Abstract
In present study free radical scavenging potential of aerial parts and root of Phyllanthus fraternus was investigated. Extraction was done in water and ethanol. Total antioxidant capacity was measured by DPPH free radical scavenging method; ethanolic extract of aerial part was most potent in activity with 50% inhibition at 258 μg/mL concentration. Lipid peroxidation (LPO) was measured in terms of thiobarbituric acid-reactive substances (TBARS) by using egg-yolk homogenates as lipid-rich media with EC50 of aerial part (ethanolic) 1522 μg/mL which was found to be most active. Superoxide (SO) radical scavenging activity was measured using riboflavin-light-nitroblue tetrazolium assay. Ethanolic and aqueous extract of both aerial part and root was almost similar in superoxide radical scavenging activity. Reducing power was determined on the basis of Fe3+-Fe2+ transformation in the presence of extract. Total phenolic and flavonoid contents were also measured by spectroscopic method. Results showed that the ethanolic fraction of aerial part is most active towards antioxidant potential and this activity is related to its polyphenolic content and reducing potential. Thus, P. fraternus extract can be used as potent natural antioxidant.
1. Introduction
Natural antioxidants have generated considerable interest in preventive medicine. Free radicals play very crucial role in occurrence of various neurodegenerative diseases and also accelerate aging [1, 2]. Free radicals associated with oxygen known as reactive oxygen species (ROS) are continuously produced during normal physiological processes. There are so many scavengers such as glutathione (GSH), super oxide dismutase (SOD), catalase (CAT), and so forth present in body for balancing of free radicals. In pathological conditions free radical formation is very high and oxidative stress cause damage of DNA, protein, lipid, and so forth. In this condition, internal antioxidants become insufficient for balancing of free radicals so external antioxidants are needed to prevent the oxidative damages by directly reacting with ROS, quenching them and/or chelating catalytic metal ions and also by scavenging free oxygen [3]. Plants contain a wide variety of free radical scavenging molecules, such as flavonoids, anthocyanins, carotenoids, dietary glutathione, vitamins, and endogenous metabolites [4, 5]. Among the various natural antioxidants, phenolic compounds possess significant antioxidant potential because it has characteristic of quenching oxygen-derived free radicals by donating a hydrogen atom or an electron to the free radicals [6].
Phyllanthus fraternus is a medicinal herb widely distributed in most tropical and subtropical countries [7]. It is extensively used in folk medicine in India and most other countries in the treatment of a broad spectrum of diseases, such as disturbances of the kidney and urinary bladder, intestinal infections, diabetes, and hepatitis for thousands of years [8, 9]. It also possesses significant astringent, deobstruent, diuretic, and antiseptic activity. There is no extensive study regarding antioxidant activity of different plant parts of P. fraternus. Koffuor and Amoateng [10] have reported antioxidant and anticoagulant property of fruit parts of P. fraternus. So, we have studied the antioxidant activity of ethanol and aqueous extracts derived from its aerial part and root.
2. Materials and Methods
2.1. Chemicals
1,1-Diphenyl,2-picryl hydrazyl (DPPH), nitroblue tetrazolium (NBT), riboflavin, L-methionine, thiobarbituric acid (TBA), ethylenediaminetetraacetic acid (EDTA), ascorbic acid, gallic acid, rutin and trichloroacetic acid (TCA), potassium ferricyanide (“K3[Fe(CN)6]”), and ferric chloride (“FeCl3”) were purchased from Hi-Media Ltd. All reagents were of analytical grade.
2.2. Plant Collection and Extract Preparation
Plants of P. fraternus were collected from the campus of Banaras Hindu University, Varanasi, during months of August and September. The plant was taxonomically identified by Professor N. K. Dubey, Botany Department, Banaras Hindu University. For preparation of extract, aerial parts and roots were washed thoroughly under running tap water, oven dried at 50–60°C for two days, and then powdered in a mechanical grinder. For aqueous extract preparation, about 250 g of the powdered material was boiled in 500 mL distilled water for 30 minutes, kept for 3 days with intermittent shaking [11]. For preparation of ethanolic extract each powdered sample (100 g) was extracted with 250 mL of ethanol by using a soxhlet extractor [12]. Extract was then filtered and evaporated to dryness at a 45°C with rotary evaporator. Their % yield (w/w) was calculated with original amount of coarse powder used for extraction. It was about 20.89 and 33% for ethanolic and aqueous aerial part, respectively. For root extract % yield was 10.56 and 13% for ethanolic and aqueous fraction, respectively.
2.3. DPPH Radical Scavenging Activity
The free radical scavenging activity of the extracts, based on the scavenging activity of the stable 1, 1-diphenyl-2-picrylhydrazyl (DPPH) free radical, was determined by slightly modified method described by Brand-Williams et al. [13]. Different concentration of plant extract were added to 3 mL of a 0.004% methanolic solution of DPPH and incubated for 15 minutes at room temperature. Absorbance was recorded at 517 nm by using spectrophotometer (Thermo Scientific UV1).
2.4. Lipid Peroxidation Assay
A modified thiobarbituric acid-reactive species (TBARS) assay [14] was used to measure the lipid peroxide formed, using egg-yolk homogenates as lipid-rich media [15]. Malondialdehyde (MDA), a secondary product of the oxidation of polyunsaturated fatty acids, reacts with two molecules of thiobarbituric acid (TBA), yielding a pinkish red chromogen with an absorbance maximum at 532 nm [16]. Egg homogenate (250 μL, 10% in distilled water, v/v) and 50 μL of extract were mixed in a test tube and the volume was made up to 500 μL, by adding distilled water. Finally, 25 μL “FeSO4” (0.07 M) was added to the above mixture and incubated for 30 min, to induce lipid peroxidation. Thereafter, 750 μL of 20% acetic acid (pH 3.5) and 750 μL of 0.8% TBA (w/v) (prepared in 1.1% sodium dodecyl sulphate) and 25 μL 20% TCA were added, vortexed, and then heated in a boiling water bath for 60 min. After cooling, 3.0 mL of 1-butanol was added to each tube and centrifuged at 3000 rpm for 10 min. The absorbance of the organic upper layer was measured against 3 mL butanol at 532 nm. For the blank 50 μL of distilled water was used in place of the extract.
2.5. Superoxide Radical Scavenging Property
This assay was based on the capacity of the extract to inhibit the photochemical reduction of nitroblue tetrazolium (NBT) [17]. In brief, each 3 mL reaction mixture contained 0.01 M phosphate buffer solution (PBS) (pH 7.8), 130 mM methionine, 60 μM riboflavin, 0.5 mM EDTA, 0.75 mM NBT, and 0.5 mL of test sample solution. It was kept in front of fluorescent light for 6 minutes and absorbance was taken at 560 nm. Identical tubes containing reaction mixture were kept in the dark and served as controls. The percentage inhibition of superoxide generation was measured by comparing the absorbance of the control and those of the reaction mixture containing test sample. The blank was 0.01 M PBS.
2.6. Reducing Power (RP)
The reducing power of both aqueous and ethanolic extracts of P. fraternus was determined according to the method described by [18]. Different concentrations (50–400 μg) of extracts were mixed with phosphate buffer (2.5 mL, 0.2 M, pH 6.6) and potassium ferricyanide [“K3Fe(CN)6”] (2.5 mL, 1%). The mixture was incubated at 37°C for 20 min after that 2.5 mL of trichloroacetic acid (TCA, 10%) was added to the mixture which was then centrifuged at 1000 rpm for 10 min. The upper organic layer of solution (2.5 mL) was taken and mixed with distilled water (2.5 mL) and “FeCl3” (0.5 mL, 0.1%), and the absorbance of reaction mixture was measured at 700 nm. Increased absorbance of the reaction mixture indicated high reducing power. Ascorbic acid was used as standard.
2.7. Measurement of Total Phenolics (TP)
TP concentration was measured by Folin-Ciocalteu assay [19]. Briefly, 1 mL of distilled water, 0.1 mL of 1 mg/mL sample, and 0.2 mL of Folin-Ciocalteu reagent were added in test tube; then contents were mixed and allowed to stand for 5–8 min at room temperature. Next, 2 mL of 7% sodium carbonate solution were added, followed by 0.7 mL distilled water to make 3 mL reaction mixture. Solutions were mixed and allowed to stand at room temperature for 15 min, and then absorbance was recorded at 750 nm. Phenolic contents were estimated by using a standard curve obtained from various concentration of gallic acid and expressed as milligrams per gram of gallic acid equivalents (GAE).
2.8. Measurement of Total Flavonoids (TF)
AlCl3 colorimetric method was used for TF determination [20]. Each plant extract (0.1 mL of 10 mg/mL) in ethanol was mixed with 0.1 mL of 2% AlCl3, 0.1 mL of 1 M potassium acetate, and 2.7 mL of ethanol. The reaction mixture was kept at room temperature for 30 min and absorbance was taken at 415 nm. TF content was calculated using rutin as standard and expressed as milligrams per gram of rutin equivalents (RE).
2.9. Determination of Percentage Inhibition and Statistical Analysis
Percentage inhibition was calculated as [A 0 − A t/A 0 × 100]. A 0 is the absorbance of the control and A t is the absorbance of the test samples/standard. All experiments were performed in triplicate and data are expressed as means ± SE. Statistical comparisons were made by means of one-way ANOVA test followed by post hoc analysis with Dunnett test by using SPSS (version 16). P values ≤0.001 were considered highly significant. EC50 values were calculated from linear regression analysis.
3. Results
3.1. DPPH Radical Scavenging Assay
From Table 1 it is evident that all extracts have significant free radical scavenging activity. Ethanolic extract of aerial part have greater scavenging activity (EC50 = 258 μg/mL) than aqueous extract (EC50 = 360 μg/mL). Similarly, ethanolic extracts of roots also have greater activity (EC50 = 337 μg/mL) than its aqueous extract (EC50 = 3038 μg/mL).
Table 1.
DPPH free radical scavenging activity of P. fraternus aqueous and ethanolic extracts of aerial part and root.
| Aerial part | Root | ||||
|---|---|---|---|---|---|
| Concentration (µg/mL) | Aqueous extract | Ethanol extract | Ethanol extract | Concentration (µg/mL) | Aqueous extract |
| Percentage inhibition (mean ± SE) |
Percentage inhibition (mean ± SE) |
Percentage inhibition (mean ± SE) |
Percentage inhibition (mean ± SE) |
||
| 50 | 10.28 ± 0.72 | 8.47 ± 0.47 | 5.67 ± 0.68** | 100 | 8.47 ± 0.28** |
| 100 | 15.09 ± 0.51** | 18.64 ± 0.99** | 11.72 ± 1.36 | 500 | 15.29 ± 0.67** |
| 200 | 31.23 ± 1.54** | 45.99 ± 1.15** | 32.85 ± 0.54** | 1000 | 24.35 ± 1.11** |
| 300 | 40.16 ± 1.33** | 61.18 ± 1.32** | 39.28 ± 1.68** | 2000 | 35.46 ± 0.61** |
| 400 | 54.04 ± 1.91** | 73.96 ± 1.01** | 57.77 ± 1.50** | 3000 | 48.40 ± 0.52** |
| 500 | 62.92 ± 1.89** | 94.59 ± 1.10** | 64.91 ± 1.33** | 4000 | 63.86 ± 0.78** |
| 5000 | 77.98 ± 0.76** | ||||
| EC50 | 360 | 258 | 337 | EC50 | 3038 |
EC50 of Ascorbic acid is 88.96.
**Significant at P < 0.001.
3.2. Superoxide Scavenging Assay
Both the aqueous and ethanolic extract of aerial part exhibited potent scavenging activity for superoxide radicals in a concentration dependent manner with almost similar EC50 values of 52 and 55 μg/mL, respectively (Table 2). Root extracts (aqueous and alcoholic) also exhibited good scavenging activity. Ethanolic extract of root is more potent in activity than its aqueous extract with EC50 values of 201 μg/mL which is less than EC50 of latter (391 μg/mL) (Table 2).
Table 2.
Superoxide radical scavenging activity of P. fraternus aqueous and ethanolic extracts of aerial part and root.
| Aerial part | Root | ||||
|---|---|---|---|---|---|
| Concentration (µg/mL) | Aqueous extract | Ethanol extract | Concentration (µg/mL) | Aqueous extract | Ethanol extract |
| Percentage inhibition (mean ± SE) |
Percentage inhibition (mean ± SE) |
Percentage inhibition (mean ± SE) |
Percentage inhibition (mean ± SE) |
||
| 20 | 1.81 ± 0.32 | 6.64 ± 1.53 | 50 | 14.08 ± 0.93** | 7.6 ± 0.88** |
| 40 | 48.04 ± 0.86** | 30.06 ± 1.36** | 100 | 17.73 ± 0.10** | 29.20 ± 0.46** |
| 60 | 64.51 ± 0.32** | 77.32 ± 2.95** | 200 | 24.27 ± 1.21** | 62.14 ± 1.08** |
| 80 | 80.32 ± 0.46** | 88.81 ± 0.56** | 400 | 49.78 ± 0.51** | 88.09 ± 0.94** |
| 100 | 99.98 ± 0.03** | — | 600 | 78.02 ± 0.20** | 95.55 ± 0.48** |
| 800 | 93.72 ± 0.49** | — | |||
| EC50 | 52 | 55 | EC50 | 391 | 201 |
EC50 of Copper sulphate is 4.18.
**Significant at P < 0.001.
3.3. Lipid Peroxidation Assay
Both extracts of P. fraternus inhibited lipid peroxidation, induced by ferrous sulfate in egg-yolk homogenates in a concentration dependent manner. Interestingly, there was no significant difference in the EC50 values of both ethanolic and aqueous extract of aerial part which is about 1522 μg/mL and 1533 μg/mL, respectively (Table 3). Aqueous extracts of root have less lipid peroxidation inhibitory activity than ethanolic extract with EC50 value of 3547 μg/mL and 1957 μg/mL, respectively (Table 3).
Table 3.
Lipid peroxidation activity of P. fraternus aqueous and ethanolic extracts of aerial part and root.
| Concentration (µg/mL) | Percentage inhibition (mean ± SE) | |||
|---|---|---|---|---|
| Aerial Part | Root | |||
| Aqueous extract | Ethanol extract | Aqueous extract | Ethanol extract | |
| 500 | 18.22 ± 1.05 | 20.76 ± 1.05 | — | 15.97 ± 0.62** |
| 1000 | 42.0 ± 1.73** | 47.92 ± 3.84** | 9.85 ± 4.66 | 39.00 ± 0.41** |
| 2000 | 62.34 ± 1.52** | 63.22 ± 0.75** | 31.16 ± 1.18** | 54.83 ± 0.27** |
| 3000 | 81.07 ± 0.64** | 83.45 ± 0.54** | 45.54 ± 0.12** | 69.74 ± 0.82** |
| 4000 | 93.34 ± 0.48** | 96.55 ± 0.27** | 57.04 ± 1.63** | 83.92 ± 0.62** |
| 6000 | — | — | 85.57 ± 0.30** | — |
| EC50 | 1533 |
1521 |
3547 | 1957 |
EC50 of Ascorbic acid is 643.89.
**Significant at P < 0.001.
3.4. Reducing Power Assay
The reducing power of both aqueous and ethanolic extracts increased in concentration dependant manner. Ethanolic extracts of both aerial part and root have greater reducing power in comparison to their aqueous extract. There is no significant difference in reducing power of both extracts of aerial parts while aqueous extracts of root have lesser reducing capacity in comparison to its ethanolic extract (Figures 1(a) and 1(b)).
Figure 1.
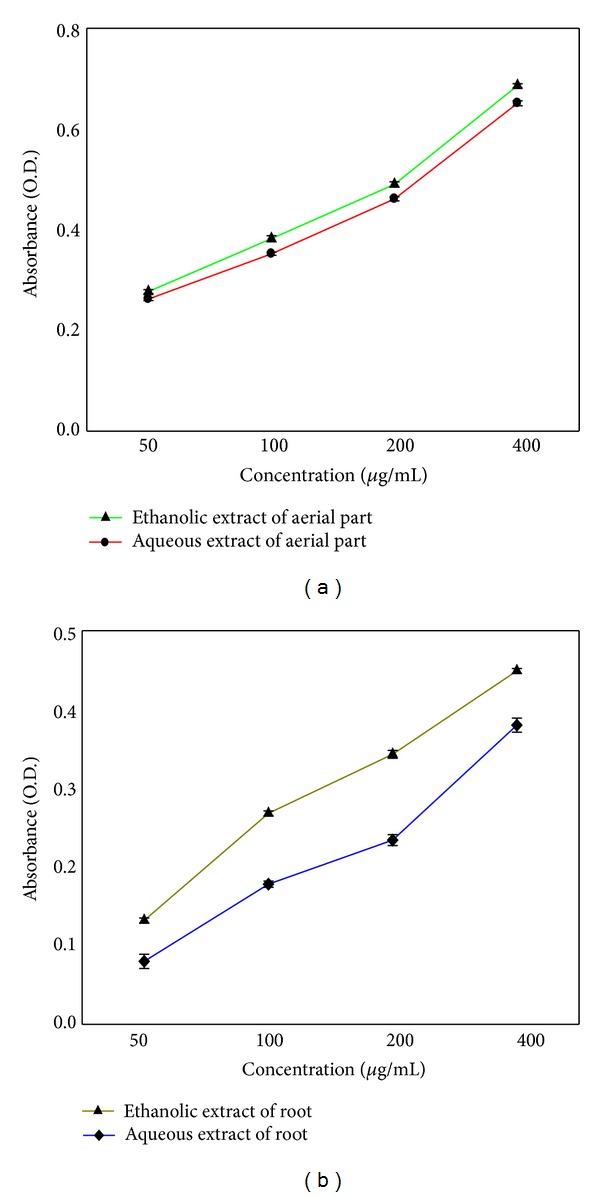
Reducing power of extracts of P. fraternus. (a) Ethanolic and aqueous extracts of aerial part. (b) Ethanolic and aqueous extracts of root. Results are mean ± SE of three independent experiments.
3.5. Total Phenolic Content
TP content, as determined by Folin-Ciocalteu's method, is reported as GAE by reference to standard curve (y = 0.026x, R 2 = 0.993). Phenolic content is higher in the ethanolic extract of aerial part than its aqueous extract which is about 230.85 ± 0.59 mg/g and 161.92 ± 14.12 mg/g GAE, respectively. Similarly, ethanolic extract of root is greater in phenolic content (118.94 ± 2.69 mg/g GAE) than aqueous extract (98.37 ± 5.47 mg/g GAE) (Figure 2).
Figure 2.
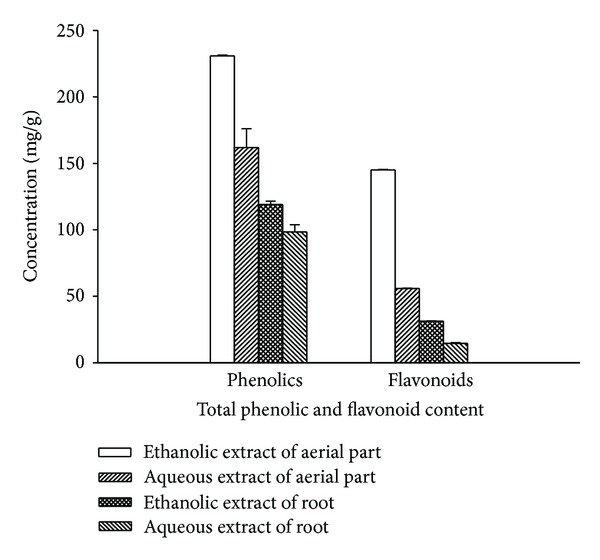
Total phenolic and flavonoid content in P. fraternus aerial part and root.
3.6. Total Flavonoid Content
Total flavonoid content was determined by AlCl3 colorimetric method and reported as RE by reference to standard curve (y = 0.009x, R 2 = 0.998). Total flavonoids are higher in ethanolic extract of both plant parts than aqueous extract. Ethanolic extracts of aerial part have flavonoid content of 145.03 ± 0.26 mg/g RE while aqueous extracts have 55.83 ± 0.31 mg/g RE. Total flavonoid content is about 31.23 ± 0.14 mg/g and 14.60 ± 0.52 mg/g RE in ethanolic and aqueous part of root, respectively (Figure 2).
3.7. Correlation between Total Antioxidant Activity and Polyphenolic Contents (TP&TF)
Very close correlation was obtained between total antioxidant activity and polyphenolic contents (TP&TF) of various extracts which is clear from their correlation coefficient (R 2) values (Figure 3). A very high linear correlation was established between antioxidant activities and TP and TF content of different extracts (R 2 values ≥ 0.975).
Figure 3.
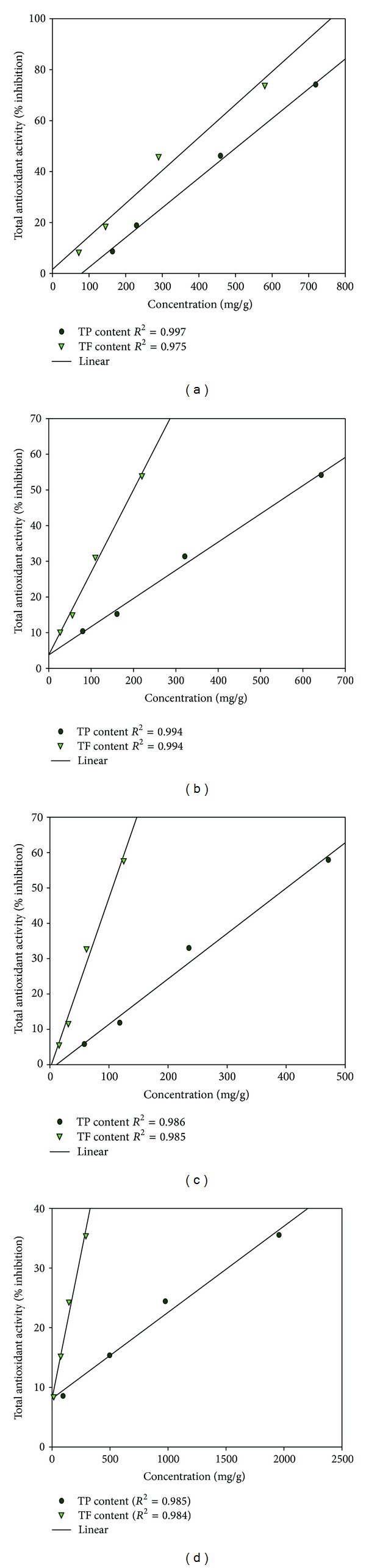
Correlation between total antioxidant activity and polyphenolic (TP&TF) contents of different plant parts of P. fraternus. (a) Ethanolic extract of aerial Part. (b) Aqueous extract of aerial part. (c) Ethanolic extract of root. (d) Aqueous extract of root.
4. Discussion
In present study two different solvents, namely, water and ethanol, were used for extraction of dried aerial parts and roots of P. fraternus. The DPPH method is an easy, rapid, stable, and sensitive way to determine the antioxidant activity of a specific compound or plant extracts [21]. In this assay, DPPH free radical accepts hydrogen and gets reduced by an antioxidant. Both aqueous and ethanolic extract of aerial parts and roots have shown steady increase in percentage inhibition by DPPH radicals with increasing concentration (Table 1). Ethanolic extract of both plant parts have greater inhibitory activity than aqueous extract. The radical scavenging activity of aqueous extract of root was much lower than the ethanol extract suggesting that the antioxidants in the aqueous extract of root are weak radical-scavengers and required extremely high concentration to have a significant effect.
Superoxide radical is known to be a very harmful species to cellular components because they are precursor of more reactive oxygen species [22]. The superoxide radical is known to be produced in vivo and can result in the formation of H2O2 via dismutation reaction. Moreover, the conversion of superoxide and H2O2 into more reactive species, for example, the hydroxyl radical (OH•), has been thought to be one of the unfavourable effects caused by superoxide radicals [23]. In our study, extracts of both plant parts are found to be an efficient scavenger of superoxide radical generated in riboflavin-NBT-light system in vitro and their activity increased with increase in concentration of extract (Table 2). There is no significant difference in scavenging activity of both aqueous and ethanolic extract of aerial part (Table 2). However, ethanolic extracts of root have greater scavenging activity than its aqueous extract which might be due to weak scavenging activity of compounds in aqueous extract. Lipid peroxidation is a free radical-initiated oxidative chain reaction in which one lipid molecule after another becomes oxidized to the maximum possible extent or so as to form lipid peroxide. Normally, this chain reaction is terminated when the substrate is depleted. Other condition includes the combination of two radicals to form nonradical product or reaction with antioxidants, which provide easily donatable hydrogen for abstraction by peroxyl radicals. This assay is done by either enzymatic (Fe/NADPH) or nonenzymatic (Fe/ascorbic acid) method. Since we have used egg-yolk as a substrate it could be suggested that P. fraternus is active against nonenzymatic oxidation. Pandey et al. [24] have also reported non enzymatic method of lipid peroxidation by using different fractions of tubers from Pueraria tuberosa Linn. In our study both extract of aerial parts causes similar inhibition of lipid peroxidation. However, ethanolic extracts of root have greater inhibitory activity than aqueous extract. In reducing power assay, the yellow colour of the test solution changes to various shades of green and blue, depending on the reducing power of extract. The presence of reducers in sample causes the reduction of the Fe3+/ferricyanide complex to the Fe2+ form. Thus, the higher the reducer concentration, the higher the amount of Fe2+ which becomes evident with high absorbance of sample. These reducers show their antioxidant action by breaking the free radical chain by donating a hydrogen atom [25] and also react with certain precursors of peroxide, which in turn prevents peroxide formation. Some earlier workers [26] have observed a direct correlation between antioxidant activity and reducing power of certain plant extracts. In the present study also, reducing power of all extracts increases with increase in concentration which is in close correlation with its observed antioxidant activity. Thus, these reducers must be responsible for antioxidant property of extracts.
Phenolic compounds are secondary metabolites of plants and can act as antioxidants by many potential pathways such as free radical scavenging, oxygen radical absorbance, and chelation of metal ions [27]. Phenolic content is greater in ethanolic extract of both plant parts than its aqueous extract which might be responsible for high antioxidant activity and reducing power of respective extract. Some earlier workers have also found good correlation in phenolic content and antioxidant activity of plant extract [28, 29]. Flavonoids are well-known antioxidant constituents of plants and possess a broad spectrum of chemical and biological activity, including radical scavenging properties [30]. Therefore, flavonoid content of the extracts was also analyzed. There was a good correlation between antioxidant activity of plant extracts, and its TP and TF content.
5. Conclusions
In conclusion, extracts of P. fraternus have potent antioxidant activity and reducing capacity. They are rich source of polyphenols like phenolics and flavonoids. Their antioxidant activities are quite correlated with their polyphenolic contents and reducing power. Among both plant parts, namely, aerial parts and root, aerial part shows greater antioxidant property which is possibly due to its higher polyphenolic content. Higher antioxidant property of ethanolic extract than aqueous extracts might be due to greater solubility of active constituents in ethanol than in water. So, these extracts could be used as new sources of natural antioxidants for treatment of oxidative stress induced diseases. It can be supplemented in nutraceutical products as dietary supplements. It may be used as preservatives in food and cosmetics as well as to prevent the degradation of rubber and gasoline.
Acknowledgment
Author (Richa Upadhyay) is highly thankful to Council of Scientific and Industrial Research (CSIR), New Delhi, for providing fellowship as senior research fellow.
Conflict of Interests
No contributing author has a conflict of interests in the publication of this study.
References
- 1.Beal MF. Aging, energy, and oxidative stress in neurodegenerative diseases. Annals of Neurology. 1995;38(3):357–366. doi: 10.1002/ana.410380304. [DOI] [PubMed] [Google Scholar]
- 2.Muramatsu H, Kogawa K, Tanaka M, et al. Superoxide dismutase in SAS human tongue carcinoma cell line is a factor defining invasiveness and cell motility. Cancer Research. 1995;55(24):6210–6214. [PubMed] [Google Scholar]
- 3.Buyukokuroglu ME, Gulcin I, Oktay M, Kufrevioglu OI. In vitro antioxidant properties of dantrolene sodium. Pharmacological Research. 2001;44(6):491–495. doi: 10.1006/phrs.2001.0890. [DOI] [PubMed] [Google Scholar]
- 4.Larson RA. The antioxidants of higher plants. Phytochemistry. 1988;27(4):969–978. [Google Scholar]
- 5.Zheng W, Wang SY. Antioxidant activity and phenolic compounds in selected herbs. Journal of Agricultural and Food Chemistry. 2001;49(11):5165–5170. doi: 10.1021/jf010697n. [DOI] [PubMed] [Google Scholar]
- 6.Wanasundara PKJPD, Shahidi F. Optimization of hexametaphosphate-assisted extraction of flaxseed proteins using response surface methodology. Journal of Food Science. 1996;61(3):604–607. [Google Scholar]
- 7.Abedin S, Mossa JS, AI-Said MS, AI-Yahya MA. Flora of kingdom of Saudi Arabia. In: Chaudhary SA, editor. National Agriculture and Water Research Centre. 2001. [Google Scholar]
- 8.Calixto JB, Santos AR, Cechinel Filho V, Yunes RA. A review of the plants of the genus Phyllanthus: their chemistry, pharmacology and therapeutic potential. Medicinal Research Review. 1998;18(4):225–258. doi: 10.1002/(sici)1098-1128(199807)18:4<225::aid-med2>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 9.Unander DW, Webster GL, Blumberg BS. Usage and bioassays in Phyllanthus (Euphorbiaceae) - IV: clustering of antiviral uses and other effects. Journal of Ethnopharmacology. 1995;45(1):1–18. doi: 10.1016/0378-8741(94)01189-7. [DOI] [PubMed] [Google Scholar]
- 10.Koffuor GA, Amoateng P. Antioxidant and anticoagulant properties of Phyllanthus fraternus GL Webster (Family: Euphorbiaceae) Journal of Pharmacology and Toxicology. 2011;6(7):624–636. [Google Scholar]
- 11.Malar HLV, Bai SMM. Hepatoprotective activity of Phyllanthus emblica against paracetamol induced hepatic damage in wister albino rats. African Journal of Basic Applied Sciences. 2009;1:21–25. [Google Scholar]
- 12.Naaz F, Javed S, Abdin MZ. Hepatoprotective effect of ethanolic extract of Phyllanthus amarus Schum. et Thonn. on aflatoxin B1-induced liver damage in mice. Journal of Ethnopharmacology. 2007;113(3):503–509. doi: 10.1016/j.jep.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 13.Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. Food Science and Technology. 1995;28(1):25–30. [Google Scholar]
- 14.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Analytical Biochemistry. 1979;95(2):351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 15.Ruberto G, Baratta MT, Deans SG, Dorman HJD. Antioxidant and antimicrobial activity of Foeniculum vulgare and Crithmum maritimum essential oils. Planta Medica. 2000;66(8):687–693. doi: 10.1055/s-2000-9773. [DOI] [PubMed] [Google Scholar]
- 16.Janero DR. Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radical Biology and Medicine. 1990;9(6):515–540. doi: 10.1016/0891-5849(90)90131-2. [DOI] [PubMed] [Google Scholar]
- 17.Beauchamp C, Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Analytical Biochemistry. 1971;44(1):276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- 18.Oyaizu M. Studies on products of browning reactions: antioxidative activities of products of browning reaction prepared from glucosamine. Japanese Journal of Nutrition. 1986;44(6):307–315. [Google Scholar]
- 19.Lister E, Wilson P. Measurement of Total Phenolics and ABTS Assay For Antioxidant Activity (Personal Communication) Lincoln, Mass, USA: Crop Research Institute; 2001. [Google Scholar]
- 20.Chang C-C, Yang M-H, Wen H-M, Chern J-C. Estimation of total flavonoid content in propolis by two complementary colometric methods. Journal of Food and Drug Analysis. 2002;10(3):178–182. [Google Scholar]
- 21.Koleva II, Van Beek TA, Linssen JPH, De Groot A, Evstatieva LN. Screening of plant extracts for antioxidant activity: a comparative study on three testing methods. Phytochemical Analysis. 2002;13(1):8–17. doi: 10.1002/pca.611. [DOI] [PubMed] [Google Scholar]
- 22.Valko M, Morris H, Cronin MTD. Metals, toxicity and oxidative stress. Current Medicinal Chemistry. 2005;12(10):1161–1208. doi: 10.2174/0929867053764635. [DOI] [PubMed] [Google Scholar]
- 23.Halliwell B. Reactive oxygen species in living systems: source, biochemistry, and role in human disease. American Journal of Medicine. 1991;91(3):14–22. doi: 10.1016/0002-9343(91)90279-7. [DOI] [PubMed] [Google Scholar]
- 24.Pandey N, Chaurasia JK, Tiwari OP, Tripathi YB. Antioxidant properties of different fractions of tubers from Pueraria tuberosa Linn. Food Chemistry. 2007;105(1):219–222. [Google Scholar]
- 25.Gordon MH. The mechanism of antioxidant action in vitro . In: Hudson BJF, editor. Food Antioxidants. London, UK: Elsevier Applied Science; 1990. pp. 1–18. [Google Scholar]
- 26.Ferreira ICFR, Baptista P, Vilas-Boas M, Barros L. Free-radical scavenging capacity and reducing power of wild edible mushrooms from northeast Portugal: individual cap and stipe activity. Food Chemistry. 2007;100(4):1511–1516. [Google Scholar]
- 27.Halliwell B, Aeschbach R, Löliger J, Aruoma OI. The characterization of antioxidants. Food and Chemical Toxicology. 1995;33(7):601–617. doi: 10.1016/0278-6915(95)00024-v. [DOI] [PubMed] [Google Scholar]
- 28.Miliauskas G, Venskutonis PR, Van Beek TA. Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chemistry. 2004;85(2):231–237. [Google Scholar]
- 29.Chaurasia JK, Tripathi YB. Chemical characterization of various fractions of leaves of Cinnamomum tamala Linn toward their antioxidant, hypoglycemic, and anti-inflammatory property. Immunopharmacology and Immunotoxicology. 2011;33(3):466–472. doi: 10.3109/08923973.2010.538850. [DOI] [PubMed] [Google Scholar]
- 30.Chirinos R, Pedreschi R, Rogez H, Larondelle Y, Campos D. Phenolic compound contents and antioxidant activity in plants with nutritional and/or medicinal properties from the Peruvian Andean region. Industrial Crops and Products. 2013;47:145–152. [Google Scholar]


