Abstract
Information about potential risks of iron nanomaterials is still limited, while a wide variety of applications are expected. We recently reported acute phase responses of male and female Fischer 344 rats after a single intratracheal spray instillation of Fe3O4 nanoparticles (magnetite), clearly showing dose-dependent pulmonary inflammatory changes (Tada et al., J Toxicol Pathol 25, 233–239, 2012). The present study assessed long-term responses of male and female Fischer 344 rats to multiple administrations of magnetite. Ten-week-old male and female Fischer 344 rats (n=20/group) were exposed to a total of 13 quadweekly intermittent intratracheal spray instillations of magnetite during the experimental period of 52 weeks, at doses of 0, 0.2 (low), 1.0 (medium) and 5.0 (high-dose) mg/kg body weight per administration. Absolute and relative lung weights of the high-dose group were significantly higher than those of the control group. Macroscopically, slight enlargement and scattered black patches were recognized in the lungs and the lung-associated lymph nodes of the high-dose group. Histopathologically, infiltration of macrophages phagocytosing magnetite (all dose groups) and of chronic inflammatory cells (medium- and high-dose males and high-dose females), alveolar bronchiolization and granuloma (high-dose group) were observed. In addition, alveolar hyperplasias were observed in some rats of the high-dose group, and cytoplasmic overexpression of β-catenin protein was immunohistochemically found in such lesions. The present results clearly show that instilled magnetite causes chronic inflammatory responses in the lung. These responses occur in a dose-dependent manner without apparent differences among sexes
Keywords: magnetite, Fe3O4, nanoparticles, lung, intratracheal spray instillation, Fischer 344 rat
Introduction
Engineered nanoparticles can have unique photonic and catalytic properties that display great differences from those of over-nanoscaled materials with the same composition. The superb biological and environmental reactivities of nanoparticles have led to their wide and considerable use in disease treatment, pollutant degradation and so forth1. Iron nanomaterials are a typical example and are attracting attentions due to their superparamagnetic characteristics and high catalytic abilities. It is expected that iron nanomaterials can be applied to a wide variety of fields such as environmental catalysis, magnetic storage, biomedical imaging1, magnetic target drug delivery2 and hyperthermia3,4.
While information about potential risks of magnetite is still limited, there are several reports describing some toxicologic findings for magnetite5,6,7,8,9,10,11,12,13. In in vitro studies using L-929 murine fibroblastic cells, iron oxide nanoparticles affected cell viability and DNA stability5, while in human alveolar epithelial-like type-II cells (A549), a micronucleus test and comet assay showed genotoxicity of magnetite6. In vivo studies with Fe3O4 particles caused inflammatory changes in rats exposed to pigment-sized particles through inhalation7,8. Magnetic nanoparticles of Fe3O4 affected the immune system of ICR mice, enhancing production of cytokines like interleukin (IL)-2, interferon-γ and IL-10 (but not IL-4) in the peripheral blood9. A high amount of magnetite, 15 mg × 15 intratracheal instillations, unexpectedly resulted in development of lung tumors in female Wistar rats10, while chronic exposure to iron oxide did not increase the incidence of pulmonary tumors of rats7,11. Recently, we reported acute phase responses of male and female Fischer 344 rats 14 days after a single intratracheal spray instillation of magnetite (Fe3O4) nanoparticles, clearly showing that magnetite caused foreign body inflammatory and granulating lesions in the lung in a dose-dependent manner12. Our histopathological findings showed that magnetite-laden macrophages were seen not only in the alveolar space but also in the alveolar septa or interstitial space, even in the low-dose group given 5.0 mg/kg body weight, indicating the possibility of severe injury resulting from intratracheal administration of magnetite12.
Iron is a transition metal that is considered to play a pivotal role in modulating oxidative stress and other biological responses13,14, which are speculated to be the critical mechanisms in eliciting the adverse effects of iron particulate matter exposure. The Organization for Economic Cooperation and Development (OECD) has generated a list of 14 commercially important nanoparticulate materials, in order to explore the possible health effects and regulate occupational and nonoccupational exposure scenarios, and iron oxide is included in the list15. In this context, it is apparently important to accumulate data regarding the toxicity/safety of magnetite, especially for its chronic influence on the living organisms. The present study was thus conducted to assess pulmonary responses to long-term intermittent intratracheal spray instillations of Fe3O4 nanoparticles (magnetite) in male and female Fischer 344 rats.
Materials and Methods
Ethical considerations
The current study was performed principally in conformity with the Guidelines for the Toxicity Testing of Pharmaceuticals released by the Ministry of Health, Labour and Welfare of Japan (http://www.pmda.go.jp/ich/s/s4_93_8_10.pdf; http://www.pmda.go.jp/ich/s/s4a_99_4_5.pdf). The experimental protocol was approved by the Experiments Regulation Committee and Animal Experiment Committee of the Tokyo Metropolitan Institute of Public Health, prior to its execution. All the animals were handled in accordance with the Japanese Government Animal Protection and Management Law, Japanese Government Notification on Feeding and Safekeeping of Animals and the Guidelines for Animal Experimentation issued by the Japanese Association for Laboratory Animal Science16.
Animals
A total of 168 male and female specific pathogen-free Fischer 344 (F344/DuCrlCrlj) rats were purchased at 8 weeks of age from Charles River Laboratories Japan, Inc. (Kanagawa, Japan). The rats were housed individually in stainless steel cages and were kept under controlled conditions of temperature (22–24°C), relative humidity (50–60%) and ventilation (more than 10 times/hour) with a 12-hr light/dark cycle. They were allowed free access to pelleted chow CE-2 (CLEA Japan, Inc., Tokyo, Japan) and drinking water throughout both the acclimation and experimental periods. After confirming normal health status at the end of the 2-week acclimation period, 80 rats of each sex were selected for use and randomly allocated to 4 groups of 20 rats. The rats were observed twice daily, and clinical signs and mortality were recorded. Body weight and food intakes were monitored weekly during the first 13 weeks, and were monitored every four weeks thereafter.
Test chemical and animal treatments
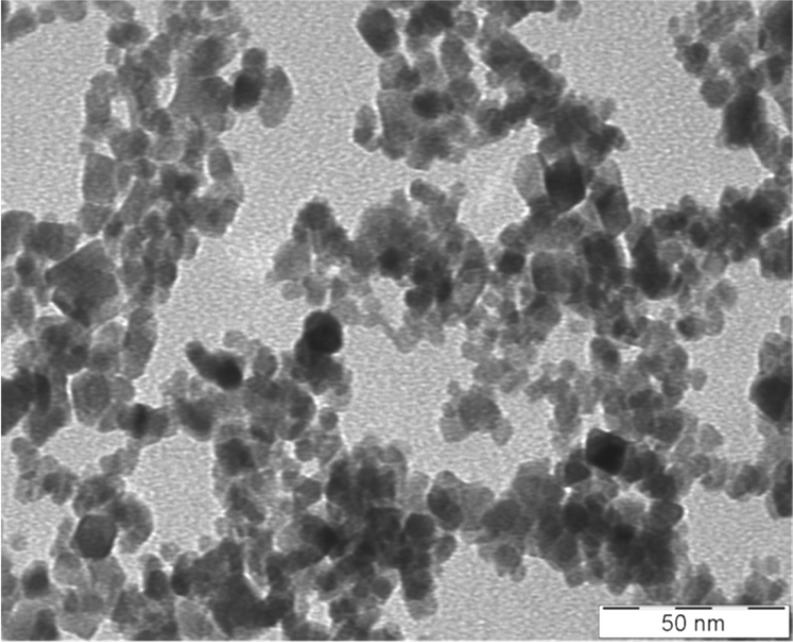
The magnetite slurry (Fe3O4 nanoparticle suspension; lot numbers, 90828 and 100316) was generously supplied by Toda Kogyo Corp. (Hiroshima, Japan). A representative transmission electron microscopic view of magnetite particles is shown in Fig. 1, and the primary particle size of the prepared sample was estimated to be 5–15 nm in diameter. The specific surface areas were determined to be 116.0 and 122.0 m2/g for lot numbers 90828 and 100316, respectively, by the BET method. The purity of the test chemical was determined by an energy dispersive X-ray spectrometer, and only iron and oxygen were detected. Magnetite particles are poorly soluble in water. According to our previous report12, magnetite slurry was diluted in sterile ultrapure water (vehicle: Milli-Q water, 18.2 MΩ) and adjusted to pH 7.4 with 0.1 M hydrochloric acid.
Fig. 1.
Representative transmission electron microscopic view of magnetite nanoparticles. The estimated primary particle size is about 5–15 nm in diameter.
The intratracheal instillation technique was performed according to the recommendations of Driscoll et al.17. Before intratracheal spray instillation, the rats were anesthetized with diethyl ether and placed in a supine position on an angled board with their necks extended. Magnetite suspension was placed in an ultrasonication bath (Sonorex RK31, Bandelin Electronic, Berlin, Germany) set at a high frequency (35 kHz) and then inserted via the mouth into the bifurcation of trachea by a sterile stainless steel tube (IA-1B MicroSprayer, Penn-Century, Inc., Wyndmoor, PA, USA) at the concentrations of 0 (control: vehicle, 1.0 mL/kg body weight), 0.2 (low), 1.0 (medium) and 5.0 (high) mg/kg body weight, and this was followed by insufflation of 0.2 mL of air. The doses were decided based on the finding of our acute single-dose toxicity study12 showing that 15 mg/kg body weight of magnetite cannot be well dispersed in the lung and thus aggregated and filled in the alveoli. The rats were given a total of 13 quadweekly intermittent exposures during the experimental period of 52 weeks.
Animal sacrifice and assessments
Four weeks after the last instillation, all rats were deprived of food (but not water) overnight, and fresh urine samples were obtained for urinalysis of urobilinogen, occult blood, bilirubin, ketone, glucose, protein and pH using test papers (N-Multistix, Siemens Healthcare Diagnostics Inc., Tokyo, Japan). The rats were then lightly anesthetized by diethyl ether and sacrificed by exsanguination after collecting blood samples via the abdominal aorta. The blood for hematology was collected into tubes treated with dipotassium ethylenediaminetetraacetate. The hematological examination was carried out using an automatic analyzer (Sysmex KX-21NV, Sysmex Corporation, Hyogo, Japan) to determine the red blood cell count (RBC), hemoglobin concentration (HGB), hematocrit level (HCT), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), white blood cell count (WBC) and platelet count (PLT). A serum biochemistry analysis was performed with an automatic analyzer (TBA-120FR, Toshiba Medical Systems Corporation, Tokyo, Japan) to determine the levels of total protein (TP), albumin (ALB), albumin/globulin ratio (A/G), glucose (GLU), total cholesterol (T-CHO), triglyceride (TG), total bilirubin (T-BIL), aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), blood urea nitrogen (BUN), creatinine (CRE), uric acid (UA), sodium (Na), potassium (K), chlorine (Cl) and calcium (Ca).
Upon sacrifice, the rats were macroscopically examined and subjected to a full autopsy. The brain, thyroids (with parathyroids), adrenal glands, lungs (including bronchi, fixed by inflation with fixative), heart, spleen, liver, kidneys, testes, ovaries and uterus were weighed and fixed in 10% neutrally buffered formalin. As well as these organs, the pituitary gland, sciatic nerve, spinal cords (cervical, mid-thoracic and lumbar), eyes, harderian glands, Zymbal’s glands, thoracic aorta, nasal cavity, trachea, thymus, salivary glands, tongue, esophagus, stomach, duodenum, jejunum, ileum, caecum, rectum, pancreas, urinary bladder, epididymides, seminal vesicles, prostate, preputial glands, oviducts, vagina, skin with mammary glands, skeletal muscle, lymph nodes (submandibular and mesenteric), bones (femur and sternum) with bone marrow and all gross lesions of each animal were fixed. Paraffin-embedded sections were routinely prepared and histopathologically examined after being stained with hematoxylin and eosin, Azan-Mallory and Berlin blue procedures.
Immunohistochemistry was performed on 4-μm sections of the formalin-fixed paraffin embedded tissues, using a monoclonal mouse antibody to β-catenin (610153, BD Biosciences, San Jose, CA, USA). Heat-induced antigen retrieval with 10 mM citrate buffer, pH 6.0, was followed by incubation with the primary antibody (diluted to 1:100) at room temperature for 30 minutes. Detection of primary antibody binding was carried out with an EnVision antimouse/horseradish peroxidase-labeled polymer detection system (Dako North America Inc., Carpinteria, CA, USA) followed by visualization with 3,3’-diaminobenzidine (DAB) (Dako) and counterstaining with Mayer’s hematoxylin. Negative control slides were subjected to the same staining protocol modified by the substitution of mouse normal IgG for the primary antibody.
Statistical analysis
For numerical data such as body and organ weights and hematological and serological outcomes, equality was assessed by Bartlett’s test. Homogeneity of variance was then analyzed by one-way analysis of variance, and finally differences between the values of the control group and those of each treated group were evaluated by Dunnett’s test. If the Bartlett’s test was significant, the data were subjected to the Kruskal-Wallis test and Dunnett’s-type rank sum test. For contingent data such as incidences of histopathological lesions, differences between the values of the control group and those of each treated group were evaluated by Fisher’s exact probability test18. Statistical processing was conducted using the StatLight software (Yukms Co., Ltd., Tokyo, Japan). Intergroup differences were considered statistically significant, when P values less than 0.05 were obtained.
Results
General findings
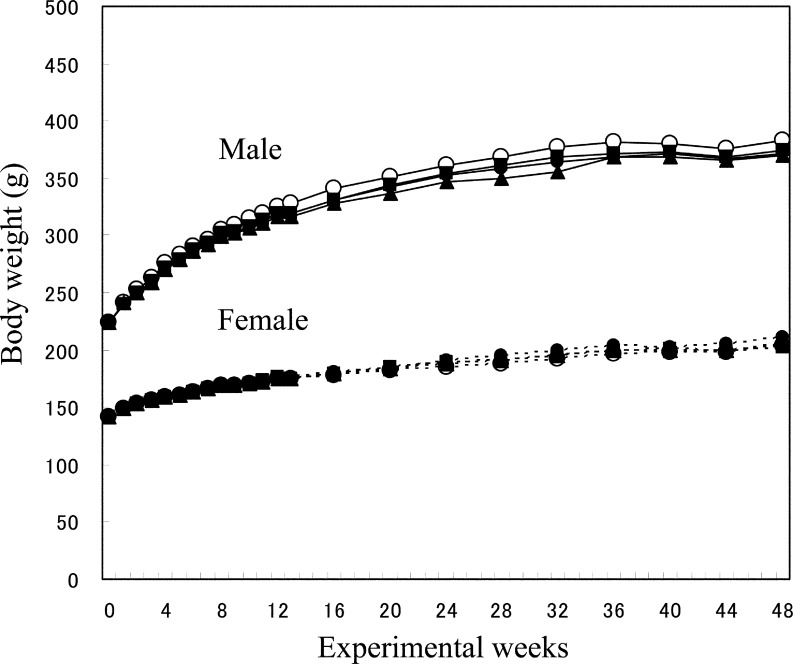
Rats given magnetite (especially at the high dose) became less active than controls after instillation but recovered relatively soon. During the study, however, 3 low-dose males, 3 control females and 1, 2 and 4 low-, medium- and high-dose females, respectively, died due to deep anesthesia following magnetite administration. In addition, accidental deaths due to suffocation with the diet occurred in 3 medium-dose males, 3 control females and 2, 1 and 4 low-, medium- and high-dose females, respectively. As a result, the final effective numbers of rats were 20, 17, 17 and 20 for males and 14, 17, 17 and 12 for females in the control group and low-, medium- and high-dose groups, respectively. There were no significant differences in average body weights among groups throughout the experimental period for either sex (Fig. 2).
Fig. 2.
Changes in mean body weights determined quadweekly for 52 weeks. The doses of magnetite were 0 (control) ○, 0.2 ●, 1.0 ▲ or 5.0 ■ mg/kg body weight per administration.
Urinalysis, hematology and serum biochemistry
In the urinalysis, no significant or treatment-related changes were observed in any analyzed parameters.
In the hematology, the MCHC values of the female control and low-, medium- and high-dose groups were 34.1 ± 0.8, 34.3 ± 0.6, 34.0 ± 0.6 and 33.4 ± 0.3 g/dL, respectively. Although the female high-dose value was significantly lower than the female control value, the change must lack a biological meaning or relation to the magnetite exposure, because the slight decrease was not accompanied by any other hematological, serological or pathological relatedness. The WBC values of the male control and low-, medium- and high-dose groups were 32.8 ± 7.3, 30.0 ± 7.0, 30.0 ± 6.7 and 37.1 ± 10.7 ×102/μL, respectively. The WBC values of the female control and low-, medium- and high-dose groups were 21.7 ± 6.1, 23.1 ± 6.6, 18.1 ± 3.5 and 21.8 ± 5.4 × 102/μL, respectively. There were no significant differences in WBC data between the control and treated groups. No significant or treatment-related changes were observed in any other analyzed parameters.
In the serum biochemistry, the GLU values of the male control and low-, medium- and high-dose groups were 155.1 ± 16.6, 142.8 ± 15.5, 144.9 ± 15.5 and 139.1 ± 13.2 mg/dL, respectively, and the male low- and high-dose values were significantly lower than the male control value. The Na values of the male control and low-, medium- and high-dose groups were 140.8 ± 1.1, 141.3 ± 1.9, 141.7 ± 1.7 and 143.0 ± 1.6 mmol/L, respectively, and the male high-dose value was significantly higher than the male control value. The Na values of the female control and low-, medium- and high-dose groups were 141.4 ± 1.8, 142.6 ± 2.0, 144.7 ± 1.7 and 143.8 ± 1.3 mmol/L, respectively, and the female medium- and high-dose values were significantly higher than the female control group value. The Cl values of the male control and low-, medium- and high-dose groups were 104.6 ± 1.3, 105.8 ± 0.6, 105.4 ± 0.8 and 106.8 ± 1.0 mmol/L, respectively, and the male low- and high-dose values were significantly higher than the male control value. The Cl values of the female control and low-, medium- and high-dose groups were 105.8 ± 1.7, 106.2 ± 2.1, 108.2 ± 2.8 and 107.7 ± 1.6 mmol/L, respectively, and the female medium-dose value was significantly higher than the female control value. The Ca values of the female control and low-, medium- and high-dose groups were 10.2 ± 0.3, 10.5 ± 0.3, 10.4 ± 0.3 and 10.4 ± 0.3 mg/dL, respectively, and the female low- and medium- dose values were significantly higher than the female control value. These slight changes must lack a biological meaning or relation to the magnetite exposure, because they were not accompanied any other hematological, serological or pathological relatedness, and frequently did not show first-order dose-dependency. No significant or treatment-related changes were observed in any other analyzed parameters.
Pathology
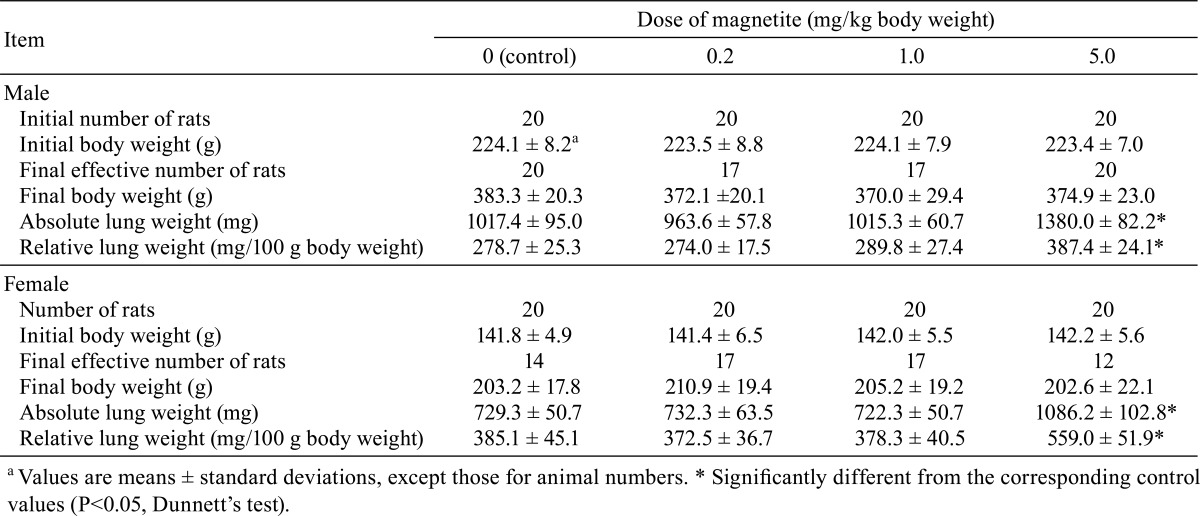
While initial or final body weights were not different among groups, both absolute and relative lung weights of the high-dose group were significantly higher than the control value in both sexes (Table 1). The absolute weights of the thyroid glands of the male control and low-, medium- and high-dose groups were 21.0 ± 3.5, 17.7 ± 2.5, 21.4 ± 5.3 and 18.6 ± 2.2 mg, while their relative weights were 5.7 ± 0.8, 5.0 ± 0.7, 6.1 ± 1.7 and 5.2 ± 0.6 mg/100 g body weight, respectively, and the male low-dose values were significantly lower than the respective male control values. On the other hand, the absolute weights of the thyroid glands of the female control and low-, medium- and high-dose groups were 13.2 ± 3.0, 12.6 ± 3.0, 15.7 ± 3.1 and 13.1 ± 1.9 mg, while their relative weights were 6.9 ± 1.2, 6.4 ± 1.4, 8.2 ± 1.6 and 6.7 ± 0.8 mg/100 g body weight, respectively, and the female medium-dose values were significantly higher than the respective female control values. These slight changes in the thyroid gland weights must lack a biological meaning or relation to the magnetite exposure, because they were not accompanied any other hematological, serological or pathological relatedness, and did not show first-order dose-dependency. No significant or treatment-related changes were observed in any other organ weights.
Table 1. Initial and Final Body Weights and Lung Weights.
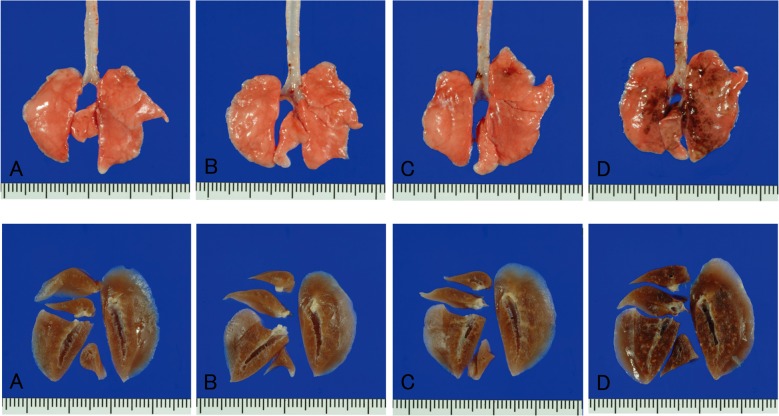
An apparent macroscopic finding was obtained at necropsy in the high-dose groups of both sexes, with the lungs being slightly enlarged in association with dark brown patches scattered in almost all their lobes (Fig. 3). Additionally, dark brown patches were also observed in the parathymic lymph nodes of male and female rats in the high-dose groups. No significant or treatment-related changes were macroscopically observed in any other organs or tissues.
Fig. 3.
Representative gross views (upper row) and cross-sections of the formalin-fixed tissues (lower row) of the lungs. The doses of magnetite were 0 (control) (A), 0.2 (B), 1.0 (C) and 5.0 (D) mg/kg body weight per administration.
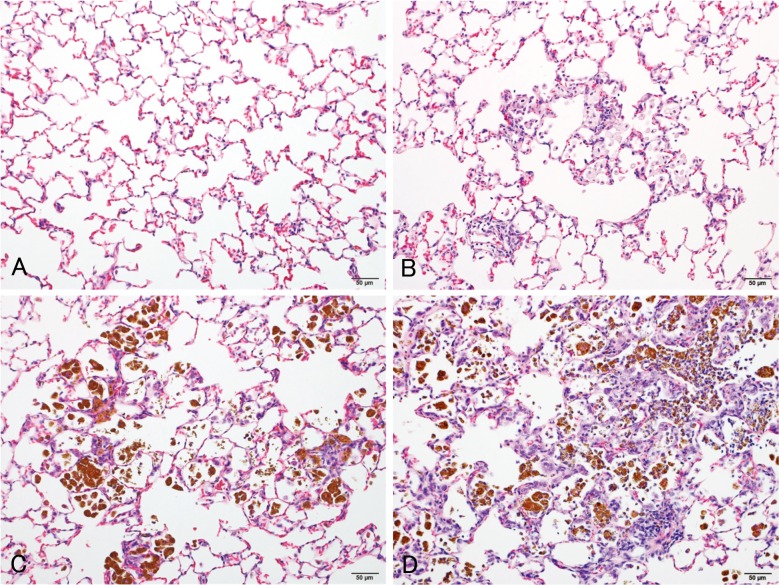
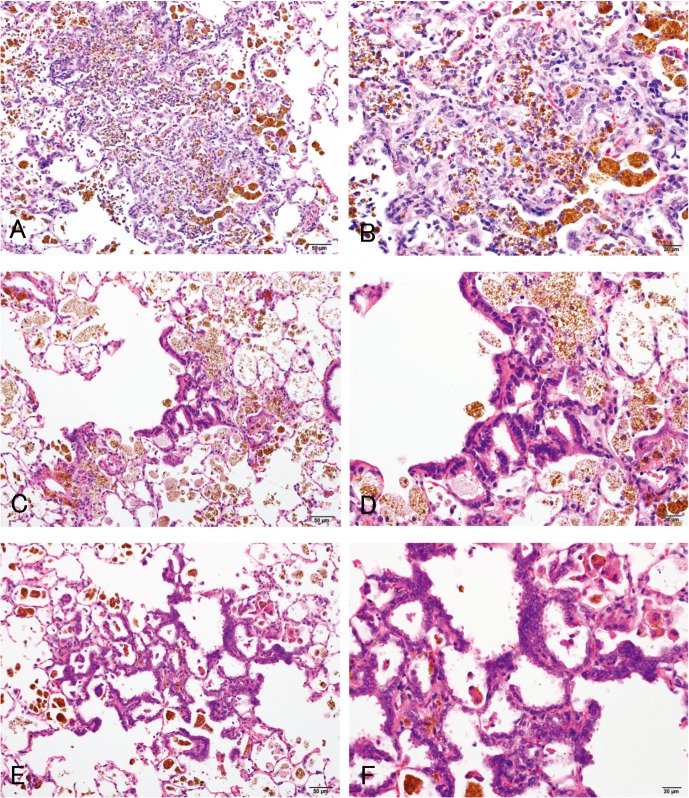
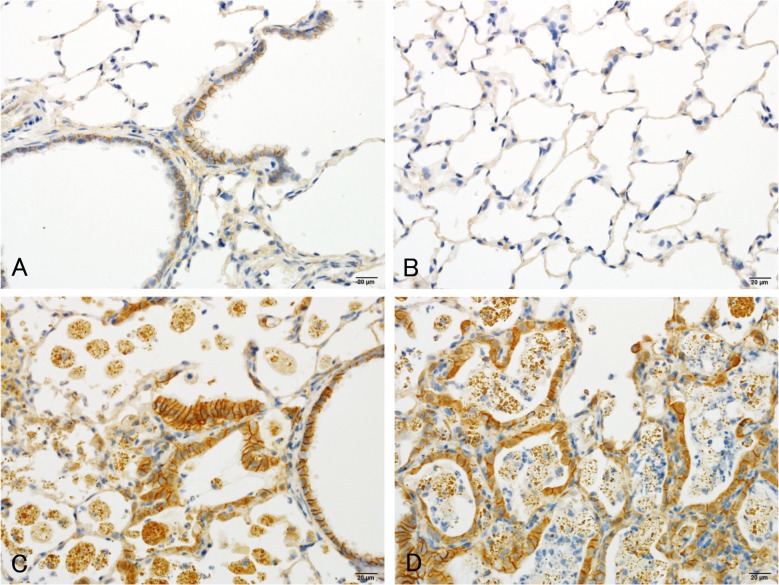
The histopathological changes detected in the lungs are summarized in Table 2. In the lungs of all rats in all magnetite-treated groups, infiltration of brownishly pigmented macrophages phagocytosing magnetite was observed in the alveolar spaces, walls and interstitium (Figs. 4B, 4C, 4D). Such infiltration was not present in the control groups, and among the magnetite-treated groups, the severity of this change generally increased with the increasing doses of magnetite. Chronic inflammation was observed in 4/17 (23.5%) and 20/20 (100%) of the medium- and high-dose males and 3/17 (17.6%; statistically insignificant), 3/17 (17.6%; statistically insignificant) and 12/12 (100%) of the low-, medium- and high-dose females, respectively. The chronic inflammation was evidenced by an infiltration of inflammatory cells, such as lymphocytes or neutrophils, and fibrosis. This chronic inflammation was frequently associated with macrophages (either phagocytosing magnetite or not) diffusely scattered within the alveolar lumens, and thence multiple focal clusters of alveoli filled with inflammatory cells (mostly macrophages but occasionally mixed with small to moderate numbers of neutrophils) were commonly observed with debris (Figs. 5A, 5B). Hypertrophy and hyperplasia of alveolar epithelial cells (presumably type II pneumocytes) were usually seen in alveoli containing such inflammatory exudates, which were not considered separate/independent lesions but regenerative responses secondary to the inflammation. Alveolar bronchiolization, identified by the lining of normal or thickened alveolar walls with cells resembling the bronchiolar epithelium (Figs. 5C, 5D), was observed in 2/17 (11.8%; statistically insignificant) and 15/20 (75.0%) of the medium- and high-dose males and 3/17 (17.6%; statistically insignificant) and 12/12 (100%) of the medium- and high-dose females, respectively. The bronchiolized epithelium consisted of a single layer of ciliated and nonciliated columnar cells, and extended from the terminal bronchiole onto the septa of the adjacent alveoli (Figs. 5C, 5D). As an additional inflammatory change, granuloma was observed in 7/20 (35%) and 6/12 (50%) of the high-dose males and females, respectively. On the other hand, alveolar hyperplasia was observed in 2/20 (10%; statistically insignificant) and 2/12 (16.7%; statistically insignificant) of the high-dose males and females, respectively, as well as 3/20 (15%; statistically insignificant) of the control males. While the alveolar hyperplasias did not compress the surrounding parenchyma, those observed in the control males were focal, and those found in the high-dose males and females tended to extend to the surroundings with poor demarcation and were accompanied by inflammation (Figs. 5E, 5F). The hyperplastic alveolar epithelial cells (presumably type II pneumocytes) were cuboidal and round-to-oval shaped hyperchromatic nuclei (Figs. 5E, 5F), and cytoplasmic overexpression of β-catenin protein was immunohistochemically found (Figs. 6C, 6D), whereas the protein was mainly localized at the membranes of the cell-cell borders in the background lung tissue irrespective of magnetite exposure (Figs. 6A, 6B).
Table 2. Histological Findings of the Lungs and Parathymic Lymph Nodes.
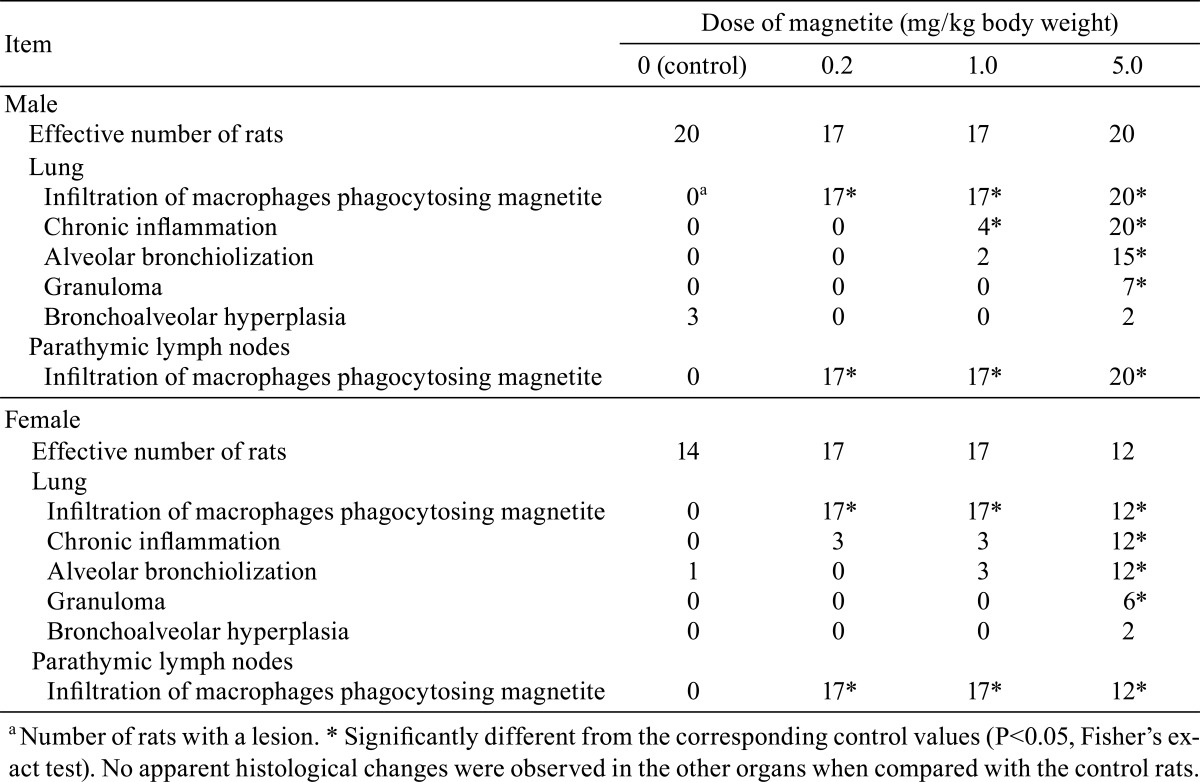
Fig. 4.
Representative microscopic views of the lungs of the control (A) and low- (B), medium- (C) and high-dose (D) groups (hematoxylin and eosin).
Fig. 5.
Representative lesions observed in the lungs of the high-dose group (hematoxylin and eosin): infiltration of macrophage phagocytosing magnetite (A–F), chronic inflammation (A, B), alveolar bronchiolization (C, D) and alveolar hyperplasia (E, F). The right row shows higher magnification versions of the left row images. The bronchiolization was identified as a non-proliferative lesion, in which the lining of alveolar walls was replaced by cells resembling the bronchiolar epithelium. The alveolar hyperplasia was identified as a lesion consisting of proliferative alveolar epithelial cells.
Fig. 6.
Representative results of β-catenin immunohistochemistry of the lungs of the control (A, B) and high-dose (C, D) groups. The β-catenin protein was localized at the membranes of the cell-cell borders in the background lung tissue (A–D), whereas cytoplasmic overexpression of the protein was found in the hyperplastic alveolar epithelial cells (C, D).
In the parathymic lymph nodes, infiltration of macrophages phagocytosing magnetite was observed in all rats in all magnetite-treated groups (Fig. 7) but not in the control animals, as shown in the lungs (Table 2). No significant or treatment-related changes were histopathologically observed in any other organs or tissues, whereas sporadic spontaneous lesions were observed identically in the control and treated rats.
Fig. 7.
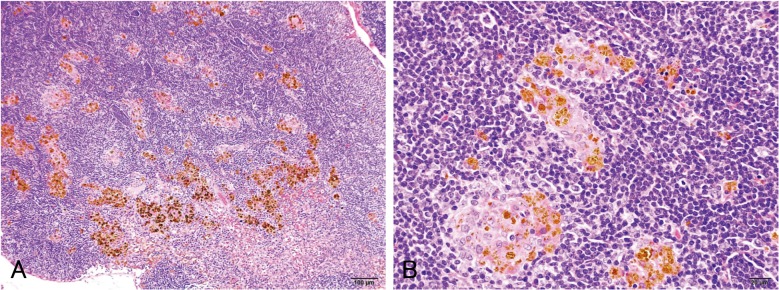
Representative microscopic views of the parathymic lymph node of the high-dose group (hematoxylin and eosin). Infiltration of macrophages phagocytosing magnetite and deposits of magnetite particles are evident.
Discussion
The present study clearly revealed that a total of 13 quadweekly intermittent intratracheal spray instillations of magnetite (Fe3O4) nanoparticles for 52 weeks at doses of 0, 0.2, 1.0 and 5.0 mg/kg body weight per administration caused dose-dependent inflammatory changes in the lung and parathymic lymph node of male and female Fischer 344 rats but not in the other organs or tissues. A large amount of the administered magnetite remained in the lung, and some of the magnetite was distributed into the regional lymph nodes. Histopathologically, infiltration of macrophages phagocytosing magnetite and of chronic inflammatory cells, alveolar bronchiolization and granuloma were observed in the lungs of treated rats.
Alveolar bronchiolization is identified as cells resembling a bronchiolar epithelia line on normal or thickened alveolar walls, which often manifest an acinar formation19. This lesion is thought to arise from the “colonization” of alveolar walls within the bronchiolar epithelium either via cell migration through alveolar pores or from the transformation of alveolar type II cells into bronchiolar-type epithelium19. This is seen in various types of experimental lung injury caused by viral infection20 or exposure to ozone21, chromate19 and paraquat22; it is also seen in human lung cancer cases23. Jensen-Taubman et al. examined the incidence and association of alveolar bronchiolization with non-small cell lung cancer (NSCLC) in lung resection specimens from 2 patient groups, those with NSCLC and those diagnosed with a variety of non-neoplastic lung conditions23. They observed that alveolar bronchiolization occurs in up to 8% (1/12) of non-lung-cancer cases and 12% (5/42) of NSCLC cases. They described that alveolar bronchiolization does not necessarily occur only in adenocarcinoma cases, suggesting that this form of alveolar metaplasia may somehow be associated with a spectrum of NSCLC histologic subtypes. The precise roles of alveolar bronchiolization in lung carcinogenesis are not known at this moment, and thus this lesion is currently considered to be an inflammatory response.
In line with the content of the last paragraph, alveolar hyperplasia was observed in male and female rats exposed to the high dose of magnetite, whereas the incidences were low and not significantly different from those in the respective control groups. A similar lesion was observed also in control males (but not females), but the histopahological appearances of the lesions seen in the treated groups were somewhat qualitatively different from those seen in male control animals. A “primary” alveolar hyperplasia is considered a precursor lesion of lung cancers and must be differentiated from regenerative hyperplasia. A preneoplastic hyperplasia is usually not associated with inflammation or necrosis, and the parenchymal architecture is maintained24. While alveolar hyperplasias seen in the high-dose groups of the present study were accompanied by chronic inflammation, a number of molecular pathways activated in chronic inflammation have been indicated to contribute to lung carcinogenesis25. Alveolar epithelial cells contribute to the initiation and modulation of inflammatory responses, which in turn may result in the release of cytokines, growth factors and other peptide mediators that may predispose epithelial cells themselves to a proliferative response26,27. Foreign particles, infectious agents and chemicals can induce an inflammatory response in the lung leading to oxidative stress. Also with regard to magnetite, exposure to it has been shown to induce oxidative stress and deplete antioxidant levels in the lung epithelial cells, stimulating the apoptotic pathway for cell death14. Furthermore, magnetite particles have been revealed to induce concentration-dependent DNA damage and enhance reactive oxygen species production6 and micronuclei induction28. It may be particularly of importance that β-catenin protein was overexpressed in the epithelial cells of the presently observed alveolar hyperplasia in the high magnetite dose groups, while the protein was localized in the membranes of the cell-cell borders of the background lung tissue irrespective of magnetite exposure. It is very well known that β-catenin protein is a versatile component of homotypic cell adhesion and signaling, the subcellular localization and cytoplasmic levels of which are tightly regulated by adenomatous polyposis coli (APC) protein. Mutations occurring in the β-catenin and/or APC genes result in aberrant overexpression of β-catenin protein first in the cytoplasms and then its translocation into the nuclei, causing nuclear accumulation of the protein and in turn improper gene activation29. Typically, for instance, the accumulated β-catenin protein in the nuclei interacts with the Tcf family members, resulting in the acquisition of growth advantage of target genes30. Likewise, immunohistochemical studies of N-nitrosobis (2-hydroxypropyl) amine-induced rat lung tumors30 and of azoxymethane-induced rat colon tumors31,32 have revealed frequent translocation of the β-catenin protein from the cell membranes to the cytoplasms and then the nuclei in adenomas and adenocarcinomas, but not in hyperplasia, suggesting the mechanistic involvement of the nuclear accumulation of the protein in those chemical carcinogenesis processes. In this context, the presently observed alveolar hyperplasias in rats exposed to the high dose of magnetite might possess a potent preneoplastic potential. When considering the potential risk for humans, therefore, further studies are apparently warranted to elucidate whether or not intratracheally administered magnetite has the potency to cause lung carcinogenesis, and such studies are being conducted in our laboratories.
In conclusion, the present results clearly show that instilled magnetite causes chronic inflammatory responses in the lung. These long-term pulmonary responses occur in a dose-dependent manner without apparent differences among sexes.
Acknowledgments
The authors would like to express their gratitude to Dr. Yukari Totsuka (Cancer Prevention Basic Research Project, National Cancer Center Research Institute, Tokyo, Japan) for arrangement of the supply of magnetite and helpful suggestions. We also thank Mr. Toshiyuki Hakata and the staff of Toda Kogyo Co., Inc. (Hiroshima, Japan), for their generous supply of magnetite and its technical information. This work was supported in part by a Health and Labour Sciences Research Grant (awarded to Dai Nakae) from the Ministry of Health, Labour and Welfare of Japan.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives (by-nc-nd) License <http://creativecommons.org/licenses/by-nc-nd/3.0/>.
References
- 1.Hood E. Nanotechnology: looking as we leap. Environ Health Perspect. 112: A740–A749 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin BL, Shen XD, and Cui S. Application of nanosized Fe3O4 in anticancer drug carriers with target-orientation and sustained-release properties. Biomed Mater. 2: 132–134 2007. [DOI] [PubMed] [Google Scholar]
- 3.Kikumori T, Kobayashi T, Sawaki M, and Imai T. Anti-cancer effect of hyperthermia on breast cancer by magnetite nanoparticle-loaded anti-HER2 immunoliposomes. Breast Cancer Res Treat. 113: 435–441 2009. [DOI] [PubMed] [Google Scholar]
- 4.Hilger I, and Kaiser WA. Iron oxide-based nanostructures for MRI and magnetic hyperthermia. Nanomedicine (Lond). 7: 1443–1459 2012. [DOI] [PubMed] [Google Scholar]
- 5.Hong SC, Lee JH, Lee J, Kim HY, Park JY, Cho J, Lee J, and Han DW. Subtle cytotoxicity and genotoxicity differences in superparamagnetic iron oxide nanoparticles coated with various functional groups. Int J Nanomedicine. 6: 3219–3231 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Könczöl M, Ebeling S, Goldenberg E, Treude F, Gminski R, Gieré R, Grobéty B, Rothen-Rutishauser B, Merfort I, and Mersch-Sundermann V. Cytotoxicity and genotoxicity of size-fractionated iron oxide (magnetite) in A549 human lung epithelial cells: Role of ROS, JNK, and NF-κB. Chem Res Toxicol. 24: 1460–1475 2011. [DOI] [PubMed] [Google Scholar]
- 7.Slesinski RS, and Turnbull D. Chronic inhalation exposure of rats for up to 104 weeks to a non-carbon-based magnetite photocopying toner. Int J Toxicol. 27: 427–439 2008. [DOI] [PubMed] [Google Scholar]
- 8.Pauluhn J. Subchronic inhalation toxicity of iron oxide (magnetite, Fe3O4) in rats: pulmonary toxicity is determined by the particle kinetics typical of poorly soluble particles. J Appl Toxicol. 32: 488–504 2012. [DOI] [PubMed] [Google Scholar]
- 9.Chen BA, Jin N, Wang J, Ding J, Gao C, Cheng J, Xia G, Gao F, Zhou Y, Chen Y, Zhou G, Li X, Zhang Y, Tang M, and Wang X. The effect of magnetic nanoparticles of Fe3O4 on immune function in normal ICR mice. Int J Nanomedicine. 5: 593–599 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pott F, Ziem U, Reiffer FJ, Huth F, Ernst H, and Mohr U. Carcinogenicity studies on fibres, metal compounds, and some other dusts in rats. Exp Pathol. 32: 129–152 1987. [DOI] [PubMed] [Google Scholar]
- 11.Steinhoff D, Mohr U, and Hahnemann S. Carcinogenesis studies with iron oxides. Exp Pathol. 43: 189–194 1991. [DOI] [PubMed] [Google Scholar]
- 12.Tada Y, Yano N, Takahashi H, Yuzawa K, Ando H, Kubo Y, Nagasawa A, Ogata A, and Nakae D. Acute phase pulmonary responses to a single intratracheal spray instillation of magnetite (Fe3O4) nanoparticles in Fischer 344 rats. J Toxicol Pathol. 25: 233–239 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh N, Jenkins GJ, Asadi R, and Doak SH. Potential toxicity of superparamagnetic iron oxide nanoparticles (SPION). Nano Rev. 1: 5358–5373 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramesh V, Ravichandran P, Copeland CL, Gopikrishnan R, Biradar S, Goornavar V, Ramesh GT, and Hall JC. Magnetite induces oxidative stress and apoptosis in lung epithelial cells. Mol Cell Biochem. 363: 225–234 2012. [DOI] [PubMed] [Google Scholar]
- 15.Organisation for Economic Co-operation and Development (OECD). List of Manufactured Nanomaterials and List of Endpoints for Phase One of the Sponsorship Programme for the Testing of Manufactured Nanomaterials: Revision. Paris. 1–16. 2010 [Google Scholar]
- 16.Japanese Association for Laboratory Animal Science (JALAS) Guidelines for animal experimentation. Exp Anim. 36: 285–288 1987 [Google Scholar]
- 17.Driscoll KE, Costa DL, Hatch G, Henderson R, Oberdorster G, Salem H, and Schlesinger RB. Intratracheal instillation as an exposure technique for the evaluation of respiratory tract toxicity: uses and limitations. Toxicol Sci. 55: 24–35 2000. [DOI] [PubMed] [Google Scholar]
- 18.Gad SC, and Weil CS. Statistics for toxicologist. In: Principles and Methods of Toxicology, 3rd ed. AW Hayes (ed). Raven Press, New York. 221–274. 1994 [Google Scholar]
- 19.Nettesheim P, and Szakal AK. Morphogenesis of alveolar bronchiolization. Lab Invest. 26: 210–219 1972. [PubMed] [Google Scholar]
- 20.Baskerville A, Thomas G, Wood M, and Harris WJ. Histology and ultrastructure of metaplasia of alveolar epithelium following infection of mice and hamsters with influenza virus. Br J Exp Pathol. 55: 130–137 1974. [PMC free article] [PubMed] [Google Scholar]
- 21.Pinkerton KE, Dodge DE, Cederdahl-Demmler J, Wong VJ, Peake J, Haselton CJ, Mellick PW, Singh G, and Plopper CG. Differentiated bronchiolar epithelium in alveolar ducts of rats exposed to ozone for 20 months. Am J Pathol. 142: 947–956 1993. [PMC free article] [PubMed] [Google Scholar]
- 22.Fukuda Y, Takemura T, and Ferrans VJ. Evolution of metaplastic squamous cells of alveolar walls in pulmonary fibrosis produced by paraquat. Virchows Arch B. 58: 27–43 1989. [DOI] [PubMed] [Google Scholar]
- 23.Jensen-Taubman SM, Steinberg SM, and Linnoila RI. Bronchiolization of the alveoli in lung cancer: pathology, patterns of differentiation and oncogene expression. Int J Cancer. 75: 489–496 1998. [DOI] [PubMed] [Google Scholar]
- 24.Dixon D, Herbert RA, Sills RC, and Boorman GA. Lungs, pleura, and mediastinum. In: Pathology of the Mouse: Reference and Atlas. RR Maronpot, GA Boorman, and BW Gaul (eds). Cache River, Vienna. 293-332. 1999 [Google Scholar]
- 25.Ballaz S, and Mulshine JL. The potential contributions of chronic inflammation to lung carcinogenesis. Clin Lung Cancer. 5: 46–62 2003. [DOI] [PubMed] [Google Scholar]
- 26.Simon RH, and Paine R. Participation of pulmonary alveolar epithelial cells in lung inflammation. J Lab Clin Med. 126: 108–118 1995. [PubMed] [Google Scholar]
- 27.Shacter E, and Weitzman SA. Chronic inflammation and cancer. Oncology. 16: 217–226, 229–232 2002. [PubMed] [Google Scholar]
- 28.Kawanishi M, Ogo S, Ikemoto M, Totsuka Y, Ishino K, Wakabayahsi K, and Yagi T. Genotoxicity and reactive oxygen species production induced by magnetite nanoparticles in mammalian cells. J Toxicol Sci. 2013; in press. [DOI] [PubMed] [Google Scholar]
- 29.Ilyas M, and Tomlinson IPM. The interactions of APC, E-cadherin and β-catenin in tumor development and progression. J Pathol. 182: 128–137 1997. [DOI] [PubMed] [Google Scholar]
- 30.Tsujiuchi T, Tsutsumi M, Sasaki Y, Murata N, and Konishi Y. Mutations of adenomatous polyposis coli and beta-catenin genes during progression of lung tumors induced by N-nitrosobis(2-hydroxypropyl)amine in rats. Cancer Res. 60: 6611–6616 2000. [PubMed] [Google Scholar]
- 31.Takahashi M, Fukuda K, Sugimura T, and Wakabayashi K. Beta-catenin is frequently mutated and demonstrates altered cellular location in azoxymethane-induced rat colon tumors. Cancer Res. 58: 42–46 1998. [PubMed] [Google Scholar]
- 32.Takahashi M, Mutoh M, Kawamori T, Sugimura T, and Wakabayashi K. Altered expression of beta-catenin, inducible nitric oxide synthase and cyclooxygenase-2 in azoxymethane-induced rat colon carcinogenesis. Carcinogenesis. 21: 1319–1327 2000. [PubMed] [Google Scholar]