Abstract
Endothelial activation characterized by the expression of multiple chemokines and adhesive molecules is a critical initial step of vascular inflammation, which results in recruitment of leucocytes into the sub-endothelial layer of the vascular wall and triggers vascular inflammatory diseases such as atherosclerosis. Although inhibiting endothelial inflammation has already been well recognized as a therapeutic strategy in vascular inflammatory diseases, the therapeutic targets are still elusive. In the present study we found that Zc3h12c (zinc finger CCCH-type-containing 12C), a recently discovered CCCH zinc finger-containing protein, significantly inhibited the endothelial cell inflammatory response in vitro. Overexpression of Zc3h12c significantly attenuated TNFα (tumour necrosis factor α)-induced expression of chemokines and adhesive molecules, and thus reduced monocyte adherence to HUVECs (human umbilical vein endothelial cells). Conversely, siRNA (small interfering RNA)-mediated knockdown of Zc3h12c increased the TNFα- induced expression of chemokines and adhesive molecules in HUVECs. Furthermore, forced expression of Zc3h12c decreased TNFα-induced IKKα/β [IκB (inhibitor of nuclear factor κB) kinase α/β], IκBα phosphorylation and p65 nuclear translocation, suggesting that Zc3h12c exerted its anti-inflammatory function probably by suppressing the NF-κB (nuclear factor κB) pathway. Thus Zc3h12c is an endogenous inhibitor of TNFα-induced inflammatory signalling in HUVECs and might be a therapeutic target in vascular inflammatory diseases.
Keywords: human umbilical vein endothelial cell (HUVEC), inflammation, nuclear factor κB (NF-κB), vascular cell adhesion molecule-1 (VCAM-1), zinc finger CCCH-type-containing 12C (Zc3h12c)
INTRODUCTION
Endothelial activation is a critical process involved in many vascular diseases including atherosclerosis. Dysfunctional endothelial cells are strongly related to and promote vascular inflammation [1–3]. Upon inflammatory stimulation, such as with TNFα (tumour necrosis factor α), the endothelial cells express many chemokines and adhesive molecules [4–6]. IL (interleukin)-8 [7] and MCP-1 (monocyte chemotactic protein-1) [8] have been proved to be two critical chemokines that endothelial cells secrete to recruit neutrophils and monocytes respectively. After that highly expressed adhesion molecules, including ICAM-1 (intercellular adhesion molecule-1), VCAM-1 (vascular cell adhesion molecule-1) and E-selectin (endothelial selectin), direct the leucocytes roll and adherence to the endothelial cells and migration into the sub-endothelial region [9]. The infiltrated leucocytes cause vascular inflammation, lipid accumulation and tissue damage, which contribute to the development of vascular inflammatory diseases.
The expression of endothelial inflammatory chemokines and adhesive molecules is mainly attributed to the NF-κB (nuclear factor κB) pathway. This pathway is triggered by phosphorylation of IKK [IκB (inhibitor of NF-κB) kinase] β, a kinase dedicated to phosphorylate IκBα. IκBα is an endogenous inhibitor of the NF-κB pathway. After its phosphorylation by IKKβ, IκBα undergoes ubiquitination and then degradation. The active NF-κB transcription factors p65 and p50 are released and then translocate to the nucleus to direct their target gene expression [10,11]. NF-κB is critically important for the transcription of many inflammatory genes, including the endothelial chemokines and adhesive molecules [10,11]. Although the NF-κB pathway has been well defined as a promising therapeutic target in inflammatory diseases, possible regulators are still elusive.
We and others identified a novel CCCH-zinc finger-containing protein family, which includes four members of MCPIP1 (MCP-induced protein 1)/Zc3h12 (zinc finger CCCH-type-containing 12) a, Zc3h12b, Zc3h12c and Zc3h12d [12–15]. Previous studies demonstrated that MCPIP1 is a negative regulator in LPS (lipopolysaccharide)-induced macrophage activation [12]. These findings were further confirmed by observations in MCPIP1-deficient mice [13,14]. MCPIP1-deficient mice suffered from spontaneous inflammatory diseases characterized by multi-organ inflammation, splenomegaly, heightened production of inflammatory cytokines and premature death [13,14]. Furthermore, we and others have shown that MCPIP1 is a multifunctional protein that actively participates in several distinct signalling pathways. For example, MCPIP1 down-regulates LPS-induced inflammatory responses by acting as an RNase [14]. MCPIP1 inhibits JNK (c-Jun N-terminal kinase) and NF-κB signalling by interfering with the ubiquitination of upstream signalling molecules, such as TRAFs (TNF-receptor-associated factors) [13]. These results collectively suggest that MCPIP1 is a novel anti-inflammatory protein that can negatively regulate both innate and adaptive immunity, and that its selective expression in lymphoid and inflamed tissues suppresses hyper-responsiveness, thereby contributing to the maintenance of immune homoeostasis.
Zc3h12c is a 99 kDa protein comprising 883 amino acids. Similar to MCPIP1/Zc3h12a, Zc3h12c also contains a single CCCH-zinc finger domain at the middle of the protein and an RNase domain at the N-terminus before the CCCH-zinc finger domain. The Zc3h12c gene has been mapped to chromosome 9 in the mouse and the equivalent gene in humans, ZC3H12C, has been mapped to chromosome 11q22.3. Although Zc3h12c is evolutionarily close to MCPIP1/Zc3h12a on the basis of their sequence homology, the function of Zc3h12c remains unknown.
In the present study, we have shown that Zc3h12c expression is induced by the inflammatory cytokine TNFα and that overexpression of Zc3h12c suppresses TNFα-induced expression of chemokines and adhesive molecules such as MCP-1, IL-8, VCAM-1, ICAM-1 and E-selectin, and by this mechanism it attenuated monocyte adhesion to human endothelial cells. These results indicate that Zc3h12c negatively regulates the pro-inflammatory activation of vascular endothelial cells.
EXPERIMENTAL
Cell culture and reagents
HUVECs (human umbilical vein endothelial cells) were acquired from Lonza Walkersville and cultured in M199 medium supplemented with 20% fetal bovine serum, 20 mM Hepes (pH 7.4), 1 ng/ml recombinant human fibroblast growth factor and 90 µg/ml heparin and antibiotics. In all experiments, cells were used within five passages. The human acute monocytic leukaemia cell line THP-1 was obtained from the A.T.C.C. and was grown in RPMI 1640 medium containing 10%fetal bovine serum. Human recombinant TNFα was purchased from Sigma.
Adenovirus preparation and infection
Adenovirus was prepared as described previously [12]. In brief, the human Zc3h12c cDNA was cloned into AdTrack vector, an adenovirus shuttle vector, and co-transformed with AdEasy into the BJ5183 bacteria strain (Agilent Technologies) for generating adenovirus vector carrying Zc3h12c. The adenovirus vector Ad-Zc3h12c was transfected into the Ad293 packing cell line by Lipofectamine™ 2000 (Life Technologies) to produce adenovirus. The recombinant adenoviruses were purified by CsCl density-gradient ultracentrifugation and adenovirus titration was performed using the Adeno-X™ quantitative PCR Titration kit (Clontech). In the present study the HUVECs were infected with recombinant adenovirus [5 pfu (plaque-forming units)/cell].
Q-PCR (quantitative real-time reverse transcription PCR) and Western blotting
HUVECs were infected with Ad/Zc3h12c or Ad/EGFP (5 pfu/cell). At 24 h later, the infected cells were exposed to the indicated stimuli. RNA was harvested for Q-PCR analysis with the RNeasy Mini kit (Qiagen). Total RNA (2 µg) was reverse-transcribed into cDNA with the SuperScript III First-Strand Synthesis system (Invitrogen) and oligo(dT) used as a primer. The real-time PCR used SYBR Green dye and Taq polymerase. At total of 40 cycles were conducted as follows: 95°C for 20 s, 60°C for 15 s, and 72°C for 20 s, preceded by 3 min at 95°C for polymerase activation. The primer sequences for all of the genes we measured in this report are available upon request. Quantification was performed by the delta cycle-time method, with GAPDH (glyceraldehyde-3-phosphate dehydrogenase) used for normalization.
Total protein was harvested for Western blotting as described previously [12]. NE-PER (R) Nuclear and Cytoplasmic Extraction/NE-P kit (Thermo)was used to extract cytoplasmic and nuclear proteins according to the manufacturer’s instructions. Anti-Zc3h12c (sc-241749), anti-VCAM-1 (sc-13160), anti-ICAM-1 (sc-1511-R), anti-E-selectin (sc-14011), anti-IKKα/β (sc-7607), anti-NF-κB p65 (sc-372), anti-Lamin A/C (sc-7292) and anti-actin (sc-1616) antibodies were purchased from Santa Cruz Biotechnology. Anti-phospho-IκBα mouse (catalogue number 9246) and anti-phospho-IKKα/β rabbit (catalogue number 2687) monoclonal antibodies were purchased from Cell Signaling Technology.
Luciferase assay
HEK (human embryonic kidney)-293 cells were seeded into 24-well plates and transfected with Lipofectamine™ 2000 (Invitrogen) following the manufacturer’s instructions. The total amount of plasmid DNA was kept constant within each experiment. The human Zc3h12c expression plasmid was kindly provided by Dr Hiroshi Suzuki (University of Tokyo, Tokyo, Japan) [12]. The human VCAM-1-Luc plasmid was kindly provided by DrMukesh K. Jain (Case Western Reserve University, Cleveland, OH, U.S.A.). Luciferase activity was measured by a dual-luciferase reporter assay system (Promega) according to the manufacturer’s instructions. In the DLR™ assay, the activities of firefly (Photinus pyralis) and Renilla (Renilla reniformis or sea pansy) luciferases are measured sequentially from a single sample. The firefly luciferase reporter was measured first by adding Luciferase Assay Reagent II (LAR II) to generate a luminescent signal lasting at least 1 min. After quantifying the firefly luminescence, the reaction was quenched and the Renilla luciferase reaction was initiated simultaneously by adding Stop & Glo® Reagent to the same sample. The plasmid expressing Renilla luciferase (pRL-TK) was co-transfected to normalize the transfection efficiency. All transfections were performed in triplicate and repeated at least two times.
siRNA (short interfering RNA)-mediated Zc3h12c knockdown
The pre-designed siRNA targeting the human Zc3h12c as well as its negative control were purchased from Ambion. The siRNA was transfected into HUVECs by using Lipofectamine™ RNAiMAX (Invitrogen) following the manufacturer’s instructions. At 24 h later, the cells were treated with TNFα (2.5 ng/ml) for 4 h. Then the cells were harvested and total RNA was isolated to assess for Zc3h12c knockdown and the expression of endothelial inflammatory chemokines and adhesive molecules by Q-PCR.
Monocyte adhesion assay
HUVECs were infected with Ad/Zc3h12c for 24 h in six-well plates and then stimulated with TNFα (2.5 ng/ml) for 4 h. Medium containing TNFα was removed and fresh medium was added after washing twice with PBS. THP-1 cells were activated by PMA (120 ng/ml) for 4 h at 37°C and then labelled with blue-fluorescence dye, Hoechst 33258 (10 µg/ml; excitation/emission maxima approximately 350/461 nm respectively when bound to DNA), for 30 min and gently washed twice. Finally, 5×105 fluorescence dye-labelled THP-1 cells were added to each well and allowed to interact with HUVECs for 30 min at 37°C. Unbound THP-1 cells were removed by gently washing with ice-cold PBS. Images were taken using a Nikon fluorescence microscope (Zeiss). Adherent THP-1 cells were counted.
Statistics
Results are means ± S.D. Statistical analysis between two groups was performed using an unpaired Student’s t test. Data from three groups or more were analysed by one-way ANOVA followed by an unpaired Student’s t test. P < 0.05 was considered statistically significant.
RESULTS
TNFα-induced Zc3h12c expression in HUVECs
We previously showed that MCPIP1/Zc3h12a functioned as a negative-feedback regulator in macrophage and endothelial inflammation [12,16]. To investigate the role of Zc3h12c in vascular endothelial cells, we first examined the expression of Zc3h12c in HUVECs in response to TNFα stimulation. We found that TNFα strongly up-regulated Zc3h12c expression in HUVECs in both time- and dose-dependent manners (Figure 1), as determined by Q-PCR analysis. TNFα increased Zc3h12c expression at 2 h and peaked at 4 h, then gradually declined and returned to the baseline value after 16 h of treatment (Figure 1A). At concentrations as low as 2.5 ng/ml, TNFα induced Zc3h12c expression 3-fold. The maximal induction of TNFα on Zc3h12c expression was observed at a concentration of 5 ng/ml, with increased Zc3h12c expression by 3.6-fold (Figure 1B). Our observation that Zc3h12c can be induced in cultured endothelial cells by TNFα suggests that it may regulate cellular responses to this stimulus.
Figure 1. TNFα-induced Zc3h12c expression in HUVECs.
(A) HUVECs were stimulated with 2.5 ng/ml TNFα and then the RNA was collected at the time points indicated. (B) HUVECs were stimulated with TNFα at different doses as indicated and the RNA was collected 4 h later. The mRNA level of Zc3h12c was detected by Q-PCR. Results are means ± S.D., n =4. *P < 0.05 and **P < 0.01 compared with the untreated group.
Overexpression of Zc3h12c inhibited inflammatory gene expression in HUVECs
Next, we examined the effect of Zc3h12c overexpression on TNFα-induced expression of inflammatory chemokines and adhesive molecules in HUVECs. The HUVECs were stimulated with or without TNFα for 4 h after infection with Ad/GFP or Ad/Zc3h12c. The RNA and cell lysates were harvested from the treated cells for Q-PCR and Western blot analysis. As shown in Figure 2(A), TNFα induced the expression of VCAM-1, ICAM-1, E-selectin and IL-8 by several-hundred-fold and MCP-1 by approximately 30-fold in HUVECs. Overexpression of Zc3h12c markedly attenuated the TNFα-induced expression of VCAM-1, ICAM-1, E-selectin and IL-8, as determined by Q-PCR (Figure 2A). Western blot analysis further confirmed that Zc3h12c overexpression suppressed TNFα- induced VCAM-1 and E-selectin expression, but did not affect ICAM-1 protein expression (Figure 2B). Zc3h12c overexpression in HUVECs was confirmed by both Q-PCR and Western blotting (Figure 2). These results suggest that Zc3h12c functions as a negative regulator in endothelial inflammation.
Figure 2. Overexpression of Zc3h12c inhibited inflammatory gene expression in HUVECs.
(A) HUVECs were infected with 5 pfu/per cells of Ad/Zc3h12c or Ad/GFP and then treated with or without 2.5 ng/ml TNF-α for 4 h. The relative mRNA level of Zc3h12c, VCAM-1, ICAM-1, E-selectin, IL-8 and MCP-1 was detected by Q-PCR. **P < 0.01. (B) HUVECs were treated as above and the protein levels of Zc3h12c, VCAM-1, ICAM-1 and E-selectin were detected by Western blotting. Results are means ± S.D.
Knocking down of Zc3h12c increased inflammatory gene expression in HUVECs
To further study the significance of Zc3h12c in endothelial activation, we performed a loss-of-function study using RNA interference. Transfection of Zc3h12c siRNA substantially inhibited Zc3h12c expression by approximately 50%, which resulted in an increased expression of VCAM-1, ICAM-1, IL- 8 and MCP-1 after TNFα treatment in HUVECs (Figure 3). These results suggest further the involvement of Zc3h12c in the regulation of cytokine-induced endothelial activation.
Figure 3. Knockdown of Zc3h12c increased inflammatory gene expression in HUVECs.
HUVECs were transfected with Zc3h12c siRNA or control siRNA. The transfected cells were treated with or without 2.5 ng/ml TNFα for 4 h. The relative mRNA level of Zc3h12c, VCAM-1, ICAM-1, E-selectin, IL-8 andMCP-1 was detected by Q-PCR. Results are means ± S.D., n =4. *P < 0.05 and **P < 0.01 compared with the control siRNA group.
Zc3h12c inhibited monocyte adherence to HUVECs
To determine the functional consequence of Zc3h12c’s effect on the expression of inflammatory chemokines and adhesive molecules, we examined the effect of Zc3h12c on THP-1 monocyte adhesion to the activated HUVECs. When HUVECs were stimulated with TNFα (2.5 ng/ml), THP-1 cell adhesion was substantially increased and the increased adhesion of THP-1 cells to the TNFα-stimulated HUVECs was suppressed 80% by Zc3h12c overexpression (Figure 4). These results suggest further that Zc3h12c functions as a negative regulator of the cytokineinduced inflammatory responses in vascular endothelial cells.
Figure 4. Zc3h12c inhibited monocyte adhesion to HUVECs.
(A) HUVECs were transfected with Ad/Zc3h12c or Ad/GFP and then cultured with Hoechst-labelled THP-1 cells. At 30 min later the floating THP-1 cells were washed away and the adhesive THP-1 cells were detected by fluorescence microscopy. (B) The adhesive THP-1 cells were counted and analysed. Results are means ± S.D from three independent experiments. DAPI, 4′,6-diamidino-2-phenylindole.
Zc3h12c inhibited the promoter activation of the human VCAM-1 gene
We then asked whether Zc3h12c regulates the promoter activity of VCAM-1. For these studies, we cloned a 1.3 kb fragment of the human VCAM-1 promoter into the pGL4.10 vector (Promega). The promoter contains all of the regulatory elements necessary for TNFα responsiveness in vivo [13,16]. As shown in Figure 5(A), the forced expression of Zc3h12c inhibited TNFα-induced promoter activity of VCAM-1, but did not affect the p65-induced promoter activation of VCAM-1 (Figure 5B), suggesting that Zc3h12c may target the upstream signal of the NF-κB pathway.
Figure 5. Zc3h12c inhibited human VCAM-1 promoter activity.
(A) HEK-293 cells were transfected with the human VCAM-1 promoter reporter plasmid along with FLAG control or FLAG–Zc3h12c plasmids. At 24 h later, the transfected cells were treated with or without 2.5 ng/ml TNFα. At 24 h after the treatment, the luciferase (Luc.) activity was measured. (B) HEK-293 cells were transfected with the human VCAM-1 promoter reporter and pcDNA3 or pcDNA3-p65 plasmids as indicated. At 24 h later the luciferase activity was measured. Results are means ± S.D., n =4. *P < 0.05 compared with the FLAG control group.
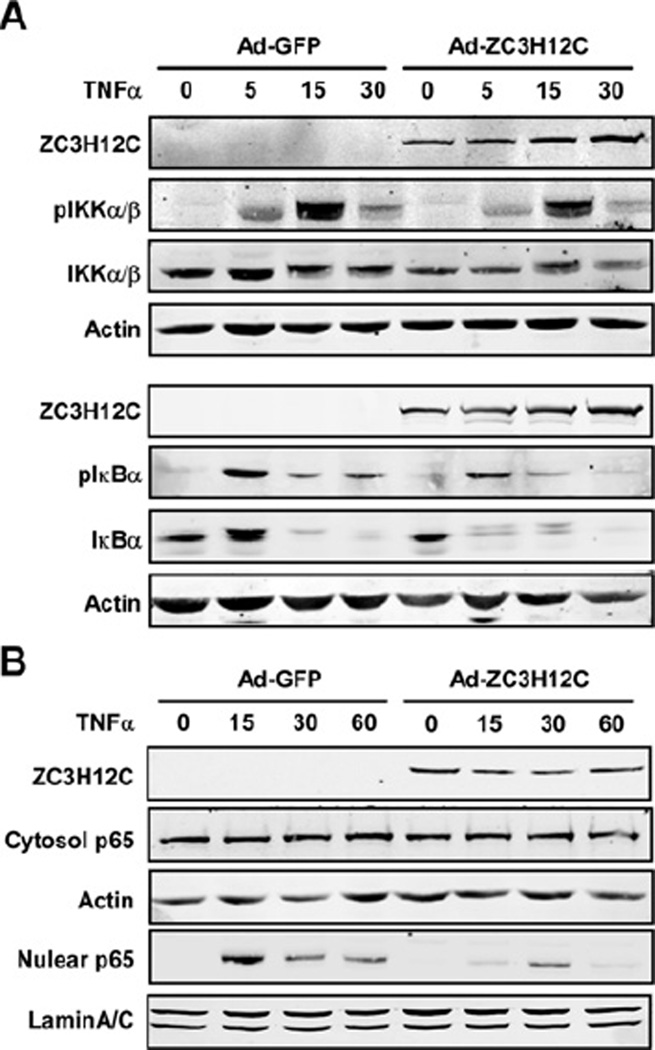
Zc3h12c inhibited the NF-κB signalling pathway
To further define the mechanisms by which Zc3h12c suppresses the expression of inflammatory chemokines and adhesive molecules in HUVECs, we analysed the effect of Zc3h12c overexpression on the activation of the NF-κB signalling pathway. As shown in Figure 6(A), overexpression of Zc3h12c significantly attenuated TNFα-induced phosphorylation of IKKα/β and IκBα in HUVECs. Consistently, Zc3h12c expression also attenuated TNFα-induced p65 nuclear translocation in HUVECs (Figure 6B). These results suggested that Zc3h12c feedback suppressed the cytokine-induced NF-κB signalling pathway by which it negatively regulates endothelial inflammation. Interestingly, overexpression of Zc3h12c also significantly attenuated the total protein level of IκBα, especially after a 5 min TNFα treatment (Figure 6A). The results have been repeatedly observed in three independent experiments.
Figure 6. Zc3h12c inhibited the NF-κB signalling pathway.
(A) HUVECs were infected with Ad/Zc3h12c or Ad/GFP and then treated with 2.5 ng/ml of TNFα for 0, 5, 15 and 30 min as indicated. The cell lysates were extracted and Western blotting was performed to detect the phosphorylation of IKKα/β and IκBα with specific antibodies as indicated. (B) HUVECs were infected with Ad/Zc3h12c or Ad/GFP and then treated with 2.5 ng/ml TNFα for 0, 15, 30 and 60 min as indicated. The cytosol and nuclear fraction was isolated and Western blotting was performed to detect the p65 protein level in the cytosol and nuclear fractions. Three independent experiments showed similar results.
DISCUSSION
Vascular disease such as atherosclerosis involves predominantly macrophages, T-cells, endothelial cells and smooth muscle cells that interact with each other in the vessel wall [17,18]. Endothelial cell activation is the initial step in vascular inflammatory diseases, including atherosclerosis [1–3]. We have previously reported that MCPIP1, a protype member of the Zc3h12 protein family, functioned as a critical regulator of the pro-inflammatory activation of macrophages and vascular endothelial cells [12,16]. Although Zc3h12c is evolutionarily close to MCPIP1 on the basis of their sequence homology, no function of Zc3h12c has been reported. In the present study, we have found that Zc3h12c was induced in response to TNFα stimulation in HUVECs and the expression of Zc3h12c feedback suppressed the expression of chemokines and adhesive molecules including IL-8, MCP-1, VCAM-1, ICAM-1 and E-selectin, and attenuated the adhesion of monocytes to endothelial cells. Further studies suggest that Zc3h12c may target to the upstream signalling molecules of the NF-κB signalling pathway and inhibit the cytokine-induced transcription of chemokines and adhesive molecules, although other mechanisms may also exist. Many risk factors, such as hyperhomocysteinaemia [19], diabetes [20] and oscillatory shear stress [21], result in atherosclerosis by triggering chemokine and adhesive molecule expression in vascular endothelial cells. Zc3h12c therefore may be a potential therapeutic target for vascular inflammatory diseases including atherosclerosis.
NF-κB is an important signalling pathway in directing inflammatory gene expression, including chemokine and adhesive molecules in endothelial cells [10,11]. Our previous findings revealed that Zc3h12a, another member of the Zc3h12 family, inhibited endothelial cell inflammation through suppressing the NF-κB pathway [16]. In the present study we found that Zc3h12c expression was markedly induced by TNFα. Overexpression of Zc3h12c inhibited TNFα-induced IKKα/β phosphorylation, IκBα phosphorylation and p65 nuclear translocation. These results defined a Zc3h12c-mediated negative-feedback loop to control the activation of NF-κB signalling and endothelial activation.
Interestingly, we observed that the overexpression of Zc3h12c significantly attenuated the total protein level of IκBα. It has been reported that Zc3h12a acts as an RNase and selectively promotes the mRNA degradation of IL-6, IL-2 and IL-12b [14,22]. As Zc3h12c shares a similar RNase domain with Zc3h12a, Zc3h12c may also act as an RNase and promote the mRNA degradation of IκBα, thereby reducing the protein pool of IκBα.
In summary, the present study revealed an anti-inflammatory function of Zc3h12c in HUVECs in vitro and pointed out that the regulation of Zc3h12c expression and activity might be a potential therapeutic strategy in vascular inflammatory diseases. The physiological role of Zc3h12c in vivo needs to be further examined with efficient animal models. Although we have shown that Zc3h12c was an inhibitor of the NF-κB pathway, the exact molecular mechanism by which Zc3h12c inhibited NF-κB remains to be discovered.
ACKNOWLEDGEMENTS
We thank Dr Mukesh K. Jain and Dr Hiroshi I. Suzuki for providing plasmids.
FUNDING
This work was partially supported by the National Institutes of Health [grant numbers HL068878 and HL089544 (to Y.E.C) and HL098794 (to M.F.)]. Y.E.C. is an Established Investigator of the American Heart Association [grant number 0840025N].
Abbreviations used
- E-selectin
endothelial selectin
- HEK
human embryonic kidney
- HUVEC
human umbilical vein endothelial cell
- IκB
inhibitor of nuclear factor κB
- ICAM-1
intercellular adhesive molecule-1
- IKK
IκB kinase
- IL
interleukin
- LPS
lipopolysaccharide
- MCP-1
monocyte chemotactic protein-1
- MCPIP1
MCP-induced protein 1
- NF-κB
nuclear factor κB
- pfu
plaque-forming unit
- Q-PCR
quantitative real-time reverse transcription PCR
- siRNA
short interfering RNA
- TNFα
tumour necrosis factor α
- VCAM-1
vascular cell adhesive molecule-1
- Zc3h12
zinc finger CCCH-type-containing 12.
Footnotes
AUTHOR CONTRIBUTION
Ling Liu performed most of the experiments. Zhou Zhou, Shengping Huang, Yanhong Guo, Yanbo Fan and Ji Zhang performed the other experiments. Ling Liu and Zhou Zhou contributed to writing the paper. Jifeng Zhang and Y. Eugene Chen supervised the experimental work. Mingui Fu and Jifeng Zhang wrote the paper.
REFERENCES
- 1.Kinlay S, Libby P, Ganz P. Endothelial function and coronary artery disease. Curr. Opin. Lipidol. 2001;12:383–389. doi: 10.1097/00041433-200108000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Landmesser U, Hornig B, Drexler H. Endothelial function: a critical determinant in atherosclerosis? Circulation. 2004;109:II27–II33. doi: 10.1161/01.CIR.0000129501.88485.1f. [DOI] [PubMed] [Google Scholar]
- 3.Bonetti PO, Lerman LO, Lerman A. Endothelial dysfunction: a marker of atherosclerotic risk. Arterioscler., Thromb., Vasc. Biol. 2003;23:168–175. doi: 10.1161/01.atv.0000051384.43104.fc. [DOI] [PubMed] [Google Scholar]
- 4.Li WG, Gavrila D, Liu X, Wang L, Gunnlaugsson S, Stoll LL, McCormick ML, Sigmund CD, Tang C, Weintraub NL. Ghrelin inhibits proinflammatory responses and nuclear factor-κB activation in human endothelial cells. Circulation. 2004;109:2221–2226. doi: 10.1161/01.CIR.0000127956.43874.F2. [DOI] [PubMed] [Google Scholar]
- 5.Eto M, Kouroedov A, Cosentino F, Luscher TF. Glycogen synthase kinase-3 mediates endothelial cell activation by tumor necrosis factor-α. Circulation. 2005;112:1316–1322. doi: 10.1161/CIRCULATIONAHA.105.564112. [DOI] [PubMed] [Google Scholar]
- 6.Ouchi N, Kihara S, Arita Y, Okamoto Y, Maeda K, Kuriyama H, Hotta K, Nishida M, Takahashi M, Muraguchi M, et al. Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-κB signaling through a cAMP-dependent pathway. Circulation. 2000;102:1296–1301. doi: 10.1161/01.cir.102.11.1296. [DOI] [PubMed] [Google Scholar]
- 7.Yuan L, Nikolova-Krstevski V, Zhan Y, Kondo M, Bhasin M, Varghese L, Yano K, Carman CV, Aird WC, Oettgen P. Antiinflammatory effects of the ETS factor ERG in endothelial cells are mediated through transcriptional repression of the interleukin-8 gene. Circ. Res. 2009;104:1049–1057. doi: 10.1161/CIRCRESAHA.108.190751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li D, Mehta JL. Antisense to LOX-1 inhibits oxidized LDL-mediated upregulation of monocyte chemoattractant protein-1 and monocyte adhesion to human coronary artery endothelial cells. Circulation. 2000;101:2889–2895. doi: 10.1161/01.cir.101.25.2889. [DOI] [PubMed] [Google Scholar]
- 9.Mestas J, Ley K. Monocyte–endothelial cell interactions in the development of atherosclerosis. Trends Cardiovasc. Med. 2008;18:228–232. doi: 10.1016/j.tcm.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Martin R, Hoeth M, Hofer-Warbinek R, Schmid JA. The transcription factor NF-κ B and the regulation of vascular cell function. Arterioscler., Thromb., Vasc. Biol. 2000;20:E83–E88. doi: 10.1161/01.atv.20.11.e83. [DOI] [PubMed] [Google Scholar]
- 11.Monaco C, Paleolog E. Nuclear factor κB: a potential therapeutic target in atherosclerosis and thrombosis. Cardiovasc. Res. 2004;61:671–682. doi: 10.1016/j.cardiores.2003.11.038. [DOI] [PubMed] [Google Scholar]
- 12.Liang J, Wang J, Azfer A, Song W, Tromp G, Kolattukudy PE, Fu M. A novel CCCH-zinc finger protein family regulates proinflammatory activation of macrophages. J Biol. Chem. 2008;283:6337–6346. doi: 10.1074/jbc.M707861200. [DOI] [PubMed] [Google Scholar]
- 13.Liang J, Saad Y, Lei T, Wang J, Qi D, Yang Q, Kolattukudy PE, Fu M. MCP-induced protein 1 deubiquitinates TRAF proteins and negatively regulates JNK and NF-κ B signaling. J Exp. Med. 2010;207:2959–2973. doi: 10.1084/jem.20092641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsushita K, Takeuchi O, Standley DM, Kumagai Y, Kawagoe T, Miyake T, Satoh T, Kato H, Tsujimura T, Nakamura H, Akira S. Zc3h12a is an RNase essential for controlling immune responses by regulating mRNA decay. Nature. 2009;458:1185–1190. doi: 10.1038/nature07924. [DOI] [PubMed] [Google Scholar]
- 15.Huang S, Qi D, Liang J, Miao R, Minagawa K, Quinn T, Matsui T, Fan D, Liu J, Fu M. The putative tumor suppressor Zc3h12d modulates toll-like receptor signaling in macrophages. Cell. Signalling. 2012;24:569–576. doi: 10.1016/j.cellsig.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qi Y, Liang J, She ZG, Cai Y, Wang J, Lei T, Stallcup WB, Fu M. MCP-induced protein 1 suppresses TNF α-induced VCAM-1 expression in human endothelial cells. FEBS Lett. 2010;584:3065–3072. doi: 10.1016/j.febslet.2010.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 18.Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat. Immunol. 2011;12:204–212. doi: 10.1038/ni.2001. [DOI] [PubMed] [Google Scholar]
- 19.Wang G, Woo CW, Sung FL, Siow YL, Karmin O. Increased monocyte adhesion to aortic endothelium in rats with hyperhomocysteinemia: role of chemokine and adhesion molecules. Arterioscler., Thromb., Vasc. Biol. 2002;22:1777–1783. doi: 10.1161/01.atv.0000035404.18281.37. [DOI] [PubMed] [Google Scholar]
- 20.Tikellis C, Jandeleit-Dahm KA, Sheehy K, Murphy A, Chin-Dusting J, Kling D, Sebokova E, Cooper ME, Mizrahi J, Woollard KJ. Reduced plaque formation induced by rosiglitazone in an STZ-diabetes mouse model of atherosclerosis is associated with downregulation of adhesion molecules. Atherosclerosis. 2008;199:55–64. doi: 10.1016/j.atherosclerosis.2007.10.038. [DOI] [PubMed] [Google Scholar]
- 21.Sucosky P, Balachandran K, Elhammali A, Jo H, Yoganathan AP. Altered shear stress stimulates upregulation of endothelial VCAM-1 and ICAM-1 in a BMP-4- and TGF-β 1-dependent pathway. Arterioscler., Thromb., Vasc. Biol. 2009;29:254–260. doi: 10.1161/ATVBAHA.108.176347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li M, Cao W, Liu H, Zhang W, Liu X, Cai Z, Guo J, Wang X, Hui Z, Zhang H, et al. MCPIP1 down-regulates IL-2 expression through an ARE-independent pathway. PLoS ONE. 2012;7:e49841. doi: 10.1371/journal.pone.0049841. [DOI] [PMC free article] [PubMed] [Google Scholar]