Abstract
The nucleoside analog ganciclovir (GCV) elicits cytotoxicity in tumor cells via a novel mechanism in which drug incorporation into DNA produces minimal disruption of replication, but numerous DNA double strand breaks occur during the second S-phase after drug exposure. We propose that homologous recombination (HR), a major repair pathway for DNA double strand breaks, can prevent GCV-induced DNA damage, and that inhibition of HR will enhance cytotoxicity with GCV. Survival after GCV treatment in cells expressing a herpes simplex virus thymidine kinase was strongly dependent on HR (>14-fold decrease in IC50 in HR-deficient vs. HR-proficient CHO cells). In a homologous recombination reporter assay, the histone deacetylase inhibitor, suberoylanilide hydroxamic acid (SAHA; vorinostat), decreased HR repair events up to 85%. SAHA plus GCV produced synergistic cytotoxicity in U251tk human glioblastoma cells. Elucidation of the synergistic mechanism demonstrated that SAHA produced a concentration-dependent decrease in the HR proteins Rad51 and CtIP. GCV alone produced numerous Rad51 foci, demonstrating activation of HR. However, the addition of SAHA blocked GCV-induced Rad51 foci formation completely and increased γH2AX, a marker of DNA double strand breaks. SAHA plus GCV also produced synergistic cytotoxicity in HR-proficient CHO cells, but the combination was antagonistic or additive in HR-deficient CHO cells. Collectively, these data demonstrate that HR promotes survival with GCV and compromise of HR by SAHA results in synergistic cytotoxicity, revealing a new mechanism for enhancing anticancer activity with GCV.
Keywords: Homologous recombination, Histone deacetylase inhibitor, DNA repair, DNA damage, anticancer drug
1. Introduction
Nucleoside analog antitumor drugs have a well established role in cancer chemotherapy. Cytarabine, fludarabine and the more recently approved clofarabine are mainstays of treatment in leukemias, whereas gemcitabine is first line treatment in pancreatic and non-small cell lung cancer [1–3]. The hallmark of these drugs is that each requires activation by cellular kinases to the corresponding 5’-triphosphate, which then serves as a fraudulent substrate for DNA replication. In a more selective approach to chemotherapy, nucleoside analogs that are not activated by human enzymes have been coupled with intratumoral gene transfer of an exogenous activating enzyme, as in suicide gene therapy using the herpes simplex virus thymidine kinase (HSV-TK) to activate the antiviral drug ganciclovir (GCV) [4].
For most nucleoside analogs, incorporation into DNA with consequent disruption of DNA replication is primarily responsible for their antitumor activity. However the exact molecular mechanism by which cell death occurs is not known. Studies have revealed that exposure of cells to nucleoside analogs, such as cytarabine and gemcitabine, activate the S-phase checkpoint protein, Chk1, via phosphorylation by ATR, an important regulator of the DNA damage response pathway [5;6]. Prolonged stalling of DNA replication by these analogs may lead to a DNA double strand break (DSB), a lesion that activates the other major regulator of the DNA damage response, ATM [7]. Evaluation of the role of Chk1 in cytotoxicity with antimetabolites demonstrated that Chk1 activation contributes to cell survival [6;8]. These mechanisms predicted that depletion or chemical inhibition of Chk1 would enhance cytotoxicity with nucleoside analogs, which has been demonstrated in vitro thus encouraging clinical trials [6;8–11].
While studies have focused on the ability of cell cycle checkpoint inhibitors to increase activity of anticancer nucleoside analogs, data are sparse on the role of DNA repair pathways in response to DNA damage with these drugs. Evaluation of mismatch repair (MMR) in the cytotoxicity of antimetabolites has demonstrated that cells defective in MMR are more sensitive to the nucleobase analog 6-thioguanine [12]. Sensitivity to nucleoside analogs cytarabine, fludarabine and clofarabine was dependent on the specific MMR protein that was mutated, in which hMSH6 enhanced cytotoxicity but hMSH2 decreased cytotoxicity [13]. These reports illustrate the important yet complex effects of MMR in antimetabolite cytotoxicity, and justify further exploration of repair pathways in the mechanism of action for this important class of drugs.
The nucleoside analog GCV, utilized in a suicide gene therapy approach with transfer of HSV-TK into tumor cells, has shown excellent anticancer activity in preclinical models [14–16]. It is widely studied in clinical trials, with promising results when combined with other modalities in patients with prostate cancer [17;18]. Ganciclovir is of particular interest because of its high cytotoxicity and novel mechanism of action compared to other nucleoside analogs. In cells engineered to activate GCV, it is readily incorporated into tumor cell DNA with minimal disruption of DNA replication, followed by arrest in the second S-phase after drug incubation and cell death [15;19]. Cytotoxicity with GCV is associated with the production of DNA double strand breaks (DSBs) [20;21] concurrent with activation of the DNA repair pathway of homologous recombination (HR) [21], suggesting a causal link between the two processes. Prior studies by Thust et al have indirectly implicated HR through the observation that GCV produced sister chromatid exchanges in CHO cells, a process that requires HR [22;23]. Although these studies indicated that HR was activated in response to GCV, it was not known whether HR assisted cells in circumventing the cytotoxic insult of GCV, or whether HR mediated cell death similar to the futile attempt to repair cisplatin lesions in DNA by mismatch repair [24]. Previous studies suggested that yeast strains deficient in HR were more sensitive to GCV than the corresponding wild-type strains [25]. However, because HR plays a greater role in DNA repair in yeast compared to mammalian cells [26], extrapolation to human tumor cells was not clear.
Here we propose that HR promotes survival of mammalian cells after GCV exposure. Furthermore, we hypothesize that inhibition of HR will enhance GCV-mediated cytotoxicity. To test these ideas, we have undertaken studies in matched HR proficient and deficient CHO cell lines. To explore the pharmacologic inhibition of HR, we used the histone deacetylase (HDAC) inhibitor, suberoylanilide hydroxamic acid (SAHA; vorinostat), which has been reported to decrease expression of the HR-required protein Rad51 in human tumor cells [27]. We demonstrate that HR promotes survival in response to GCV in mammalian cells, providing a mechanism to be exploited pharmacologically to enhance GCV mediated cytotoxicity. The results further illustrate that SAHA decreases HR activity, and blocks HR-mediated repair of GCV induced DNA damage. Importantly, GCV and SAHA synergize only in HR proficient cells, demonstrating that the observed synergy is specifically due to inhibition of HR by SAHA.
2. Materials and methods
2.1. Cell Culture
U251 human glioblastoma cells that stably express HSV-TK [15] were maintained in RPMI 1640 supplemented with 10% calf serum (GIBCO) and L-glutamine (Fisher Scientific). The CHO cell lines, AA8 and irs1SF, were maintained in MEMα supplemented with 10% FBS (Gibco) and L-glutamine (Fisher Scientific). HeLa cells containing the green fluorescent protein recombination substrate (DR-GFP) construct [28] were maintained in DMEM containing 10% FBS and L-glutamine. All other chemicals were obtained from Fisher Scientific. AA8 and irs1SF cells were transduced to express HSV-TK or β-galactosidase (LacZ) using a lentivirus vector containing the cDNA for LacZ, and monoclonal sublines were developed for each. HSV-TK expressing HeLa DR-GFP were generated by a similar approach with retrovirus as previously described [15]. Cell survival was measured in colony forming assays with exponentially growing cells [29]. Interactions between GCV and SAHA were evaluated by isobologram analysis [30]. Each experiment was carried out in triplicate and performed at least twice.
2.2. Western blots and immunohistochemistry
Primary antibodies used were: Rad51 and actin (Calbiochem), histone 3, histone 3 acetyl-lysine 9, H2AX and CtIP (Cell Signaling), γH2AX (Millipore) and HSV-TK (a generous gift from Margaret Black [31]). Western blots and Rad51 foci analysis were performed as previously described [21]. For Rad51 foci, images of representative cell populations were captured by laser confocal microscopy, and Rad51 positive cells were scored visually (positive cell identified as ≥10 Rad51 foci) [21].
2.3. Analysis of GCV metabolism and incorporation
U251tk or CHO cells were incubated with GCV for up to 24 hr. [3H]GCV (Moravek Biochemicals, Inc., Brea, CA) was used in studies measuring ganciclovir 5’-triphosphate (GCVTP) or its incorporation into DNA. At the conclusion of the incubation, cells were harvested for analysis of cellular deoxynucleotides by HPLC [15] and incorporation into DNA by scintillation counting [29].
2.4. Cell cycle analysis
Exponentially growing U251tk cells were treated with GCV and/or SAHA at the indicated concentrations and time periods. Cells were harvested by trypsinization and prepared for dual parameter flow cytometry (propidium iodide and bromodeoxyuridine) as described [16]. Analysis was performed on a BD FacsCalibur at the University of Michigan Flow Cytometry Core Facility. At least 10,000 cells were evaluated for each condition, and the experiment was performed at least two times.
2.5. Measurement of HR activity
HR activity was evaluated using the DR-GFP recombination substrate with a recognition site for the I-SceI endonuclease in one copy of the GFP gene [32]. Exponentially growing HeLa DR-GFPtk cells were treated with SAHA and/or adenovirus containing the cDNA for the I-SceI endonuclease for 24hr [33]. After growth for an additional 24 hr in fresh media, cells were harvested by trypsinization and resuspended in 1% formaldehyde (Fisher Scientific). Cells were analyzed for GFP expression by flow cytometry. Data was analyzed using WinMDI software (version 2.9).
3. Results
3.1. Homologous Recombination Promotes Survival in Response to GCV
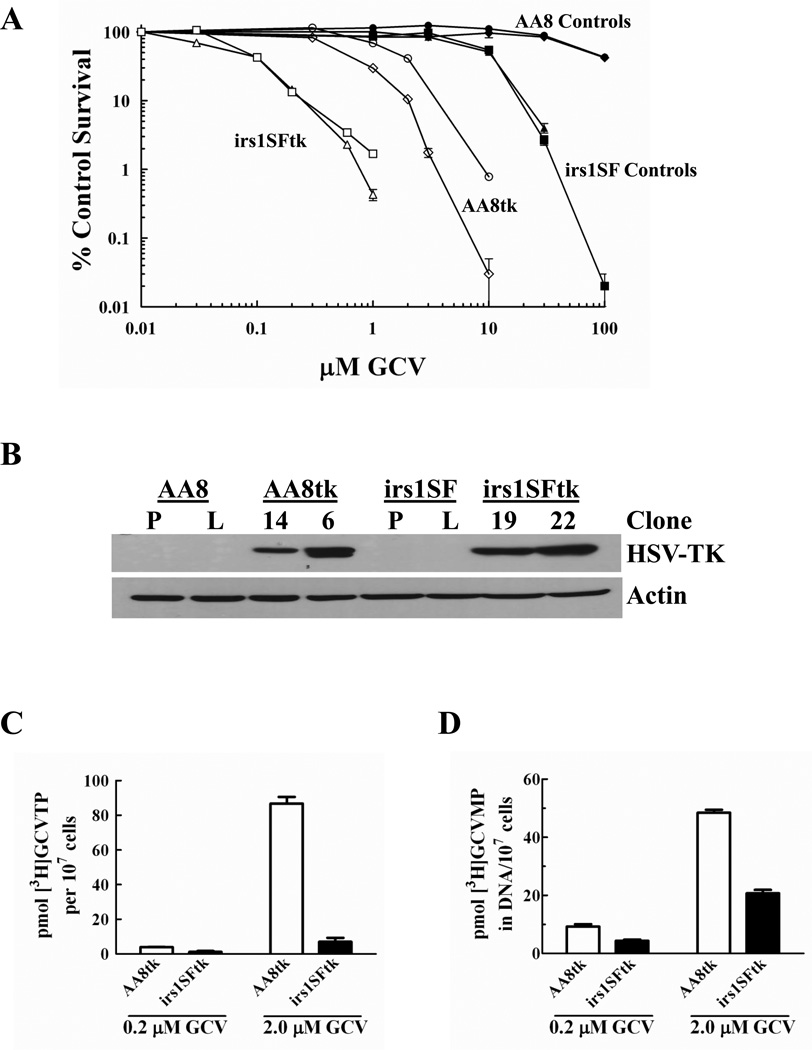
The role of HR on GCV-mediated cytotoxicity was evaluated using matched CHO cell lines that are either proficient (AA8) or deficient in HR (irs1SF) [32;34;35], and transduced to stably express HSV-TK (for activation of GCV) or LacZ (control). In two separate monoclonal sublines of HR proficient and deficient cell lines, the HR-deficient irs1SFtk cells were >14-fold more sensitive to GCV than HR-proficient AA8tk cells (IC50 = 0.08 ± 0.011 µM vs. 1.14 ± 0.694 µM, respectively; p = 0.013; Fig. 1A). The CHO cell lines expressed similar levels of HSV-TK (Fig. 1B). Importantly, the HR-deficient irs1SFtk cells were more sensitive even though the AA8tk clones accumulated similar or greater levels of the active metabolite GCVTP (Fig. 1C). The amount of dGTP, the endogenous competitor of GCVTP for incorporation into DNA, was similar in the absence of drug and did not change significantly in response to GCV treatment in either cell line (data not shown). Furthermore, AA8tk cells incorporated as much or more of the analog into DNA compared to irs1SFtk cells (Fig. 1D; data not shown), consistent with the higher levels of GCVTP in the AA8tk cells. In CHO cells not expressing HSV-TK, activation and incorporation of [3]GCV was <5% of that in HSV-TK-expressing cells (data not shown). Collectively, these data establish that decreased activation of GCV, decreased dGTP or decreased GCVTP incorporation into DNA cannot explain the differences in cytotoxicity observed between the cell lines. Thus, these data demonstrate that HR strongly promotes survival in the AA8tk cells.
Fig. 1. GCV cytotoxicity in HR proficient and deficient CHO cells.
A, Exponentially growing CHO cells that are HR proficient (AA8) and HR deficient (irs1SF) and sublines stably expressing HSV-TK or LacZ were incubated with GCV for 16 hr, and survival was measured by a colony formation assay. Cell lines, ♦ AA8, ● AA8LacZ, ▲ irs1SF, ■ irs1SFLacZ, ○ AA8tk clone 14, ◊ AA8tk clone 6, □ irs1SFtk clone 22, △irs1SFtk clone 19. Points represent the mean number of colonies of at least three wells from a representative experiment repeated at least twice. Bars represent standard error of the mean (SEM). B, Western blot analysis of HSV-TK expression in cell lines. P = parental (not transduced); L = LacZ transduction control; numbers represent monoclonal sublines of HSV-TK expressing cells. C and D, Cells were incubated with [3H]GCV for 16 hr, extracted with perchloric acid and analyzed for [3H]GCVTP in the cytosol by HPLC (C) or [3H]GCVMP in DNA from the acid-insoluble pellet (D). Bars represent mean ± SEM.
3.2. Synergistic cytotoxicity with GCV and SAHA
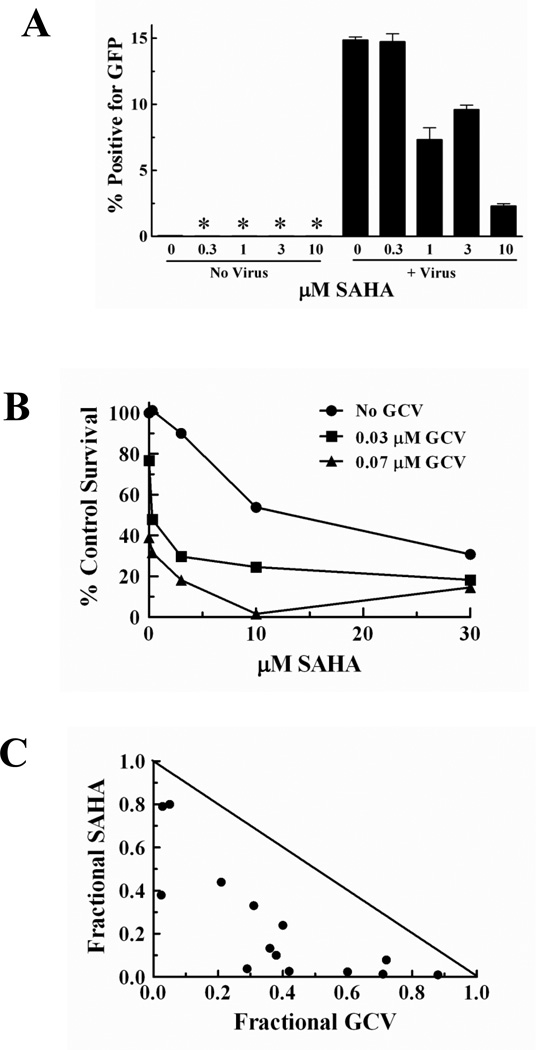
Based on the increased sensitivity to GCV observed in the HR deficient CHO cells, we hypothesized that pharmacologic inhibition of HR in HR-proficient human tumor cells would enhance cytotoxicity with GCV. SAHA was recently reported to decrease expression of the required HR protein, Rad51, in tumor cells [27;36]. We proposed that the SAHA-mediated decrease in Rad51 would result in a decrease in HR similar to previous studies with another hydroxamic acid HDAC inhibitor, PCI-24781 [37]. Using a cell based recombination assay in which functional GFP is expressed only when HR successfully repairs an I-SceI induced DSB [32], SAHA decreased HR events by 50% to 85% at concentrations of 1, 3 and 10 µM (Fig. 2A).
Fig. 2. SAHA inhibits HR repair and synergistically enhances GCV cytotoxicity in HR proficient cells.
A, HeLa-D-GFP-TK cells were incubated with SAHA alone or with the AdNGUS24i adenovirus containing the cDNA for the I-SceI endonuclease. After 48 hr, cells were assessed for GFP expression by flow cytometry. Bars represent a mean of at least three experiments performed in triplicate ± SEM. *, GFP not detectable. B, Cytotoxicity of SAHA and GCV in U251tk glioblastoma cells. C, Synergy with GCV and SAHA identified by isobologram analysis; diagonal line, isoeffective line of additivity.
With the demonstration that SAHA could decrease HR, we next evaluated the effect of SAHA on GCV cytotoxicity using U251tk human glioblastoma cells. Concentrations of SAHA that inhibited HR were combined with GCV concentrations that decreased clonogenic cell survival by 25% to 50%. SAHA decreased cytotoxicity with GCV in a concentration-dependent manner (Fig. 2B). Isobologram analysis demonstrated that the combination of these two drugs resulted in synergistic cytotoxicity over the entire range of concentrations used (Fig. 2C).
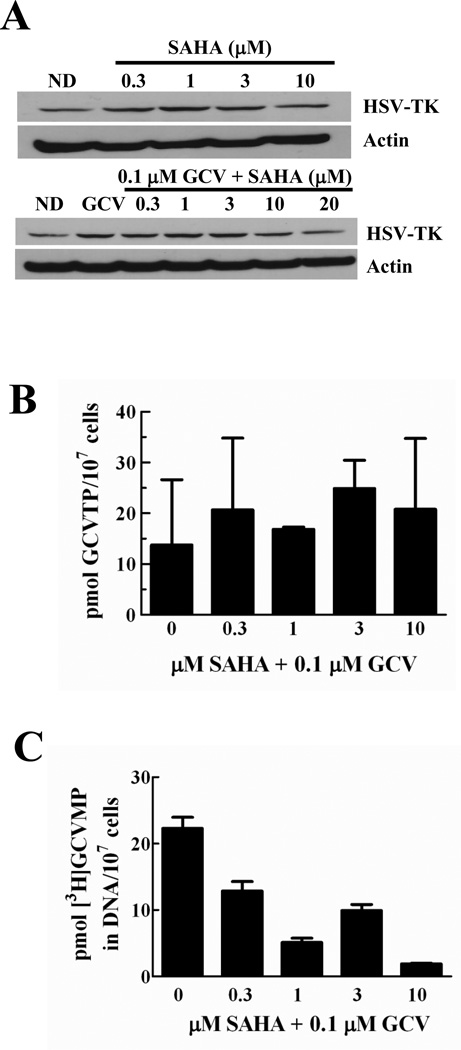
To elucidate the mechanism of synergistic cytotoxicity with SAHA and GCV, we evaluated the effects of SAHA on HSV-TK and activation of GCV, which is crucial for GCV cytotoxicity. A previous report demonstrated that HDAC inhibition increased mRNA for HSV-TK in cells that stably expressed the enzyme [38], which could result in increased GCV activation and cytotoxicity. Over the range of concentrations that produced synergy with GCV, SAHA alone resulted in a modest increase in HSV-TK expression when administered either alone (1.5-fold) or combined with GCV (2.2-fold) (Fig. 3A). We then asked whether this increase in HSV-TK expression would increase GCVTP levels sufficiently to increase its incorporation into DNA, thus effecting greater cytotoxicity. Metabolic studies demonstrated that SAHA did not significantly increase GCVTP levels after a 24 hr incubation (Fig. 3B). Notably, SAHA actually decreased incorporation of GCVTP into DNA, with a 12-fold reduction at 10 µM SAHA (Fig. 3C). Furthermore, SAHA did not significantly alter the amount of dGTP or other deoxynucleoside triphosphates in U251tk cells when administered with GCV (data not shown). These data eliminate the possibility that the synergistic cytotoxicity observed with GCV and SAHA is due to altered metabolism of GCV or endogenous deoxynucleotides, or increased incorporation of GCVTP into DNA.
Fig. 3. SAHA does not enhance GCV phosphorylation or incorporation into DNA.
A, effect of a 24 hr incubation with SAHA and GCV on HSV-TK expression in U251tk cells. B, effect of a 24 hr incubation with SAHA and GCV (unlabeled or 3H-labeled) on GCVTP accumulation, and on [3H]GCVMP incorporation into DNA (C). Bars represent mean ± SEM for triplicate values.
3.3. Decrease in HR-required proteins with SAHA
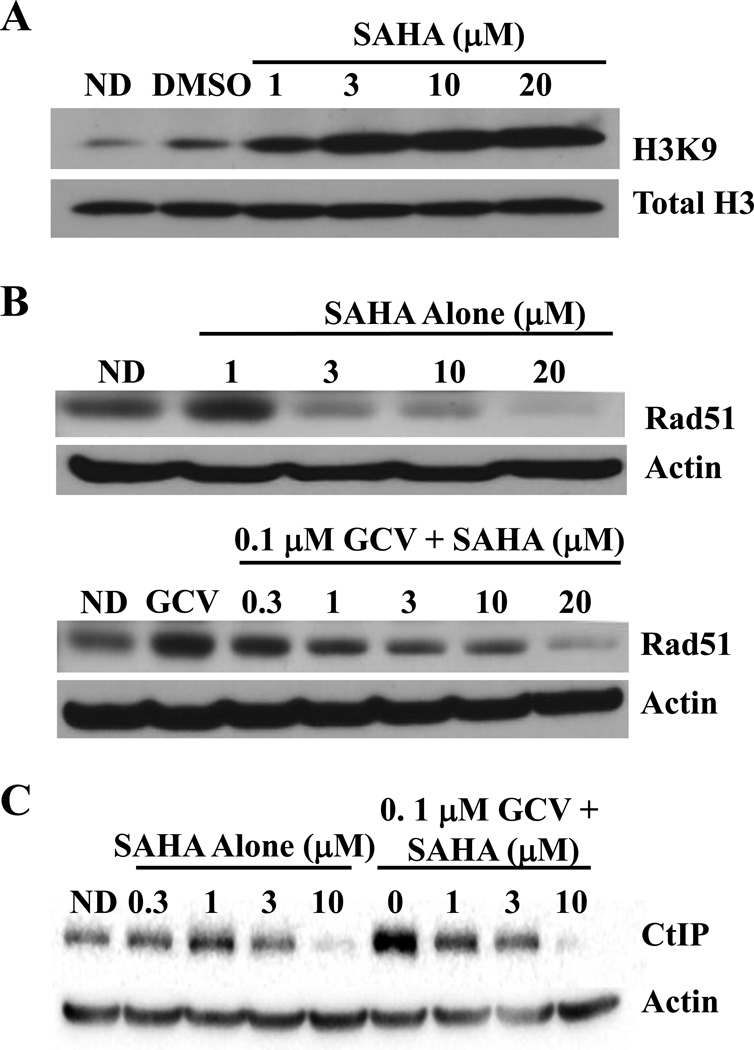
To further investigate the mechanism by which SAHA synergistically increased GCV cytotoxicity, we evaluated its ability to block expression of HR required proteins. The concentrations of SAHA that produced synergistic cytotoxicity resulted in higher levels of acetylation of histone H3 lysine 9 by 8 hr after drug addition in U251tk cells, thus demonstrating its HDAC inhibitory property (Fig. 4A). While increased histone acetylation is associated with increased gene transcription, previous studies have demonstrated that there are also decreases in transcribed genes. For example, one study demonstrated that an HDAC inhibitor decreased protein levels of HR required proteins such as Rad51 [37]. Previously we have established a correlation between Rad51 and cytotoxicity with GCV [21]. To determine whether the synergy with SAHA and GCV could be due to inhibition of expression of HR-required proteins, we measured the effects of the drugs alone or together on expression of Rad51 as well as CtIP, a required exonuclease for DNA resection during HR [39]. The results demonstrated that SAHA alone decreased Rad51 at concentrations as low as 3 µM, whereas CtIP was decreased only at 10 µM SAHA (Figs. 4B, C). However, GCV alone produced a strong increase in both of these HR proteins, possibly due to S-phase accumulation of cells. This GCV-induced increase in protein expression was blocked by SAHA in a concentration dependent manner at concentrations as low as 1 µM. At the highest concentration of SAHA tested, Rad51 and CtIP were decreased to as little as 4% and 5%, respectively, of the levels observed after treatment with GCV alone. Thus, SAHA alone or with GCV decreased expression of Rad51 and CtIP.
Fig. 4. SAHA inhibits expression of Rad51, CtIP and Rad51 foci in response to GCV and increases γH2AX.
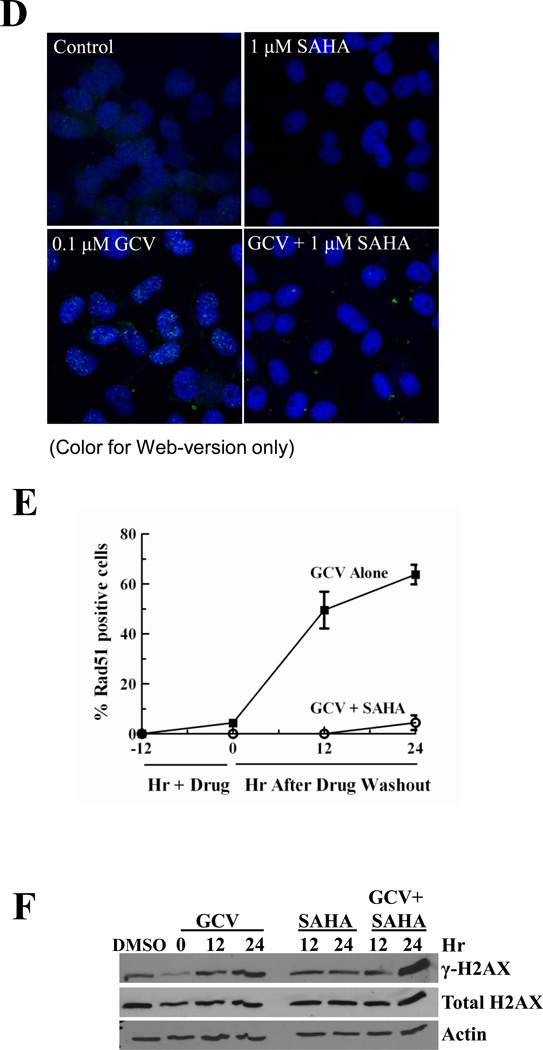
A, U251tk cells were incubated with SAHA for 8 hr then assessed for histone H3 lysine 9 acetylation by Western blot analysis. B and C, cells were incubated with SAHA alone or with GCV for 24 hr, then expression of Rad51 (B) and CtIP (C) was determined by Western blot analysis. D, E and F, effect of a 24 hr incubation with 0.1 µM GCV and 1 µM SAHA alone or together on: D, Rad51 foci formation, evaluated by immunocytochemistry; E, number of Rad51 foci (points represent mean of triplicate measurements ± SEM); F, γH2AX by Western blot analysis. For Western blots, total H3, actin or H2AX were used as loading controls. DMSO, dimethyl sulfoxide; ND, no drug.
To further evaluate the ability of SAHA to inhibit HR, we measured its effect on GCV-induced Rad51 foci, which form at sites of HR repair of DNA damage [40]. The addition of 1 µM SAHA to 0.1 µM GCV markedly decreased the number of Rad51 foci positive cells (Fig. 4D). Whereas GCV alone increased Rad51 foci by 16.5-fold compared to control untreated cells, the addition of SAHA resulted in no significant increase in Rad51 foci compared to controls through 24 hr after drug washout (Fig. 4E). Strikingly, there were no Rad51 positive cells after treatment with 0.1 µM GCV plus 3 or 10 µM SAHA, or with any concentration of SAHA alone at any of the time points evaluated (data not shown). In addition, cells treated with SAHA and GCV exhibited a greater increase in γH2AX, a marker of DSBs, than cells treated with either drug alone (Fig. 4F). Thus, the inhibition of HR activity by SAHA appears to be due, at least in part, to a direct effect on two required HR proteins, Rad51 and CtIP. These results further support the hypothesis that SAHA produces synergistic cytotoxicity with GCV through decreasing HR activity.
3.4. Cell cycle effects with GCV and SAHA
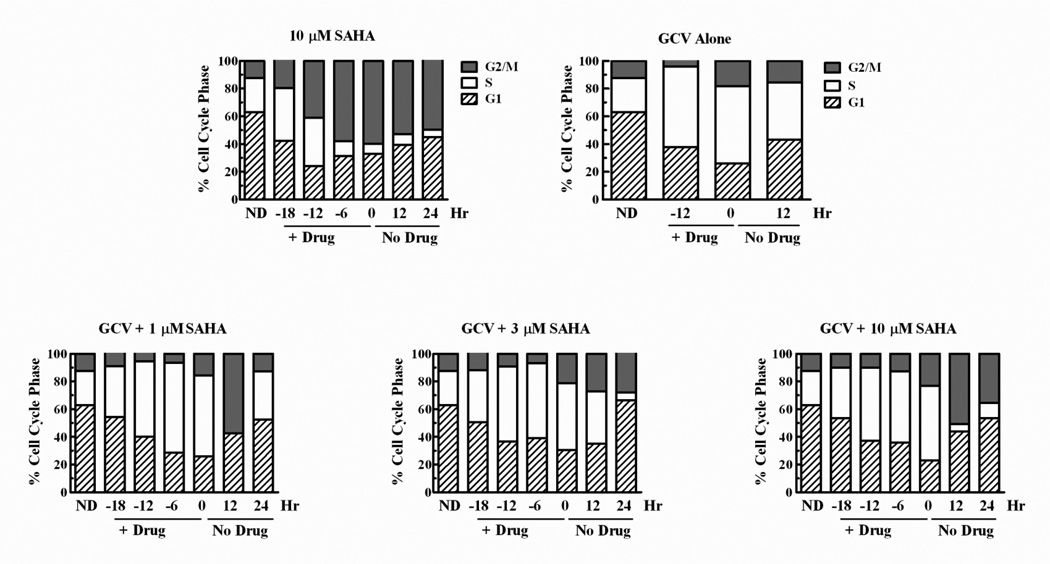
HR is used for DNA repair primarily in S and G2 phases of the cell cycle, thus the apparent decrease in HR activity after SAHA exposure could occur as a result of drug-mediated cell cycle arrest in G1. Evaluation of cell cycle distribution in U251tk cells demonstrated that, during the first 12 hr of incubation, SAHA alone did not decrease the percentage of cells in S or G2, while it did decrease the percentage of cells in G1 (Fig. 5). Thereafter, there was a decrease in cells in S with increases in G2/M and G1, concomitant with a decrease in cell number. When SAHA was combined with GCV, the number of S-phase cells increased as observed with GCV alone throughout the drug incubation period, with decreases in G1 cells. Following drug washout, G1 cells increased as S-phase cells decreased. Thus, our findings that SAHA decreased Rad51 expression and HR events in a cell based assay cannot be explained simply by a G1 arrest.
Fig. 5. SAHA does not increase S or G2/M accumulation with GCV.
U251tk cells were incubated with 10 µM SAHA alone, 0.1 µM GCV alone or with 1, 3 or 10 µM SAHA for 24 hr. Cells were harvested periodically and cell cycle position was evaluated by dual parameter (bromodeoxyuridine and propidium iodide) flow cytometry.
3.5. Synergistic cell killing occurs only in HR proficient cells with GCV and SAHA
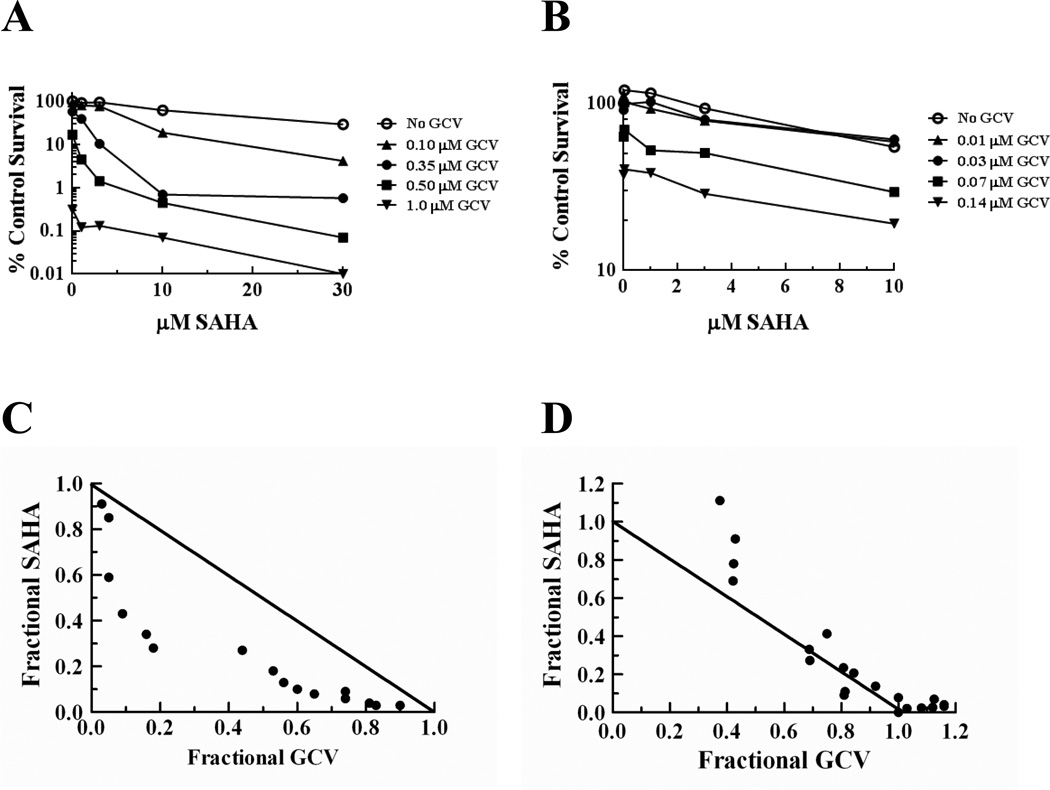
If the synergy observed with GCV and SAHA is due to decreasing HR activity, then this drug combination should show synergy in HR-proficient cells but not in cells lacking HR. To test this, we evaluated the drug interaction in the HR proficient and deficient CHO cell lines. As observed in the U251tk cells, the combination of SAHA and GCV elicited synergistic cytotoxicity in the HR-proficient AA8tk cells (Fig. 6A). In contrast, in the irs1SFtk HR-deficient cells cytotoxicity with GCV and SAHA was additive at best with evidence of antagonism at some concentrations (Fig. 6B), despite the finding that the irs1SFtk cells were more sensitive to SAHA as a single agent compared to the AA8tk cells (IC50 = 10 and 17 µM, respectively; data not shown). These data further demonstrate that the synergistic cytotoxicity observed with GCV and SAHA in two HR-proficient cell lines is due to SAHA-mediated effects on HR in response to GCV-induced DNA damage.
Fig. 6. Synergistic cytotoxicity with SAHA and GCV occurs only in HR proficient cells.
AA8tk (A,C) or irs1SFtk (B,D) cells were incubated with GCV (0.1, 0.35, 0.5, 1.0 µM for AA8tk cells and 0.01, 0.03, 0.07 and 0.14 µM for irs1SFtk cells) and/or SAHA (1, 3, 10 and 30 µM for AA8tk cells and 0.3, 1, 3, and 10 µM irs1SFtk cells) for 16 hr. Clonogenic survival graphs (A,B) were used to generate isobolograms (C,D). Data points on survival graphs represent means of triplicate determinations from a typical experiment. Diagonal line on isobolograms represents the isoeffective line of additivity.
4. Discussion
Nucleoside analogs are S-phase specific agents that elicit anticancer activity by inducing DNA damage, and numerous studies have focused on the type and amount of DNA damage these compounds produce. GCV potently produces tumor cell death in an S-phase specific manner via induction of DSBs [20;21]. Because HR is a major mechanism of DSB repair for cells in S phase, we evaluated the role of HR in cell death with GCV. Here we have demonstrated that cells treated with GCV require functional HR for survival. Furthermore, we have demonstrated that SAHA synergistically enhances the cytotoxicity of GCV through SAHA-mediated inhibition of HR, providing a previously unknown mechanism to improve outcomes with HSV-TK/GCV suicide gene therapy.
These results firmly establish HR as an important mechanism that promotes survival for cells treated with GCV. Prior reports have suggested that HR is activated following cell exposure to GCV, but whether HR plays a role in either survival or cell death with GCV, or is merely an unrelated occurrence, was not known. For example, Thust et al demonstrated that GCV produced sister chromatid exchanges in CHO cells [22], and previously we have correlated late induction of DNA double strand breaks with an increase in Rad51 foci [21]. Although these studies suggested an association between HR and GCV, there was no clear proof. Yeast studies suggested that HR promoted cell survival with GCV [25], however yeast have greatly increased HR activity compared to human cells [26] so extrapolation to mammalian cells was uncertain. Studies presented here with matched HR-proficient and HR-deficient cell lines conclusively demonstrated that HR promotes survival with GCV.
Based on the large increase in cell killing with GCV in the HR-deficient cells, we hypothesized that pharmacologic inhibition of HR would result in synergistic cell killing with GCV. With reports that SAHA as well as another hydroxamic acid analog decreased expression of HR proteins in cell culture and animal models [27;36;37;41], SAHA was used for these studies. We demonstrated that SAHA alone or with GCV decreased HR activity through decreased expression of the HR-required proteins Rad51 and CtIP, resulting in increased DNA damage. These results, combined with the findings that SAHA synergistically increased GCV cytotoxicity in two HR-proficient cell lines but not in an HR-deficient cell line, strongly support our conclusion that the drug synergy is due to the SAHA-mediated decrease in HR activity.
Recent reports have demonstrated that other HDAC inhibitors can increase cell killing with GCV due to other mechanisms. Valproic acid and FR901228 enhanced transgene expression in cells treated with an adenovirus encoding the HSV-TK cDNA [42;43]. Other investigators reported that phenylbutyrate and valproic acid enhanced HSV-TK/GCV efficacy through increasing gap junctional communication, which mediates bystander killing through transfer of cytotoxic GCV phosphates from HSV-TK expressing cells to neighboring cells [38;43;44]. The ability of HDAC inhibitors to increase expression of the coxsackie and adenovirus receptor has also been attributed as a mechanism of synergy between valproic acid and GCV with adenovirus-delivered HSV-TK [43]. While a phenylbutyrate-mediated increase mRNA expression of HSV-TK was reported in stably expressing cells [38], in our studies only a modest increase in HSV-TK protein expression occurred with SAHA which was not sufficient to increase GCVTP or its incorporation into DNA, thus eliminating increased transgene expression as a mechanism of synergy. Because all cells in our studies expressed HSV-TK, effects on gap junctional communication to increase bystander killing also was not a factor in the synergy. Thus, this is the first demonstration of synergy with GCV and a HDAC inhibitor due to inhibition of HR. Considering that decreased Rad51 expression has been reported with two HDAC inhibitors [27;37], the synergy observed with GCV and a HDAC inhibitor in other studies [38;42–44] may have been due, at least in part, to HR impairment.
We recognize that SAHA, as with other HDAC inhibitors, affects expression of numerous proteins in addition to those studied here that can lead to cytotoxicity [45], and which could potentially account for the synergy with GCV. For example, other investigators have reported that SAHA mediated the production of reactive oxygen species SAHA that contributed to cell death [46;47]. We hypothesized that, if the mechanism of synergy was due primarily to the SAHA-mediated inhibition of HR, then SAHA would synergistically enhance GCV cytotoxicity in the HR-proficient but not in the HR-deficient CHO cells. Indeed, the results demonstrated that synergy was observed only in the HR-proficient cells, indicating that the synergistic cytotoxicity with GCV and SAHA depends on functional HR and is not due to non-HR related mechanisms, such as the production of reactive oxygen species. Taken together, the results presented here demonstrate that synergistic tumor cell killing with GCV in HR-proficient cells is due to inhibition of expression of HR proteins by SAHA.
Because HR is a major pathway for repair of DNA double strand breaks during S-phase, other chemotherapeutic drugs that cause such damage may also benefit from co-administration with SAHA or other HDAC inhibitors. Indeed it has been reported that CNDAC, which can directly cause a DSB once incorporated into DNA, also relies on HR for repair of the DSB [48]. In addition, HDAC inhibitors have been reported to enhance cell killing with ionizing radiation [27;36], which causes cell death through generation of DSBs. In view of the excellent synergy observed with GCV and SAHA in these studies through inhibition of HR, this may be an effective mechanism to enhance cytotoxicity with other DNA-damaging drugs and modalities.
Based on these results, we propose the following mechanism for the role of HR in cytotoxicity with GCV. As we have described previously, incorporation of GCVTP into tumor cell DNA produces only modest inhibition of cell growth, with the majority of cells able to complete DNA replication and divide [15]. Cells enter the second S-phase after GCV addition with GCV monophosphate in the template and replication is stopped during S phase, leading to cell death. We now propose that the presence of GCV 5’-monophosphate (GCVMP) in the template causes stalling of DNA replication, which may be due to the inability of the replication fork to utilize drug in the template as a substrate, or accommodate the drug-induced conformational change in DNA [49]. Replication fork stalling leads to a DSB with HR attempting to repair the break, which then activates exonucleases, such as CtIP, to resect DNA in the region of the DSB. We propose that GCVMP can inhibit the ability of CtIP to resect DNA, thus preventing Rad51 foci formation and, ultimately, HR cannot repair the DSB. If CtIP does not encounter GCVMP, then strand invasion and completion of HR repair can occur. Crossover resolution of the Holliday junction would result in the sister chromatid exchanges observed by Thust [22]. Consistent with this model, we have previously observed that GCVMP is exceptionally difficult to excise from isolated tumor cell DNA with spleen phosphodiesterase (unpublished data). If human exonucleases have an equally difficult time excising GCVMP from DNA, it would prevent establishment of HR at a DSB site. While GCVMP in template DNA may inhibit the function of HR proteins resulting in failed repair and cell death, decreasing these proteins with SAHA may compromise HR to further enhance the ability of GCVMP to inhibit HR.
These and other studies demonstrate that HDAC inhibitors have multiple mechanisms by which they can enhance cell killing with chemotherapeutic agents. One advantage to SAHA is that it may selectively affect tumor compared to non-transformed cells [50]. In addition, it is used clinically with an excellent safety profile [51], thus should it be an effective adjunct to HSV-TK/GCV it could be readily combined in clinical trials. Here, we demonstrate that SAHA can synergistically increase cell killing with GCV by a previously unknown mechanism: inhibition of HR-mediated repair of GCV-induced DNA damage. Further studies are warranted to evaluate this property of SAHA, and HDAC inhibitors in general, with other DSB-inducing drugs. Moreover, these studies further emphasize the importance of HR as a target for cancer drugs that induce DSBs.
Highlights.
Homologous recombination deficiency enhances ganciclovir cytotoxicity >14-fold
Vorinostat inhibits homologous recombination repair events
Vorinostat + ganciclovir is synergistic only in cells with homologous recombination
Vorinostat does not alter ganciclovir metabolism or cell cycle distribution
Ganciclovir synergy is due to vorinostat suppression of Rad51, Rad51 foci and CtIP
Acknowledgements
The authors thank Dr. Margaret Black for providing the generous gift of the HSV-TK antibody. This work was supported in part by National Cancer Institute grants CA076581 and CA083081 to D.S.S., and CA133046 to C.E.C., and the National Institute of General Medical Sciences grant GM007767 to B.L. and J.K.H. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institute of General Medical Sciences. Support was also provided in part by the National Institutes of Health through the University of Michigan’s Cancer Center Support Grant (P30 CA046592) by the use of the University of Michigan Biomedical Research Core Flow Cytometry and Vector Facilities.
Abbreviations
- DSB
DNA double strand break
- DR-GFP
green fluorescent protein recombination substrate
- GCV
ganciclovir
- GCVMP
ganciclovir 5’-monophosphate
- GCVTP
ganciclovir 5’-triphosphate
- HDAC
histone deacetylase
- HR
homologous recombination
- HSV-TK
herpes simplex virus thymidine kinase
- LacZ
β-galactosidase
- MMR
mismatch repair
- SAHA
suberoylanilide hydroxamic acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement: The authors report no conflicts of interest.
References
- 1.Burnett A, Wetzler M, Lowenberg B. Therapeutic advances in acute myeloid leukemia. J.Clin.Oncol. 2011;29:487–494. doi: 10.1200/JCO.2010.30.1820. [DOI] [PubMed] [Google Scholar]
- 2.Hidalgo M. Pancreatic cancer. N.Engl.J.Med. 2010;362:1605–1617. doi: 10.1056/NEJMra0901557. [DOI] [PubMed] [Google Scholar]
- 3.Azzoli CG, Temin S, Aliff T, Baker S, Jr, Brahmer J, Johnson DH, Laskin JL, Masters G, Milton D, Nordquist L, Pao W, Pfister DG, Piantadosi S, Schiller JH, Smith R, Smith TJ, Strawn JR, Trent D, Giaccone G. 2011 Focused Update of 2009 American Society of Clinical Oncology Clinical Practice Guideline Update on Chemotherapy for Stage IV Non-Small-Cell Lung Cancer. J.Clin.Oncol. 2011;29:3825–3831. doi: 10.1200/JCO.2010.34.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duarte S, Carle G, Faneca H, de Lima MC, Pierrefite-Carle V. Suicide gene therapy in cancer: where do we stand now? Cancer Lett. 2012;324:160–170. doi: 10.1016/j.canlet.2012.05.023. [DOI] [PubMed] [Google Scholar]
- 5.Zhao H, Piwnica-Worms H. ATR-mediated checkpoint pathways regulate phosphorylation and activation of human Chk1. Mol.Cell Biol. 2001;21:4129–4139. doi: 10.1128/MCB.21.13.4129-4139.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karnitz LM, Flatten KS, Wagner JM, Loegering D, Hackbarth JS, Arlander SJ, Vroman BT, Thomas MB, Baek YU, Hopkins KM, Lieberman HB, Chen J, Cliby WA, Kaufmann SH. Gemcitabine-induced activation of checkpoint signaling pathways that affect tumor cell survival. Mol.Pharmacol. 2005;68:1636–1644. doi: 10.1124/mol.105.012716. [DOI] [PubMed] [Google Scholar]
- 7.Burma S, Chen BP, Murphy M, Kurimasa A, Chen DJ. ATM phosphorylates histone H2AX in response to DNA double-strand breaks. J.Biol.Chem. 2001;276:42462–42467. doi: 10.1074/jbc.C100466200. [DOI] [PubMed] [Google Scholar]
- 8.Cho SH, Toouli CD, Fujii GH, Crain C, Parry D. Chk1 is essential for tumor cell viability following activation of the replication checkpoint. Cell Cycle. 2005;4:131–139. doi: 10.4161/cc.4.1.1299. [DOI] [PubMed] [Google Scholar]
- 9.Matthews DJ, Yakes FM, Chen J, Tadano M, Bornheim L, Clary DO, Tai A, Wagner JM, Miller N, Kim YD, Robertson S, Murray L, Karnitz LM. Pharmacological abrogation of S-phase checkpoint enhances the anti-tumor activity of gemcitabine in vivo. Cell Cycle. 2007;6:104–110. doi: 10.4161/cc.6.1.3699. [DOI] [PubMed] [Google Scholar]
- 10.Sampath D, Shi Z, Plunkett W. Inhibition of cyclin-dependent kinase 2 by the Chk1-Cdc25A pathway during the S-phase checkpoint activated by fludarabine: dysregulation by 7-hydroxystaurosporine. Mol.Pharmacol. 2002;62:680–688. doi: 10.1124/mol.62.3.680. [DOI] [PubMed] [Google Scholar]
- 11.Karp JE, Thomas BM, Greer JM, Sorge C, Gore SD, Pratz KW, Smith BD, Flatten KS, Peterson K, Schneider P, Mackey K, Freshwater T, Levis MJ, McDevitt MA, Carraway HE, Gladstone DE, Showel MM, Loechner S, Parry DA, Horowitz JA, Isaacs R, Kaufmann SH. Phase I and pharmacologic trial of cytosine arabinoside with the selective checkpoint 1 inhibitor Sch 900776 in refractory acute leukemias. Clin.Cancer Res. 2012;18:6723–6731. doi: 10.1158/1078-0432.CCR-12-2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hawn MT, Umar A, Carethers JM, Marra G, Kunkel TA, Boland CR, Koi M. Evidence for a connection between the mismatch repair system and the G2 cell cycle checkpoint. Cancer Res. 1995;55:3721–3725. [PubMed] [Google Scholar]
- 13.Fordham SE, Matheson EC, Scott K, Irving JA, Allan JM. DNA mismatch repair status affects cellular response to Ara-C and other anti-leukemic nucleoside analogs. Leukemia. 2011;25:1046–1049. doi: 10.1038/leu.2011.38. [DOI] [PubMed] [Google Scholar]
- 14.Moolten FL. Tumor chemosensitivity conferred by inserted herpes thymidine kinase genes: paradigm for a prospective cancer control strategy. Cancer Res. 1986;46:5276–5281. [PubMed] [Google Scholar]
- 15.Rubsam LZ, Davidson BL, Shewach DS. Superior cytotoxicity with ganciclovir compared with acyclovir and 1-beta-D-arabinofuranosylthymine in herpes simplex virus-thymidine kinase-expressing cells: a novel paradigm for cell killing. Cancer Res. 1998;58:3873–3882. [PubMed] [Google Scholar]
- 16.Boucher PD, Ostruszka LJ, Murphy PJ, Shewach DS. Hydroxyurea significantly enhances tumor growth delay in vivo with herpes simplex virus thymidine kinase/ganciclovir gene therapy. Gene Ther. 2002;9:1023–1030. doi: 10.1038/sj.gt.3301730. [DOI] [PubMed] [Google Scholar]
- 17.Rainov NG. A phase III clinical evaluation of herpes simplex virus type 1 thymidine kinase and ganciclovir gene therapy as an adjuvant to surgical resection and radiation in adults with previously untreated glioblastoma multiforme. Hum.Gene Ther. 2000;11:2389–2401. doi: 10.1089/104303400750038499. [DOI] [PubMed] [Google Scholar]
- 18.Freytag SO, Movsas B, Aref I, Stricker H, Peabody J, Pegg J, Zhang Y, Barton KN, Brown SL, Lu M, Savera A, Kim JH. Phase I Trial of Replication-competent Adenovirus-mediated Suicide Gene Therapy Combined with IMRT for Prostate Cancer. Mol.Ther. 2007;15:1016–1023. doi: 10.1038/mt.sj.6300120. [DOI] [PubMed] [Google Scholar]
- 19.Thust R, Tomicic M, Klocking R, Voutilainen N, Wutzler P, Kaina B. Comparison of the genotoxic and apoptosis-inducing properties of ganciclovir and penciclovir in Chinese hamster ovary cells transfected with the thymidine kinase gene of herpes simplex virus-1: implications for gene therapeutic approaches. Cancer Gene Ther. 2000;7:107–117. doi: 10.1038/sj.cgt.7700106. [DOI] [PubMed] [Google Scholar]
- 20.Tomicic MT, Thust R, Kaina B. Ganciclovir-induced apoptosis in HSV-1 thymidine kinase expressing cells: critical role of DNA breaks, Bcl-2 decline and caspase-9 activation. Oncogene. 2002;21:2141–2153. doi: 10.1038/sj.onc.1205280. [DOI] [PubMed] [Google Scholar]
- 21.Ladd B, O'Konek JJ, Ostruszka LJ, Shewach DS. Unrepairable DNA double-strand breaks initiate cytotoxicity with HSV-TK/ganciclovir. Cancer Gene Therapy. 2011;18:751–759. doi: 10.1038/cgt.2011.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thust R, Schacke M, Wutzler P. Cytogenetic genotoxicity of antiherpes virostatics in Chinese hamster V79-E cells. I. Purine nucleoside analogues. Antiviral Res. 1996;31:105–113. doi: 10.1016/0166-3542(96)00961-8. [DOI] [PubMed] [Google Scholar]
- 23.Wilson DM, III, Thompson LH. Molecular mechanisms of sister-chromatid exchange. Mutat.Res. 2007;616:11–23. doi: 10.1016/j.mrfmmm.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 24.Papouli E, Cejka P, Jiricny J. Dependence of the cytotoxicity of DNA-damaging agents on the mismatch repair status of human cells. Cancer Res. 2004;64:3391–3394. doi: 10.1158/0008-5472.CAN-04-0513. [DOI] [PubMed] [Google Scholar]
- 25.O'Konek JJ, Boucher PD, Iacco AA, Wilson TE, Shewach DS. MLH1 deficiency enhances tumor cell sensitivity to ganciclovir. Cancer Gene Ther. 2009 doi: 10.1038/cgt.2009.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paques F, Haber JE. Multiple pathways of recombination induced by double-strand breaks in Saccharomyces cerevisiae. Microbiol.Mol.Biol.Rev. 1999;63:349–404. doi: 10.1128/mmbr.63.2.349-404.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chinnaiyan P, Vallabhaneni G, Armstrong E, Huang SM, Harari PM. Modulation of radiation response by histone deacetylase inhibition. Int.J.Radiat.Oncol.Biol.Phys. 2005;62:223–229. doi: 10.1016/j.ijrobp.2004.12.088. [DOI] [PubMed] [Google Scholar]
- 28.Weinstock DM, Nakanishi K, Helgadottir HR, Jasin M. Assaying double-strand break repair pathway choice in mammalian cells using a targeted endonuclease or the RAG recombinase. Methods Enzymol. 2006;409:524–540. doi: 10.1016/S0076-6879(05)09031-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boucher PD, Im MM, Freytag SO, Shewach DS. A novel mechanism of synergistic cytotoxicity with 5-fluorocytosine and ganciclovir in double suicide gene therapy. Cancer Res. 2006;66:3230–3237. doi: 10.1158/0008-5472.CAN-05-3033. [DOI] [PubMed] [Google Scholar]
- 30.Machado SG, Robinson GA. A direct, general approach based on isobolograms for assessing the joint action of drugs in pre-clinical experiments. Stat.Med. 1994;13:2289–2309. doi: 10.1002/sim.4780132202. [DOI] [PubMed] [Google Scholar]
- 31.Kokoris MS, Sabo P, Adman ET, Black ME. Enhancement of tumor ablation by a selected HSV-1 thymidine kinase mutant. Gene Ther. 1999;6:1415–1426. doi: 10.1038/sj.gt.3300966. [DOI] [PubMed] [Google Scholar]
- 32.Pierce AJ, Johnson RD, Thompson LH, Jasin M. XRCC3 promotes homology-directed repair of DNA damage in mammalian cells. Genes Dev. 1999;13:2633–2638. doi: 10.1101/gad.13.20.2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharma S, Hicks JK, Chute CL, Brennan JR, Ahn JY, Glover TW, Canman CE. REV1 and polymerase zeta facilitate homologous recombination repair. Nucleic Acids Res. 2012;40:682–691. doi: 10.1093/nar/gkr769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fuller LF, Painter RB. A Chinese hamster ovary cell line hypersensitive to ionizing radiation and deficient in repair replication. Mutat.Res. 1988;193:109–121. doi: 10.1016/0167-8817(88)90041-7. [DOI] [PubMed] [Google Scholar]
- 35.Tebbs RS, Zhao Y, Tucker JD, Scheerer JB, Siciliano MJ, Hwang M, Liu N, Legerski RJ, Thompson LH. Correction of chromosomal instability and sensitivity to diverse mutagens by a cloned cDNA of the XRCC3 DNA repair gene. Proc.Natl.Acad.Sci.U.S.A. 1995;92:6354–6358. doi: 10.1073/pnas.92.14.6354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen X, Wong P, Radany EH, Stark JM, Laulier C, Wong JY. Suberoylanilide hydroxamic acid as a radiosensitizer through modulation of RAD51 protein and inhibition of homology-directed repair in multiple myeloma. Mol.Cancer Res. 2012;10:1052–1064. doi: 10.1158/1541-7786.MCR-11-0587. [DOI] [PubMed] [Google Scholar]
- 37.Adimoolam S, Sirisawad M, Chen J, Thiemann P, Ford JM, Buggy JJ. HDAC inhibitor PCI-24781 decreases RAD51 expression and inhibits homologous recombination. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:19482–19487. doi: 10.1073/pnas.0707828104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ammerpohl O, Thormeyer D, Khan Z, Appelskog IB, Gojkovic Z, Almqvist PM, Ekstrom TJ. HDACi phenylbutyrate increases bystander killing of HSV-tk transfected glioma cells. Biochem.Biophys.Res.Commun. 2004;324:8–14. doi: 10.1016/j.bbrc.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 39.Sartori AA, Lukas C, Coates J, Mistrik M, Fu S, Bartek J, Baer R, Lukas J, Jackson SP. Human CtIP promotes DNA end resection. Nature. 2007;450:509–514. doi: 10.1038/nature06337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wyman C, Ristic D, Kanaar R. Homologous recombination-mediated double-strand break repair. DNA Repair (Amst) 2004;3:827–833. doi: 10.1016/j.dnarep.2004.03.037. [DOI] [PubMed] [Google Scholar]
- 41.Premkumar DR, Jane EP, Agostino NR, Didomenico JD, Pollack IF. Bortezomib-induced sensitization of malignant human glioma cells to vorinostat-induced apoptosis depends on reactive oxygen species production, mitochondrial dysfunction, Noxa upregulation, Mcl-1 cleavage, and DNA damage. Mol.Carcinog. 2011 doi: 10.1002/mc.21835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamamoto S, Yamano T, Tanaka M, Hoon DS, Takao S, Morishita R, Aikou T, Kaneda Y. A novel combination of suicide gene therapy and histone deacetylase inhibitor for treatment of malignant melanoma. Cancer Gene Ther. 2003;10:179–186. doi: 10.1038/sj.cgt.7700551. [DOI] [PubMed] [Google Scholar]
- 43.Kothari V, Joshi G, Nama S, Somasundaram K, Mulherkar R. HDAC inhibitor valproic acid enhances tumor cell kill in adenovirus-HSVtk mediated suicide gene therapy in HNSCC xenograft mouse model. Int.J.Cancer. 2010;126:733–742. doi: 10.1002/ijc.24700. [DOI] [PubMed] [Google Scholar]
- 44.Ryu CH, Park KY, Kim SM, Jeong CH, Woo JS, Hou Y, Jeun SS. Valproic acid enhances anti-tumor effect of mesenchymal stem cell mediated HSV-TK gene therapy in intracranial glioma. Biochem.Biophys.Res.Commun. 2012;421:585–590. doi: 10.1016/j.bbrc.2012.04.050. [DOI] [PubMed] [Google Scholar]
- 45.Lane AA, Chabner BA. Histone deacetylase inhibitors in cancer therapy. J.Clin.Oncol. 2009;27:5459–5468. doi: 10.1200/JCO.2009.22.1291. [DOI] [PubMed] [Google Scholar]
- 46.Ruefli AA, Ausserlechner MJ, Bernhard D, Sutton VR, Tainton KM, Kofler R, Smyth MJ, Johnstone RW. The histone deacetylase inhibitor and chemotherapeutic agent suberoylanilide hydroxamic acid (SAHA) induces a cell-death pathway characterized by cleavage of Bid and production of reactive oxygen species. Proc.Natl.Acad.Sci.U.S.A. 2001;98:10833–10838. doi: 10.1073/pnas.191208598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gospodinov A, Popova S, Vassileva I, Anachkova B. The inhibitor of histone deacetylases sodium butyrate enhances the cytotoxicity of mitomycin C. Mol.Cancer Ther. 2012;11:2116–2126. doi: 10.1158/1535-7163.MCT-12-0193. [DOI] [PubMed] [Google Scholar]
- 48.Liu X, Wang Y, Benaissa S, Matsuda A, Kantarjian H, Estrov Z, Plunkett W. Homologous recombination as a resistance mechanism to replication-induced double-strand breaks caused by the antileukemia agent CNDAC. Blood. 2010;116:1737–1746. doi: 10.1182/blood-2009-05-220376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Foti M, Marshalko S, Schurter E, Kumar S, Beardsley GP, Schweitzer BI. Solution structure of a DNA decamer containing the antiviral drug ganciclovir: combined use of NMR, restrained molecular dynamics, and full relaxation matrix refinement. Biochemistry. 1997;36:5336–5345. doi: 10.1021/bi962604e. [DOI] [PubMed] [Google Scholar]
- 50.Lee JH, Choy ML, Ngo L, Foster SS, Marks PA. Histone deacetylase inhibitor induces DNA damage, which normal but not transformed cells can repair. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:14639–14644. doi: 10.1073/pnas.1008522107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rangwala S, Zhang CL, Duvic M. HDAC inhibitors for the treatment of cutaneous T-cell lymphomas. Future Medicinal Chemistry. 2012;4:471–486. doi: 10.4155/fmc.12.6. [DOI] [PubMed] [Google Scholar]