Abstract
Background
Management of occult primary breast cancer (OPBC), including the role of magnetic resonance imaging (MRI), is controversial. We conducted a pooled analysis of OPBC patients and a meta-analysis of MRI accuracy in OPBC in order to elucidate current practices.
Methods
A literature search yielded 201 studies. Patient-level data for clinically/mammographically OPBC from studies published after 1993 and from our institution were pooled; logistic regression examined associations between patient/study data and outcomes, including treatments and recurrence. We report adjusted odds ratios (OR) and 95 % confidence intervals (95 % CI) significant at 2-tailed p<0.05. Meta-analysis included data for patients who received MRIs for workup of clinically/mammographically OPBC. We report pooled sensitivity and specificity with 95 % CIs.
Results
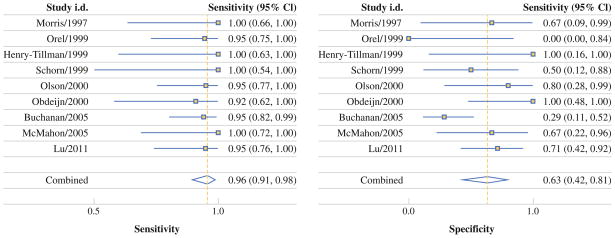
The pooled analysis included 92 patients (15 studies [n = 85] plus our institution [n = 7]). Patients from Asia were more likely to receive breast surgery (OR = 5.98, 95 % CI = 2.02–17.65) but not chemotherapy (OR = 0.32, 95 % CI = 0.13–0.82); patients from the United States were more likely to receive chemotherapy (OR = 13.08, 95 % CI = 2.64–64.78). Patients from studies published after 2003 were more likely to receive radiotherapy (OR = 3.86, 95 % CI = 1.41–10.55). Chemotherapy recipients were more likely to have distant recurrence (OR = 9.77, 95 % CI = 1.10–87.21). More patients with positive MRIs received chemotherapy than patients with negative MRIs (10 of 12 [83.3 %] vs 5 of 13 [38.5 %]; p = 0.0414). In the MRI-accuracy meta-analysis (10 studies, n = 262), pooled sensitivity and specificity were 96 % (95 % CI = 91–98 %) and 63 % (95 % CI = 42–81 %), respectively.
Conclusions
OPBC management varied geographically and over time. We recommend establishing an international OPBC patient registry to facilitate longitudinal study and develop global treatment standards.
Management of occult primary breast cancer (OPBC), which first presents through regional or distant disease and without clinical or radiographic evidence of disease in the breast, has been a subject of controversy since initially described by Halsted in 1907.1 Most OPBC cases involve presentation with axillary lymphadenopathy and are estimated to represent<1 % of all breast cancers.2–7 The rarity of OPBC has made it difficult to develop evidence-based diagnostic and treatment standards. The first article examining the potential role of magnetic resonance imaging (MRI) in the diagnosis and management of clinically and mammographically OPBC was published in 1994,8 and since then, breast MRI has been increasingly incorporated into the diagnostic and surveillance practices of many breast oncologists. However, financial, technical, and personnel limitations have precluded the widespread adoption of breast MRI.9
In the wake of disparate but increasing use of breast MRI, we sought to explore how diagnosis and management of OPBC differs internationally and has changed over time. Here, we report the results of a pooled analysis of OPBC patients and a meta-analysis of MRI accuracy in OPBC. Although systematic reviews on breast MRI use in OPBC diagnosis10 and on management of axillary nodal metastases from carcinoma of unknown primary (CUP)11 were recently published, ours is the first study to examine international and temporal trends in the management of and outcomes in OPBC using pooled patient-level data and the first to provide a meta-analysis of breast MRI accuracy in diagnosing clinically and mammographically OPBC.
METHODS
Data Collection
A medical librarian developed search strategies (see Appendix) for English-language studies published through July 2012 in these databases: Medline/PubMed, EMBASE, Scopus, ClinicalTrials.gov, the Cochrane Database of Systematic Reviews, the Cochrane Central Register of Controlled Trials, and the Health Economic Evaluations Database. We used a combination of standard index terms and plain language to cover the concept of OPBC as comprehensively as possible.
Study Selection
For the pooled analysis, we limited study inclusion to OPBC case series and case reports that were published in or after 1994—when the first article examining the potential role of MRI in the diagnosis and management of OPBC was published8—and that provided patient-level data specifying treatment for female patients without a previous history of breast cancer who were diagnosed with clinically (on exam) and radiographically (mammogram and/or ultrasound) OPBC. We also conducted a retrospective review of all breast-cancer patients at our institution diagnosed with clinically and radiographically OPBC between March 1999 and September 2010 for inclusion in the pooled analysis. This review was approved by the institutional review board at our medical center. Publications without patient-level data and conference abstracts were excluded.
For the MRI-accuracy meta-analysis, we included observational studies and case series that (1) reported the number of female patients with clinically and mammographically OPBC who received MRIs as part of their workup, (2) explicitly stated the results of these patients’ MRIs, and (3) noted whether these results were confirmed by pathology, subsequent imaging, and/or disease progression. Patients in the meta-analysis were allowed to have a previous history of breast cancer and either axillary lymphadenopathy or distant metastases at the time the MRI was performed but could not have any clinical or radiographic evidence of intramammary cancer. Publication bias was assessed using linear regression of log odds ratios on inverse root of effective sample sizes as a test for funnel plot asymmetry in diagnostic meta-analyses; a nonzero slope coefficient would be suggestive of significant small-study bias, with 2-tailed p<0.10 considered significant.12
Data Extraction
Data were independently abstracted and verified by 2 coders. Patient-level demographic information (country of origin and age) and clinical information (mode of breast-cancer detection, treatments received, mortality, and recurrence: locoregional and distant/metastatic) were extracted for patients in the OPBC pooled analysis. For the MRI accuracy meta-analysis, we abstracted both results and confirmatory information (indicating whether a given MRI result was a true-positive [TP], false-positive [FP], true-negative [TN], or false-negative [FN] reading or whether its accuracy was not confirmed by pathology or additional imaging).
Statistical Analysis
For the OPBC pooled analysis, patients’ demographic and clinical characteristics—including geographic region (United States [U.S.], Europe, or Asia), year of study publication (during or before 2003 or after 2003, the year in which the American College of Radiology first published a BIRADS [Breast Imaging-Reporting and Data System] MRI lexicon13), radiographic modality of cancer detection, and treatments received (surgical, systemic, radiation, and/or endocrine/receptor-targeted)—were initially examined using Chi-square tests. Multivariate logistic regression models were constructed to examine associations between clinical characteristics and binary measures of each outcome: type of treatment and recurrence. We report proportions and adjusted odds ratios (OR) with 95 % confidence intervals (95 % CI) significant at 2-tailed p<0.05. Chi-square tests and regression analyses were conducted using SAS 9.2 (SAS Institute Inc., Cary, NC).
For the MRI accuracy meta-analysis, reported MRI results for each study were examined and rates of TP, TN, FP, and FN results were determined using patient-level data; the term “positive” was defined as the identification of a presumptively malignant tumor on a radiographic examination, while “negative” was defined as not identifying a cancer on imaging. Bivariate random-effects meta-analyses were conducted to calculate pooled sensitivity (i.e., TPs/[TPs + FNs]), pooled specificity (i.e., TNs/[TNs + FPs]), pooled positive likelihood ratio (LR+, i.e., sensitivity/[1-specificity], which is the ratio of the TP rate to the FP rate), pooled negative likelihood ratio (LR−, i.e., [1-sensitivity]/specificity, which is the ratio of FN rate to the TN rate), and pooled diagnostic odds ratio (DOR; i.e., LR+/LR−), all of which we report with 95 % CIs. In addition, sensitivity analyses were conducted to address MRI results for which a confirmation of accuracy was not reported; accordingly, for each pooled accuracy measure, we report 3 point estimates: 1 in which only confirmed results are included, 1 in which unconfirmed findings are included and presumed to be true (best-case scenario), and 1 in which unconfirmed findings are included but presumed to be false (worst-case scenario). Meta-analyses were conducted in STATA 12 (Stata, College Station, TX).
RESULTS
Study Selection
The initial search yielded 201 unique reports. Abstracts for all 201 reports were screened, and of these, 70 reports—which either had primary data on patients with OPBC or were reviews of OPBC, breast MRI, and/or CUP—had their bibliographies reviewed and were read in full to assess for inclusion in either the pooled analysis of OPBC patients or in the MRI meta-analysis.
A total of 15 studies14–28 (all case series and case reports, n = 85) were selected for the pooled analysis (Table 1), and we found no significant publication bias (p = 0.356). We also included 7 patients from our institutional review who met criteria,29 for a total of 92 patients. Also, 11 studies5,18,30–37 met eligibility criteria for the MRI-accuracy meta-analysis, but 1 was excluded because it included no negative MRIs, which precluded the calculation of accuracy measures;38 of the remaining 10 studies (all observational studies and case series, n = 262), 118 was only included in the best-case (all unconfirmed results are presumed to be true) and worst-case (all unconfirmed results are presumed to be false) sensitivity analyses because neither of the reported negative MRIs was confirmed. Accordingly, 10 studies were ultimately included in the best-case and worst-case sensitivity analyses of MRI accuracy, but the primary pooled estimates were derived from 9 studies (n = 250, Table 2).
TABLE 1.
Studies included in occult primary breast cancer pooled analysis
| Study | Year | Country | Institution | N = (F/U) |
|---|---|---|---|---|
| Abe et al.14 | 2000 | Japan | Shiga University of Medical Science, Shiga | 1 (60 months) |
| Abe et al.15 | 2010 | Japan | Shiga University of Medical Science, Shiga | 1 (29 months) |
| Agarwal et al.16 | 2000 | India | Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow | 1 (5 months) |
| Capobianco et al.17 | 2007 | Italy | University of Sassari | 1 (72 months) |
| Fayanju et al.29 | 2013 | United States | Siteman Cancer Center, St. Louis | 7 (91 months)a |
| Ko et al.18 | 2007 | South Korea | Samsung Medical Center, Seoul | 12 (42 months)a,b |
| Lanitis et al.19 | 2009 | United Kingdom | St. Mary’s Hospital, London | 3 (40 months)a |
| Lloyd et al.20 | 2001 | United Kingdom | The Royal Marsden Hospital, Sutton | 6 (57 months)a |
| Matsuoka et al.21 | 2003 | Japan | The National Shikoku Cancer Center, Matsuyama City | 11 (88 months)a |
| Medina-Franco et al.22 | 2002 | United States | University of Alabama, Birmingham | 10 (49 months)a |
| Misra et al.23 | 2001 | India | Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow | 4 (13 months)a,c |
| Piekarski et al.24 | 2003 | Poland | Medical University of Lodz | 22 (37 months)a |
| Steunebrink et al.25 | 2005 | The Netherlands | Albert Schweitzer Hospital, Dordrecht | 1 (42 months) |
| Tamiolakis et al.26 | 2008 | Greece | General Hospital of Chania, Crete | 1 (12 months) |
| Teodossiu et al.27 | 2009 | Italy | University Hospital, Bari | 1 (48 months) |
| Varadarajan et al.28 | 2006 | United States | Roswell Park Cancer Institute, Buffalo | 10 (57 months)d |
F/U time to follow-up
Mean
Follow-up information only available for 2 of 12 patients
Follow-up information only available for 3 of 4 patients
Median
TABLE 2.
Studies included in meta-analysis of MRI accuracy in occult primary breast cancer
| Study | Year | Country | Institution | Year range | No. who received MRIs | No. with confirmed MRIsa |
|---|---|---|---|---|---|---|
| Buchanan et al.30 | 2005 | United States | Memorial Sloan-Kettering Cancer Center, New York | 1995–2001 | 69b | 58 |
| Henry-Tillman et al.31 | 1999 | United States | University of Arkansas, Little Rock | 1994–1998 | 10 | 10 |
| Ko et al.18 | 2007 | South Korea | Samsung Medical Center, Seoul | 2001–2006 | 12 | 10 |
| Lu et al.32 | 2011 | China | Tianjin Medical University Cancer Institute and Hospital, Tianjin | 2005–2010 | 35 | 35 |
| McMahon et al.33 | 2005 | Australia | Greenslopes Private Hospital, Brisbane | 2000–2004 | 18 | 17 |
| Morris et al.34 | 1997 | United States | Memorial Sloan-Kettering Cancer Center, New York | 1995–1996 | 12 | 12 |
| Obdeijn et al.35 | 2000 | The Netherlands | Dr. Daniel den Hoed Cancer Center, University Hospital Rotterdam | 1995–1998 | 30b | 17 |
| Olson et al.5 | 2000 | United States | Memorial Sloan-Kettering Cancer Center, New York | 1994–1998 | 40b | 27 |
| Orel et al.36 | 1999 | United States | University of Pennsylvania, Philadelphia | 1993–1997 | 22 | 22 |
| Schorn et al.37 | 1999 | Germany | Klinikum der Georg-August- Universität Göttingen | NR | 14 | 12 |
MRIs magnetic resonance imaging
Number of participants whose breast MRIs were confirmed by pathology, subsequent imaging, disease progression, or disease response to
treatment
Includes some patients with a previous history of breast cancer
Pooled Analysis of OPBC Patients
Mean and median age of included patients was 56 years (range, 28–88 years). Mean and median times to follow-up were 49 and 42 months, respectively (range, 4–310 months). All 92 patients presented with unilateral axillary lymphadenopathy. There were 43 patients (46.7 %) who received no breast surgery. For 4 patients who underwent breast conservation therapy (BCT; i.e., lumpectomy and radiation therapy [XRT]), the authors did not specify whether or to what extent an axillary operation was performed. Also, 80 patients (87.0 %) underwent an axillary lymph node dissection (ALND), either as the only operation (n = 35) or in conjunction with either modified radical mastectomy (MRM; n = 33) or lumpectomy (n = 12). There were 5 patients who received axillary biopsies but not complete dissections, and none of these 5 patients underwent breast surgery. There were 3 patients who received no breast or axillary surgery of any kind; 2 of these 3 patients received radiation therapy, and all 3 received chemotherapy. Of the 92 patients, 62 (67.4 %) received chemotherapy, and 27 (29.3 %) received hormone therapy. Also, 54 (58.7 %) received XRT, 12 in conjunction with lumpectomy. There were 4 instances of locoregional recurrence and 16 instances of distant/metastatic recurrence in 19 patients (1 patient had both locoregional and distant/metastatic recurrence). Of the 92 patients, 10 (10.9 %) in the pooled analysis ultimately died, all of them of advanced breast cancer.
In Chi-square tests, a smaller proportion of patients from the United States (17 of 27, 63.0 %) and Europe (20 of 35, 57.1 %) received breast surgery when compared with patients from Asia (24 of 30, 80.0 %), where breast operations were predominantly MRMs (14 of 24, 58.3 %, p = 0.0043). A greater proportion of patients from studies published in 2004 or later received XRT (28 of 37, 75.7 %) when compared with patients from studies published during or before 2003 (26 of 55, 47.3 %, p = 0.0067). Compared with those who underwent MRM (9 of 33, 27.3 %), a greater proportion of patients who underwent lumpectomy (12 of 16, 75.0 %) or no breast surgery (33 of 43, 76.7 %) received XRT (p<0.0001). Among patients who received no breast surgery (n = 43), there was no difference in rates of recurrence or death between those patients who received XRT (8 of 33 [24.2 %] recurred or died) and those who did not (2 of 10 [20.0 %] recurred or died, p = 1.0000). A greater proportion of U.S.-based patients (25 of 27, 92.6 %) received chemotherapy when compared with patients from Europe (21 of 35, 60.0 %) and Asia (14 of 30, 46.7 %, p<0.0001). When compared with those who received lumpectomy (6 of 16, 37.5 %), a greater proportion of the patients who underwent no breast surgery (31 of 43, 72.1 %) or MRM (23 of 33, 69.7 %) received chemotherapy (p = 0.0368).
Of the 92 patients in the pooled analysis, 25 had individual- level MRI results reported, and all 25 patients were from studies published in 2004 or later. Of these 25 patients, 13 had positive MRIs, and 12 had negative breast MRIs. Of the 13 with positive MRIs, a greater proportion received lumpectomy (8 of 13, 61.5 %) versus MRM (3 of 13, 23.1 %) or no breast surgery (2 of 13, 15.4 %, p = 0.0011). Compared with patients who had positive MRIs, chemotherapy was received by a greater proportion of patients with negative breast MRIs (10 of 12 [83.3 %] vs 5 of 13 [38.5 %]; p = 0.0414).
In multivariate logistic regression (Table 3), patients receiving XRT were less likely to receive breast surgery; similarly, patients receiving breast surgery were less likely to receive XRT. Patients from Asia were more likely to receive breast surgery and less likely to receive chemotherapy, but patients from the United States were more likely to receive chemotherapy. Patients from studies published in 2004 or later were more likely to receive XRT. Among the 61 patients for whom recurrence information was provided, patients who received chemotherapy were more likely to experience a distant recurrence.
TABLE 3.
Multivariate logistic regression model results for occult primary breast cancer pooled analysis
| Regression model outcome/independent variable | Odds ratio (OR) | 95 % CI | p value |
|---|---|---|---|
| Receiving breast surgery | |||
| Being from Asiaa | 5.98 | 2.02–17.65 | 0.0012 |
| Receiving radiation therapyb | 0.23 | 0.09–0.59 | 0.0033 |
| Receiving chemotherapy | |||
| Being from Asiac | 0.32 | 0.13–0.82 | 0.0167 |
| Being from the United Statesd | 13.08 | 2.64–64.78 | 0.0016 |
| Receiving radiation therapy | |||
| Study published ≥ 2004e | 3.86 | 1.41–10.55 | 0.0084 |
| Receiving breast surgerye | 0.23 | 0.09–0.60 | 0.0027 |
| Distant/metastatic recurrence | |||
| Receiving chemotherapyf | 9.77 | 1.10–87.21 | 0.0412 |
CI confidence interval
Multivariate logistic regression model included the following variables (variable values in parentheses with reference group italicized): geography (from Asia, not from Asia), year of publication (≥2004, ≤2003), chemotherapy (received, did not receive), radiation therapy (received, did not receive)
Multivariate logistic regression model included the following variables (variable values in parentheses with reference group italicized): geography (from United States, not from United States), year of publication (≥2004, ≤2003), chemotherapy (received, did not receive), radiation therapy (received, did not receive). Similar point estimates and CIs obtained from models in which geography variable values were Asia versus Not from Asia and Europe versus Not from Europe
Multivariate logistic regression model included the following variables (variable values in parentheses with reference group italicized): geography (from Asia, not from Asia), year of publication (≥2004, ≤2003), breast surgery (received, did not receive), radiation therapy (received, did not receive)
Multivariate logistic regression model included the following variables (variable values in parentheses with reference group italicized): geography (from United States, not from United States), year of publication (≥2004, ≤2003), breast surgery (received, did not receive), radiation therapy (received, did not receive)
Multivariate logistic regression model included the following variables (variable values in parentheses with reference group italicized): geography (from United States, not from United States), year of publication (≥2004, ≤2003), breast surgery (received, did not receive), chemotherapy (received, did not receive). Similar point estimates and CIs obtained from models in which geography variable values were Asia versus Not from Asia and Europe versus Not from Europe
Multivariate logistic regression model included the following variables (variable values in parentheses with reference group italicized): geography (from United States, not from United States), year of publication (≥2004, ≤2003), breast surgery (received, did not receive), chemotherapy (received, did not receive), radiation therapy (received, did not receive). Similar point estimates and CIs obtained from models in which geography variable values were Asia versus Not from Asia and Europe versus Not from Europe
Meta-Analysis of MRI Accuracy
Of the 262 patients in the 10 included studies, 220 patients had an MRI study with a confirmed result. Pooled accuracy estimates (Fig. 1) as well as best-case and worst-case sensitivity analyses are reported in Table 4. Pooled sensitivity was 96 % but as low as 89 % when all unconfirmed positive MRIs were assumed to be FPs; pooled MRI specificity was 63 % but as low as 55 % when all unconfirmed negative MRIs were assumed to be FNs. Pooled LR+ was 2.6, and pooled LR− was 0.06. DOR was 41, indicating that breast MRI has a high, prevalence-independent level of diagnostic accuracy with regard to OPBC.
FIG. 1.
Meta-analysis of MRI accuracy in diagnosis of occult primary breast cancer
TABLE 4.
Pooled measures in meta-analysis of MRI accuracy in occult primary breast cancer
| Measure | Point estimate (95 % CI) | Best-case point estimate (95 % CI) | Worst-case point estimate (95 % CI) |
|---|---|---|---|
| Sensitivity | 0.96 (0.91–0.98) | 0.96 (0.92–0.98) | 0.89 (0.71–0.96) |
| Specificity | 0.63 (0.42–0.81) | 0.77 (0.55–0.90) | 0.55 (0.34–0.74) |
| LR+ | 2.6 (1.5–4.6) | 4.2 (1.9–9.1) | 2.0 (1.2–3.2) |
| LR− | 0.06 (0.03–0.16) | 0.05 (0.02–0.11) | 0.20 (0.06–0.62) |
| DOR | 41 (12–143) | 89 (24–338) | 10 (2–42) |
CI confidence interval, LR+ positive likelihood ratio, LR− negative likelihood ratio, DOR diagnostic odds ratio
DISCUSSION
Our review confirms previous findings3,4,11,24,39–44 of significant heterogeneity in the management of OPBC. Historically, OPBC presenting with axillary lymphadenopathy has been thought to have a similar natural history and biological profile (e.g., histology, receptor status) to non-occult node-positive breast cancers.11,44 Thus, one would expect OPBC treatment to focus on local control of axillary disease, and this approach was largely but not completely observed among the patients in the studies we reviewed, the vast majority of whom underwent ALND. The 5-year survival rate for non-occult node-positive breast cancer is~84 %,45 but in our pooled analysis, nearly 90 % of patients were alive at follow-up. Furthermore, most published case series of OPBC patients have demonstrated outcomes superior to those of non-occult, node-positive patients.3,4,6,20–22,28,46–48 Indeed, in our institutional experience, all 7 OPBC patients were alive with no evidence of disease after a median follow-up of 86 months.29 Thus, it appears that OPBC patients, on the whole, may actually have a better prognosis than non-occult node-positive breast-cancer patients, and this difference in outcomes may be related to the paucity or absence of macroscopic intramammary disease in OPBC patients.
Thus, the extent to which OPBC warrants breast surgery is unclear. Studies have reported occult-tumor rates of 45–82 % in OPBC mastectomy specimens.3,39,44,46 The authors of 1 review recommended mastectomies for all patients with OPBC,11 while others maintain that mastectomy is unwarranted24 or advocate localized radiation therapy as a primary method of OPBC treatment.39,43,49 In our pooled analysis, we found that patients in Asia were more likely to receive mastectomy, a finding in line with other studies demonstrating increased rates of mastectomy over BCT among both Asian-American breast-cancer patients treated in the United States and among breast-cancer patients in Asia.50–53 Possible reasons for this trend include higher instances of clinical contraindications to lumpectomy, including high tumor-size-to-breast-size ratios and increased rates of multicentricity and multifocality, as well as many Asian patients’ reported reluctance to undergo XRT.50 Furthermore, the geographic variation in our analysis may reflect not only resource limitations in different countries with regard to the quality and availability of chemotherapy, XRT, and breast MRI but also country-specific differences in patient prognosis for node-positive breast cancer, local physician treatment practice patterns, and/or culturally informed perceptions of risk by patients. In trying to understand why OPBC patients in Asia were more likely to undergo mastectomy, both clinical and cultural contributions to medical decision making would need to be further explored.
In our pooled analysis and other studies,5,30,32,33,54–56 MRI findings were significantly associated with the types of treatment patients received: OPBC patients with cancers putatively identified on MRI were more likely to receive lumpectomies, which have been estimated to have a pathological tumor yield of 95 %.5 In contrast, patients with negative MRIs were more likely to receive MRMs and systemic therapy, both of which are associated with significant morbidity. A meta-analysis examining chemotherapeutic regimens for CUP, of which OPBC is a subset, found that no type of chemotherapy conferred a definitive survival benefit. 57 Given its equivocal effect on prognosis and increased use of receptor-targeted therapy, it is unclear what if any role chemotherapy should play in OPBC treatment.
In our analysis, there was a trend toward increased XRT use in OPBC treatment over time. By indicating a focus of disease, MRI diagnosis of OPBC enables the performance of BCT and, concomitantly, receipt of XRT. At least 1 study has reported improved disease-free survival in association with ipsilateral breast irradiation as treatment for OPBC.39 Thus, MRI not only facilitates receipt of a less morbid and invasive procedure, but, by enabling lumpectomy and concomitant XRT, also potentially decreases the risk of locoregional recurrence.58
In our meta-analysis of MRI accuracy in OPBC, we found MRI to be very sensitive, but not particularly specific, in line with accuracy estimates for other types of breast cancer.56 The relatively low specificity of MRI means that many women will undergo additional procedures to follow up false-positive MRI findings.13,55,59,60 Furthermore, despite its high sensitivity, it is unclear whether increased use of MRI has changed the incidence of OPBC, with some studies reporting a decline10 and others reporting no change.42
As previously noted, it appears that OPBC patients presenting with axillary lymphadenopathy may actually have superior outcomes to non-occult node-positive patients, but a longitudinal study of a large cohort of OPBC patients would be needed to confirm this observation. Furthermore, approaches to treatment must be informed by increasing evidence of significant heterogeneity among OPBC patients, with a recent study reporting outcome differences between OPBC patients with different immunohistochemical profiles.7 If, as some would argue,61 CUP is an orphan disease within medicine, OPBC is certainly an orphan disease within breast oncology. As with other rare diseases, evidence-based approaches to OPBC diagnosis and treatment would benefit from initiation of a prospectively accrued international patient registry. The National Institutes of Health Office of Rare Diseases Research62 and the Coordination of Rare Diseases at Sanford63 both provide models for a registry that might facilitate the collection of data on OPBC patients. Provided through the American Society of Breast Surgeons (ASBS), the Mastery of Breast Surgery Program represents a potential platform for maintaining an information repository of OPBC patients’ management and outcomes, in much the same way this is already being done for patients who have undergone nipple-sparing mastectomy.64 Information from such a database ideally would be available to ASBS members in de-identified form, thereby facilitating self-review of individual and institutional practices as well as the type of longitudinal research on OPBC needed to answer persistent questions about OPBC epidemiology, management, and post-treatment outcomes.
Our study had some limitations. The studies included in both our analyses were retrospective, raising the concern for selection bias. Furthermore, our pooled analysis was limited by what individual-level data could be gleaned from published reports. However, we have confidence in the thoroughness of our literature review, which was initiated by a clinical librarian and completed by 2 coders with expertise in meta-analysis. The geographic diversity of the studies included in both analyses further attests to the wide scope of our search despite its having been limited to English-language studies. Furthermore, because we worked with patient-level data in the pooled analysis, we were able to adjust for anticipated covariates through multivariate regression, thereby minimizing the influence of potential confounders on our outcomes. Our MRI meta-analysis included all of the relevant studies from a recent systematic review on breast MRI in OPBC diagnosis10 as well as 3 additional studies, further illustrating the completeness of our literature review. Our study is the only meta-analysis of MRI accuracy in OPBC, and our findings are in keeping with published assessments of MRI accuracy in the diagnosis of non-occult breast cancer.13,55,56,65,66
In conclusion, management of OPBC varied significantly with geographic location. It is unclear whether receipt of mastectomy, XRT, or chemotherapy provides long-term benefits. We recommend the use of breast MRI for patients who present with axillary lymphadenopathy but no evidence of an intramammary tumor on clinical exam, mammography, or ultrasound. MRI sensitivity for OPBC is high, and MRI findings may have an impact on the type of treatments patients with OPBC ultimately receive. However, we caution that OPBC incidence has not definitively decreased since the introduction of MRI, and the relatively modest specificity of MRI all but ensures that many women will undergo unnecessary procedures based on false-positive results. Given the rarity of this condition, we recommend the establishment of an international OPBC patient registry to facilitate longitudinal study and eventual development of global treatment standards.
Acknowledgments
Dr. Fayanju was supported by the NIH Ruth L. Kirschstein National Research Service Award Institutional Research Training Grant 5T32CA009621-22. Ms. Stoll and Dr. Colditz were supported by the Foundation for Barnes-Jewish Hospital. Dr. Jeffe was supported in part by the NCI Cancer Center Support Grant (P30 CA091842) to the Alvin J. Siteman Cancer Center at Barnes-Jewish Hospital and Washington University School of Medicine in St. Louis, Missouri. Portions of this study’s findings were presented at the American Society of Breast Surgeons 14th Annual Meeting, May 1–5, 2013.
APPENDIX: PUBMED DATABASE SEARCH STRATEGY FOR META-ANALYSIS OF OCCULT PRIMARY BREAST CANCER AND MRI ACCURACY
“Neoplasms, Unknown Primary”[Mesh] OR “occult primary cancer” OR “occult cancers” OR “occult primary cancers” OR “Occult Primary Neoplasms” OR “Occult Primary Neoplasm” OR “Unknown Primary Neoplasms” OR “Unknown Primary Neoplasm”OR “Unknown Primary Tumors” OR “Unknown Primary Tumor” OR “occult cancer” OR “occult carcinoma”) AND (“Breast Neoplasms”[Mesh] OR “Breast Neoplasm” OR “Breast Tumors”OR”Breast Tumor”OR”Mammary Carcinomas” OR “Mammary Carcinoma” OR “Mammary Neoplasm” OR “Mammary Neoplasms” OR “Breast Cancer” OR “Cancer of the Breast”OR”Cancer of Breast”OR”mamma cancer” OR “mammary cancer” OR “mammary gland cancer” OR “Mammary Ductal Carcinomas” OR “Mammary Ductal Carcinoma”) AND (“Axilla”[Mesh] OR axilla OR axillas OR underarm) NOT ((“Animals”[Mesh]) NOT (“Animals”[Mesh] AND “Humans”[Mesh]).
Footnotes
CONFLICT OF INTEREST None of the authors has any financial interests or conflicts to disclose.
References
- 1.Halsted WS. The result of radical operations for the cure of carcinoma of the breast. Ann Surg. 1907;46:1–19. doi: 10.1097/00000658-190707000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patel J, Nemoto T, Rosner D. Axillary lymph node metastasis from an occult breast cancer. Cancer. 1981;47:2923–7. doi: 10.1002/1097-0142(19810615)47:12<2923::aid-cncr2820471231>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 3.Baron PL, Moore MP, Kinne DW, Candela FC, Osborne MP, Petrek JA. Occult breast cancer presenting with axillary metastases. Updated management. Arch Surg. 1990;125:210–14. doi: 10.1001/archsurg.1990.01410140088014. [DOI] [PubMed] [Google Scholar]
- 4.Rosen PP, Kimmel M. Occult breast carcinoma presenting with axillary lymph node metastases: a follow-up study of 48 patients. Hum Pathol. 1990;21:518–23. doi: 10.1016/0046-8177(90)90008-s. [DOI] [PubMed] [Google Scholar]
- 5.Olson JA, Morris EA, Van Zee KJ, Linehan DC, Borgen PI. Magnetic resonance imaging facilitates breast conservation for occult breast cancer. Ann Surg Oncol. 2000;7:411–5. doi: 10.1007/s10434-000-0411-4. [DOI] [PubMed] [Google Scholar]
- 6.Vlastos G, Jean ME, Mirza AN, Mirza NQ, Kuerer HM, Ames FC, et al. Feasibility of breast preservation in the treatment of occult primary carcinoma presenting with axillary metastases. Ann Surg Oncol. 2001;8:425–31. doi: 10.1007/s10434-001-0425-6. [DOI] [PubMed] [Google Scholar]
- 7.Montagna E, Bagnardi V, Rotmensz N, Viale G, Cancello G, Mazza M, et al. Immunohistochemically defined subtypes and outcome in occult breast carcinoma with axillary presentation. Breast Cancer Res Treat. 2011;129:867–75. doi: 10.1007/s10549-011-1697-6. [DOI] [PubMed] [Google Scholar]
- 8.Davis PL, Julian TB, Staiger M, Harris KB, Borochovitz D, Klementaviciene J, et al. Magnetic resonance imaging detection and wire localization of an ‘occult’ breast cancer. Breast Cancer Res Treat. 1994;32:327–30. doi: 10.1007/BF00666010. [DOI] [PubMed] [Google Scholar]
- 9. [Accessed 10 April 2013];Emerging Areas in Early Detection. 2013 http://ww5.komen.org/BreastCancer/EmergingAreasinEarlyDetection.html#mri.
- 10.de Bresser J, de Vos B, van der Ent F, Hulsewe K. Breast MRI in clinically and mammographically occult breast cancer presenting with an axillary metastasis: a systematic review. Eur J Surg Oncol. 2010;36:114–9. doi: 10.1016/j.ejso.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 11.Pentheroudakis G, Lazaridis G, Pavlidis N. Axillary nodal metastases from carcinoma of unknown primary (CUPAx): a systematic review of published evidence. Breast Cancer Res Treat. 2010;119:1–11. doi: 10.1007/s10549-009-0554-3. [DOI] [PubMed] [Google Scholar]
- 12.Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol. 2005;58:882–93. doi: 10.1016/j.jclinepi.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 13.DeMartini W, Lehman C, Partridge S. Breast MRI for cancer detection and characterization: a review of evidence-based clinical applications. Acad Radiol. 2008;15:408–16. doi: 10.1016/j.acra.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 14.Abe H, Naitoh H, Umeda T, Shiomi H, Tani T, Kodama M, et al. Occult breast cancer presenting axillary nodal metastasis: a case report. Jpn J Clin Oncol. 2000;30:185–7. doi: 10.1093/jjco/hyd047. [DOI] [PubMed] [Google Scholar]
- 15.Abe H, Shimizu T, Cho H, Kubota Y, Umeda T, Kurumi Y, et al. Occult breast cancer with EDTA-dependent pseudothrombocytopenia— a case report. Jpn J Cancer Chemother. 2010;37(5):915–918. [PubMed] [Google Scholar]
- 16.Agarwal G, Mishra SK, Dutta NR, Jain M. Occult squamous cell carcinoma of the axillary tail of breast presenting as isolated axillary lymph node mass. Eur J Surg. 2000;166:177–9. doi: 10.1080/110241500750009564. [DOI] [PubMed] [Google Scholar]
- 17.Capobianco G, Spaliviero B, Dessole S, Rocca PC, Cherchi PL, Ambrosini G, et al. Lymph node axillary metastasis from occult contralateral infiltrating lobular carcinoma arising in accessory breast: MRI diagnosis. Breast J. 2007;13:305–7. doi: 10.1111/j.1524-4741.2007.00428.x. [DOI] [PubMed] [Google Scholar]
- 18.Ko EY, Han BK, Shin JH, Kang SS. Breast MRI for evaluating patients with metastatic axillary lymph node and initially negative mammography and sonography. Korean J Radiol. 2007;8:382–9. doi: 10.3348/kjr.2007.8.5.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lanitis S, Behranwala KA, Al-Mufti R, Hadjiminas D. Axillary metastatic disease as presentation of occult or contralateral breast cancer. Breast. 2009;18:225–7. doi: 10.1016/j.breast.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 20.Lloyd MS, Nash AG. ‘Occult’ breast cancer. Ann R Coll Surg Engl. 2001;83:420–4. [PMC free article] [PubMed] [Google Scholar]
- 21.Matsuoka K, Ohsumi S, Takashima S, Saeki T, Aogi K, Mandai K. Occult breast carcinoma presenting with axillary lymph node metastases: follow-up of eleven patients. Breast Cancer. 2003;10:330–4. doi: 10.1007/BF02967653. [DOI] [PubMed] [Google Scholar]
- 22.Medina-Franco H, Urist MM. Occult breast carcinoma presenting with axillary lymph node metastases. Rev Invest Clin. 2002;54:204–8. [PubMed] [Google Scholar]
- 23.Misra AK, Mishra A, Agrawal G, Agarwal A, Mishra SK. Occult breast carcinoma: a report of four cases and review of literature. Indian J Cancer. 2001;38:49–54. [PubMed] [Google Scholar]
- 24.Piekarski J, Pluta P, Nejc D, Jeziorski A. Mastectomy is an overtreatment in patients with occult breast cancer. Nowotwory. 2003;53:630–4. [Google Scholar]
- 25.Steunebrink M, Schnater JM, Storm RK, van Ingen G, Vegt PA, Plaisier PW. Bilateral axillary metastases of occult breast carcinoma: report of a case with a review of the literature. Breast. 2005;14:165–8. doi: 10.1016/j.breast.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 26.Tamiolakis D, Antoniou C. Axillary nodal metastasis of occult breast primary cancer. Chirurgia (Bucur) 2008;103:467–71. [PubMed] [Google Scholar]
- 27.Teodossiu G, Andrikou K, Lazzaro R, Filiotis N, Triggiani E. Occult breast cancer presenting with axillary nodal metastasis: a case report. Eur J Oncol. 2009;14:109–13. [Google Scholar]
- 28.Varadarajan R, Edge SB, Yu J, Watroba N, Janarthanan BR. Prognosis of occult breast carcinoma presenting as isolated axillary nodal metastasis. Oncology. 2006;71:456–9. doi: 10.1159/000107111. [DOI] [PubMed] [Google Scholar]
- 29.Fayanju OM, Jeffe DB, Margenthaler JA. Occult primary breast cancer at a comprehensive cancer center. J Surg Res. 2013 doi: 10.1016/j.jss.2013.06.020. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buchanan CL, Morris EA, Dorn PL, Borgen PI, Van Zee KJ. Utility of breast magnetic resonance imaging in patients with occult primary breast cancer. Ann Surg Oncol. 2005;12:1045–53. doi: 10.1245/ASO.2005.03.520. [DOI] [PubMed] [Google Scholar]
- 31.Henry-Tillman RS, Harms SE, Westbrook KC, Korourian S, Klimberg VS. Role of breast magnetic resonance imaging in determining breast as a source of unknown metastatic lymphadenopathy. Am J Surg. 1999;178:496–500. doi: 10.1016/s0002-9610(99)00221-4. [DOI] [PubMed] [Google Scholar]
- 32.Lu H, Xu YL, Zhang SP, Lang RG, Zee CS, Liu PF, et al. Breast magnetic resonance imaging in patients with occult breast carcinoma: Evaluation on feasibility and correlation with histopathological findings. Chin Med J. 2011;124:1790–5. [PubMed] [Google Scholar]
- 33.McMahon K, Medoro L, Kennedy D. Breast magnetic resonance imaging: an essential role in malignant axillary lymphadenopathy of unknown origin. Australas Radiol. 2005;49:382–9. doi: 10.1111/j.1440-1673.2005.01499.x. [DOI] [PubMed] [Google Scholar]
- 34.Morris EA, Schwartz LH, Dershaw DD, Van Zee KJ, Abramson AF, Liberman L. MR imaging of the breast in patients with occult primary breast carcinoma. Radiology. 1997;205:437–40. doi: 10.1148/radiology.205.2.9356625. [DOI] [PubMed] [Google Scholar]
- 35.Obdeijn IMA, Brouwers-Kuyper EMJ, Tilanus-Linthorst MMA, Wiggers T, Oudkerk M. MR imaging-guided sonography followed by fine-needle aspiration cytology in occult carcinoma of the breast. AJR Am J Roentgenol. 2000;174:1079–84. doi: 10.2214/ajr.174.4.1741079. [DOI] [PubMed] [Google Scholar]
- 36.Orel SG, Weinstein SP, Schnall MD, Reynolds CA, Schuchter LM, Fraker DL, et al. Breast MR imaging in patients with axillary node metastases and unknown primary malignancy. Radiology. 1999;212:543–9. doi: 10.1148/radiology.212.2.r99au40543. [DOI] [PubMed] [Google Scholar]
- 37.Schorn C, Fischer U, Luftner-Nagel S, Westerhof JP, Grabbe E. MRI of the breast in patients with metastatic disease of unknown primary. Eur Radiol. 1999;9:470–3. doi: 10.1007/s003300050694. [DOI] [PubMed] [Google Scholar]
- 38.Tilanus-Linthorst MMA, Obdeijn AIM, Bontenbal M, Oudkerk M. MRI in patients with axillary metastases of occult breast carcinoma. Breast Cancer Res Treat. 1997;44:179–82. doi: 10.1023/a:1005774009740. [DOI] [PubMed] [Google Scholar]
- 39.Barton SR, Smith IE, Kirby AM, Ashley S, Walsh G, Parton M. The role of ipsilateral breast radiotherapy in management of occult primary breast cancer presenting as axillary lymphadenopathy. Eur J Cancer. 2011;47:2099–106. doi: 10.1016/j.ejca.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 40.Brill KL, Brenin DR. Occult breast cancer and axillary mass. Curr Treat Options Oncol. 2001;2:149–55. doi: 10.1007/s11864-001-0057-x. [DOI] [PubMed] [Google Scholar]
- 41.Carr JA. Treating occult breast malignancy presenting as axillary node metastases. J Am Coll Surg. 2007;204:517–8. doi: 10.1016/j.jamcollsurg.2006.12.028. [DOI] [PubMed] [Google Scholar]
- 42.Foroudi F, Tiver KW. Occult breast carcinoma presenting as axillary metastases. Int J Radiat Oncol Biol Phys. 2000;47:143–7. doi: 10.1016/s0360-3016(99)00542-8. [DOI] [PubMed] [Google Scholar]
- 43.Masinghe SP, Faluyi OO, Kerr GR, Kunkler IH. Breast radiotherapy for occult breast cancer with axillary nodal metastases— does it reduce the local recurrence rate and increase overall survival? Clin Oncol. 2011;23:95–100. doi: 10.1016/j.clon.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 44.Pavlidis N, Pentheroudakis G. Cancer of unknown primary site. Lancet. 2012;379:1428–35. doi: 10.1016/S0140-6736(11)61178-1. [DOI] [PubMed] [Google Scholar]
- 45.Giuliano AE, Hunt KK, Ballman KV, Beitsch PD, Whitworth PW, Blumencranz PW, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA. 2011;305:569–75. doi: 10.1001/jama.2011.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Merson M, Andreola S, Galimberti V, Bufalino R, Marchini S, Veronesi U. Breast carcinoma presenting as axillary metastases without evidence of a primary tumor. Cancer. 1992;70:504–8. doi: 10.1002/1097-0142(19920715)70:2<504::aid-cncr2820700221>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 47.van Ooijen B, Bontenbal M, Henzen-Logmans SC, Koper PC. Axillary nodal metastases from an occult primary consistent with breast carcinoma. Br J Surg. 1993;80:1299–300. doi: 10.1002/bjs.1800801026. [DOI] [PubMed] [Google Scholar]
- 48.Vezzoni P, Balestrazzi A, Bignami P. Axillary lymph node metastases from occult carcinoma of the breast. Tumori. 1979;65:87–91. doi: 10.1177/030089167906500109. [DOI] [PubMed] [Google Scholar]
- 49.Yang T-IJ, Yang Q, Haffty BG, Moran MS. Prognosis for mammographically occult, early-stage breast cancer patients treated with breast-conservation therapy. Int J Radiat Oncol Biol Phys. 2010;76:79–84. doi: 10.1016/j.ijrobp.2009.01.039. [DOI] [PubMed] [Google Scholar]
- 50.Feigelson HS, James TA, Single RM, Onitilo AA, Aiello Bowles EJ, Barney T, et al. Factors associated with the frequency of initial total mastectomy: results of a multi-institutional study. J Am Coll Surg. 2013;216:966–75. doi: 10.1016/j.jamcollsurg.2013.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gelber RP, McCarthy EP, Davis JW, Seto TB. Ethnic disparities in breast cancer management among Asian Americans and Pacific Islanders. Ann Surg Oncol. 2006;13:977–84. doi: 10.1245/ASO.2006.08.036. [DOI] [PubMed] [Google Scholar]
- 52.Lim SE, Back M, Quek E, Iau P, Putti T, Wong JEL. Clinical observations from a breast cancer registry in Asian women. World J Surg. 2007;31:1387–92. doi: 10.1007/s00268-007-9086-3. [DOI] [PubMed] [Google Scholar]
- 53.Pham JT, Allen LJ, Gomez SL. Why do Asian-American women have lower rates of breast conserving surgery: results of a survey regarding physician perceptions. BMC Public Health. 2009;9:246. doi: 10.1186/1471-2458-9-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Padhani AR, Ah-See MLW, Makris A. MRI in the detection and management of breast cancer. Expert Rev Anticancer Ther. 2005;5:239–52. doi: 10.1586/14737140.5.2.239. [DOI] [PubMed] [Google Scholar]
- 55.Morrow M. Magnetic resonance imaging for screening, diagnosis, and eligibility for breast-conserving surgery: promises and pitfalls. Surg Oncol Clin North Am. 2010;19:475–92. doi: 10.1016/j.soc.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 56.Morrow M, Waters J, Morris E. MRI for breast cancer screening, diagnosis, and treatment. Lancet. 2011;378:1804–11. doi: 10.1016/S0140-6736(11)61350-0. [DOI] [PubMed] [Google Scholar]
- 57.Golfinopoulos V, Pentheroudakis G, Salanti G, Nearchou AD, Ioannidis JP, Pavlidis N. Comparative survival with diverse chemotherapy regimens for cancer of unknown primary site: multiple-treatments meta-analysis. Cancer Treat Rev. 2009;35:570–3. doi: 10.1016/j.ctrv.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 58.Morrow M. MRI for breast cancer screening and staging. Menopause. 2011;18:1337. [Google Scholar]
- 59.Bloom S, Morrow M. A clinical oncologic perspective on breast magnetic resonance imaging. Magn Reson Imaging Clin N Am. 2010;18:277–94. doi: 10.1016/j.mric.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 60.Orel S. Who should have breast magnetic resonance imaging evaluation? J Clin Oncol. 2008;26:703–11. doi: 10.1200/JCO.2007.14.3594. [DOI] [PubMed] [Google Scholar]
- 61.Krämer A, Hübner G, Schneeweiss A, Folprecht G, Neben K. Carcinoma of unknown primary—an orphan disease? Breast Care. 2008;3:164–70. doi: 10.1159/000136001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. [Accessed 10 April 2013];The National Institutes of Health Office of Rare Diseases Research. 2013 http://www.rarediseases.info.nih.gov/
- 63. [Accessed 10 April 2013];Coordination of Rare Diseases at Sanford (CoRDS) 2013 http://www.sanfordresearch.org/cords/
- 64. [Accessed 10 June 2013];The American Society of Breast Surgeons Mastery of Breast Surgery Program. 2013 http://www.breastsurgeons.org/mastery/
- 65.El-Barhoun EN, Pitman AG. Impact of breast MR in nonscreening Australian clinical practice: Audit data from a singlereader single-centre site. J Med Imaging Radiat Oncol. 2011;55:461–73. doi: 10.1111/j.1754-9485.2011.02302.x. [DOI] [PubMed] [Google Scholar]
- 66.Bartella L, Smith CS, Dershaw DD, Liberman L. Imaging breast cancer. Radiol Clin North Am. 2007;45:45–67. doi: 10.1016/j.rcl.2006.10.007. [DOI] [PubMed] [Google Scholar]