Abstract
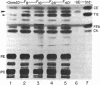
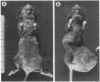
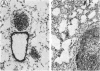
A series of closely related mouse fibroblast cell lines that differ in their content of neutral ether-linked glycerolipid and fatty acids has been used to investigate the relationship between lipid composition and tumorigenicity. Although these cell lines, derived from the same parental culture, were selected without reference to transformation or tumorigenicity, their ability to form tumors in irradiated mice was found to be closely correlated with ether-lipid content. The cell line with the highest level of ether-lipid (designated F40) produces more tumors, the tumors appear more rapidly than when parental cells are injected, and the number of F40 cells required for tumor induction is less by a factor of approximately equal to 1000. F40 tumors are highly invasive, readily metastasize, and rarely regress, in contrast to the occasional benign tumors produced by the parental cell line. Cell lines that are intermediate in their lipid composition appear to be intermediate in tumorigenicity. This panel of graded cell lines provides a useful model system for both in vitro and in vivo studies on the acquisition of tumorigenicity and malignancy in cultured cells.
Full text
PDF

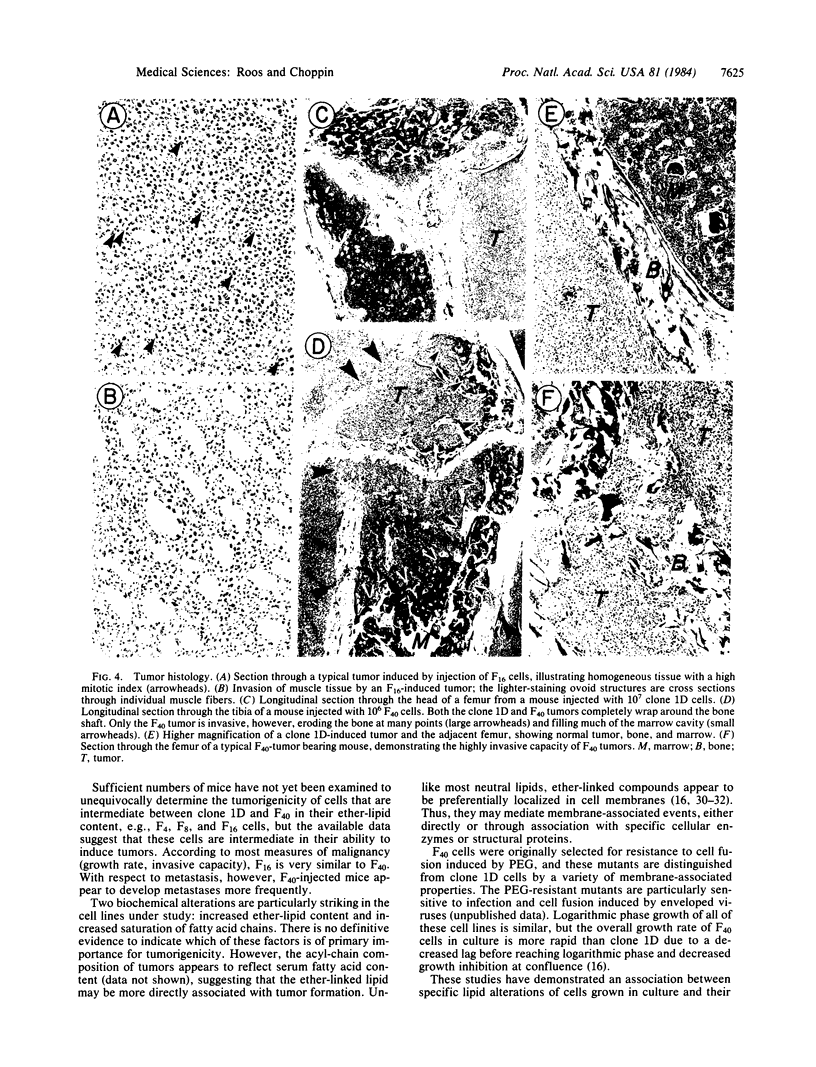

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert D. H., Anderson C. E. Ether-linked glycerolipids in human brain tumors. Lipids. 1977 Feb;12(2):188–192. doi: 10.1007/BF02533292. [DOI] [PubMed] [Google Scholar]
- Anderson R. E., Cumming R. B., Walton M., Snyder F. Lipid metabolism in cells grown in tissue culture: O-alkyl, O-alk-I-enyl, and acyl moieties of L-M cells. Biochim Biophys Acta. 1969 Apr 29;176(3):491–501. doi: 10.1016/0005-2760(69)90216-1. [DOI] [PubMed] [Google Scholar]
- Avilès D., Ritz E., Jami J. Chromosomes in tumors derived from mouse tumor x diploid cell hybrids obtained in vitro. Somatic Cell Genet. 1980 Mar;6(2):171–186. doi: 10.1007/BF01538794. [DOI] [PubMed] [Google Scholar]
- Bollinger J. N. The isolation and tentative identification of diacylglyceryl ethers from the walker 256 carcinoma of the rat and a human lymphosarcoma. Lipids. 1967 Mar;2(2):143–148. doi: 10.1007/BF02530914. [DOI] [PubMed] [Google Scholar]
- Brown R. C., Blank M. L., Kostyu J. A., Osburn P., Kilgore A., Snyder F. Analysis of tumor-associated alkyldiacylglycerols and other lipids during radiation-induced thymic leukemogenesis. Proc Soc Exp Biol Med. 1975 Jul;149(3):808–813. doi: 10.3181/00379727-149-38904. [DOI] [PubMed] [Google Scholar]
- Cabot M. C., Welsh C. J. Ether lipid studies in mouse C3H/10T1/2 cells and a 3-methylcholanthrene-transformed clone. Arch Biochem Biophys. 1981 Oct 1;211(1):240–244. doi: 10.1016/0003-9861(81)90450-1. [DOI] [PubMed] [Google Scholar]
- Chen T. R. In situ detection of mycoplasma contamination in cell cultures by fluorescent Hoechst 33258 stain. Exp Cell Res. 1977 Feb;104(2):255–262. doi: 10.1016/0014-4827(77)90089-1. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Friedberg S. J., Halpert M. Ehrlich ascites tumor cell surface membranes: an abnormality in ether lipid content. J Lipid Res. 1978 Jan;19(1):57–64. [PubMed] [Google Scholar]
- GRAY G. M. The lipid composition of tumour cells. Biochem J. 1963 Feb;86:350–357. doi: 10.1042/bj0860350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HSU T. C., MERCHANT D. J. Mammalian chromosomes in vitro. XIV. Genotypic replacement in cell populations. J Natl Cancer Inst. 1961 May;26:1075–1083. [PubMed] [Google Scholar]
- Howard B. V., Morris H. P., Bailey J. M. Ether-lipids, -glycerol phosphate dehydrogenase, and growth rate in tumors and cultured cells. Cancer Res. 1972 Jul;32(7):1533–1538. [PubMed] [Google Scholar]
- KIT S., DUBBS D. R., PIEKARSKI L. J., HSU T. C. DELETION OF THYMIDINE KINASE ACTIVITY FROM L CELLS RESISTANT TO BROMODEOXYURIDINE. Exp Cell Res. 1963 Aug;31:297–312. doi: 10.1016/0014-4827(63)90007-7. [DOI] [PubMed] [Google Scholar]
- Lin H. J., Ho F. C., Lee C. L. Abnormal distribution of O-alkyl groups in the neutral glycerolipids from human hepatocellular carcinomas. Cancer Res. 1978 Apr;38(4):946–949. [PubMed] [Google Scholar]
- Pfleger R. C., Anderson N. G., Snyder F. Lipid class and fatty acid composition of rat liver plasma membranes isolated by zonal centrifugation. Biochemistry. 1968 Aug;7(8):2826–2833. doi: 10.1021/bi00848a019. [DOI] [PubMed] [Google Scholar]
- Robinson J. M., Roos D. S., Davidson R. L., Karnovsky M. J. Membrane alterations and other morphological features associated with polyethylene glycol-induced cell fusion. J Cell Sci. 1979 Dec;40:63–75. doi: 10.1242/jcs.40.1.63. [DOI] [PubMed] [Google Scholar]
- Roos D. S., Davidson R. L. Isolation of mouse cell lines resistant to the fusion-inducing effect of polyethylene glycol. Somatic Cell Genet. 1980 May;6(3):381–390. doi: 10.1007/BF01542790. [DOI] [PubMed] [Google Scholar]
- Roos D. S., Robinson J. M., Davidson R. L. Cell fusion and intramembrane particle distribution in polyethylene glycol-resistant cells. J Cell Biol. 1983 Sep;97(3):909–917. doi: 10.1083/jcb.97.3.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushton A. R. The biarmed chromosomes of mouse cell line LMTK-. Can J Genet Cytol. 1973 Dec;15(4):791–799. doi: 10.1139/g73-092. [DOI] [PubMed] [Google Scholar]
- SANFORD K. K., HOBBS G. L., EARLE W. R. The tumor-producing capacity of strain L mouse cells after 10 years in vitro. Cancer Res. 1956 Feb;16(2):162–166. [PubMed] [Google Scholar]
- Schroeder F., Vagelos P. R. Effects of phospholipid base analogues on the subcellular membrane ether composition of suspension cultured LM cells. Biochim Biophys Acta. 1976 Aug 23;441(2):239–254. doi: 10.1016/0005-2760(76)90167-3. [DOI] [PubMed] [Google Scholar]
- Snyder F., Blank M. L., Morris H. P. Occurrence and nature of O-alkyl and O-alk-I-enyl moieties of glycerol in lipids of Morris transplanted hepatomas and normal rat liver. Biochim Biophys Acta. 1969 Apr 29;176(3):502–510. doi: 10.1016/0005-2760(69)90217-3. [DOI] [PubMed] [Google Scholar]
- Snyder F., Wood R. Alkyl and alk-1-enyl ethers of glycerol in lipids from normal and neoplastic human tissues. Cancer Res. 1969 Jan;29(1):251–257. [PubMed] [Google Scholar]
- Wood R., Snyder F. Characterization and identification of glyceryl ether diesters present in tumor cells. J Lipid Res. 1967 Sep;8(5):494–500. [PubMed] [Google Scholar]