Abstract
The resting behavior of Aedes albopictus was evaluated by aspirating diurnal resting mosquitoes from common landscape vegetation in residential communities in St. Augustine, FL. Energy reserves of the resting mosquitoes were analyzed to determine if there was a correlation between mosquito resting habitat and energy accumulation. Six species of plants were selected and 9 collections of resting mosquitoes were aspirated from each plant using a modified John W. Hock backpack aspirator during June and July 2012. Eight mosquito species were collected, with Ae. albopictus representing 74% of the overall collection. The number of Ae. albopictus collected varied significantly with the species of vegetation. When comparing the vegetation and abundance of resting mosquitoes, the highest percentages of Ae. albopictus were collected resting on Ruellia brittoniana (Mexican petunia), Asplenium platyneuron (fern), Gibasis geniculate (Tahitian bridal veil), followed by Plumba goauriculata (plumbago), Setcreasea pallida (purple heart), and Hibiscus tiliaceus (hibiscus). There were significant differences in lipid and glycogen accumulation based on type of vegetation Ae. albopictus was found resting in. Resting mosquitoes' sugar reserves were not influenced by species of vegetation. However, there was an overall correlation between vegetation that serves as a resting habitat and energy reserve accumulation. The results of our study demonstrate the potential to target specific vegetation for control of diurnal resting mosquitoes.
Keywords: Sugar feeding, mosquito control, landscaping, energy reserves, diurnal resting, Aedes albopictus
Introduction
The distribution of Aedes albopictus (Skuse) spatially and temporally has amplified the risk of arbovirus transmission and is a major public health concern (Lambrechts et al. 2010) as this species is considered the main vector in the global resurgence of dengue (Gubler 1998). Aedes albopictus is opportunistic and thrives in heavily vegetated habitats, making this species difficult to control (Hawley 1988, Braks et al. 2003). Combined with the reemergence of locally acquired dengue cases in Florida (Radke et al. 2012) and insufficient control methods, understanding the biology and behavior of this species is imperative for protection of public health.
Aedes albopictus needs regular sugar meals for nutrition and energy (Braks et al. 2006). Male and female mosquitoes ingest plant sugars in the form of floral and extrafloral nectar and fruit for flight energy and survival (Yuval 1992, Foster 1995, Müller et al. 2011). While sugar feeding preference of mosquitoes in nature is still largely unclear (Harada et al. 1975, 1976), this behavior more recently has been used to maximize vector control strategies (Xue et al. 2006, Müller et al. 2010a, Beier et al. 2012). Improving the knowledge of the diurnal resting and sugar feeding behavior of important vector or nuisance species can synergize with current control programs, providing more targeted application of insecticides. Therefore, we investigated the diurnal resting behavior of Ae. albopictus by aspirating resting mosquitoes from landscape vegetation found in residential communities in St. Augustine, FL. To better understand nonbloodfeeding behaviors of mosquitoes, the link between diurnal resting and sugar feeding on mosquito energy accumulation was analyzed.
Materials and Methods
Study area
We selected sites in and around residential communities in St. Augustine, FL, to sample diurnal resting mosquitoes from flowering and nonflowering plants commonly used in landscaping (Table 1). Landscape vegetation that dominated the yards and woods at the sample sites were palmetto (Seronoa repens Bartram), ornamental plants, live oak (Quercus virginiana Mill), as well as fern (Polystichum munitum Kaulfuss) and palm (Livistona chinensis Jacq.). Nine collections of resting mosquitoes from the 6 species of plants (Table 1) were conducted from mid-June to July, 2012.
Table 1.
Information on the vegetation sampled for resting mosquitoes.
| Scientific name and family | Common name | Plant type |
|---|---|---|
| Plumbago auriculata (Plumbaginaceae) | Blue Plumbago, Cape Plumbago, Cape Leadwort, Skyflower | Ornamental shrub, native to Tropical Africa Evergreen perennial shrub with vine-like habit |
| Hibiscus tiliaceus (Malvaceae) | Sea Hibiscus, Beach Hibiscus, Coastal (or Coast) Hibiscus, Coastal (or Coast) Cottonwood, Green Cottonwood, Native Hibiscus, Native Rosella, Cottonwood Hibiscus, Kurrajong, Sea Rosemallow | Native to the Old World tropics The leaves are alternate, ovate to lanceolate, often with a toothed or lobed margin. The flowers are large, trumpet-shaped, with 5 or more petals. The flowers of H. tiliaceus are bright yellow with a deep red center upon opening but, over the course of the day, gradually turns orange and finally red before falling |
| Ruellia brittoniana (Acanthaceae) | Mexican Petunia | Native of Mexico, the Caribbean, and South America. It has become a widespread invasive plant in Florida They have greenish-purple stems clad with sword-shaped green leaves. They thrive in a variety of conditions, ranging from wet pond margins to average garden soils |
| Setcreasea purpurea (Commelinaceae) | Purple Heart | Native to the Gulf Coast region of eastern Mexico Vibrant purple foliage with delicate lavender blooms and stems when grown in full sun |
| Asplenium platyneuron (Aspleniaceae) | Fern | This fern has erect, dark, evergreen fronds 6 to 20 in. tall. It is native throughout South Carolina Prefers some sun to light shade |
| Gibasis geniculate (Commelinaceae) | Tahitian Bridal Veil | Invasive in tropical areas A creeping plant with long, trailing stems that carry slender, lance-shaped leaves that are olive green on top and purple underneath. Small, airy, white flowers are held well out from the foliage and bloom from spring to fall |
Collections
A battery-modified Centers for Disease Control and Prevention backpack aspirator (Model 1412; John W. Hock Company, Gainesville, FL) was used to collect resting adult mosquitoes from vegetation. Mosquitoes were collected between 08:00 and 10:00 a.m. Before collecting the sample, a volunteer conducted a 1-min landing rate count (LRC) to determine the relative abundance of mosquitoes. The LRC provided information on mosquito populations and their distributions relative to plant vegetation. Mosquito aspirations were conducted if after the 1-min LRC mosquitoes were noticed flying or landing. A collection cup was inserted into the aspirating end of the suction vacuum and replaced with a new cup every time a new plant was sampled. The vegetation was aspirated for 5 min. After the collection, the cup was closed before turning the aspirator off. The collected mosquitoes were then brought back to the laboratory on dry ice, enumerated, sexed, and identified to species. The mosquitoes were saved in individual micro-centrifuge tubes at −70°C for later detection of energy reserves.
Laboratory sample preparation
The mosquitoes were transferred to 15-ml glass centrifuge tubes (Fisher Scientific, Pittsburg, PA) for extraction of sugar (fructose), glycogen, and lipid fractions to determine if the resting mosquitoes collected had energy reserves. Male and female mosquitoes were crushed individually with a pellet pestle motor in 0.2 ml of 2% sodium sulfate (NaSO4) solution and then 1.5 ml of a 1:2 chloroform–methanol solution was added and mixed vigorously. Next, the samples were centrifuged at 14,000 × g for 5 min, and equal volumes of supernatant were divided into 16- × 100-mm glass tubes for lipid and fructose analysis. The remaining precipitate was used for glycogen determination. At the respective wavelength for detection, each sample had a single lipid, sugar, and glycogen value as determined using the anthrone–sulfuric acid and vanillin–phosphoric reagent test (Van Handel 1985a, 1985b; Van Handel and Day 1988; Kaufmann and Brown 2008). The absorbance was read using a spectrophotometer that determined the micrograms per mosquito set at specific wavelengths (Jenway Model 6700, London, United Kingdom).
Statistical analysis
The number of resting Ae. albopictus was analyzed with a generalized linear model, using a negative binomial link. Planned comparisons were made among the various plants. The amount of glycogen, lipids, and fructose detected in the mosquitoes was highly variable. Therefore, we used a generalized linear model to fit a negative binomial model to the data. This provided a better fit to the data than a general linear model. The data from this analysis are presented as means and standard errors. The 0.05 significance level was used to determine statistical significance using SAS 9.3 (SAS Institute, Inc. 2012) for all analyses.
Results
Mosquito collections
Eight mosquito species were collected, with Ae. albopictus representing 74% of the overall collection (122/164). The other mosquito species were collected in low numbers, but Ae. taeniorhynchus (Wiedemann) was found resting in 4 of the 6 plants sampled and represented 10% of the collection (Table 2). Females represented 74.4% of the collections, with 65.0% being Ae. albopictus. All of the male mosquitoes collected were Ae. albopictus with the exception of 1 Culex quinquefasciatus (Say) male found resting in the Mexican petunia (Ruellia brittoniana Leonard). Although the females were collected twice as often as males, the collection of males and females were significantly different only in the fern (Asplenium platyneuron L.) (t = 2.88, P < 0.013; Fig. 1).
Table 2.
Number of female mosquitoes by species collected resting in the different vegetation sampled in St. Augustine, FL.1
| Mosquito species | Vegetation sampled | ||||
|---|---|---|---|---|---|
|
| |||||
| Mexican petunia | Tahitian bridal veil | Hibiscus | Fern | Plumbago | |
| Aedes atlanticus | 2 | ||||
| Ae. infirmatus | 3 | 7 | |||
| Ae. sollicitans | 5 | 6 | |||
| Ae. taeniorhynchus | 1 | 6 | 4 | 6 | |
| Ae. vexans | 2 | ||||
All mosquitoes collected except for Aedes albopictus, 1 male Culex quinquefasciatus collected on the Mexican petunia, and 1 Psorophora ferox female collected resting in the hibiscus.
Fig. 1.
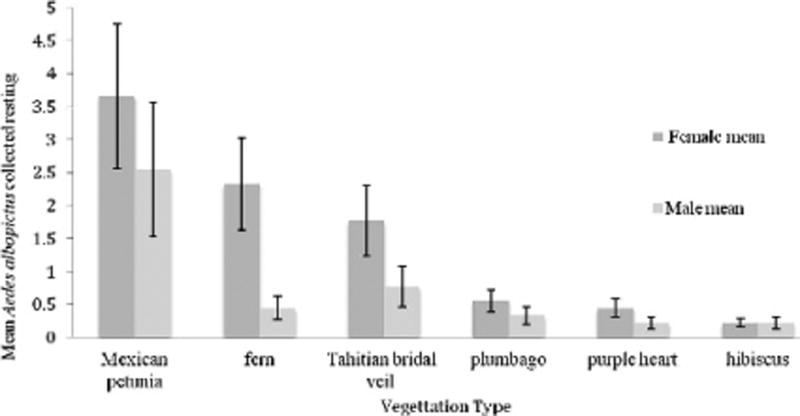
Numbers (mean ± SE) of female and male resting Aedes albopictus collected on the different vegetation sampled (n = 9).
The number of resting Ae. albopictus collected varied significantly with the type of vegetation (F = 2.47, df1,2 = 5, 48; P = 0.045). There were significantly more Ae. albopictus resting in the Mexican petunia and the fern, followed by the Tahitian bridal veil (Gibasis geniculate Jacq.), plumbago (Plumbago auriculata Lam), purple heart (Setcreasea pallida Rose), and hibiscus (Hibiscus tiliaceus L.).
Energy reserves
There were significant differences found in the glycogen reserves detected in resting Ae. albopictus (F = 5.16, df1,2 = 5, 116; P < 0.01; Table 3). Glycogen accumulation was highest in Ae. albopictus found resting in hibiscus, plumbago, and Mexican petunia, followed by Tahitian bridal veil, the fern, and purple heart. There were significant differences found in the amount of lipid reserves detected in resting Ae. albopictus (F = 4.69, df1,2 = 5, 116; P = 0.001; Table 3). Lipid accumulation was the highest in Ae. albopictus found resting in the Mexican petunia and hibiscus, followed by the fern, Tahitian bridal veil, plumbago, and purple heart. Fructose accumulation of Ae. albopictus was not significantly different (Table 3). A strong correlation was detected between accumulation of energy reserves and resting habitat of Ae. albopictus (r = 0.06, P < 0.001).
Table 3.
Mean concentration (μg) ± SE of glycogen, lipid, and sugar reserves detected in collected resting Aedes albopictus on the different vegetation sampled.
| Plant | Glycogen | Lipids | Fructose | ||||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| n | Mean1 | SE | Mean1 | SE | Mean1 | SE | |
| Mexican petunia | 56 | 33.2 a | 5.0 | 33.2 a | 4.7 | 28.0 | 3.4 |
| Purple heart | 6 | 9.6 b | 4.1 | 10.0 b | 4.4 | 11.9 | 4.7 |
| Fern | 25 | 13.0 b | 3.0 | 13.0 b | 3.0 | 19.7 | 4.1 |
| Hibiscus | 4 | 42.0 a | 21.4 | 24.0 a | 13.1 | 23.8 | 11.1 |
| Tahitian bridal veil | 23 | 15.0 b | 3.0 | 15.0 b | 3.2 | 18.4 | 3.4 |
| Plumbago | 8 | 35.3 a | 13.0 | 11.1 b | 4.2 | 20.0 | 7.1 |
Columns with different letters indicate significant differences at P < 0.05 using a generalized linear model.
Discussion
This study demonstrates that Ae. albopictus will rest during the daytime in vegetation commonly found in and around residential areas, thus suggesting that landscaping can influence species distribution within an area. Certain plants provide a more suitable resting habitat as shown in this study in St. Augustine, FL. Aedes albopictus attack rates tend to increase as the proximity to plants increases, highlighting the importance of vegetation with vector–human contact (personal observations of the authors). Further identifying the importance of the mosquito–vegetation relationship, we found a correlation between vegetation that serves as a resting habitat and energy reserve accumulation. This suggests that targeting vegetation that serves as a resting site could increase control efficacy and reduce the potential that vegetation serves in increasing vector–human contact.
This is the 1st report of energy reserves detected in field-collected Ae. albopictus. Day-to-day activities of the mosquito require an energy budget, and studies have demonstrated that energy reserves accumulated by laboratory mosquitoes deplete rapidly (Nayer and Sauerman 1975). Since laboratory-reared mosquitoes generally have higher energy reserves than their field conspecifics, depletion of these reserves should be consistent with what has been observed in the laboratory if not more rapidly (Day and Van Handel 1986). These reserves are replenished after the mosquito locates and feeds on a sugar meal. Studies have demonstrated that mosquitoes have flowering preferences for sugar feeding (Grimstad and DeFoliart 1974, Müller and Schlein 2005, Schlein and Müller 2008, Gouagna et al. 2010, Müller et al. 2010b). In our study, we established resting preferences by Ae. albopictus and a correlation with energy accumulation. Manda et al. (2007) showed that female Anopheles gambiae Meigen preference for different plants correlated with the relative fitness-related benefits the mosquito derives from feeding on the selected plant species. These findings suggest that mosquitoes preferentially feed on plants with a high sugar content, which in turn will confer a greater fitness advantage. Interestingly, Ae. albopictus acquired the greatest amount of total energy from the same plant this species was collected in greatest abundance, which supports the assumption of Manda et al. (2007).
Although we do not know for certain that energy reserves were acquired on the plants sampled, resting and sugar feeding within the same vegetation would also allow for an increase in fitness through energy conservation. This conservation of energy could in turn be used for other behaviors such as host seeking and oviposition. Manda et al. (2007) suggested that mosquitoes land on plants only to acquire a sugar meal. Thus, we assume that the number of mosquitoes caught per plant reflects the preference of the mosquitoes to feed on them as individual sugar sources. Another option may be that certain vegetation provides a more suitable resting habitat, i.e., holds more moisture, provides more shade from ultraviolet exposure, etc., and is therefore more favorable for the conservation of energy reserves acquired by the mosquito during sugar feeding. Most likely in different environments there are different plant–mosquito associations that need to be explored to provide a better understanding of how these behaviors can be exploited for effective control.
Furthermore, our findings support the targeting of diurnal resting behavior of mosquitoes. Based on our observations, mosquitoes were collected resting more often in thick, dense vegetation as seen with the Mexican petunia, fern, and Tahitian bridal veil. Barrier application of pyrethroids or attractive toxic sugar baits (ATSB) to the preferred resting vegetation of Ae. albopictus could be a viable option for this difficult-to-control species. The ATSB application for Ae. albopictus control has been successful in residential communities in St. Augustine, FL (Naranjo et al. 2013). Based on the current study findings, targeting nonflowering vegetation, like the fern, with combinations of barrier applications and spot target treatments that employ an attract-and kill strategy could synergize by targeting 2 specific nonbloodfeeding behaviors of mosquitoes. Thus, reducing area-wide applications of insecticides to control host-seeking mosquitoes overall will provide some cost benefit to control programs (Xue 2006, Qualls et al. 2012).
The resting behavior of Ae. albopictus deserves further research, as new approaches for integrated vector management for this species are urgently needed (Townson et al. 2005). Current control strategies of adulticiding and larviciding are not efficacious against this species as seen by its spread within the USA (Reiter and Gubler 1997). Comparable studies of mosquito resting behavior in other ecosystems are needed to determine how barrier treatment and ATSB applications can be maximized by targeting the diurnal resting mosquitoes.
Acknowledgments
The authors are grateful for the support of the Anastasia Mosquito Control District employees and the residents of the communities. This research was supported by the National Institutes of Health under Award Numbers R01AI100968 and R01GM093345. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References Cited
- Beier JC, Müller GC, Gu W, Arheart KL, Schlein Y. Attractive toxic sugar bait (ATSB) methods decimate populations of Anopheles malaria vectors in arid environments regardless of the local availability of favored sugar-source blossoms. Malar J. 2012;11:31. doi: 10.1186/1475-2875-11-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braks MAH, Honoria NA, Lourenco-de-Oliveira R, Juliano SA, Lounibos LP. Convergent habitat segregation of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) in southeastern Brazil and Florida. J Med Entomol. 2003;40:785–794. doi: 10.1603/0022-2585-40.6.785. [DOI] [PubMed] [Google Scholar]
- Braks MAH, Julian SA, Lounibos LP. Superior reproductive success on human blood without sugar is not limited to highly anthropophilic mosquito species. Med Vet Entomol. 2006;21:53–59. doi: 10.1111/j.1365-2915.2006.00612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day JF, Van Handel E. Differences between the nutritional reserves of laboratory-maintained and field-collected adult mosquitoes. J Am Mosq Control Assoc. 1986;2:154–157. [PubMed] [Google Scholar]
- Foster WA. Mosquito sugar feeding and reproductive energetics. Annu Rev Entomol. 1995;40:443–474. doi: 10.1146/annurev.en.40.010195.002303. [DOI] [PubMed] [Google Scholar]
- Gouagna LC, Poueme RS, Dabiré KR, Ouedraogo JB, Fontenille D, Simard F. Pattern of sugar feeding and host plant preferences in adult males of Anopheles gambiae (Diptera: Culicidae) J Vector Ecol. 2010;35:267–276. doi: 10.1111/j.1948-7134.2010.00082.x. [DOI] [PubMed] [Google Scholar]
- Grimstad PR, DeFoliart GR. Nectar sources of Wisconsin mosquitoes. J Med Entomol. 1974;11:331–341. doi: 10.1093/jmedent/11.3.331. [DOI] [PubMed] [Google Scholar]
- Gubler DJ. Dengue and dengue hemorrhagic fever. Clin Microbiol Rev. 1998;11:480–496. doi: 10.1128/cmr.11.3.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada F, Moriya K, Yabe T. Observations on the survival and longevity of Culex and Aedes mosquitoes fed on the flowers of nectar plants (IV) Jpn J Sanit Zool. 1975;26:193–201. [Google Scholar]
- Harada F, Moriya K, Yabe T. Observations on the survival and longevity of Culex and Aedes mosquitoes fed on the flowers of nectar plants (IV supplement) Jpn J Sanit Zool. 1976;27:307–309. [Google Scholar]
- Hawley WA. The biology of Aedes albopictus. J Am Mosq Control Assoc. 1988;1:1–39. [PubMed] [Google Scholar]
- Kaufmann C, Brown MR. Regulation of carbohydrate metabolism and flight performance by a hypertrehalosaemic hormone in the mosquito Anopheles gambiae. J Insect Physiol. 2008;54:367–377. doi: 10.1016/j.jinsphys.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrechts L, Scott TW, Gubler DJ. Consequences of the expanding global distribution of Aedes albopictus for dengue virus transmission. PLoS Neglected Trop Dis. 2010;4:e646. doi: 10.1371/journal.pntd.0000646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manda H, Gouagna LC, Nyandat E, Kabiru EW, Jackson RR, Foster WA, Githure JI, Beier JC, Hassanali A. Discriminative feeding behavior of Anopheles gambiae s.s. on endemic plants in western Kenya. Med Vet Entomol. 2007;21:103–111. doi: 10.1111/j.1365-2915.2007.00672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller GC, Beier JC, Traore SF, Toure MB, Traore MM, Bah S, Doumbia S, Schlein Y. Successful field trial of attractive toxic sugar bait (ATSB) plant-spraying methods against malaria vectors in the Anopheles gambiae complex in Mali, West Africa. Malar J. 2010a;9:210. doi: 10.1186/1475-2875-9-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller GC, Schlein Y. Plant tissues: the frugal diet of mosquitoes in adverse conditions. Med Vet Entomol. 2005;19:413–422. doi: 10.1111/j.1365-2915.2005.00590.x. [DOI] [PubMed] [Google Scholar]
- Müller GC, Xue RD, Schlein Y. Seed pods of the carbo tree Ceratonia siliqua are a favored sugar source for the mosquito Aedes albopictus in coastal Israel. Acta Trop. 2010b;116:235–239. doi: 10.1016/j.actatropica.2010.08.014. [DOI] [PubMed] [Google Scholar]
- Müller GC, Xue RD, Schlein Y. Differential attraction of Aedes albopictus in the field to flowers, fruits, and honeydew. Acta Trop. 2011;118:45–49. doi: 10.1016/j.actatropica.2011.01.009. [DOI] [PubMed] [Google Scholar]
- Naranjo DP, Qualls WA, Müller GC, Samson DM, Roque D, Alimi TO, Arheart K, Beier JC, Xue RD. Evaluation of boric acid sugar baits against Aedes albopictus (Diptera: Culicidae) in tropical environments. Parasitol Res. 2013;112:1583–1587. doi: 10.1007/s00436-013-3312-8. [DOI] [PubMed] [Google Scholar]
- Nayar JK, Sauerman DMJ. The effects of nutrition on survival and fecundity in Florida mosquitoes. 1. Utilization of sugar for survival. J Med Entomol. 1975;12:92–98. doi: 10.1093/jmedent/12.1.92. [DOI] [PubMed] [Google Scholar]
- Qualls WA, Smith ML, Müller GC, Zhao TY, Xue RD. Field evaluation of a large-scale barrier application of bifenthrin on a golf course to control floodwater mosquitoes. J Am Mosq Control Assoc. 2012;28:219–224. doi: 10.2987/12-6255R.1. [DOI] [PubMed] [Google Scholar]
- Radke EG, Gregory CJ, Kintziger KW, Sauber-Schatz EK, Hunsperger EA, Gallagher GR. Dengue outbreak in Key West, Florida, USA, 2009. Emerg Infect Dis. 2012;18:135–137. doi: 10.3201/eid1801.110130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter P, Gubler DJ. Surveillance and control of urban dengue vectors. In: Gubler DJ, Kuno G, editors. Dengue and dengue hemorrhagic fever. Wallingford, United Kingdom: CABI Publishing; 1997. pp. 1–22. [Google Scholar]
- SAS Institute, Inc. SAS 9.3 for Windows. Cary, NC: SAS Institute, Inc; 2012. [Google Scholar]
- Schlein Y, Müller G. An approach to mosquito control: using the dominant attraction of flowering tamarix jordanis trees against Culex pipiens. J Med Entomol. 2008;45:384–390. doi: 10.1603/0022-2585(2008)45[384:aatmcu]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Townson H, Nathan MB, Zaim M, Guillet P, Manga L, Bos R, Kindhauser M. Exploiting the potential of vector control for disease prevention. Bull WHO. 2005;83:942–947. [PMC free article] [PubMed] [Google Scholar]
- Van Handel E. Rapid determination of glycogen and sugar in mosquitoes. J Am Mosq Control Assoc. 1985a;1:299–301. [PubMed] [Google Scholar]
- Van Handel E. Rapid determination of total lipids in mosquitoes. J Am Mosq Control Assoc. 1985b;1:302–304. [PubMed] [Google Scholar]
- Van Handel E, Day JF. Assay of lipids, glycogen and sugars in individual mosquitoes: correlations with wing length in field-collected Aedes vexans. J Am Mosq Control Assoc. 1988;4:549–550. [PubMed] [Google Scholar]
- Xue RD. Adult mosquito behavior in relation to operational mosquito control programs mosquitoes. Tech Bull Fla Mosq Control Assoc. 2006;7:17–21. [Google Scholar]
- Xue RD, Kline DL, Ali A, Barnard DR. Application of boric acid baits to plant foliage for adult mosquito control. J Am Mosq Control Assoc. 2006;22:497–500. doi: 10.2987/8756-971X(2006)22[497:AOBABT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Yuval B. The other habit: sugar feeding by mosquitoes. Bull Soc Vector Ecol. 1992;17:150–156. [Google Scholar]


