Abstract
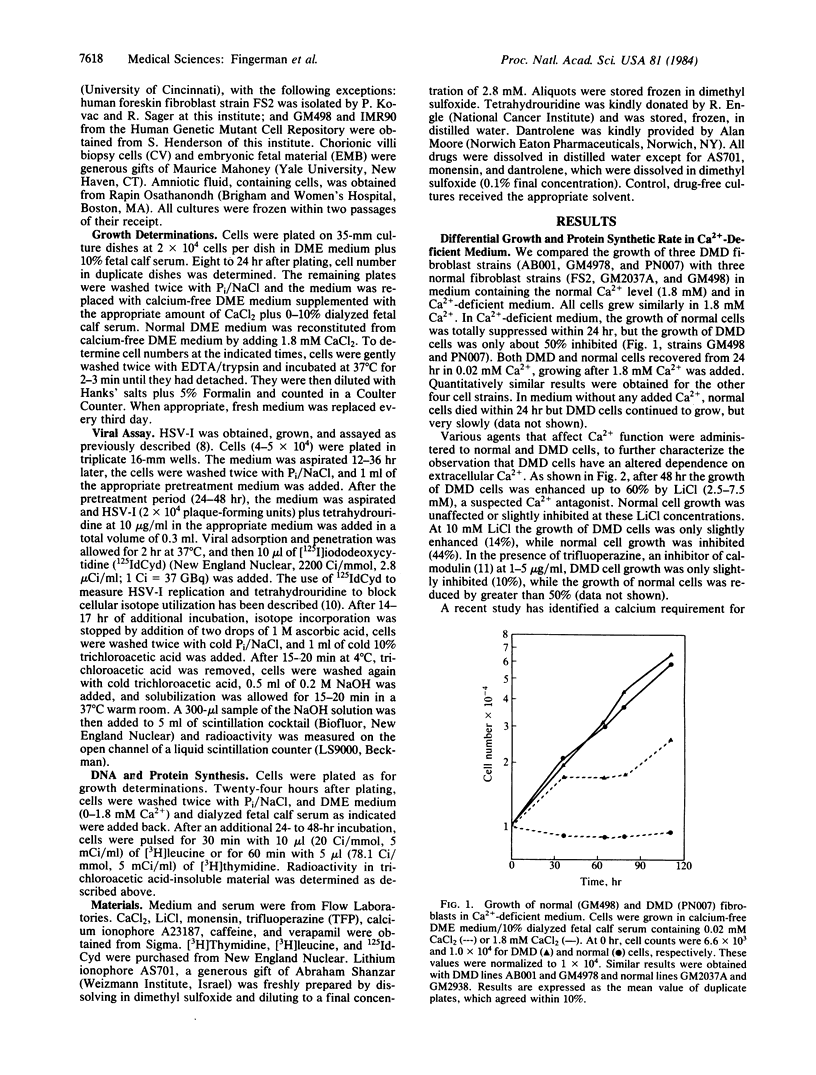
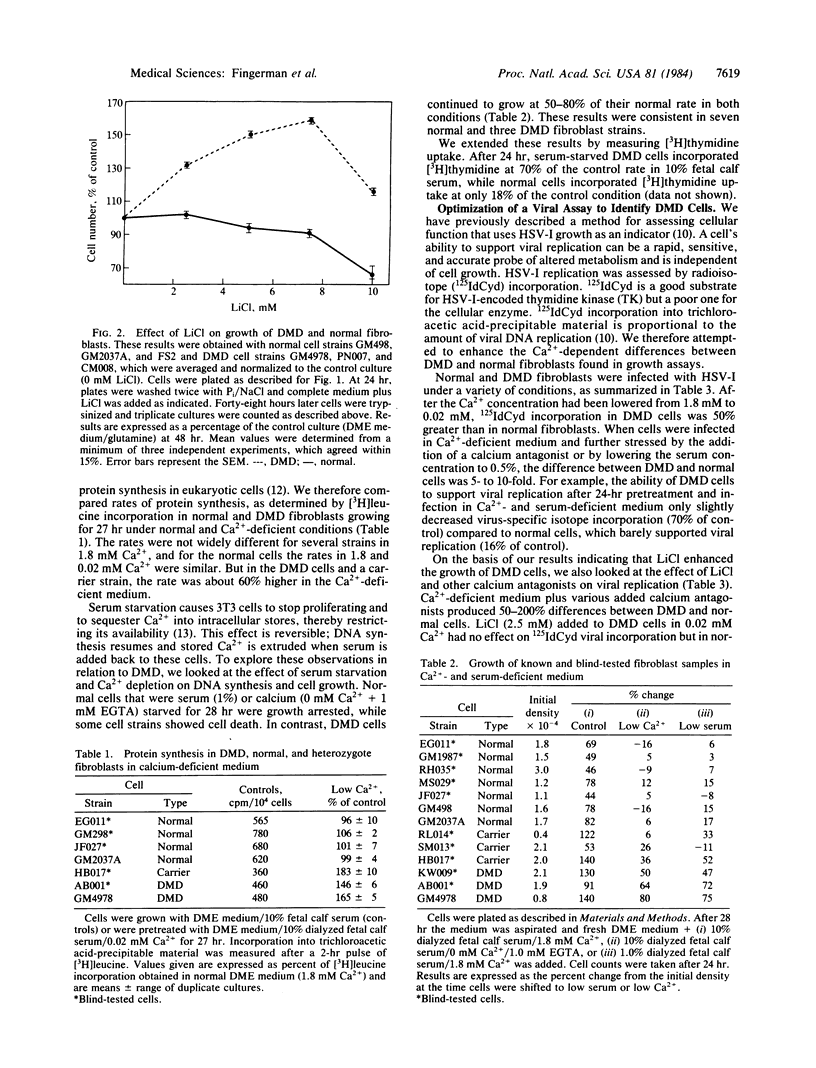
Normal fibroblasts in medium containing 0.02 mM CaCl2 arrested growth within 24 hr, whereas Duchenne muscular dystrophy fibroblasts continued to grow for 5 days, albeit at 40% of their rate in standard medium (1.8 mM CaCl2). Moreover, Duchenne cells in calcium-deficient medium showed an enhanced rate of protein synthesis (60% over the rate in standard medium), whereas normal cells were unaffected. Previously we described a general assay for detection of mutant cells by using herpes simplex virus I replication as a probe of cellular function. By altering the growth medium, one can elicit changes in viral DNA replication that depend upon cellular differences. Duchenne fibroblasts in calcium-deficient low-serum (0.5%) medium supported viral replication at a rate 7- to 10-fold greater than did normal cells infected under the same conditions. Using this viral assay, we have successfully identified all 10 samples of a blind coded set of Duchenne muscular dystrophy, normal, and heterozygote cells. In addition, differences of a lower magnitude were found between these cell strains as measured by cellular growth or protein synthesis. Therefore, a cell's ability to grow and support viral replication in calcium-deficient medium can be used to readily distinguish Duchenne muscular dystrophy fibroblasts from normal ones. These results suggest that the viral assay could be used as a prenatal diagnostic test. A defect related to calcium metabolism may be fundamental to this disease.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brostrom C. O., Bocckino S. B., Brostrom M. A. Identification of a Ca2+ requirement for protein synthesis in eukaryotic cells. J Biol Chem. 1983 Dec 10;258(23):14390–14399. [PubMed] [Google Scholar]
- Campisi J., Hafner J., Boorstein R., Pardee A. B. Hereditary orotic aciduria, Lesch-Nyhan syndrome, and xeroderma pigmentosum probed by herpes simplex virus: 125I-iododeoxycytidine incorporation as an assay for viral growth. J Cell Physiol. 1983 Jan;114(1):21–28. doi: 10.1002/jcp.1041140105. [DOI] [PubMed] [Google Scholar]
- Campisi J., Pardee A. B. Cellular mutations and drug resistance probed by herpes simplex virus. J Cell Physiol. 1981 Dec;109(3):469–480. doi: 10.1002/jcp.1041090313. [DOI] [PubMed] [Google Scholar]
- Emery A. E., Burt D. Intracellular calcium and pathogenesis and antenatal diagnosis of Duchenne muscular dystrophy. Br Med J. 1980 Feb 9;280(6211):355–357. doi: 10.1136/bmj.280.6211.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata M., Suematsu E., Koga T. Calmodulin antagonists inhibit Ca2+ uptake of mitochondria of guinea pig peritoneal macrophages. Biochem Biophys Res Commun. 1982 Apr 14;105(3):1176–1181. doi: 10.1016/0006-291x(82)91093-2. [DOI] [PubMed] [Google Scholar]
- Jones G. E., Witkowski J. A. Membrane abnormalities in Duchenne muscular dystrophy. J Neurol Sci. 1983 Feb;58(2):159–174. doi: 10.1016/0022-510x(83)90214-9. [DOI] [PubMed] [Google Scholar]
- Lucy J. A. Is there a membrane defect in muscle and other cells? Br Med Bull. 1980 May;36(2):187–192. doi: 10.1093/oxfordjournals.bmb.a071636. [DOI] [PubMed] [Google Scholar]
- Mahoney M. J., Haseltine F. P., Hobbins J. C., Banker B. Q., Caskey C. T., Golbus M. S. Prenatal diagnosis of Duchenne's muscular dystrophy. N Engl J Med. 1977 Nov 3;297(18):968–973. doi: 10.1056/NEJM197711032971803. [DOI] [PubMed] [Google Scholar]
- Margalit R., Shanzar A. New Li+-selective ionophores with the potential ability to mediate Li+-transport in vivo. Ionic selectivity and relative potencies, studied in model membranes. Pflugers Arch. 1982 Nov 1;395(2):87–92. doi: 10.1007/BF00584719. [DOI] [PubMed] [Google Scholar]
- Margalit R., Shanzer A. A study of Li+-selective permeation through lipid bilayer membranes mediated by a new ionophore (AS701). Biochim Biophys Acta. 1981 Dec 7;649(2):441–448. doi: 10.1016/0005-2736(81)90434-x. [DOI] [PubMed] [Google Scholar]
- McKeehan W. L. Control of normal and transformed cell proliferation by growth factor-nutrient interactions. Fed Proc. 1984 Jan;43(1):113–115. [PubMed] [Google Scholar]
- Pato C. N., Davis M. H., Doughty M. J., Bryant S. H., Gruenstein E. Increased membrane permeability to chloride in Duchenne muscular dystrophy fibroblasts and its relationship to muscle function. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4732–4736. doi: 10.1073/pnas.80.15.4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland L. P. Biochemistry of muscle membranes in Duchenne muscular dystrophy. Muscle Nerve. 1980 Jan-Feb;3(1):3–20. doi: 10.1002/mus.880030103. [DOI] [PubMed] [Google Scholar]
- Schneider E. L., Stanbridge E. J., Epstein C. J. Incorporation of 3H-uridine and 3H-uracil into RNA: a simple technique for the detection of mycoplasma contamination of cultured cells. Exp Cell Res. 1974 Mar 15;84(1):311–318. doi: 10.1016/0014-4827(74)90411-x. [DOI] [PubMed] [Google Scholar]
- Tupper J. T., Del Rosso M., Hazelton B., Zorgniotti F. Serum-stimulated changes in calcium transport and distribution in mouse 3T3 cells and their modification by dibutyryl cyclic AMP. J Cell Physiol. 1978 Apr;95(1):71–84. doi: 10.1002/jcp.1040950110. [DOI] [PubMed] [Google Scholar]



