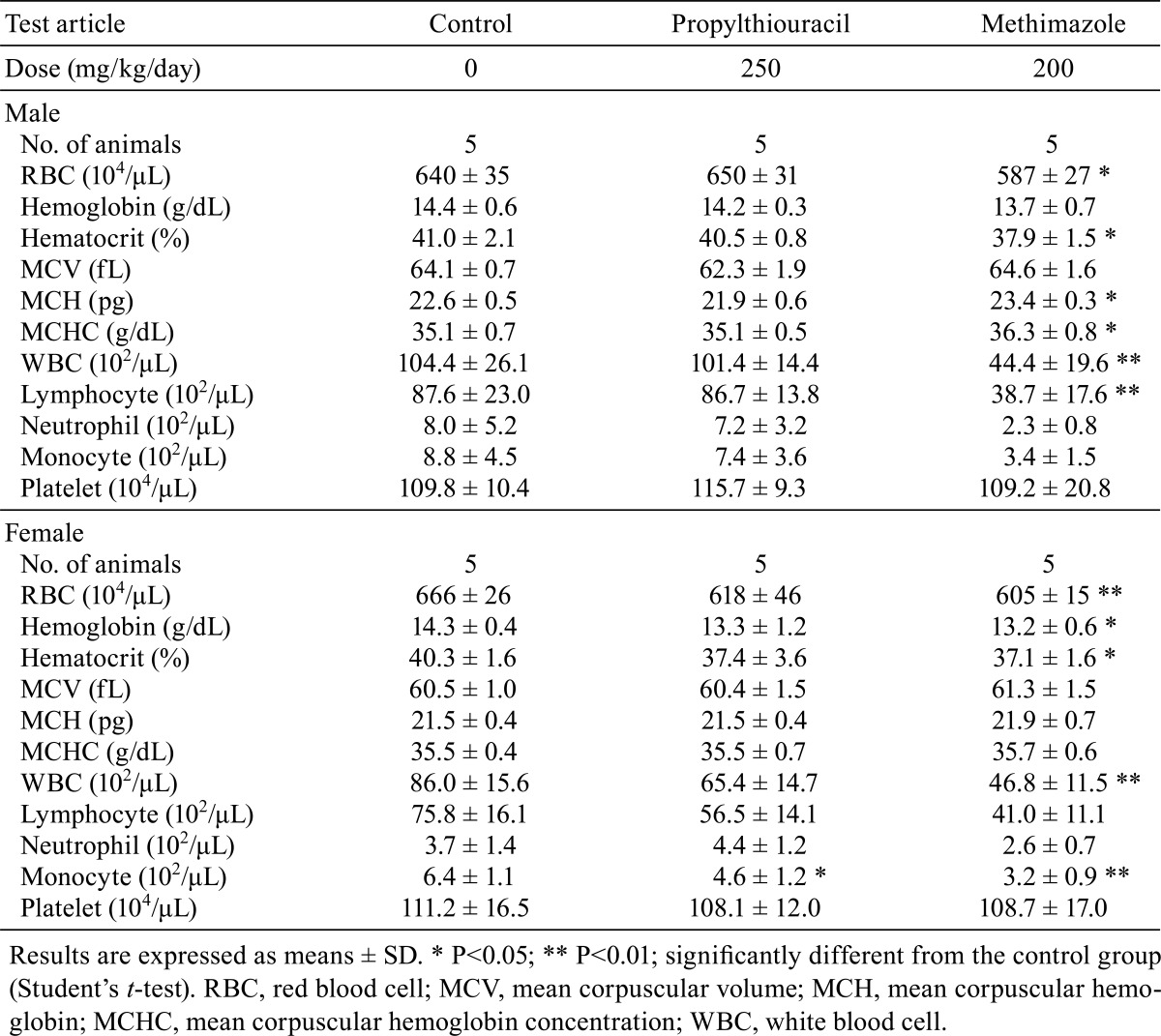
The results of the hematological examination are shown in Table 5. In the PTU-treated rats, only monocytes were significantly decreased in females; no changes were noted in males. In the MMI-treated rats, RBC and WBC counts and hematocrit were significantly lower in both males and females. Lymphocytes were decreased significantly in MMI-treated males and tended to decrease but were statistically nonsignificant in females. MCH and MCHC were increased in the MMI-treated males only; in females, hemoglobin and monocytes were decreased significantly.
Table 5. Hematological Findings in Rats Treated with Antithyroid Drugs.
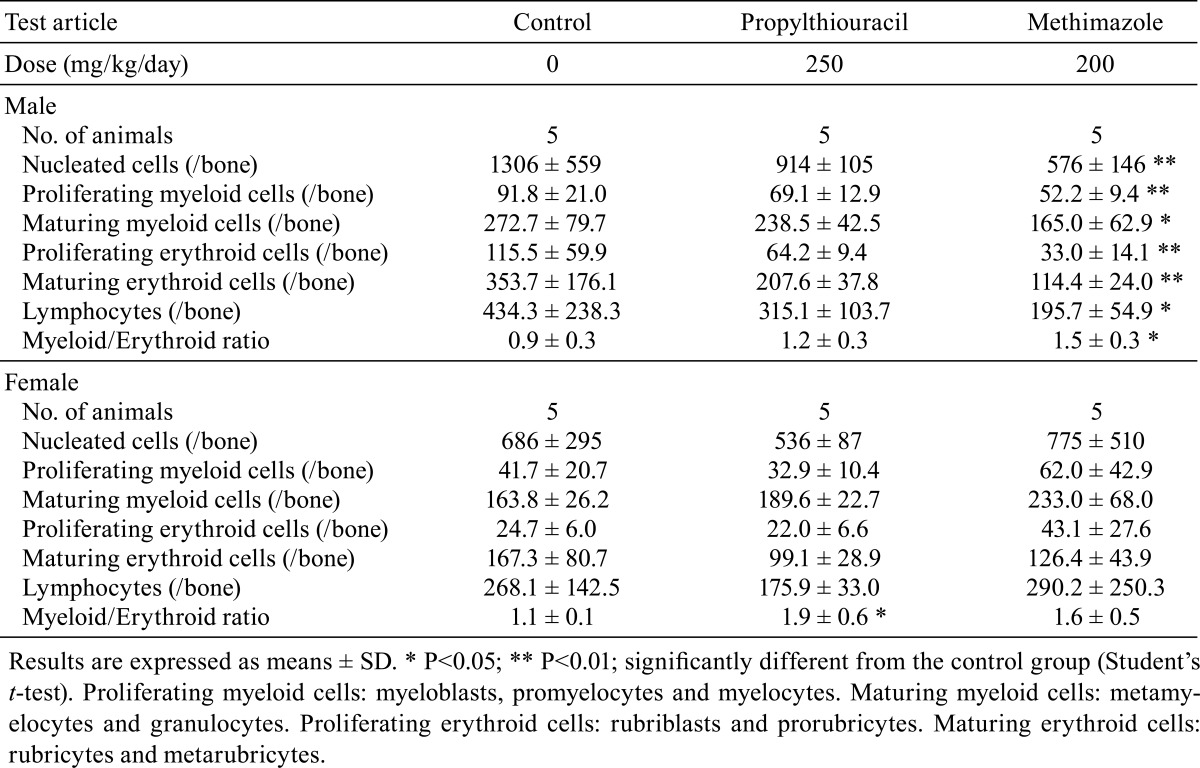
Bone marrow examination
The results of bone marrow examination are shown in Table 6. The myeloid/erythroid (M/E) ratios were significantly increased in the PTU-treated females and MMI-treated males. Furthermore, the number of nucleated cells along with maturing and proliferating populations of both myeloid and erythroid cells was significantly lower in the MMI-treated males. No drug-related changes were noted in PTU-treated males and MMI-treated females.
Table 6. Bone Marrow Findings in Rats Treated with Antithyroid Drugs.
Histopathology of the spleen
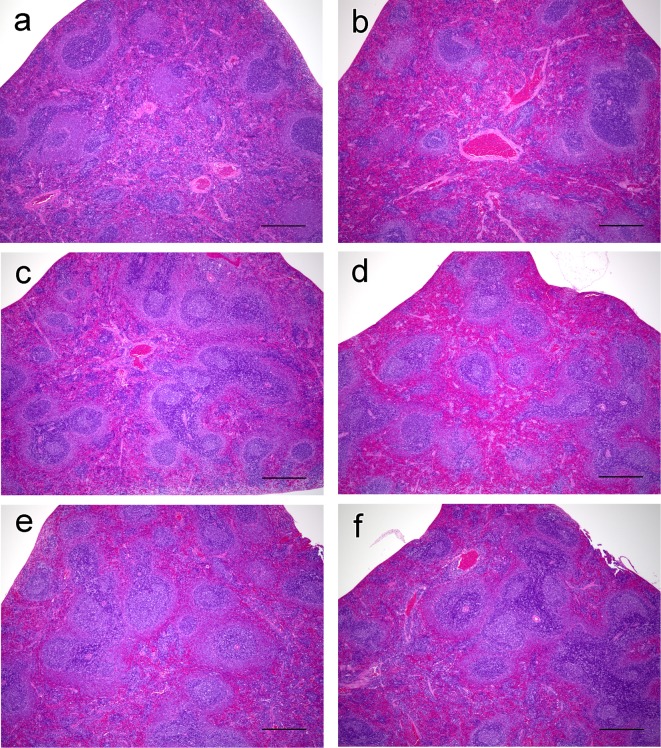
No or few germinal centers were noted in the spleens of control rats; however, development of germinal centers was observed in both the ATD-treated groups (Fig. 2). Increases in the numbers of germinal centers were observed in the male and female MMI-treated rats; increases in the PTU-treated rats did not reach statistical significance (Fig. 3). The cellularities of the periarterial lymphatic sheath and the marginal zone were not affected by treatment with either of the ATDs. Conversely, in the red pulp, the cellularities of lymphocytes and hematopoietic cells were slightly decreased in both the ATD-treated groups, consistent with the decreased splenic weights noted in these rats. In addition, in the MMI-treated group, mononuclear cells with eosinophilic cytoplasm resembling histiocytes were found to have accumulated in the perivascular and peritrabecular areas of the red pulp (Fig. 4).
Fig. 2.
Micrographs of spleens from rats treated with antithyroid drugs (ATDs) for 14 days. a, control male; b, control female; c, propylthiouracil (PTU)-treated male; d, PTU-treated male; e, methimazole (MMI)-treated male; and f, MMI-treated female. Development of germinal centers is noted in ATD-treated rats (c, d, e and f). HE stain. Bar: 500 µm.
Fig. 3.
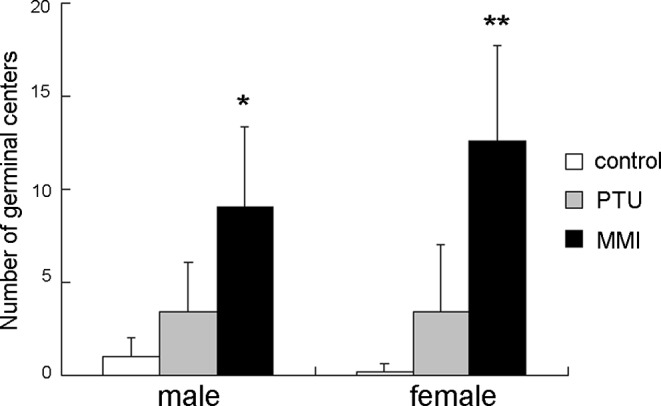
The number of germinal centers in the spleen of male and female rats treated with antithyroid drugs for 14 days. Results are expressed as means ± SD. PTU, propylthiouracil; MMI, methimazole. *P<0.05; **P<0.01; significantly different from the control group (Aspin-Welch test).
Fig. 4.
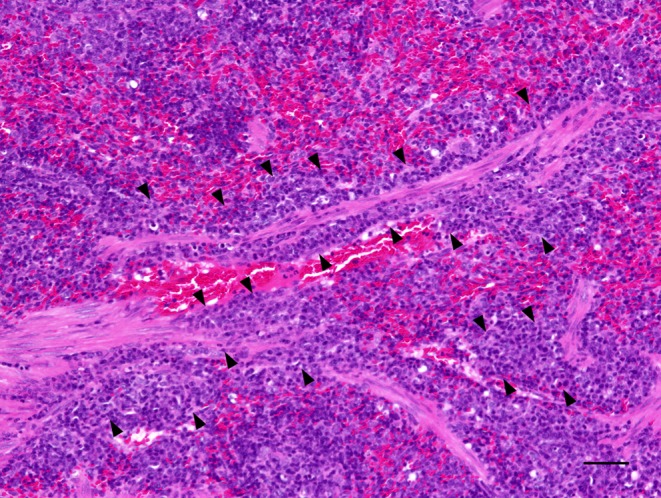
Micrograph of the spleen from a female rat treated with methimazole for 14 days. Accumulated histiocyte-resembling cells with eosinophilic cytoplasm are noted in the perivascular and peritrabecular areas. HE stain. Bar: 50 µm.
Immunohistochemistry of the spleen
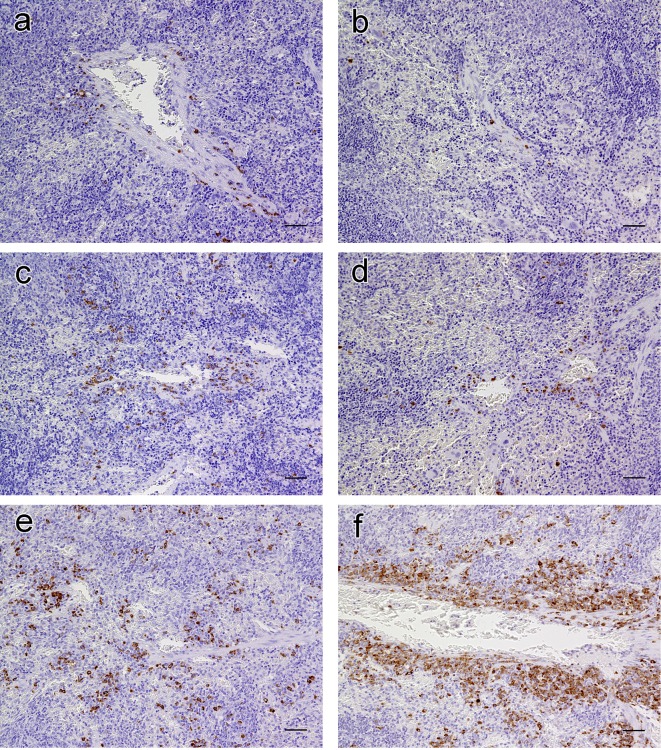
We used immunohistochemistry to characterize the increased cells resembling histiocytes in the perivascular and peritrabecular areas of the splenic red pulp in the MMI-treated females. IgG Fcγ-positive mononuclear cells, suggestive of IgG antibody-secreting plasma cells, were sparse in the control rats (Fig. 5a and 5b) and only slightly higher in number in the PTU-treated rats of both sexes (Fig. 5c and 5d). In the MMI-treated rats, however, increased numbers of IgG Fcγ-positive plasma cells had accumulated mainly in the perivascular and peritrabecular areas; this was more evident in spleens from females than in males (Fig. 5e and 5f). Conversely, IgM μ chain-positive lymphoid cells, judged as B cells, were diffusely present in the lymphoid follicles and marginal zones and were only scattered in the red pulp along with sporadic IgM-positive plasma cells (Fig. 6), consistent with results reported by Rehg et al.8. However, there were no inter-group differences in the distribution of IgM-positive cells (Fig. 6). Furthermore, CD68 (ED-1)- or CD163 (ED-2)-positive cells were scattered diffusively within the red pulp; no differences in their distribution in the splenic red pulp, including perivascular and peritrabecular areas, were found between the ATD-treated and control rats (data not shown). Therefore, the increased cells resembling histiocytes accumulated in the splenic red pulp observed in the MMI-treated rats were determined to be IgG-positive plasma cells.
Fig. 5.
Immunohistochemistry for IgG Fcγ in the spleens of rats treated with antithyroid drugs for 14 days. a, control male; b, control female; c, propylthiouracil (PTU)-treated male; d, PTU-treated female; e, methimazole (MMI)-treated male; and f, MMI-treated female. In the control group, cells stained positive for IgG Fcγ are extremely sparse in the red pulp (a and b). In the PTU-treated group, there is a slight increase in the number of positively stained cells compared with the control group (c and d). In the MMI-treated group, accumulation of positively stained cells can be seen in the perivascular and peritrabecular areas (e and f). Bar: 50 µm.
Fig. 6.
Immunohistochemistry for IgM μ chain in the spleens of rats treated with antithyroid drugs for 14 days. a, control male; b, control female; c, propylthiouracil (PTU)-treated male; d, PTU-treated female; e, methimazole (MMI)-treated male; and f, MMI-treated female. Positive staining is seen in follicles and in the marginal zone. In the red pulp, the distribution of positively stained cells is diffuse. There are no inter-group differences. Bar: 500 µm.
Discussion
We found that treatment of rats for 2 weeks with MMI or PTU at higher doses induced spleen-specific development of germinal centers and increase in the number of IgG-positive plasma cells mainly in the perivascular and peritrabecular areas of the splenic red pulp. These findings were more evident in the MMI-treated animals and more so in females than in males.
Antigenic stimulation such as vaccination induces germinal centers to develop in the regional lymphoid tissues drained by the primed antigens, i.e., the mesenteric lymph nodes and the spleen react to antigens drained from the gastrointestinal tract and blood, respectively9,10. Therefore, spleen-specific development of germinal centers in the ATD-treated animals might reflect antibody responses against blood-borne antigens. These antigens are most likely self-proteins and not pathogens, given that microbiological examination of sentinel animals and histopathology revealed no evidence of infection. After antigen stimulation, proliferating antigen-specific IgM-B cells form germinal centers, undergo immunoglobulin class switching from IgM to IgG or IgA and exhibit an increase in affinity to specific antigens due to mutations of immunoglobulin genes and clonal selection. Thereafter, activated B cells enter the peripheral circulation and differentiate into plasma cells or memory B cells6,11,12.
Certain ADRs associated with ATDs, such as agranulocytosis and vasculitis, rarely occur; however, autoantibodies such as ANCAs are frequently detected in ATD-treated patients, suggesting that autoantibodies may lead to these ADRs1,2. The formation of reactive metabolites following the interaction of ATDs might result from reaction with their primary target enzyme, thyroid peroxidase, and reaction with MPO is thought to be a trigger for ATD-induced autoimmunity1.
Considering these facts together, the spleen-specific germinal center development in conjunction with the increase in the number of IgG-positive plasma cells in the splenic red pulp observed in the ATD-treated rats in the present study suggests the induction of T-cell-dependent IgG antibody production in response to some blood-borne self-proteins; however, the antigenic proteins remain unclear at present. Because ANCAs are frequently detected in serum from ATD-treated patients, it stands to reason that cytoplasmic proteins from blood cells, especially neutrophils, modified by reactive metabolites from thiourea derivatives of MMI and PTU via MPO may act as modified self-antigens in the ATD-treated rats as well as in humans1,13,14.
ATDs are also reported to have immunosuppressive effects. Despite inducing a transient increase in the number of circulating CD8-positive suppressor/cytotoxic T cells, ATDs reduce the number of CD4-positive helper T cells, CD19-positive B cells and natural killer cells1,15. The immunosuppressive effects of the ATDs might contribute at least in part to decreases in the levels of autoantibodies to thyroid stimulating hormone receptors in patients with Graves’ disease1. Correspondingly, in the present study, the ATD-treated rats displayed evidence of immunosuppression, including thymic atrophy and decreased cellularity in the splenic red pulp (MMI- and PTU-treated rats) and leukocytopenia and lymphopenia (MMI-treated rats). These changes were also noted in the rats with the doses of ATDs that did not affect body weights in experiments 1 and 2, and no significant findings were observed in the adrenal glands including organ weights and histopathology in the ATD-treated rats (data not shown); therefore, these changes in immune organs were attributed to primary effects related to treatment with the ATDs.
Development of germinal centers with an increased number of IgG-positive plasma cells in the splenic red pulp was more evident in the MMI-treated rats than in the PTU-treated rats, and in MMI-treated rats, these splenic findings were more evident in females than males. In the bone marrow examination, myelotoxicity with decreased nucleated cells and all populations of erythroid and myeloid cells was found in the MMI-treated rats with a sex difference of more evident changes in males than in females. Hematological examination revealed leukopenia and erythropenia in the MMI-treated males and females, but no clear sex differences were noted. However, decreases in peripheral blood lymphocytes and splenic weights accompanying decreased lymphocytic cells in the red pulp were slightly more evident in MMI-treated males compared with females. Considering these findings together, MMI might induce minor B cell responses in the spleen in males compared with females attributable to decreases in the numbers of peripheral lymphocytes in males. Additionally, in general, females are more susceptible to autoimmune diseases in humans and animal models with spontaneous or induced autoimmunity compared with males, and estrogens are prone to induce Th2-type cytokine production to enhance humoral immunity16,17. Considering these facts, another possible factor concerning the sex difference in the splenic B cell reaction in MMI-treated rats might be ascribable to sex differences in immune responses.
MPO-ANCAs have been detected more frequently in patients taking PTU compared with MMI2; conversely, our findings suggesting enhanced antibody production against blood-borne self-proteins was more evident in the MMI-treated rats. The nature of the species differences in autoantibody production is unclear because it is unknown which proteins serve as antigens in the MMI- and PTU-treated rats; thus, it is difficult to directly compare findings from humans with those from rats.
To date, few studies have addressed the induction of splenic lesions in ATD-treated rats. Cano-Europa et al.18 found that the white pulp was smaller in MMI-treated male rats (60 mg/kg/day, 4 weeks); Kariya et al.19 noted congestion of the red pulp in PTU-treated male rats (255 mg/kg, 4 weeks). However, neither of these studies reported findings related to enhanced antibody responses, including germinal center development. Differences between previous studies and this study may have been caused by doses or sex.
In conclusion, this is the first study to demonstrate that ATDs induce spleen-specific B-cell reactions in rats.
References
- 1.Cooper DS. Antithyroid drugs. N Engl J Med. 352: 905–917 2005. [DOI] [PubMed] [Google Scholar]
- 2.Arimura Y. Drug-induced ANCA-associated vasculitis. Saishin Igaku. 68: 252–258 2013 [Google Scholar]
- 3.Uetrecht J. Immune-mediated adverse drug reactions. Chem Res Toxicol. 22: 24–34 2009. [DOI] [PubMed] [Google Scholar]
- 4.Putman E, Van Loveren H, Bode G, Dean J, Hastings K, Nakamura K, Verdier F, and Van Der Laan J-W. Assessment of the immunotoxic potential of human pharmaceuticals: A workshop report. Drug Inform J. 36: 417–427 2002 [Google Scholar]
- 5.ICH. Immunotoxicity Studies for Human Pharmaceuticals ICH S8 Guideline Step 5. [Google Scholar]
- 6.Allen CD, Okada T, and Cyster JG. Germinal center organization and cellular dynamics. Immunity. 27: 190–202 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuper CF, de Heer E, Van Loveren H, and Vos JG. Immune system. In: Handbook of Toxicologic Pathology, 2nd ed. WM Hascheck, CG Rousseaux, and MA Walling (eds). Academic Press, San Diego. 585–646. 2002 [Google Scholar]
- 8.Rehg JE, Bush D, and Ward JM. The utility of immunohistochemistry for the identification of hematopoietic and lymphoid cells in normal tissues and interpretation of proliferative and inflammatory lesions of mice and rats. Toxicol Pathol. 40: 345–374 2012. [DOI] [PubMed] [Google Scholar]
- 9.Elmore SA. Histopathology of the lymph nodes. Toxicol Pathol. 34: 425–454 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cesta MF. Normal structure, function, and histology of the spleen. Toxicol Pathol. 34: 455–465 2006. [DOI] [PubMed] [Google Scholar]
- 11.Elmore SA. Enhanced histopathology of the spleen. Toxicol Pathol. 34: 648–655 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mebius RE, and Kraal G. Structure and function of the spleen. Nat Rev Immunol. 5: 606–616 2005. [DOI] [PubMed] [Google Scholar]
- 13.Uetrecht JP. The role of leukocyte-generated reactive metabolites in the pathogenesis of idiosyncratic drug reactions. Drug Metab Rev. 24: 299–366 1992. [DOI] [PubMed] [Google Scholar]
- 14.Wolford A, McDonald TS, Eng H, Hansel S, Chen Y, Bauman J, Sharma R, and Kalgutkar AS. Immune-mediated agranulocytosis caused by the cocaine adulterant levamisole: a case for reactive metabolite(s) involvement. Drug Metab Dispos. 40: 1067–1075 2012. [DOI] [PubMed] [Google Scholar]
- 15.Tötterman TH, Karlsson FA, Bengtsson M, and Mendel-Hartvig I. Induction of circulating activated suppressor-like T cells by methimazole therapy for Graves’ disease. N Engl J Med. 316: 15–22 1987. [DOI] [PubMed] [Google Scholar]
- 16.Whitacre CC. Sex differences in autoimmune disease. Nat Immunol. 2: 777–780 2001. [DOI] [PubMed] [Google Scholar]
- 17.Lee TP, and Chiang BL. Sex differences in spontaneous versus induced animal models of autoimmunity. Autoimmun Rev. 11: A422–A429 2012. [DOI] [PubMed] [Google Scholar]
- 18.Cano-Europa E, Blas-Valdivia V, Franco-Colin M, Gallardo-Casas CA, and Ortiz-Butrón R. Methimazole-induced hypothyroidism causes cellular damage in the spleen, heart, liver, lung and kidney. Acta Histochem. 113: 1–5 2011. [DOI] [PubMed] [Google Scholar]
- 19.Kariya K, Dawson E, and Neal RA. Toxic effects of propylthiouracil in the rat. Res Commun Chem Pathol Pharmacol. 40: 333–336 1983. [PubMed] [Google Scholar]