Abstract
We conducted a phase II feasibility study of a 6 month behavioral weight loss intervention in postmenopausal overweight and obese women at increased risk for breast cancer and the effects of weight loss on anthropomorphic, blood, and benign breast tissue biomarkers. 67 women were screened by random peri-areolar fine needle aspiration (RPFNA), 27 were registered and 24 participated in the interventional phase. The 24 biomarker evaluable women had a median baseline BMI of 34.2 kg/m2 and lost a median of 11% of their initial weight. Significant tissue biomarker modulation after the 6 month intervention was noted for Ki-67 (if restricted to the 15 women with any Ki-67 at baseline, p=0.041), adiponectin to leptin ratio (p=0.003); and cyclin B1 (p=0.001), phosphorylated retinoblastoma (p=0.005), and ribosomal S6 (p=0.004) proteins. Favorable modulation for serum markers was observed for sex hormone binding globulin (p<0.001), bioavailable estradiol (p<0.001), bioavailable testosterone (p=0.033), insulin (p=0.018), adiponectin (p=0.001), leptin (p<0.001), the adiponectin to leptin ratio (p<0.001), C-reactive protein (p=0.002) and hepatocyte growth factor (p=0.011). When subdivided by < or > 10% weight loss, change in percent total body and android (visceral) fat, physical activity and the majority of the serum and tissue biomarkers were significantly modulated only for women with >10% weight loss from baseline. Some factors such as serum PAI-1 and breast tissue pS2 (estrogen inducible gene) mRNA were not significantly modulated overall but were when considering only those with >10% weight loss. In conclusion, a median weight loss of 11% over 6 months resulted in favorable modulation of a number of anthropomorphic, breast tissue and serum risk and mechanistic markers. Weight loss of 10% or more should likely be the goal for breast cancer risk reduction studies in obese women.
Keywords: Breast Cancer Risk Biomarkers, Weight Loss
Introduction
Adult weight gain and postmenopausal obesity are associated with an increased risk of developing breast cancer in both average and high risk women [1-6]. Chronic inflammation and excess hormone production are thought to be the likely etiologic factors. Obesity is associated with activated macrophage infiltration into breast and other adipose tissue, excess production of pro-inflammatory cytokines, increased aromatase activity and higher levels of bioavailable estrogen, and breast epithelial proliferation [7-10]. In addition, reduction in adiponectin by enlarged adipocytes [11-12] combined with pro-inflammatory cytokines results in hyperinsulinemia, insulin resistance [13-15], activation of PI3 kinase and AKT/mTOR pathways and increased risk for breast cancer [16-24]. Obese women whose excess fat is concentrated in the central abdomen and liver (often referred to as central or visceral obesity) are more likely to both exhibit hyperinsulinemia and develop breast cancer than similar weight women with excess fat primarily deposited over the buttocks and hips [25-28]. Percent visceral fat is best measured with computed tomography or MRI [29] but may be approximated by measuring android fat distribution by dual-energy X-ray absorptiometry (DEXA) [29]. Waist circumference is positively associated with visceral fat, and women with values > 88 cm are more likely to exhibit metabolic abnormalities [30].
Once obese, the minimum loss required to reduce risk has yet to be defined, although reduction of breast and other cancers has been observed with ~30% weight loss after bariatric surgery [31,32]. The National Heart Lung Blood Institute Guidelines for obesity suggest a weight loss goal of 10% over 6 months for general health [33]. A few cohort studies suggest reduced risk associated with weight loss of >10% if maintained for several years [5,34]. Weight loss with behavioral interventions aimed at changing lifestyle averages between 4 and6 % of baseline in the US with the higher percentage associated with greater number of sessions [35]. Whether this amount of weight loss is associated with a significant risk reduction for breast cancer is unclear, although weight loss of at least 5% appears to have some general health benefits [36]. Investigations into both the mechanisms by which weight loss might reduce breast cancer risk and the amount of weight or fat loss likely to be needed, are high priority topics for prevention [15].
The purpose of this pilot study was to assess the feasibility of a study design in which a 6 month behavioral weight loss intervention, previously shown to be effective in producing 10% or greater weight loss in a general overweight population [37], was combined with assessment of change in anthropomorphic, blood, and benign breast tissue biomarkers of breast cancer risk and/or mechanism of action.
Methods
Eligibility
Risk Criteria for Breast Tissue Screening and Tissue Requirements for Intervention
Postmenopausal women a) not on hormone replacement therapy b) with a BMI > 25 kg/m2 and c) at increased risk for breast cancer on the basis of family history, prior precancerous biopsy, a 5-year Gail model risk estimate of ≥1.7% (http://bcra.nci.nih.gov/brc/), or prior contralateral treated breast cancer, were potentially eligible for tissue screening by random periareolar fine needle aspiration (RPFNA) [35]. Women were initially required to have cytologic evidence of hyperplasia and minimum Ki-67 of 1.5% in 500 epithelial cells; however the minimum Ki-67 requirement was soon dropped as being too restrictive. Women were required to be in good health with no clinical history/evidence of diabetes. Protocols for RPFNA and behavioral weight loss intervention were approved by the University of Kansas Medical Center Human Subjects Committee.
Tissue Acquisition and Specimen Processing
RPFNA was performed on two sites per breast under local anaesthesia at baseline. The first aspiration pass per site (four sites total) was placed in a 2-ml cryovial containing 0.5 ml PBS, immediately immersed in liquid nitrogen and transferred to a −80°C freezer within 12 hours. Frozen tissue was thawed once for mixing of specimen from the four cryovials and then realiquoted in separate cryovials for adipocytokines, proteomics, and gene expression assays such that for these assays only one further thaw was necessary. Specimen mixing and realiquoting were performed with specimen in an ice bath. Frozen specimens were not exposed to fixative. Remaining material from all aspiration sites was pooled in a single 15 cc tube with 9 ml CytoLyt® and 1 ml of 10% buffered formalin and processed to slides for pap-staining and cytomorphology or Ki-67 as previously described [38-41]. Fasting blood for hormones and adipocytokines, obtained at baseline and conclusion of the weight loss intervention, was immediately processed to aliquots and frozen at −80°C. These specimens were not thawed prior to assay. All biomarker assays besides cytomorphology and Ki-67 were batch processed on samples stored in aliquots so that the pre-study and post-study specimens were run together.
Cytomorphology
Cytomorphology was assessed by a single cytopathologist (CMZ) and classified by both a categorical method and a semi-quantitative Masood index score [40] without knowledge of Ki-67 scores. Index scores of 11-13 generally correlate with hyperplasia without atypia, 14 borderline atypia, and 15-18 with hyperplasia with atypia.
Ki-67
Ki-67 was performed using citrate buffer antigen retrieval, a 1:20 dilution of MIB-1 (M7240; Dako Cytomation, Carpinteria, CA) and a Dako Autostainer Plus (Dako Cytomation) as previously described [39]. The number of cells with nuclear staining out of 500 cells was recorded for each of two independent readers and averaged in case of a disagreement [41].
Collection of Energy Intake, Exercise, and Anthropomorphic Variables
Potentially eligible participants delayed beginning the weight loss intervention until 5-10 women could be collected per group. Prior to start, women were asked to complete 3-day diet records (for subsequent analysis of energy and macronutrient intake using Nutrition Data System for Research) and record minutes and type of exercise per week. Height, weight, and waist circumference measurements were performed in a hospital gown. Body composition via DEXA scanning (Lunar Prodigy, GE Healthcare) was obtained.
Behavioral Weight Loss Intervention
The intervention consisted of a National Heart and Lung and Blood Institute step I diet containing 1000-1200 calories per day [42], exercise, and a weekly in-person lifestyle modification meeting with 5-10 other high risk women. The diet consisted of two shelf-stable pre-packaged meals and three low calorie high protein shakes (HMR™, Health Management Resources, Boston, MA; http://www.hmrprogram.com), five fruit and vegetable servings per day, and ad libitum beverages except those that contained large kcal loads, i.e., sugar sweetened soft drinks, alcohol and high fat dairy drinks. Moderate intensity unsupervised exercise with a goal of 300 minutes per week and attendance at the weekly in-person group lifestyle modification meeting was strongly encouraged. At the meeting, women were weighed, food and exercise logs collected, and suggestions were made for food preparation and compliance [37]. Following completion of the 6-month intervention, diet and anthropomorphic assessments and blood and tissue acquisitions were repeated. There was no structured follow-up required beyond this point but most women continued to have at least yearly visits with exam, height and weight and breast imaging in the Breast Cancer Prevention Center.
Serum Hormones, Growth Factors, and Selected Adipocytokines by ELISA
Testosterone, prolactin, SHBG, IGF-1, IGFBP3, CRP, IL-6, leptin and adiponectin were assayed in the Breast Cancer Prevention Center laboratories with commercially available ELISA kits. Adiopocytokines were also assayed by commercially available Luminex kits at the University of Texas at Austin (see below) . Serum estradiol and insulin assays were performed at the University of Virginia Center for Research Reproduction Ligand Assay and Analysis Core Laboratory (Charlottesville, VA) by ultrasensitive RIA and Immulite, respectively. Bioavailable estradiol and testosterone results were computed using values for estradiol, testosterone, and SHBG according to standard formulas [43]. Serum samples which could not be confirmed as fasting were excluded for assessment of insulin, glucose, and adipocytokines. See Supplemental Methods for assays and limits of detection.
Serum and Tissue for Adipokine and Cytokine Assay by Luminex
Serum or frozen RPFNA aspirate were utilized for adipocytokines using Luminex multiplexed assays (HADK1-61K-A & HADK2-61K-B, Millipore Corporation, Billerica, MA) according to manufacturer’s instructions by Dr. Stephen Hursting at the University of Texas at Austin. The serum for adiponectin assay was diluted 400-fold. For tissue assays, results were normalized to total protein content (Bio-Rad Protein Assay, #500-0006, Bio-Rad Laboratories, Inc., Hercules, CA).
Tissue for RT-qPCR
Total RNA was extracted from frozen RPFNA samples using Trizol® LS according to the manufacturer’s instructions. We have found that the RNA quality is far superior from frozen samples compared to fixed samples, even though the latter allows micro-dissection of epithelial cell clusters [44]. As we did not employ fixed tissue and micro-dissection, change in gene expression was reflective of change in adipocytes, stroma, and epithelial cells. Since many of the biomarkers of interest in a weight reduction trial are expressed in the adipocytes and stroma, we felt this was a better approach than attempting to micro-dissect small clusters of epithelial cells. RNA was amplified using MessageAmp™II aRNA amplification kit (Life Technologies Grand Island NY ) and reverse transcribed to cDNA using SMARTScribe™ Reverse Transcriptase (Clontech Laboratories, Inc., Mountain View, CA) and random nonamer primers. Real-time PCR (RT-qPCR) was performed in the Breast Cancer Prevention Laboratory using TaqMan chemistry as previously described [44]. Levels were expressed as relative to four reference transcripts (β-actin, cyclophilin A, β-glucuronidase, and TATA box-binding protein); and then adjusted for epithelial cell content by expression of cytokeratin 19.
Tissue Proteomics
Tissue obtained by RPFNA was immediately placed in 0.5 ml of Phosphate Buffered Saline, frozen in liquid nitrogen and processed as described above; and was utilized to assess levels of peptides and phosphopeptides at the Reverse Phase Protein Array (RPPA) core at the University of Texas MD Anderson Cancer Center [45]. The sample underwent 5 two-fold serial dilutions (final 1:16) for construction of a dilution curve. The sample was spotted onto a glass slide coated with nitrocellulose with each sample represented on the slide as a serial microdilution. Dilution series were replicated on spatially distant portions of the array. Samples from the same patient were placed on multiple spots on the same slide, on multiple slides, and in multiple runs. Each slide also contained multiple positive and negative controls with results normalized to total protein loading. If the inter slide and inter run variation for the slide resulted in an R score of less than 0.9 that slide was discarded. The coefficient of variation for technical replicates is 15% when protein is present at detectable levels. [45]. Pre- and post-intervention specimens were assessed together to produce validated results for 108 antibodies. Overall, there was sufficient sample to assess all antibodies for all specimens.
Statistical Considerations
Our original presumption was that mean Ki-67 would be 4.5% ± 3.5% based on prior studies in premenopausal women or postmenopausal women on hormone replacement. We anticipated accruing 22 evaluable subjects resulting in an 80% power with a Type I error rate to detect a 50% reduction in Ki-67. However, once the study was amended allowing entry of women with Ki-67 <1.5%, our aims were primarily that of feasibility and exploration of a variety of biomarkers for usefulness in the weight loss setting. Non-parametric approaches were used for all analyses. The Wilcoxon signed rank test was used for change in biomarkers over the course of the intervention. The Mann-Whitney test was used for comparison between groups, specifically groups defined as subjects who lost <10% weight vs. those who lost >10%. We chose this cut point because the National Heart Lung Blood Institute Guidelines [33] recommend a goal of loss of 10% of baseline weight at 6 months. However, we also assessed all data using a median weight loss cut point and arrived at the same general conclusions. All analyses were conducted by IBM, SPSS, version 20. Since analyses were considered exploratory they were not corrected for multiple comparisons.
Results
Screening and Enrollment
A total of 67 risk and BMI eligible women were screened by RPFNA and 27 were enrolled. Of those screened, 24% had cytologic atypia, 42% had both cytologic hyperplasia +/− atypia and some Ki-67 expression, but only 16% had Ki-67 of 1.5% or higher. Following RPFNA, 20 were ineligible on the basis of cytomorphology and/or cell count, and 5 were deemed ineligible due to weight or medical issues. Fifteen eligible women declined participation due to unwillingness to use one brand of shelf stable meals for 6 months (7), travel to weekly in-person meetings (6), or wait to start the behavioral weight loss intervention (5). Several women declined for more than one reason. Twenty-seven women were eligible but three dropped out prior to intervention leaving 24 evaluable for biomarker evaluation. The first of four groups began the intervention November 2007; the final group completed their repeat RPFNA July 2009. Women were subsequently followed for weight regain to January 2012.
Demographic and Risk Information
Median age for the 24 evaluable women was 57.5 years, with median BMI of 34.2 kg/m2, and median 5-year Gail risk of 4% (Table 1). All subjects were Caucasian, non-Hispanic.
Table 1.
Demographic and risk information at baseline for 24 evaluable subjects.
| Variable | median | mean ± SD | range |
|---|---|---|---|
| Age, years | 57.5 | 56.8 ± 4.8 | 46 - 66 |
| Height (cm) | 163 | 163 ± 5 | 154 - 173 |
| Weight (Kg) | 93 | 91 ± 15 | 70 - 122 |
| BMI (kg/m2) | 34.2 | 34.1 ± 5.8 | 25.7 - 42.4 |
| 5-Year Gail Risk, % | 4.0 | 4.0 ± 1.7 | 1.8 - 7.7 |
| Variable | Number | Frequency | |
| Affected 1st degree relative | 17 | 71% | |
| Prior Breast Health AH/LCIS in Biopsy | 6 | 25% | |
| Prior Breast Cancer | 4 | 17% | |
| Highest Education - High School Grad | 2 | 8% | |
| College Graduate | 13 | 54% | |
| Post Graduate Degree | 9 | 38% | |
Change in Anthropomorphic Variables, Dietary and Physical Activity
Energy intake was reduced by a median of 19%. Participants lost a median of 11% of their baseline weight with 54% losing 10% or more (Table 2). The median relative reduction in total and android fat was 8% and 10%, respectively. When participants were divided into those with <10% or >10% loss, only those with >10% weight loss showed a significant decrease in percent total fat, android fat, lean mass, and significant increase in physical activity (p<0.01 for each variable). Median energy change in the >10% loss group was −387 kcal/day versus −134 kcal/day for the <10% group (Table 2A). There was no significant difference at baseline in age, weight, BMI, or Gail risk for women losing >10% versus <10% weight (Supplement to Table 1). Using the median (11%) as the cut point instead of 10% did not alter these conclusions.
Table 2.
Change in Anthropomorphic Variables, Dietary and Physical Activity
| Variable | Median Pre- Study |
Median Post- Study |
Median Absolute Change |
Median Relative Change |
Change over Time. P-Value * |
|---|---|---|---|---|---|
| Weight, Kg | 92.8 | 79.0 | −10.0 | −11% | <0.001 |
| BMI, Kg/m2 | 34.2 | 30.2 | −3.6 | −10% | <0.001 |
| Percent body fat | 50.1 | 45.1 | −4.1 | −8% | <0.001 |
| Percent android fat | 54.0 | 48.7 | −5.2 | −10% | 0.001 |
| Percent lean mass | 48.4 | 52.8 | 3.4 | 7% | <0.001 |
| Waist circumference, cm | 98.2 | 89.6 | −9.0 | −9% | <0.001 |
| Energy (kcal; Cal) | 1840 | 1254 | −368 | −19% | 0.006 |
| Total Fat (g) | 60 | 31 | −28 | −46% | 0.001 |
| % Calories from Fat | 33 | 20 | −10 | −32% | 0.001 |
| Total Carbohydrates (g) | 199 | 178 | −13 | −6% | 0.44 |
| % Calories from Carbs | 51 | 60 | 8 | 15% | 0.010 |
| Total Proteins (g) | 59 | 65 | 3 | 6% | 0.57 |
| % Calories from Protein | 17 | 21 | 3 | 20% | 0.016 |
| Steps per week | 39625 | 63855 | 19574 | 69% | 0.002 |
| Physical Activity, minutes of exercise per week |
115 | 203 | 68 | 40% | 0.030 |
| Table 2A Change in DEXA and physical activity variables dichotomized by eventual weight loss of <10% vs >10%. |
| <10% Weight Loss Median Values (N=11) |
Change over Time. P-Value * |
>10% Weight Loss Median Values (N=13) |
Change over Time. P-Value * |
Compare Groups, P-Value □ |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Biomarker | Pre | Post | Change | Relative | P-value | Pre | Post | Change | Relative | P-value | P-Value |
| Weight, Kg | 97.4 | 92.8 | −6.8 | −7% | 0.008 | 86.9 | 76.9 | −12.8 | −16% | 0.001 | <0.001 |
| BMI, Kg/m2 | 33.6 | 32.3 | −2.7 | −7% | 0.008 | 34.8 | 29.6 | 4.9 | −16% | 0.001 | <0.001 |
| Percent body fat | 49.6 | 47.7 | 1.7 | −3% | 0.11 | 50.3 | 43.4 | −69 | −14% | 0.001 | 0.004 |
| Percent android fat | 54.4 | 53.2 | 0.6 | −1% | 0.31 | 53.1 | 46.1 | −8.2 | −14% | 0.001 | 0.002 |
| Percent lean mass | 49.0 | 51.0 | 1.5 | 3% | 0.17 | 48.2 | 54.6 | 6.2 | 14% | 0.001 | 0.007 |
| Waist circumference, cm | 98 | 98 | 7 | −7% | 0.011 | 98 | 89 | −11 | −11% | 0.001 | 0.008 |
| Energy (kcal; Cal) | 1336 | 1205 | −134 | −10% | 0.068 | 1880 | 1287 | −387 | −21% | 0.023 | 0.73 |
| Total Fat (g) | 63 | 30.5 | −30 | −40% | 0.066 | 58 | 31 | −28 | −46% | 0.004 | 0.73 |
| % Calories from Fat | 40 | 26 | −8 | −22% | 0.068 | 31 | 20 | −10 | −33% | 0.003 | 0.43 |
| Total Carbohydrates (g) | 168 | 186 | 29 | 14% | 0.72 | 213 | 178 | −25 | −15% | 0.25 | 0.50 |
| % Calories from Carbs | 45 | 52 | 9 | 19% | 0.11 | 53 | 60 | 7 | 15% | 0.039 | 0.57 |
| Total Proteins (g) | 54 | 65 | 7 | 10% | 0.72 | 85 | 66 | −7 | −8% | 0.48 | 0.57 |
| % Calories from Protein | 17 | 21 | 5 | 29% | 0.066 | 17 | 21 | 3 | 19% | 0.080 | 0.73 |
| Steps per week | 41741 | 41110 | 10300 | 33% | 0.14 | 38203 | 66670 | 38045 | 96% | 0.007 | 0.072 |
| Physical Activity | 110 | 165 | 30 | 33% | 0.68 | 120 | 230 | 90 | 75% | 0.007 | 0.25 |
Wilcoxon signed rank test (2-tailed) for assessment of change in values over time (Pre-study to Post-Study).
Wilcoxon signed rank test (2-tailed) assessment of change in values over time (Pre-study to Post-Study) within each of the two weight loss groups.
Mann-Whitney U test (2-tailed) for differences in Relative Change between the two weight loss groups. Statistically significant differences also indicated by gray shading for Relative Change values. Single underline indicates statistically significant differences in Pre-Study, Post-Study, or Absolute Change values between groups.
Change in Serum Biomarkers
Bioavailable estradiol and testosterone decreased by medians of 31% and 13%, respectively; fasting insulin by 40%, and CRP by 28% (Table 3). Favorably modulated adipocytokines (via Luminex assay) included adiponectin (increased by 19%), leptin (decreased by 37%), the ratio of adiponectin to leptin (increased by 130%), and hepatocyte growth factor which was decreased by 17%. Similar results for serum levels of adiponectin and leptin were obtained by ELISA as by Luminex (R2 of 0.87 and 0.50, respectively) (Supplement to Table 3). The 1-35% reductions observed for other serum pro-inflammatory cytokines including interleukin-6 (IL6), macrophage chemo-attractant protein (MCP-1), plasminogen activator inhibitor-1 (PAI-1), resistin, and tumor necrosis factor alpha (TNF alpha) were not significantly different when considering the group as a whole.
Table 3.
Change in Serum Biomarkers
| Biomarker | Median Pre- Study |
Median Post- Study |
Median Absolute Change |
Median Relative Change |
Change over Time. P-Value * |
|---|---|---|---|---|---|
| ELISA | |||||
| SHBG, nM | 19.3 | 27.3 | 5.2 | 39% | <0.001 |
| Estradiol, pM | 45.3 | 36.4 | −10.0 | −20% | 0.002 |
| Bioavailable Estradiol, pM | 0.91 | 0.63 | −0.24 | −31% | <0.001 |
| Testosterone, nM | 1.3 | 1.2 | 0.0 | 1.7% | 0.99 |
| Bioavailable Testosterone, pM | 23.9 | 18.5 | −2.6 | −13% | 0.033 |
| Prolactin, ng/ml | 7.1 | 7.7 | −0.4 | −6.5% | 0.15 |
| IGF−1, nM | 14.6 | 15.3 | 0.7 | 5.9% | 0.031 |
| IGFBP−3, nM | 226 | 208 | −5 | −2.0% | 0.092 |
| IGF1:IGFBP3 ratio | 0.064 | 0.075 | 0.009 | 8.8% | 0.001 |
| CRP, μg/ml | 3.1 | 1.6 | −1.2 | −28% | 0.002 |
| Glucose, mg/dL † | 93.5 | 89.5 | −4.0 | −4.2% | 0.25 |
| Insulin, μIU/ml † | 7.8 | 4.0 | −3.4 | −40% | 0.018 |
| HOMA Index † | 1.0 | 0.9 | −0.3 | −26% | 0.23 |
| Luminex | |||||
| Adiponectin, μg/ml ‡ | 10.7 | 12.1 | 1.7 | 19% | 0.001 |
| Leptin, ng/ml ‡ | 52.6 | 28.9 | −17.8 | −37% | <0.001 |
| Adiponectin:Leptin Ratio ‡ | 213 | 526 | 308 | 130% | <0.001 |
| lL−6, pg/ml ‡ | 2.3 | 1.8 | −0.8 | −35% | 0.11 |
| TNF-α, pg/ml | 8.0 | 7.8 | −0.1 | −2.6% | 0.45 |
| MCP−1, pg/ml | 392 | 412 | −6.0 | −1.4% | 0.84 |
| HGF, ng/ml | 2.3 | 2.2 | −0.4 | −17% | 0.011 |
| Resistin, ng/ml | 16.5 | 16.1 | −1.2 | −6.5% | 1.0 |
| PAI−1, ng/ml | 28.3 | 28.6 | −7.4 | −19% | 0.13 |
| Table 3A Change in serum biomarkers dichotomized by eventual weight loss of <10% vs >10%. |
| Biomarker | Median Values, <10% Weight Loss (N=11) |
Change over Time. P-Value * |
Median Values, >10% Weight Loss (N=13) |
Change over Time. P-Value * |
Compare Groups P-Value □ |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ELISA | Pre- | Post- | Change | Relative | P-value | Pre- | Post- | Change | Relative | P-value | P-Value |
| SHBG, nM | 14.2 | 15.2 | 1.7 | 11% | 0.16 | 26.4 | 49.3 | 15.0 | 57% | 0.002 | <0.001 |
| Estradiol, pM | 45.7 | 41.4 | 0.0 | 0% | 0..27 | 45.1 | 34.4 | −10.0 | −30% | 0.004 | 0.022 |
| Bioavailable Estradiol, pM |
1.0 | 0.7 | −0.0 | −5% | 0.11 | 0.9 | 0.5 | −0.3 | −36% | 0.002 | 0.007 |
| Testosterone, nM | 1.4 | 1.5 | 0.0 | 0% | 0.65 | 1.2 | 1.0 | 0.1 | 3% | 0.68 | 0.91 |
| Bioavailable Testosterone, pM |
25.0 | 29.0 | 0.1 | 2% | 0.42 | 17.7 | 13.8 | −3.3 | −17% | 0.036 | 0.17 |
| Prolactin, ng/ml | 8.3 | 7.8 | −0.4 | −6% | 0.11 | 6.6 | 7.6 | −0.4 | −7% | 0.81 | 0.39 |
| IGF−1, nM | 14.6 | 17.6 | 0.8 | 6% | 0.062 | 14.6 | 13.6 | 0.6 | 6% | 0.14 | 1.00 |
| IGFBP−3, nM | 227 | 209 | 2 | 1% | 0.93 | 218 | 201 | −7 | −4% | 0.075 | 0.15 |
| IGF1:IGFBP3 ratio | 0.67 | 0.077 | 0.004 | 8% | 0.016 | 0.063 | 0.071 | 0.011 | 13% | 0.023 | 0.42 |
| CRP, μg/ml | 2.7 | 1.8 | −1.4 | −8% | 0.21 | 3.5 | 1.4 | −1.0 | −39% | 0.001 | 0.17 |
| Glucose, mg/dL † | 98 | 91 | −6.0 | −7% | 0.35 | 91 | 89 | −3 | −3% | 0.60 | 0.39 |
| Insulin, μIU/ml † | 10.3 | 7.4 | −1.0 | −13% | 0.61 | 6.7 | 2.7 | −3.7 | −57% | 0.006 | 0.081 |
| HOMA Index † | 1.4 | 0.9 | −0.1 | −13% | 0.55 | 0.9 | 0.7 | −0.5 | −56% | 0.14 | 0.27 |
| Luminex | |||||||||||
| Adiponectin, μg/ml ‡ | 10.5 | 11.1 | 0.9 | 6% | 0.13 | 10.9 | 12.9 | 3.5 | 31% | 0.003 | 0.82 |
| Leptin, ng/ml ‡ | 72.4 | 56.2 | −18.3 | −21% | 0.050 | 36.0 | 17.7 | −17.2 | −61% | 0.002 | <0.001 |
| Adiponectin :Leptin Ratio ‡ |
120 | 259 | 73 | 50% | 0.062 | 296 | 805 | 360 | 341% | 0.002 | 0.006 |
| lL−6, pg/ml | 2.8 | 3.7 | −0.5 | −17% | 0.50 | 2.1 | 1.4 | −0.8 | −38% | 0.08 | 0.49 |
| TNF-α, pg/ml | 9.0 | 9.4 | −0.1 | −1% | 0.97 | 6.0 | 6.6 | −0.2 | −4% | 0.35 | 0.39 |
| MCP−1, pg/ml | 403 | 418 | 2 | 0.6% | 0.42 | 354 | 358 | −7 | −2% | 0.28 | 0.25 |
| HGF, ng/ml | 2.9 | 2.3 | −0.3 | −14% | 0.075 | 2.0 | 1.5 | −0.4 | −18% | 0.055 | 1.00 |
| Resistin, ng/ml | 14.5 | 15.7 | 2.1 | 34% | 0.59 | 22.2 | 16.5 | −2.4 | −8% | 0.38 | 0.49 |
| PAI−1, ng/ml | 35.7 | 37.5 | 1.3 | 6% | 0.37 | 25.1 | 22.0 | −10.5 | −36% | 0.003 | 0.005 |
Wilcoxon signed rank test (2-tailed) assessment of change in values over time (Pre-study to Post-Study).
Only included if both specimens drawn after overnight fasting.
Also performed by ELISA with similar results (See Supplement to Table 3).
Wilcoxon signed rank test (2-tailed) assessment of change in values over time (Pre-study to Post-Study) within each of the two weight loss groups.
Only included if both specimens drawn after overnight fasting.
Also performed by ELISA with similar results.
Mann-Whitney U test (2-tailed) for differences in Relative Change between the two weight loss groups. Statistically significant differences also indicated by gray shading for Relative Change values. Single underline indicates statistically significant differences in Pre-Study, Post-Study, or Absolute Change values between groups.
When dividing evaluable women into those with <10% vs >10% weight loss , a significant change for SHBG, bioavailable estradiol and testosterone, insulin, adiponectin, leptin, the ratio of adiponectin to leptin, PAI-1, and CRP was observed only for women with >10% weight loss. An increase in the ratio of IGF-1: IGFBP3 has been previously reported with weight loss [47] and was observed for both <10% and >10 % loss groups (Figure 1; Tables 3 and 3A).
Figure 1.
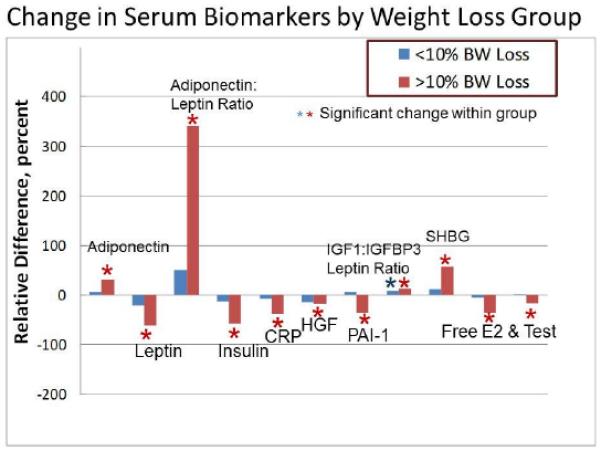
Representation of effects exhibited by various serum biomarkers. The relative difference (compared to baseline) is shown dichotomized into groups of women that lost <10% weight vs those that lost >10%. For all biomarkers (except PAI-1) there was a statistically significant effect of the intervention for the entire cohort of 24 evaluable subjects. The asterisks indicate a statistically significant change over time when analyzed separately for each of the two weight loss groups. For all biomarkers the effect was observed only in the >10% weight loss group; except for the molar ratio of IGF1:IGFBP3 where an increase was also observed for <10% weight loss.
Change in Breast Tissue Biomarkers
Change in benign breast cytomorphology and Ki-67
Ten of 24 women exhibited hyperplasia with atypia at baseline and four of 24 exhibited hyperplasia with atypia at study conclusion (p=0.070; McNemar). For the quantitative Masood score change, 14 decreased, 5 increased, and 5 stayed the same (p=0.042; Wilcoxon). For the 20 women who had a minimum of 500 cells on their Ki67 slide (as required for assessment), the median on study Ki-67 was 0.7% versus 0.3% off study. This difference was not statistically significant. For the 15 women with any Ki-67 at baseline and thus any chance of improvement, median baseline Ki-67 was 1.4% and post-intervention 0.4% (p=0.041) (Figure 2).
Figure 2.
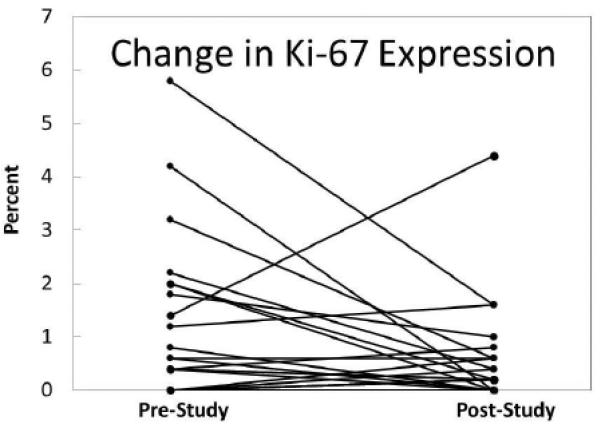
Change over the course of the study for immunocytochemical expression of Ki-67 in RPFNA specimens of benign breast tissue. Values represent the percent of cells (of 500 counted) that exhibit staining for Ki-67 antibody.
Change in benign breast adipocytokines, gene expression and proteomics
Tissue adipocytokine assessments were performed on paired frozen aliquots for 24 women and change reported only for those analytes which were measurable at baseline. The breast adiponectin to leptin ratio was increased by a median of 164% (p=0.003; Table 4) but when subdividing into < or >10% weight loss this ratio was significant different only for those with >10% loss (p=0.047) (Supplement to Table 4).
Table 4.
Changes in levels of cytokines and adipokines in RPFNA specimens, assessed by Luminex technology and expressed as relative to the amount of protein in each specimen. Analyses were confined to paired specimens where analyte was detectable in baseline sample.
| Biomarker | Median Pre- Study |
Median Post- Study |
Median Absolute Change |
Median Relative Change |
Change over Time. P-Value * |
|---|---|---|---|---|---|
| Adiponectin, pg/μg prot. | 89 | 89 | 23 | 48% | 0.11 |
| Leptin, pg/μg protein | 10.0 | 3.8 | −2.3 | −48% | 0.18 |
| Adipo:Leptin Ratio | 8.9 | 26.9 | 14.1 | 164% | 0.003 |
| TNF-α, fg/μg protein | 0.36 | 0.16 | −0.18 | −57% | 0.42 |
| MCP−1, pg/μg protein | 0.040 | 0.030 | 0.000 | −13% | 0.19 |
| HGF, ng/μg protein | 1.05 | 0.97 | −0.49 | −29% | 0.60 |
| NGF, fg/μg protein | 1.7 | 1.4 | −0.2 | −26% | 0.98 |
| IL−6, fg/μg protein | 1.8 | 0.6 | −0.4 | −31% | 0.075 |
| IL−8, fg/μg protein | 1.2 | 0.7 | 0.0 | −35% | 0.60 |
| Resistin, ng/μg protein | 1.14 | 1.16 | 0.0 | 1% | 0.75 |
| PAI−1, ng/μg protein | 0.26 | 0.15 | −0.04 | −16% | 0.55 |
Wilcoxon signed rank test (2-tailed) assessment of change in values over time (Pre-study to Post-Study).
Gene expression by RT-qPCR was performed for selected transcripts associated with proliferation, estrogen receptor activation, energy sensing and fatty acid metabolism pathways Change was noted for pS2, an estrogen inducible gene for women overall but was highly significant (p=0.005) for women with >10% weight loss. No significant differences were observed in other transcripts assessed. (Table 5) (See Supplement to Table 5 - RTqPCR for full list of transcripts).
Table 5.
Summary of favorable adipocytokine, mRNA, and proteomics changes in benign breast tissue, showing number of paired specimens exhibiting either a decrease or an increase in value.
|
Biomarker
(Assay Method) |
Total Cohort | Weight Loss < 10% | Weight Loss > 10% | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No. Dec |
No. Inc |
Change
over Time, P-Value ** |
No. Dec |
No. Inc |
Change over
Time. P-Value * |
No. Dec |
No. Inc |
Change
over Time, P-Value ** |
|
| Adiponectin:Leptin Ratio (Luminex) |
3 | 21 | 0.003 | 2 | 9 | 0.16 | 1 | 12 | 0.011 |
| pS2 (RT-qPCR) | 12 | 5 | 0.035 | 2 | 5 | 0.40 | 10 | 0 | 0.005 |
| CyclinB1 (RPPA; Epitomics 1495-1*) |
16 | 2 | 0.001 | 8 | 1 | 0.021 | 8 | 1 | 0.011 |
| Rb pS807-S811 (RPPA; CST 9308*) |
14 | 4 | 0.005 | 6 | 3 | 0.11 | 8 | 1 | 0.021 |
| S6 pS235-S236 (RPPA; CST 2211*) |
14 | 4 | 0.004 | 7 | 2 | 0.051 | 7 | 2 | 0.021 |
Antibody source and catalog number: CST=Cell Signaling Technology
Wilcoxon signed rank test (2-tailed) assessment of change in values over time (Pre-study to Post-Study).
Sufficient tissue was available for pre-post reverse phase proteomics analysis for 18 subjects The most significant changes were reduction in the proliferation associated proteins cyclin B1 (p=0.001) and phosphorylated retinoblastoma protein (p=0.005) [48-50], as well as ribosomal protein S6 (p=0.004), which is correlated with mTOR signaling [51]. All are thought to be important in development of breast cancer [49-51]. Although for cyclin B1 there was no significant difference in reduction for women with <10% vs >10% weight loss, phosphorylated retinoblastoma and S6 ribosomal protein were significantly modulated only for the >10% loss group. (Table 5) (See Supplement to Table 5- Protein Array for full list of proteins)
Correlation of Weight Loss with Biomarker Change
The relative change in serum adiponectin to leptin ratio was the variable with the greatest linear correlation with percent total weight and android fat loss (R2=0.45, p<0.001 and R2=0.53, p<0.001, respectively). Relative change in breast tissue adiponectin to leptin ratio was also correlated with percent android fat loss (R2=0.39, p=0.002).
Change in Quality of Life Indices
There were no serious study related adverse events and no significant change in quality of life as assessed by Breast Cancer Prevention Trial symptom scales, Brief Fatigue Inventory, and Mayo Hot Flash Score for the 18 subjects who completed the questionnaires at baseline and study conclusion [52-54]. The only significant change was improvement in bladder control (p<0.05).
Weight Regain after Trial Completion and Change in Tissue Biomarkers
There was no formal maintenance program and women began to regain weight at the conclusion of the intervention (Figure 3). Of the 13 subjects with >10% loss at 6 months, only five subjects at 12 months and three subjects at 24 months post study completion had maintained a >10% loss as measured during their follow-up visits to the Breast Cancer Prevention Center. Five participants had one or more RPFNAs post study completion. Tissue adiponectin to leptin ratios continued to improve for several months after initial loss but with eventual weight regain began to approach baseline values (data not shown).
Figure 3.
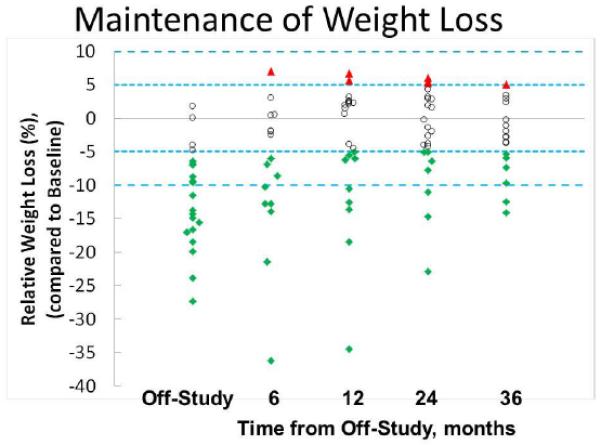
Time-dependent maintenance of weight loss following completion of the 6-month study. Weight loss is expressed as relative to the baseline weight at entry on study. Open circles represent women whose weight is within +/− 5% of baseline; solid triangles represent women whose weight is >5% of their baseline weight; and solid diamonds represent women who have achieved and maintained at least a 5% reduction of their on-study weight. Not all 24 evaluable subjects could be followed for the entire 36 months.
Discussion
Our pilot study was uniquely designed to study change in a number of breast tissue risk biomarkers along with anthropomorphic and serum variables in a homogenous group of postmenopausal women at increased risk for development of breast cancer. Weight loss was associated with favorable modulation of morphologic, proliferative, hormonal and pro-inflammatory biomarkers in benign breast tissue, as well as hormonal and pro-inflammatory biomarkers in serum When women were divided into those losing >10% or < 10% of initial weight (10% is the NHLBI suggested goal for initial weight loss), change in the majority of serum and benign breast tissue biomarkers was significant only for women with >10% weight loss. Other investigators have noted favorable serum risk biomarker change with ~10% weight loss, and greater change with more loss [55-58]; but we are the first to show the same relationship with breast tissue risk and mechanistic biomarkers.
Which markers might best serve as a primary response indicator in weight loss trials aimed at reducing risk for breast cancer? The serum adiponectin to leptin ratio, with its high linear association with weight loss, and correlation with the ratio in breast tissue, is an excellent candidate. Lower adiponectin to leptin ratios have been associated with breast cancer in cross sectional and nested case control studies within prospective cohort studies [23,59,60], but validation of the adiponectin to leptin ratio in additional prospective cohort studies or clinical trials is likely needed before it will be accepted as a surrogate response indicator for breast cancer. Serum bioavailable estradiol has been validated as a risk biomarker [9], but drawbacks to its use as the primary endpoint include lack of clarity as to the level of serum estradiol that constitutes a significant risk, intra-lab and assay variations, and the potential for non-detection in postmenopausal women unless ultra-sensitive techniques are used [61]. Higher levels of insulin PA-1 and IL6 are associated with poor prognosis in women with breast cancer [19, 24, 62, 63]. However they have not been validated in prospective cohort studies of postmenopausal women without prior breast cancer; nor do we know the levels associated with increased risk.
Although there are several good serum candidates, risk biomarkers in breast tissue are generally used as the primary response endpoint for phase II prevention trials [64]. Atypical hyperplasia or Ki-67 of 2% or higher in areas of hyperplasia are most frequently used because of the strong association with short term risk [65-67]. RPFNA is often used as a minimally invasive method to obtain benign tissue for prevention trials given the excellent tolerance, strong predictive ability of cytologic evidence of atypia in high risk women, and reproducibility in a multi-institutional setting [38,68]. Although an excellent risk marker, atypia’s qualitative and focal nature make it highly susceptible to interpretive and sampling variance. Further, RPFNA atypia was detected in only one-fourth of high risk overweight and obese postmenopausal women at baseline making it a less than ideal eligibility criteria and primary endpoint.
For postmenopausal women not on hormones, Ki-67 is impractical to use as a response endpoint given the very low level of expression. We found that only 16% of obese women had a baseline Ki-67 of 1.5% or more (our original minimum value for eligibility). The low proportion of women meeting the minimum Ki-67 forced us to amend the protocol, removing Ki-67 as an eligibility criterion and primary endpoint. Hofseth et al. reported a median Ki-67 of ~0.3% in histologically normal terminal lobular duct units adjacent to a benign breast biopsy from postmenopausal women not on hormone replacement [69]. We had previously reported a median Ki-67 of 1% in specimens obtained by RPFNA from postmenopausal women when there were sufficient cells for assessment (500 or more on slide) [41]. However, this prior cohort was not selected for BMI and the majority of women were taking hormone replacement therapy. Hormone replacement therapy was observed by Hofseth et al. to be associated with an increase in the proportion of terminal lobular duct unit cells expressing Ki-67 (median of ~0.7 % for estrogen alone and 2.5% with estrogen plus medoxyprogesterone acetate) [69]. Cyclin B1 is a proliferation associated protein is differentially expressed in normal tissue, proliferative breast disease, and cancer [50]. By reverse phase protein array it was present in all baseline breast specimens tested and thus is likely to be more prevalent than Ki-67 in postmenopausal women. If so, cyclin B1 in RPFNA specimens with atypia or hyperplasia borderline atypia (45% of our high risk cohort) would be a practical and biologically relevant primary endpoint. Change in pS2 mRNA, an indicator of estrogen response at the tissue level, is quantitative, biologically relevant and modulated with weight loss but was not detected in one-fourth of baseline samples in this postmenopausal cohort. Tissue adiponectin and leptin are quantitative, detected in all specimens, and the adiponectin to leptin ratio is significantly modulated with >10% weight loss. Breast tissue cyclin B1, pS2 and adiponectin to leptin ratio all have much to recommend them as primary response endpoints in energy balance trials focused on breast cancer risk reduction but should be validated as risk biomarkers in prospective cohort studies prior to widespread use for this purpose.
Our trial experience suggests steps that can be taken to enhance uptake of a weight management intervention. For otherwise eligible women, 36% declined participation because of either prolonged wait to start the intervention, the burden of driving to weekly in-person behavioral sessions, or use of only one brand of shelf stable meal. Our group has subsequently demonstrated the similar success of phone compared to in-person group lifestyle interventions [37,70] and good uptake and compliance with a variety of low calorie pre-packaged meals for portion control [71]. In order to avoid rapid weight regain as demonstrated for our cohort, a structured maintenance program promoting self-monitoring, consistent choice of low calorie foods, and regular physical activity daily should be built into future trials [72-75].
In summary, in addition to proliferation and atypical cytomorphology, we have identified hormonal and adipocytokine biomarkers in serum and benign breast tissue which are significantly modulated with weight loss and may be useful in future weight loss trials related to breast cancer risk. Many of these markers appear to be modulated only with a 10% or greater weight loss which should be the target for future trials aimed at breast cancer risk reduction in obese women.
Supplementary Material
Acknowledgments
Supported by grants 5R21CA121106 and U54-HD28934 from NIH, and the Breast Cancer Research Foundation (KUMC); 5R01CA129409 from NIH (UT Austin); CA16672 from NIH (MD Anderson Cancer Center). The University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core is supported by the Eunice Kennedy Shriver NICHD/NIH (SCCPIR) Grant U54-HD28934. Health Management Resources provided discounted packaged meals and shakes.
Footnotes
Contributions of Authors:
Fabian: PI responsible for recruitment, overall trial conduct performed RPFNA, writing; Kimler: regulatory, budget, initial data analysis, writing; Phillips: RT-qPCR, ELISAs and writing; Donnelley: design of weight management intervention; Sullivan: supervision of weight loss intervention; Zalles: cytomorphology; Metheny: cytomorphology preparation and Ki-67 readings; Petroff: supervision of laboratory assays; Aversman: study coordinator; Klemp: quality of life assessments; Mills: proteomics; Yeh: statistical considerations; Hursting: Luminex assays for adipocytokines. All authors were involved in manuscript editing.
Authors have no conflicts of interest to declare.
References
- 1.Huang Z, Hankinson SE, Colditz GA, et al. Dual effects of weight and weight gain on breast cancer risk. JAMA. 1997;278:1407–1411. [PubMed] [Google Scholar]
- 2.Ahn J, Schatzkin A, Lacey JV, Jr, Albanes D, Ballard-Barbash R, Adams KF, et al. Adiposity, adult weight change, and postmenopausal breast cancer risk. Arch Intern Med. 2007;167:2091–2102. doi: 10.1001/archinte.167.19.2091. [DOI] [PubMed] [Google Scholar]
- 3.Harvie M, Howell A, Vierkant RA, Kumar N, Cerhan JR, Kelemen LE, et al. Association of gain and loss of weight before and after menopause with risk of postmenopausal breast cancer in the Iowa women’s health study. Cancer Epidemiol Biomarkers Pre. 2005;14:656–661. doi: 10.1158/1055-9965.EPI-04-0001. [DOI] [PubMed] [Google Scholar]
- 4.Lahmann PH, Schulz M, Hoffmann K, Boeing H, Tjønneland A, Olsen A, et al. Long-term weight change and breast cancer risk: the European prospective investigation into cancer and nutrition (EPIC) Br J Cancer. 2005;93:582–589. doi: 10.1038/sj.bjc.6602763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eliassen AH, Colditz GA, Rosner B, Willett WC, Hankinson SE. Adult weight change and risk of postmenopausal breast cancer. JAMA. 2006;296:193–201. doi: 10.1001/jama.296.2.193. [DOI] [PubMed] [Google Scholar]
- 6.Manders P, Pijpe A, Hooning MJ, Kluijt I, Vasen HF, Hoogerbrugge N, et al. Body weight and risk of breast cancer in BRCA1/2 mutation carriers. Breast Cancer Res Treat. 2011;126:193–202. doi: 10.1007/s10549-010-1120-8. [DOI] [PubMed] [Google Scholar]
- 7.Subbaramaiah K, Howe LR, Bhardwaj P, Du B, Gravaghi C, Yantiss RK, et al. Obesity is associated with inflammation and elevated aromatase expression in the mouse mammary gland. Cancer Prev Res. 2011;4:329–346. doi: 10.1158/1940-6207.CAPR-10-0381. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8.Subbaramaiah K, Morris PG, Zhou ZK, Morrow M, Du B, Giri D, et al. Increased levels of COX-2 and prostaglandin E2 contribute to elevated aromatase expression in inflamed breast tissue of obese women. Cancer Discov. 2012;2:356–365. doi: 10.1158/2159-8290.CD-11-0241. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9.Endogenous Hormones and Breast Cancer Collaborative Group. Key TJ, Appleby PN, Reeves GK, Roddam AW, Helzlsouer KJ, Alberg AJ, et al. Circulating sex hormones and breast cancer risk factors in postmenopausal women: reanalysis of 13 studies. Br J Cancer. 2011;105:709–722. doi: 10.1038/bjc.2011.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morris PG, Hudis CA, Morrow M, Falcone DJ, Zhou XK. Inflammation and increased aromatase expression occur in the breast tissue of obese women with breast cancer. Cancer Prev Res. 2011;4:1021–1029. doi: 10.1158/1940-6207.CAPR-11-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hammarstedt A, Graham TE, Kahn BB. Adipose tissue dysregulation and reduced insulin sensitivity in non-obese individuals with enlarged abdominal adipose cells. Diabetol Metab Syndr. 2012 2012 Sep 19;4(1):42. doi: 10.1186/1758-5996-4-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coelho M, Oliveira T, Fernandes R. Biochemistry of adipose tissue: an endocrine organ. Arch Med Sci. 2013;9:191–200. doi: 10.5114/aoms.2013.33181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schairer C, Hill D, Sturgeon SR, Fears T, Pollak M, Mies C, Ziegler RG, Hoover RN, Sherman ME. Serum concentrations of IGF-I, IGFBP-3 and c-peptide and risk of hyperplasia and cancer of the breast in postmenopausal women. Int J Cancer. 2004;108:773–779. doi: 10.1002/ijc.11624. [DOI] [PubMed] [Google Scholar]
- 14.Probst-Hensch NM, Steiner JH, Schraml P, Varga Z, Zürrer-Härdi U, Storz M, Korol D, Fehr MK, Fink D, Pestalozzi BC, Lütolf UM, Theurillat JP, Moch H. IGFBP2 and IGFBP3 protein expressions in human breast cancer: association with hormonal factors and obesity. Clin Cancer Res. 2010;16:1025–1032. doi: 10.1158/1078-0432.CCR-09-0957. [DOI] [PubMed] [Google Scholar]
- 15.Hursting SD, Digiovanni J, Dannenberg AJ, Azrad M, Leroith D, Demark-Wahnefried W, Kakarala M, Brodie A, Berger NA. Obesity, energy balance, and cancer: new opportunities for prevention. Cancer Prev Res. 2012;5:1260–1272. doi: 10.1158/1940-6207.CAPR-12-0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bruning PF, Bonfrer JM, van Noord PA, Hart AA, Jong-Bakker M, Nooijen WJ. Insulin resistance and breast-cancer risk. Int J Cancer. 1992;52:511–516. doi: 10.1002/ijc.2910520402. [DOI] [PubMed] [Google Scholar]
- 17.Sieri S, Pala V, Brighenti F, Pellegrini N, Muti P, Micheli A, et al. Dietary glycemic index, glycemic load, and the risk of breast cancer in an Italian prospective cohort study. Am J Clin Nutr. 2007;86:1160–1166. doi: 10.1093/ajcn/86.4.1160. [DOI] [PubMed] [Google Scholar]
- 18.Tworoger SS, Eliassen AH, Kelesidis T, et al. Plasma adiponectin concentrations and risk of incident breast cancer. J Clin Endocrinol Metab. 2007;92:1510–1516. doi: 10.1210/jc.2006-1975. [DOI] [PubMed] [Google Scholar]
- 19.Vona-Davis L, Rose DP. Adipokines as endocrine, paracrine, and autocrine factors in breast cancer risk and progression. Endocr Relat Cancer. 2007;14:189–206. doi: 10.1677/ERC-06-0068. [DOI] [PubMed] [Google Scholar]
- 20.Becker S, Dossus L, Kaaks R. Obesity related hyperinsulinaemia and hyperglycaemia and cancer development. Arch Physiol Biochem. 2009;115:86–96. doi: 10.1080/13813450902878054. [DOI] [PubMed] [Google Scholar]
- 21.Maccio A, Madeddu C, Mantovani G. Adipose tissue as target organ in the treatment of hormone-dependent breast cancer: new therapeutic perspectives. Obesity Review. 2009;10:660–670. doi: 10.1111/j.1467-789X.2009.00592.x. [DOI] [PubMed] [Google Scholar]
- 22.Taliaferro-Smith L, Nagalingam A, Zhong D, Zhou W, Saxena NK, Sharma D. LKB1 is required for adiponectin-mediated modulation of AMPK-S6K axis and inhibition of migration and invasion of breast cancer cells. Oncogene. 2009;28:2621–2633. doi: 10.1038/onc.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grossmann ME, Ray A, Nkhata KJ, Malakhov DA, Rogozina OP, Dogan S, Cleary MP. Obesity and breast cancer: status of leptin and adiponectin in pathological processes. Cancer Metastasis Rev. 2010;29:641–653. doi: 10.1007/s10555-010-9252-1. [DOI] [PubMed] [Google Scholar]
- 24.Roberts DL, Dive C, Renehan AG. Biological mechanisms Linking Obesity and Cancer Risk: New perspectives. Ann Rev Med. 2010;61:301–316. doi: 10.1146/annurev.med.080708.082713. [DOI] [PubMed] [Google Scholar]
- 25.Mazzali G, Di Francesco V, Zoico E, Fantin F, Zamboni G, Benati C, Bambara V, Negri M, Bosello O, Zamboni M. Interrelations between fat distribution, muscle lipid content, adipocytokines, and insulin resistance: effect of moderate weight loss in older women. Am J Clin Nutr. 2006;84:1193–1199. doi: 10.1093/ajcn/84.5.1193. [DOI] [PubMed] [Google Scholar]
- 26.Harvie MN, Bokhari S, Shenton A, Ashcroft L, Evans G, Swindell R, Howell A. Adult weight gain and central obesity in women with and without a family history of breast cancer: a case control study. Fam Cancer. 2007;6:287–294. doi: 10.1007/s10689-007-9122-3. [DOI] [PubMed] [Google Scholar]
- 27.Zamboni M, Di Francesco V, Garbin U, Fratta Pasini A, Mazzali G, Stranieri C, Zoico E, Fantin F, Bosello O, Cominacini L. Adiponectin gene expression and adipocyte NF-kappaB transcriptional activity in elderly overweight and obese women: inter-relationships with fat distribution, hs-CRP, leptin and insulin resistance. Int J Obes. 2007;31:1104–1109. doi: 10.1038/sj.ijo.0803563. [DOI] [PubMed] [Google Scholar]
- 28.Karelis AD. Obesity: To be obese—does it matter if you are metabolically healthy? Nat Rev Endocrinol. 2011;7:699–700. doi: 10.1038/nrendo.2011.181. [DOI] [PubMed] [Google Scholar]
- 29.Shuster A, Patlas M, Pinthus JH, Mourtzakis M. The clinical importance of visceral adiposity: a critical review of methods for visceral adipose tissue analysis. Br J Radiol. 2012;85:1–10. doi: 10.1259/bjr/38447238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mason C, Katzmarzyk PT. Waist circumference thresholds for the prediction of cardiometabolic risk: is measurement site important? Eur J Clin Nutr. 2010;64:862–867. doi: 10.1038/ejcn.2010.82. [DOI] [PubMed] [Google Scholar]
- 31.Byers T, Sedjo RL. Does intentional weight loss reduce cancer risk? Diabetes Obes Metab. 2011;12:1063–1072. doi: 10.1111/j.1463-1326.2011.01464.x. [DOI] [PubMed] [Google Scholar]
- 32.Sjöström L, Gummesson A, Sjöström CD, Narbro K, Peltonen M, Wedel H, Bengtsson C, Bouchard C, Carlsson B, Dahlgren S, Jacobson P, Karason K, Karlsson J, Larsson B, Lindroos AK, Lönroth H, Näslund I, Olbers T, Stenlöf K, Torgerson J, Carlsson LM; Swedish Obese Subjects Study Effects of bariatric surgery on cancer incidence in obese patients in Sweden (Swedish Obese Subjects Study): a prospective, controlled intervention trial. Lancet Oncol. 2009;10:653–662. doi: 10.1016/S1470-2045(09)70159-7. [DOI] [PubMed] [Google Scholar]
- 33.Sjöström L, Gummesson A, Sjöström CD, Narbro K, Peltonen M, Wedel H, Bengtsson C, Bouchard C, Carlsson B, Dahlgren S, Jacobson P, Karason K, Karlsson J, Larsson B, Lindroos AK, Lönroth H, Näslund I, Olbers T, Stenlöf K, Torgerson J, Carlsson LM; Swedish Obese Subjects Study Executive summary of the clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. Arch Intern Med. 1998;158:1885–1867. doi: 10.1001/archinte.158.17.1855. [DOI] [PubMed] [Google Scholar]
- 34.Parker ED, Folsom AR. Intentional weight loss and incidence of obesity related cancers: the Iowa Women’s Health Study. Int J Obes Relat Metab Disord. 2003;27:1447–1452. doi: 10.1038/sj.ijo.0802437. [DOI] [PubMed] [Google Scholar]
- 35.Leblanc ES, O'Connor E, Whitlock EP, Patnode CD, Kapka T. Effectiveness of primary care-relevant treatments for obesity in adults: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2011;155:434–447. doi: 10.7326/0003-4819-155-7-201110040-00006. [DOI] [PubMed] [Google Scholar]
- 36.Moyer VA, U.S. Preventive Services Task Force Screening for and Management of Obesity in Adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157:373–378. doi: 10.7326/0003-4819-157-5-201209040-00475. [DOI] [PubMed] [Google Scholar]
- 37.Donnelly JE, Smith BK, Dunn L, Mayo MM, Jacobsen DJ, Stewart EE, Gibson C, Sullivan DK. Comparison of a phone vs clinic approach to achieve 10% weight loss. Int J Obes. 2007;31:1270–1276. doi: 10.1038/sj.ijo.0803568. [DOI] [PubMed] [Google Scholar]
- 38.Fabian CJ, Kimler BF, Zalles CM, Klemp JR, Kamel S, Zeiger S, Mayo MS. Short-term prediction of breast cancer by random peri-areolar fine needle aspiration cytology and Gail risk. J Natl Cancer Inst. 2000;92:1217–1227. doi: 10.1093/jnci/92.15.1217. [DOI] [PubMed] [Google Scholar]
- 39.Fabian CJ, Kimler BF, Zalles CM, Klemp JR, Petroff BK, Khan QJ, Sharma P, Setchell KD, Zhao X, Phillips TA, Metheny T, Hughes JR, Yeh HW, Johnson KA. Reduction in Ki-67 in benign breast tissue of high-risk women with the lignan secoisolariciresinol diglycoside. Cancer Prev Res. 2010;3:1342–1350. doi: 10.1158/1940-6207.CAPR-10-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Masood S, Frykberg ER, McLellan GL, Scalapino MC, Mitchum DG, Bullard JB. Prospective evaluation of radiologically directed fine-needle aspiration biopsy of nonpalpable breast lesions. Cancer. 1990;66:1480–1487. doi: 10.1002/1097-0142(19901001)66:7<1480::aid-cncr2820660708>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 41.Khan QJ, Kimler BF, Clark J, Metheny T, Zalles CM, Fabian CJ. Ki-67 expression in benign breast ductal cells obtained by random periareolar fine needle aspiration. Cancer Epidemiol Biomarkers Prev. 2005;14:786–789. doi: 10.1158/1055-9965.EPI-04-0239. [DOI] [PubMed] [Google Scholar]
- 42.National Task Force on the Prevention and Treatment of Obesity Dieting and the development of eating disorders in overweight and obese adults. Arch Intern Med. 2000;160:2581–2589. doi: 10.1001/archinte.160.17.2581. [DOI] [PubMed] [Google Scholar]
- 43.Vermeulen A, Verdonck G. Representativeness of a single point plasma testosterone level for the long term hormonal milieu in men. J Clin Endocrinol Metab. 1992;74:939–942. doi: 10.1210/jcem.74.4.1548361. [DOI] [PubMed] [Google Scholar]
- 44.Phillips TA, Fabian CJ, Kimler BF, Petroff BK. Assessment of RNA in human breast tissue sampled by random periareolar fine needle aspiration and ductal lavage and processed as fixed or frozen specimens. Reprod Biol. 2013;13:75–81. doi: 10.1016/j.repbio.2013.01.179. [DOI] [PubMed] [Google Scholar]
- 45.Hennessy BT, Lu Y, Gonzalez-Angulo AM, Carey MS, Myhre S, Ju Z, Davies MA, Liu W, Coombes K, Meric-Bernstam F, Bedrosian I, McGahren M, Agarwal R, Zhang F, Overgaard J, Alsner J, Neve RM, Kuo WL, Gray JW, Borresen-Dale AL, Mills GB. A Technical Assessment of the Utility of Reverse Phase Protein Arrays for the Study of the Functional Proteome in Non-microdissected Human Breast Cancers. Clin Proteomics. 2010;6:129–151. doi: 10.1007/s12014-010-9055-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moore T, Beltran L, Carbajal S, Strom S, Traag J, Hursting SD, DiGiovanni J. Dietary energy balance modulates signaling through the Akt/mammalian target of rapamycin pathways in multiple epithelial tissues. Cancer Prev Res. 2008;1:65–76. doi: 10.1158/1940-6207.CAPR-08-0022. [DOI] [PubMed] [Google Scholar]
- 47.Mason C, Xiao L, Duggan C, Imayama I, Foster-Schubert KE, Kong A, Campbell KL, Wang CY, Alfano CM, Blackburn GL, Pollack M, McTiernan A. Effects of dietary weight loss and exercise on insulin-like growth factor-1 and insulin-like growth factor binding protein-3 in postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2013;22:1457–1463. doi: 10.1158/1055-9965.EPI-13-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sidle A, Palaty C, Dirks P, Wiggan O, Kiess M, Gill RM, Wong AK, Hamel PA. Activity of the retinoblastoma family proteins, pRB, p107, and p130, during cellular proliferation and differentiation. Crit Rev Biochem Mol Biol. 1996;31:237–271. doi: 10.3109/10409239609106585. [DOI] [PubMed] [Google Scholar]
- 49.García-Tuñón I, Ricote M, Ruiz A, Fraile B, Paniagua R, Royuela M. Cell cycle control related proteins (p53, p21, and Rb) and transforming growth factor beta (TGFbeta) in benign and carcinomatous (in situ and infiltrating) human breast: implications in malignant transformations. Cancer Invest. 2006;24:119–125. doi: 10.1080/07357900500524314. [DOI] [PubMed] [Google Scholar]
- 50.Khan S, Brougham CL, Ryan J, Sahrudin A, O'Neill G, Wall D, Curran C, Newell J, Kerin MJ, Dwyer RM. miR-379 regulates cyclin B1 expression and is decreased in breast cancer. PLoS One. 2013 2013 Jul 10;8(7):e68753. doi: 10.1371/journal.pone.0068753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Magnuson B, Ekim B, Fingar DC. Regulation and function of ribosomal protein S6 kinase (S6K) within mTOR signalling networks. Biochem J. 2012;441:1–21. doi: 10.1042/BJ20110892. [DOI] [PubMed] [Google Scholar]
- 52.Stanton AL, Bernaards CA, Ganz PA. The BCPT symptom scales: a measure of physical symptoms for women diagnosed with or at risk for breast cancer. J Natl Cancer Inst. 2005;97:448–456. doi: 10.1093/jnci/dji069. [DOI] [PubMed] [Google Scholar]
- 53.Mendoza TR, Wang XS, Cleeland CS, Morrissey M, Johnson BA, Wendt JK, Huber SL. The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer. 1999;85:1186–1196. doi: 10.1002/(sici)1097-0142(19990301)85:5<1186::aid-cncr24>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 54.Sloan JA, Loprinzi CL, Novotny PJ, Barton DL, Lavasseur BI, Windschitl H. Methodologic lessons learned from hot flash studies. J Clin Oncol. 2001;19:4280–4290. doi: 10.1200/JCO.2001.19.23.4280. [DOI] [PubMed] [Google Scholar]
- 55.Klempel MC, Varaday KA. Reliability of leptin but not adiponectin as a biomarker for diet induced weight loss in humans. Nutrition Reviews. 2011;69:145–154. doi: 10.1111/j.1753-4887.2011.00373.x. [DOI] [PubMed] [Google Scholar]
- 56.Campbell KL, Foster-Schubert KE, Alfano CM, Wang CC, Wang CY, Duggan CR, Mason C, Imayama I, Kong A, Xiao L, Bain CE, Blackburn GL, Stanczyk FZ, McTiernan Reduced-calorie dietary weight loss, exercise, and sex hormones in postmenopausal women: randomized controlled trial. J Clin Oncol. 2012;30:2314–2326. doi: 10.1200/JCO.2011.37.9792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Imayama I, Ulrich CM, Alfano CM, Wang C, Xiao L, Wener MH, Campbell KL, Duggan C, Foster Schubert KE, Kong A, Mason CE, Wang CY, Blackburn GL, Bain CE, Thompson HJ, McTiernan A. Effects of a caloric restriction weight loss diet and exercise on inflammatory biomarkers in overweight/obese postmenopausal women: a randomized controlled trial. Cancer Res. 2012;72:2314–2326. doi: 10.1158/0008-5472.CAN-11-3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Abbenhardt C, McTiernan A, Alfano CM, Wener MH, Campbell KL, Duggan C, Foster-Schubert KE, Kong A, Toriola AT, Potter JD, Mason C, Xiao L, Blackburn GL, Bain C, Ulrich CM. Effects of individual and combined dietary weight loss and exercise interventions in postmenopausal women on adiponectin and leptin levels. J Intern Med. 2013;274:163–115. doi: 10.1111/joim.12062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Grossmann ME, Ray A, Dogan S, Mizuno NK, Cleary MP. Balance of adiponectin and leptin modulates breast cancer cell growth. Cell Res. 2008;18:1154–1156. doi: 10.1038/cr.2008.293. [DOI] [PubMed] [Google Scholar]
- 60.Ollberding NJ, Kim Y, Shvetsov YB, Wilkens LR, Franke AA, Cooney RV, Maskarinec G, Hernandez BY, Henderson BE, Le Marchand L, Kolonel LN, Goodman MT. Prediagnostic leptin, adiponectin, C-reactive protein, and the risk of postmenopausal breast cancer. Cancer Prev Res. 2013;6:188–195. doi: 10.1158/1940-6207.CAPR-12-0374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Santen RJ, Boyd NF, Chlebowski RT, Cummings S, Cuzick J, Dowsett M, Easton D, Forbes JF, Key T, Hankinson SE, Howell A, Ingle J; Breast Cancer Prevention Collaborative Group Critical assessment of new risk factors for breast cancer: considerations for development of an improved risk prediction model. Endocr Relat Cancer. 2007;14:169–187. doi: 10.1677/ERC-06-0045. [DOI] [PubMed] [Google Scholar]
- 62.Prieto-Hontoria PL, Pérez-Matute P, Fernández-Galilea M, Bustos M, Martínez JA, Moreno-Aliaga MJ. Role of obesity-associated dysfunctional adipose tissue in cancer: a molecular nutrition approach. Biochim Biophys Acta. 2011;807:664–678. doi: 10.1016/j.bbabio.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 63.Goodwin PJ, Ennis M, Pritchard KI, Trudeau ME, Koo J, Taylor SK, Hood N. Insulin- and obesity-related variables in early-stage breast cancer: correlations and time course of prognostic associations. J Clin Oncol 10. 2012;30:164–171. doi: 10.1200/JCO.2011.36.2723. [DOI] [PubMed] [Google Scholar]
- 64.Fabian CJ, Kimler BF, Mayo MS, Khan SA. Breast tissue sampling for risk assessment and prevention. Endocr Relat Cancer. 2005;12:185–213. doi: 10.1677/erc.1.01000. [DOI] [PubMed] [Google Scholar]
- 65.Hartmann LC, Sellers TA, Frost MH, Lingle WL, Degnim AC, Ghosh K, Vierkant RA, Maloney SD, Pankratz VS, Hillman DW, Suman VJ, Johnson J, Blake C, Tlsty T, Vachon CM, Melton LJ, Visscher DW. Benign breast disease and the risk of breast cancer. N Engl J Med. 2005;353:229–237. doi: 10.1056/NEJMoa044383. [DOI] [PubMed] [Google Scholar]
- 66.Shaaban AM, Sloane JP, West CR, Foster CS. Breast cancer risk in usual ductal hyperplasia is defined by estrogen receptor alpha and Ki-67 expression. Am J Pathol. 2002;160:597–604. doi: 10.1016/s0002-9440(10)64879-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Santisteban M, Reynolds C, Barr Fritcher EG, Frost MH, Vierkant RA, Anderson SS, Degnim AC, Visscher DW, Pankratz VS, Hartmann LC. Ki67: a time-varying biomarker of risk of breast cancer in atypical hyperplasia. Breast Cancer Res Treat. 2010;121:431–437. doi: 10.1007/s10549-009-0534-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ibarra-Drendall C, Wilke LG, Zalles C, Scott V, Archer LE, Lem S, Yee LD, Lester J, Kulkarni S, Murekeyisoni C, Wood M, Wilson K, Garber J, Gentry C, Stouder A, Broadwater G, Baker JC, Jr, Vasilatos SN, Owens E, Rabiner S, Barron AC, Seewaldt VL. Reproducibility of random periareolar fine needle aspiration in a multi-institutional Cancer and Leukemia Group B (CALGB) cross-sectional study. Cancer Epidemiol Biomarkers Prev. 2009;18:1379–1385. doi: 10.1158/1055-9965.EPI-08-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hofseth LJ, Raafat AM, Osuch JR, Pathak DR, Slomski CA, Haslam SZ. Hormone replacement therapy with estrogen or estrogen plus medroxyprogesterone acetate is associated with increased epithelial proliferation in the normal postmenopausal breast. J Clin Endocrinol Metab. 1999;84:4559–4565. doi: 10.1210/jcem.84.12.6194. [DOI] [PubMed] [Google Scholar]
- 70.Befort CA, Austin H, Klemp JR. Weight control needs and experiences among rural breast cancer survivors. Psychooncology. 2011;20:1069–1075. doi: 10.1002/pon.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Befort CA, Klemp JR, Austin HL, Perri MG, Schmitz KH, Sullivan DK, Fabian CJ. Outcomes of a weight loss intervention among rural breast cancer survivors. Breast Cancer Res Treat. 2012;132:631–639. doi: 10.1007/s10549-011-1922-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ogden J. The correlates of long-term weight loss: a group comparison study of obesity. Int J Obes Relat Metab Disord. 2000;24:1018–1025. doi: 10.1038/sj.ijo.0801354. [DOI] [PubMed] [Google Scholar]
- 73.Wing RR, Phelan S. Long-term weight loss maintenance. Am J Clin Nutr. 2005;82(1 Suppl):222S–225S. doi: 10.1093/ajcn/82.1.222S. [DOI] [PubMed] [Google Scholar]
- 74.Milsom VA, Middleton KM, Perri M. Successful long-term weight loss maintenance in a rural population. Clin Interv Aging. 2011;6:303–309. doi: 10.2147/CIA.S25389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Middleton KM, Patidar SM, Perri MG. The impact of extended care on the long-term maintenance of weight loss: a systematic review and meta-analysis. Obes Rev. 2012;13:509–517. doi: 10.1111/j.1467-789X.2011.00972.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


