Abstract
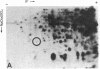
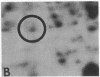
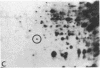
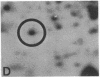
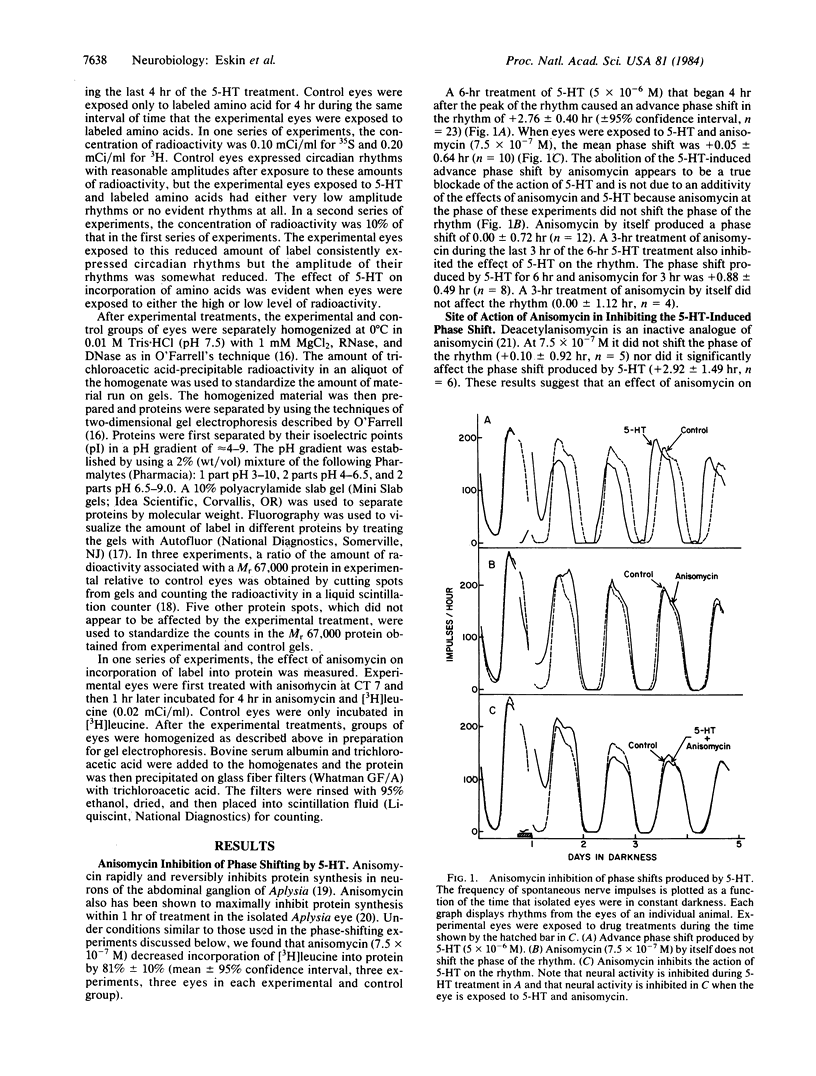
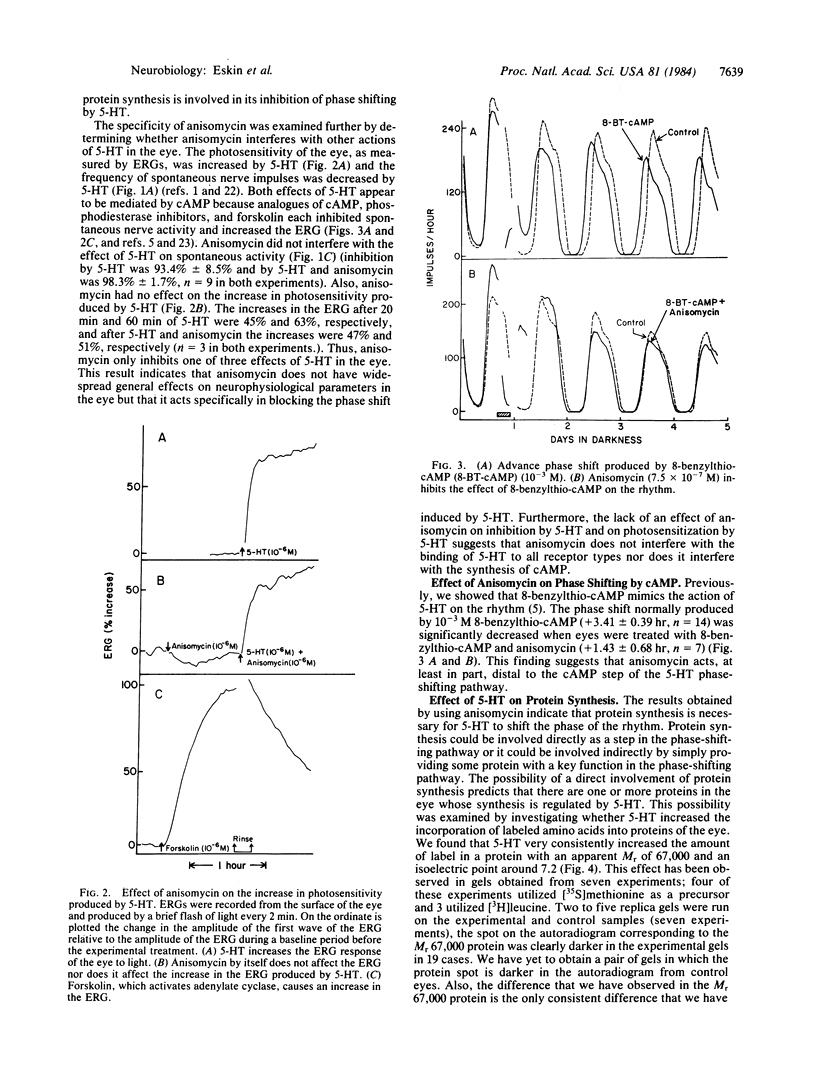
Serotonin (5-HT, 5-hydroxytryptamine) regulates the phase of a circadian pacemaker located within the eye of Aplysia. We are attempting to define the cellular and biochemical events involved in the regulatory pathway through which serotonin acts. Previously, we have shown that an activation of adenylate cyclase and an increase in cAMP are events in the 5-HT phase-shifting pathway. In this paper, we examine the role of protein synthesis in mediating the effect of 5-HT and cAMP on the phase of the circadian rhythm. Exposure of eyes to anisomycin, an inhibitor of protein synthesis, completely blocked the advance shift in phase produced by 5-HT. Although anisomycin by itself can produce phase shifts, it did not affect the rhythm at the phases where the blocking experiments were performed. The specificity of action of anisomycin was investigated in two ways. First, deacetylanisomycin, an analogue of anisomycin that is inactive in inhibiting protein synthesis, did not affect the shift in phase produced by 5-HT. Second, anisomycin did not inhibit two other effects of 5-HT on the eye that also appear to be mediated by cAMP: an inhibition of spontaneous optic nerve activity and an increase in the photosensitivity of the eye. The step in the 5-HT phase-shifting pathway that is sensitive to anisomycin appears to occur after the cAMP step because anisomycin also inhibits the ability of 8-benzylthio-cAMP to shift the phase of the rhythm. We have also examined whether 5-HT directly regulates the synthesis of any proteins in the eye. Using two-dimensional gel electrophoresis, we have found that 5-HT appears to increase the synthesis of a protein with an apparent molecular weight of 67,000. Our results indicate that protein synthesis is necessary for 5-HT to shift the phase of the rhythm and that 5-HT appears to regulate the expression of at least one protein in the eye.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Corrent G., Eskin A., Kay I. Entrainment of the circadian rhythm from the eye of Aplysia: role of serotonin. Am J Physiol. 1982 Mar;242(3):R326–R332. doi: 10.1152/ajpregu.1982.242.3.R326. [DOI] [PubMed] [Google Scholar]
- Corrent G., Eskin A. Transmitterlike action of serotonin in phase shifting a rhythm from the Aplysia eye. Am J Physiol. 1982 Mar;242(3):R333–R338. doi: 10.1152/ajpregu.1982.242.3.R333. [DOI] [PubMed] [Google Scholar]
- Corrent G., McAdoo D. J., Eskin A. Serotonin shifts the phase of the circadian rhythm from the Aplysia eye. Science. 1978 Dec 1;202(4371):977–979. doi: 10.1126/science.309655. [DOI] [PubMed] [Google Scholar]
- Eskin A., Corrent G., Lin C. Y., McAdoo D. J. Mechanism for shifting the phase of a circadian rhythm by serotonin: involvement of cAMP. Proc Natl Acad Sci U S A. 1982 Jan;79(2):660–664. doi: 10.1073/pnas.79.2.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskin A. Differential effects of amino acids on the period of the circadian rhythm from the Aplysia eye. J Neurobiol. 1982 May;13(3):231–239. doi: 10.1002/neu.480130304. [DOI] [PubMed] [Google Scholar]
- Eskin A. Increasing external K+ blocks phase shifts in a circadian rhythm produced by serotonin or 8-benzylthio-cAMP. J Neurobiol. 1982 May;13(3):241–249. doi: 10.1002/neu.480130305. [DOI] [PubMed] [Google Scholar]
- Eskin A. Neurophysiological mechanisms involved in photo-entrainment of the circadian rhythm from the Aplysia eye. J Neurobiol. 1977 May;8(3):273–299. doi: 10.1002/neu.480080310. [DOI] [PubMed] [Google Scholar]
- Eskin A., Takahashi J. S. Adenylate cyclase activation shifts the phase of a circadian pacemaker. Science. 1983 Apr 1;220(4592):82–84. doi: 10.1126/science.6298939. [DOI] [PubMed] [Google Scholar]
- Jacklet J. W. Neuronal circadian rhythm: phase shifting by a protein synthesis inhibitor. Science. 1977 Oct 7;198(4312):69–71. doi: 10.1126/science.897685. [DOI] [PubMed] [Google Scholar]
- Jacklet J. W. Protein synthesis requirement of the Aplysia circadian clock. Tested by active and inactive derivatives of the inhibitor anisomycin. J Exp Biol. 1980 Apr;85:33–42. doi: 10.1242/jeb.85.1.33. [DOI] [PubMed] [Google Scholar]
- Lamers W. H., Hanson R. W., Meisner H. M. cAMP stimulates transcription of the gene for cytosolic phosphoenolpyruvate carboxykinase in rat liver nuclei. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5137–5141. doi: 10.1073/pnas.79.17.5137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer R. A. Transcriptional regulation of the prolactin gene by ergocryptine and cyclic AMP. Nature. 1981 Nov 5;294(5836):94–97. doi: 10.1038/294094a0. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Ram J. L. High (K+) effects on the molecular weight distribution of proteins synthesized in Aplysia nervous tissue. Brain Res. 1974 Aug 16;76(2):281–296. doi: 10.1016/0006-8993(74)90460-0. [DOI] [PubMed] [Google Scholar]
- Rosenfeld M. G., Barrieux A. Regulation of protein synthesis by polypeptide hormones and cyclic AMP. Adv Cyclic Nucleotide Res. 1979;11:205–264. [PubMed] [Google Scholar]
- Rothman B. S., Strumwasser F. Phase shifting the circadian rhythm of neuronal activity in the isolated Aplysia eye with puromycin and cycloheximide. Electrophysiological and biochemical studies. J Gen Physiol. 1976 Oct;68(4):359–384. doi: 10.1085/jgp.68.4.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz J. H., Castellucci V. F., Kandel E. R. Functioning of identified neurons and synapses in abdominal ganglion of Aplysia in absence of protein synthesis. J Neurophysiol. 1971 Nov;34(6):939–953. doi: 10.1152/jn.1971.34.6.939. [DOI] [PubMed] [Google Scholar]
- Wicks W. D. Regulation of protein synthesis by cyclic AMP. Adv Cyclic Nucleotide Res. 1974;4(0):335–438. [PubMed] [Google Scholar]
- Wilson D. L., Hall M. E., Stone G. C., Rubin R. W. Some improvements in two-dimensional gel electrophoresis of proteins. Protein mapping of eukaryotic tissue extracts. Anal Biochem. 1977 Nov;83(1):33–44. doi: 10.1016/0003-2697(77)90506-1. [DOI] [PubMed] [Google Scholar]