Abstract
We developed and characterized a mouse model of primary ocular blast injury. The device consists of: a pressurized air tank attached to a regulated paintball gun with a machined barrel; a chamber that protects the mouse from direct injury and recoil, while exposing the eye; and a secure platform that enables fine, controlled movement of the chamber in relation to the barrel. Expected pressures were calculated and the optimal pressure transducer, based on the predicted pressures, was positioned to measure output pressures at the location where the mouse eye would be placed. Mice were exposed to one of three blast pressures (23.6, 26.4, or 30.4psi). Gross pathology, intraocular pressure, optical coherence tomography, and visual acuity were assessed 0, 3, 7, 14, and 28 days after exposure. Contralateral eyes and non-blast exposed mice were used as controls. We detected increased damage with increased pressures and a shift in the damage profile over time. Gross pathology included corneal edema, corneal abrasions, and optic nerve avulsion. Retinal damage was detected by optical coherence tomography and a deficit in visual acuity was detected by optokinetics. Our findings are comparable to those identified in Veterans of the recent wars with closed eye injuries as a result of blast exposure. In summary, this is a relatively simple system that creates injuries with features similar to those seen in patients with ocular blast trauma. This is an important new model for testing the short-term and long-term spectrum of closed globe blast injuries and potential therapeutic interventions.
Keywords: mouse, eye, blast, model, traumatic, injury, cell death, visual acuity
1. Introduction
An estimated 300,000 service members have traumatic brain injury as a result of exposure to improvised explosive devices in the recent wars in Iraq (http://veterans.rand.org). While improvements in body armor have led to fewer fatalities, there has been an increase in surviving service members with eye damage. Thirteen percent of all injuries treated at an in-theater hospital were to the eye (Heier et al., 1993). And during the current wars in Afghanistan and Iraq, 186,555 eye injuries were diagnosed in actively serving military personnel at fixed medical facilities (Hilber, 2011). This is despite the availability of protective eyewear, which can be explained in two ways: 1) non-compliance in the use of eyewear (Blanch and Scott, 2009; Thomas et al., 2009); and 2) lack of efficacy of the protective eyewear. Twenty four percent of soldiers with ocular blast injuries had documentation of wearing eye protection at the time of injury indicating that some explosions were so powerful that eye protection was insufficient to prevent ocular damage (Weichel and Colyer, 2008; Mader et al., 2006).
There is a lack of consensus on the ability of a blast wave to induce damage to the eye (primary blast injury). Since service members are not exposed to blasts in a sterile environment, they are often also exposed to foreign bodies in the orbit (secondary blast injury). This makes dissecting out any potential effects of the blast wave on the eye impossible. Chalioulias et al., 2007 reported on one case of primary blast injury to the eye, demonstrating that blast exposure alone may be sufficient to damage the eye. More recently others have reported ocular pathology in blast-exposed patients with closed globes including corneal abrasions, hyphemas, cataracts, corneal edema, angle recession, hemorrhage, retinal tears or detachments, macular holes, choroidal rupture, commotio retinae, and optic neuropathy (Blanch and Scott, 2009; Cockerham et al., 2011). Despite the accumulation of data implying that the blast wave by itself can induce ocular injury, there is a need for an animal model to test the effects of a pure blast wave.
Very few studies report on the effects of blast injury on the eye or visual system. Whole body exposure to a blast overpressure wave of 129-173kPa induces axonal degeneration in the central visual pathways of 83% of exposed rats (Petras et al., 1997). The retina was not analyzed. Long et al., 2009, performed similar experiments and showed diminished neuronal degeneration by covering the trunk of the rat with Kevlar, demonstrating that at least some of the damage was possibly due to air emboli. More recently a whole body mouse model of blast exposure was developed (Koliatsos et al., 2011; Cernak et al., 2011). The model induced an open wave-form primary blast that caused axonal degeneration in the optic tract 14 days after exposure to a 32psi blast and a few dying cells in the retinal ganglion cell layer of the far peripheral retina 5 days after exposure to a 29psi blast. As in the Long et al., 2009 study, less neuronal damage was detected when the trunk was protected from blast exposure. They did not assess visual function or examine other regions of the retina or eye.
To study the effects of a primary blast injury to the eye while avoiding confounding complications due to blast exposure to the body of the mouse, we developed a novel model that directs a primary blast with an open field waveform directly to the eye only. Here we characterize our mouse model of primary blast injury to the eye.
2. Materials and Methods
2.1 Blast device
A commercially available paintball gun (Invert Mini, Empire Paintball, Sewell, NJ), pressurized air tank, and x-y table were secured onto medium density fiber boards (Figure 1A). The commercial barrel was replaced with a machined barrel at half the original diameter to increase pressure output. The paintball gun has a regulated input so that the output forces can be controlled. In front of the barrel a plexiglass clamp was secured to an x-y table (Velmex, Bloomfield, NY) for fine movement. The x-y table allowed measurements to be made at increasing distances away from the barrel. A hole was machined into the tube to match the size of a mouse eye and was positioned directly in front of the barrel. A hole was also drilled into the opposite side of the pipe to fit the machined barrel of the pressure transducer. A slightly smaller PVC tube, which slides into the larger tube, was machined to create a housing chamber for the mouse (Figure 1B).
Figure 1.
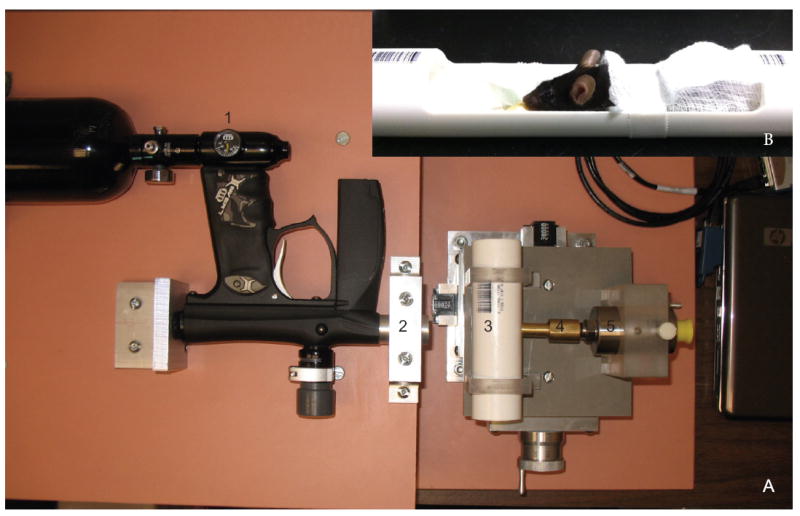
A. Image of the ocular blast injury device. B. Image of the mouse housing. Arrows indicate: 1) output pressure regulator; 2) machined barrel at the end of the paintball gun; 3) chamber with mouse eye-sized hole facing the barrel into which the mouse housing (B) slides; 4) machined barrel on the pressure transducer; 5) pressure transducer that connects to the laptop.
2.2 Measurement of output pressures
A machined pipe the same diameter as the eye hole was attached to the end of a Sensotec pressure transducer model STJE (Honeywell, Morristown, NJ) and was positioned through the PVC pipe so that the end was abutted to the eye-sized hole. This allowed for precise measurement of pressure at the future site of injury. Pressures were measured before and after exposure of each eye to a blast. The pressures detected by the pressure transducer were sent to a laptop and were recorded and analyzed using Labview software (National Instruments, Austin, TX).
2.3 Animals
Adult female C57Bl/6 mice were purchased from Jackson Laboratories (Bar Harbor, ME). Prior to blast exposure, mice were anesthetized with ketamine/xylazine (25/10mg/kg body weight) and secured into the mouse holder with surgical tape. A cushion was secured on the opposing side of the mouse housing to provide head support and modeling compound was secured to the bottom of the mouse housing next to the cushion for further head support and positioning. The mouse was positioned adjacent to the blast-side PVC, so that the mouse eye was in contact with the hole and surrounding pipe and could be visualized in the hole. Mice received 35mg/ml acetamenophen in the drinking water for a minimum of one day prior to exposure and seven days after blast exposure. In initial experiments, an average of 5% drop in body weight was noted so all remaining mice received gel food for at least 3 days post-blast. All animal studies were performed in accordance with an UTHSC Institutional Animal Care and Use Committee approved protocol and complied with the guidelines of the Association for Research in Vision and Ophthalmology. All experiments were conducted in AALAC approved laboratories. The number of mice used for each experimental condition is shown in Tables 1, 3, and 4.
Table 1.
Quantification of gross pathology findings detected after exposure to a 23.6psi blast.
| Type of Injury | 0 day (34a) | 3 days (25) | 7 days (18) | 14 days (7) | 28 days (6) |
|---|---|---|---|---|---|
| Corneal abrasions | 7 (21)b | 0 | 1 (5.6) | 0 | 0 |
| Corneal edema | 2 (5.9) | 0 | 1 (5.6) | 2 (28) | 0 |
| Corneal scarring | 0 | 0 | 0 | 1 (14) | 1 (17) |
| Traumatic cataract | 0 | 0 | 0 | 0 | 0 |
Total number of eyes examined
Number of eyes with pathology (percentage)
Note: The decrease in total eyes over time represents tissue collection and 26% mortality.
Table 3.
Quantification of gross pathology findings detected after exposure to a 26.4psi blast.
| Type of Injury | 0 day (41a) | 3 days (32) | 7 days (25) | 14 days (17) | 28 days (11) |
|---|---|---|---|---|---|
| Corneal abrasions | 1 (2.4)b | 3 (9.4) | 2 (8) | 2 (12) | 2 (18) |
| Corneal edema | 0 | 4 (12) | 2 (8) | 3 (18) | 0 |
| Corneal scarring | 0 | 0 | 1 (4) | 2 (12) | 0 |
| Torn extraocular muscles | 2 (4.9) | 0 | 0 | 0 | 0 |
| Traumatic cataract | 0 | 0 | 0 | 0 | 0 |
Total number of eyes examined
Number of eyes with pathology (percentage)
Note: The decrease in total eyes over time represents tissue collection and 22% mortality.
Table 4.
Quantification of gross pathology findings detected after exposure to a 30.4psi blast.
| Type of Injury | 0 day (24a) | 3 days (13) | 7 days (9) | 14 days (6) | 28 days (5) |
|---|---|---|---|---|---|
| Corneal abrasions | 0 | 0 | 0 | 0 | 0 |
| Corneal edema | 0 | 0 | 0 | 0 | 0 |
| Corneal scarring | 0 | 0 | 0 | 0 | 0 |
| Torn extraocular muscles | 7 (29)b | 0 | 0 | 0 | 0 |
| Optic nerve avulsion | 5 (21) | 0 | 0 | 0 | 0 |
| Traumatic cataract | 0 | 1 (7.7) | 0 | 0 | 0 |
Total number of eyes examined
Number of eyes with pathology (percentage)
Note: The decrease in total eyes over time represents tissue collection and 46% mortality.
2.4 Measurement of intraocular pressure (IOP)
The IOP was measured in awake mice pre- and post- blast using a TonoLab rebound tonometer (Colonial Medical Supply Co; Franconia, NH).
2.5 Gross Pathology
Eyes of awake mice were imaged with an SZX16 stereomicroscope and a DP71 camera (Olympus, Center Valley, PA) immediately prior to blast, immediately following blast, and 3, 7, 14, and 28 days post-blast. Eyes were examined for the presence of corneal abrasions, corneal opacity indicative of edema, stromal scarring, cataracts, hyphema, blood outside the globe, torn/ non-contractile irides, and corneal neovascularization. Any findings are reported in Tables 1, 3, and 4.
2.6 Optokinetics
Awake mice were placed on a platform inside the OptoMotry virtual reality optokinetics system to quantify the photopic visual acuity threshold (OptoMotry, Canada). A step-wise paradigm was used and the screens of contrasting bars of light were not visible to the investigator according to published methodologies (Umino et al., 2008). The spatial frequency used for the visual acuity measurements was 0.042c/d.
2.7 Ultra-high resolution optical coherence tomography (OCT)
Mice were anesthetized with 25/10 μg/g body weight ketamine/xylazine, and eyes were dilated with 1% tropicamide and kept moist with Systane Ultra. The mice were then wrapped in gauze and placed into a holder with the head stabilized by a bite bar. The retinas were imaged using the Bioptigen ultra-high resolution spectral domain OCT system and a mouse retina bore (Bioptigen, NC). The eye was repositioned and imaged with the goal of scanning as much of the peripheral retina as possible, in all quadrants, and representative images were collected.
2.8 Statistical analysis
When eyes were followed longitudinally for IOP or visual acuity measurements, a repeated ANOVA and the stringent Bonferroni post-hoc test were performed using Prism software (GraphPad, LaJolla, CA).
3. Results
3.1 Model Characterization
The blast system that was developed to produce a primary blast wave to the eye of a mouse is shown in Figure 1. Adjustment of the pressure gauge and the x-y stage provides the ability to expose the eye to a wide range of pressures. As input pressures were increased from 100 psi to 200 psi, the output pressures increased from 4psi to 67psi at 0cm from the barrel (Figure 2B). The calculated output pressures were lower than the measured output pressures (Figure 2A,B). This range includes pressures that were previously reported to induce visual system dysfunction in rats (Petras et al., 1997). The system generates an air pressure wave that mimics a simple open-field blast wave as shown in Figure 2C using an input pressure of 120psi and a distance of 0cm from the barrel. The duration of the blast wave at an input pressure of 120psi and 0cm from the barrel was 121± 21ms (Figure 2D). This increased to 180 ±18ms at input pressures of 140 to 180psi at a distance of 0cm from the barrel. The duration of the blast decreased with increased distance from the barrel. At 1cm from the barrel, a 120 psi input pressure induced a blast wave duration of 69 ± 8ms. Also, as the distance from the barrel increased, the pressures measured at the barrel decreased in a non-linear fashion (Figure 2E). An input pressure of 120psi resulted in output pressures of 26.8±0.6 (s.d), 23.6±0.6, and 19.8±0.8 at 0, 0.5, and 1cm from the barrel, respectively. In order to minimize effects of pressure loss due to dissipation, we performed all mouse experiments at 0 or 0.5 cm from the barrel.
Figure 2.
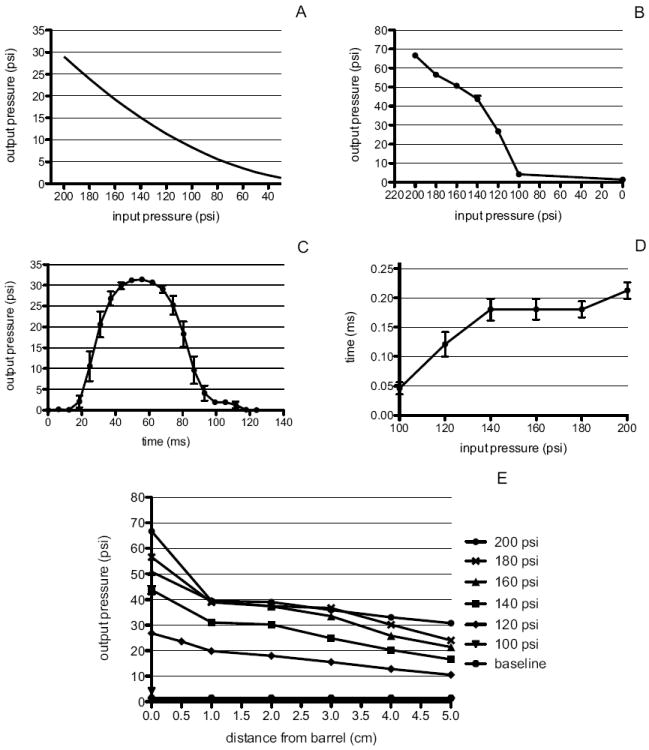
A. Predicted output pressures. B. Measured output pressures at increasing input pressures at 0cm from the barrel. C. Averaged measured waveform (10 trials) of blast pressures at 0 cm from the barrel and an input pressure of 120 psi. D. Duration of the blast at increasing input pressures measured at 0cm from the end of the barrel. E. Measured output pressures (y-axis) at increasing input pressures (legend) and distances from the barrel (x-axis). All error bars represent the standard deviation.
3.2 Identification of effective blast pressure range
Exposure to a blast of 43.8psi induced globe rupture. Exposure to a blast of 35.0psi caused extraocular muscle tears, severe bleeding, and severe bruising on the snout. Therefore studies at these pressures were aborted. The remaining pressures tested were: 23.6±0.9, 26.4±0.9, and 30.4±1.9psi. Damage was detected in two of the 101 contralateral eyes (torn iris in one and slight corneal edema in the other), all remaining eyes appeared normal (Figure 3A).
Figure 3.
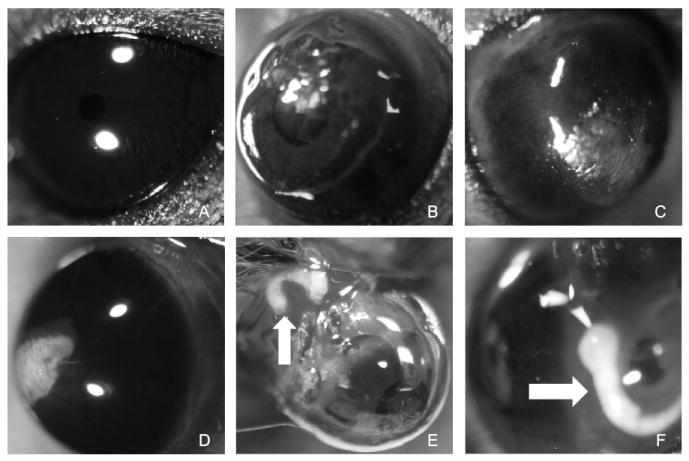
Representative images of ocular gross pathology findings after exposure to a blast. A. contralateral eye from 26psi blast; B. corneal abrasions (immediately post-26psi blast) ; C. corneal edema (3 days post-26psi blast); D. corneal scarring (14 days post-26psi blast); E. and F. torn extraocular muscles and optic nerve avulsion with intact globe (Arrows indicate optic nerve avulsion; immediately post-30psi blast).
3.3 Exposure to a 23.6±0.9 psi blast
A total of 34 mice were exposed to the 23.6psi blast. Of these, seven died immediately post-exposure, one died 24 hours after exposure, and one was euthanized 48 hours after exposure to the blast due to poor recovery. The total mortality rate was 26%. All remaining mice survived to the experimental end-point.
3.3.1 Body Weight
Despite providing gel food ad libitum for at least three days post-blast exposure, all mice lost weight (average of 4.8%), and 12 of 25 mice (48%) exhibited a loss of greater than 5% at 3 days post-blast (range of 5.5-10.5%). At 7 days post-blast exposure, all mice either maintained the same body weight or gained slightly. At 28 days post-blast, all mice had recovered their pre-blast body weight (range of -1% to +16% from baseline).
3.3.2 Gross Pathology
Seven of the 34 mice (21%) had corneal abrasions immediately post-blast, and two had corneal edema (5.9%), both of which resolved by three days post-blast in the majority of mice (Table 1; Figure 3 B, C). At 14 days post-exposure to the blast of compressed air, two eyes had persistent corneal edema, one of which also had evidence of central stromal scarring. Another eye had a central stromal scar in the absence of edema (Figure 3 D).
3.3.3 Visual Acuity, IOP, and Retinal Imaging
The averaged optokinetics data yielded no difference in photopic visual acuity threshold in the mice at any time-point post-blast (Table 2). Non-blast exposed mice had a visual acuity of 0.41±0.08 c/d. This is within the reported normal visual acuity range for a C57Bl/6 mouse using the same system (Prusky et al., 2004; Douglas et al., 2005; Umino et al., 2008). The contralateral eye of each mouse was used as an internal control. The visual acuity threshold was 0.39±0.07, 0.41± 0.05, and 0.35±0.10 c/d in contralateral eyes 3, 7, and 14 days post-blast, respectively. The visual acuity threshold was 0.45±0.08, 0.41±0.04, and 0.36±0.04c/d in blast-exposed eyes 3, 7, and 14 days post-blast exposure, respectively. Using a repeated ANOVA, there was no statistically significant decrease in visual acuity over time. However, when each mouse was analyzed separately, there appeared to be a progressive decrease in the visual acuity of the blast-exposed eyes in the 4 mice analyzed longitudinally out to 14 days post-blast that was above the variability of the optokinetic measurements (Figure 4). The percent decrease in these 4 mice ranged from 10.5% to 24% at 3 days post-blast, and 22% to 30% 14 days post blast.
Table 2.
Visual acuity thresholds of blast-exposed and contralateral eyes at increasing times post-blast.
| Blast Exposure | 3 days | 7 days | 14 days | 28 days |
|---|---|---|---|---|
| contralateral | 0.38 ± 0.08 (14) | 0.40 ± 0.06 (15) | 0.34 ± 0.10 (9) | 0.42 ± 0.07 (8) |
| 23.6 psi | 0.45 ± 0.08 (7) | 0.41 ± 0.04 (7) | 0.36 ± 0.04 (4) | 0.28 (1) |
| 26.4 psi | 0.37 ± 0.05 (6) | 0.40 ± 0.06 (7) | 0.40 ± 0.03 (4) | 0.39 ± 0.03 (5) |
| 30.4 psi | 0.25 (1) | 0.38 ± 0.13 (3) | 0.15 ± 0.09 (4) | 0.35 ± 0.08 (2) |
Values are in units of cycles/degree and are shown ± standard deviation (number of mice). The number of mice in the 30.4psi groups was very low due to the high mortality rate and likely contributed to the lack of statistical significance.
Figure 4.
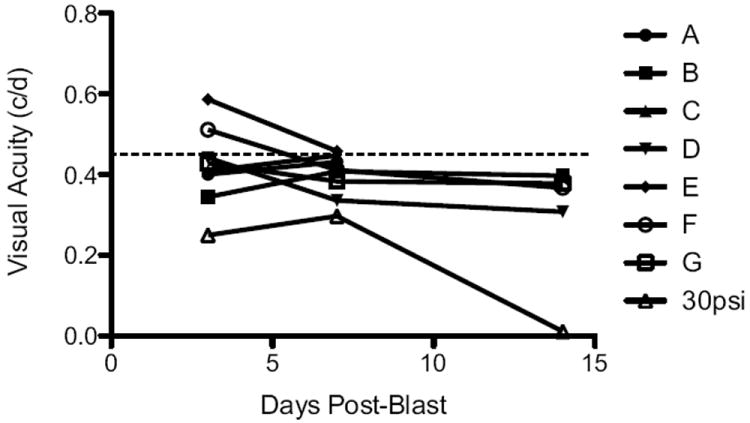
Graph of blast-exposed eyes showing a decrease in photopic visual acuity threshold post-blast in a subset of mice. Each line represents the blast-exposed eye of one mouse. All mice analyzed out to at least 7 days post-23psi blast are shown (A-G) along with one 30psi blast eye. All mice had clear corneas and lenses. The dotted line indicates the previously published average visual acuity of normal C57Bl/6 mice (Prusky et al., 2004; Douglas et al., 2005; Umino et al., 2008).
There was an overall decrease in IOP after blast-exposure (Figure 5A). The average IOP pre-blast in the exposed eye was 16±4 mmHg. At 3, 7, 14, and 28 days post-blast the IOP was 16±4, 12±3, 14±2, and 10±2 mmHg, respectively. This decrease over time was primarily due to a decrease in IOP of greater than 5 mmHg in 10 of the 25 eyes (40%). The average decrease in this subset of mice was 7±4 mmHg. Two eyes developed a slightly higher IOP after blast exposure but were still within the normal IOP range. Six eyes (24%) showed a transient rise in IOP (increase of greater than 5 mmHg from the pre-blast level) at 3 days post-blast that returned to normal levels at 7 days post-blast. Using a repeated ANOVA, the decreases in IOP at 7, 14, and 28 days post-blast were statistically significant: F= 5.2, p<0.05, df= 35; F= 5.2, p<0.01, df= 27; and F= 13.4, p<0.005, df= 17, respectively. All retinas appeared normal by OCT imaging (Figure 6B).
Figure 5.
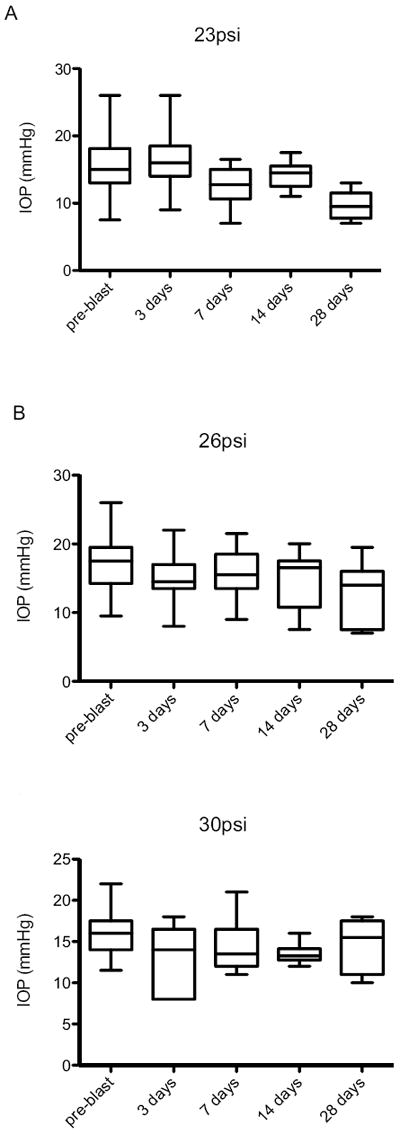
Whisker plot of IOP measurements taken pre-blast, and 3, 7, 14, and 28 days: A) 23psi; B) 26psi; C) 30psi.
Figure 6.
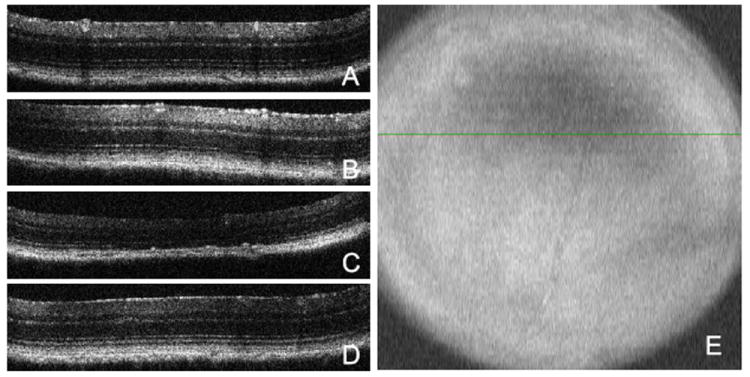
Optical coherence tomography images of representative B-line scans through superior peripheral retina of: A) contralateral eye; B) eye exposed to a 23psi blast; C) eye exposed to a 26 psi blast; and D) eye exposed to a 30psi blast. E) Fundus image of the same 26psi blast eye as shown in C). The green line indicates the location of the b-line scan through the area of retinal thinning. Note that only eyes with clear corneas and lenses were used for OCT imaging.
3.4 Exposure to a 26.4±0.9 psi blast
A total of 41 mice were exposed to a 26.4psi blast of compressed air. Nine died immediately post-blast, resulting in a 22% mortality rate. All remaining mice survived to the experimental end-point.
3.4.1 Body Weight
At 3 days post-blast, the average body weight loss was 3.9%. Average body weight stabilized at 7 and 14 days post-blast, and increased by 3.3% at 28 days post-blast. At 3 days post-blast, 8 mice (25%) had a body weight loss of greater than 5% (7.9±2.8%). Between 3 and 7 days post-blast, two mice lost an additional approximately 5% body weight.
3.4.2 Gross Pathology
Immediately post-blast, two eyes had torn extraocular muscles inferior to the globe as a result of the blast (Table 3, Figure 3 E). One of the surviving mice had corneal abrasions; there were no other gross pathology findings immediately post-blast. Four of the remaining 32 mice exhibited corneal edema 3 days post-blast (12.5%), 3, of which, also had corneal abrasions (9.4%). At seven days post-blast exposure, two of 25 mice (8%) had only corneal abrasions, two (8%) had corneal edema, and one (4%) had central corneal scarring in conjunction with edema (Figure 3 B, C, D). Corneal abrasions and edema were still present in a subset of mice at 14 and 28 days post-blast exposure, including two mice at 14 days that had central scarring (Table 3; Figure 3 D).
3.4.3 Visual Acuity, IOP, and Retinal Imaging
There was no difference in visual acuity in the blast-exposed eye versus the contralateral eye at any time-point post-blast (Table 2). The average visual acuity thresholds were 0.35±0.09, 0.38±0.05, 0.40±0.02, and 0.43±0.09 c/d in the contralateral eyes 3, 7, 14, and 28 days post-blast, respectively. The average visual acuity thresholds were 0.37±0.05, 0.40±0.06, 0.40±0.03, and 0.39±0.03c/d in blast- exposed eyes 3, 7,14, and 28 days post-blast exposure, respectively. There was a slight decrease (9%) in the visual acuity in one eye three days post-blast; this mouse was collected, so it is unknown if a further decrease over time would have been detected.
There was a trend towards a decrease in IOP after blast exposure that became statistically significant 28 days post-blast using a repeated ANOVA (F= 4.2, p<0.01, df= 54) (Figure 5 B). The average IOPs were 18±4, 15±3, 16±3, 15±4, and 13±4 mmHg in pre-blast, and 3, 7, 14, and 28 day post-blast exposed eyes, respectively. A total of 41% of mice had an IOP at least 5 mmHg lower after blast (13 mice). Within this group, the average decrease in IOP from baseline was 10±4 mmHg. However, one mouse did develop an elevated IOP–its IOP increased from 9.5 mmHg pre-blast to 14 mmHg (3 days) and 20 mmHg at 7 and 14 days post-exposure, respectively.
The central retina appeared normal by optical coherence tomography in all eyes. However, in one eye imaged 28 days post exposure to a 26.4psi blast, a large area of retinal thinning was detected in the peripheral retina (Figure 6 E). Since only eyes with clear corneas were used for imaging, this was not the result of a shadow. To confirm, multiple B-line scans were performed through this area. A representative B-line scan shows loss of RPE, rounding of the remaining RPE, and disruption of the RPE and Bruch’s membrane (Figure 6 C). More significantly there was a severe thinning of the outer nuclear layer in this area, such that it was virtually absent at the center of the affected area, indicative of significant photoreceptor cell loss.
3.5 Exposure to a 30.4±1.9 psi blast
A total of 24 mice were exposed to a 30.4psi blast of compressed air. Eleven mice died within 24 hours post-blast (a 46% mortality rate). All remaining mice survived until their experimental end-point.
3.5.1 Body Weight
Four of 13 mice (31%) lost greater than 5% of their body weight by 3 days post-blast (6.4%±0.8). At 7 days post-blast, one mouse lost an additional 4.6% body weight, but all others had stabilized or gained weight. At 14 and 28 days post-blast, all mice had gained weight or returned to their pre-blast weight.
3.5.2 Gross Pathology
There was an all or none effect of blast-exposure at this pressure level (Table 4). All of the mice that died within 24 hours post-blast except for one exhibited severe bleeding associated with severely torn extraocular muscles (7 mice; Figure 3 E), and/or avulsion of the optic nerve (5 mice; Figure 3G). Only one surviving mouse had any gross pathological findings – a cataract at 3 days post-blast. Although all mice are placed in the housing chamber in a similar manner, it is possible that the eyes were not always positioned such that they looked straight at the barrel. If the eye was at a slight angle, then the pressure from the blast may have induced torsion that caused avulsion of the optic nerve (Sponsel et al., 2011). We expect that the remaining eyes probably had subclinical corneal edema that will be evident upon histological analysis.
3.5.3 Visual Acuity, IOP, and Retinal Imaging
There was a trend for decreased visual acuity in the blast-exposed eyes, but this only reached statistical significance at the 28 day time-point (p<0.05) likely due, in part, to the high mortality rate resulting in a low n value. Only one mouse was followed longitudinally. The average visual acuity thresholds were 0.41± 0.07 and 0.35±0.08 c/d in the contralateral and blast-exposed eyes, respectively, 28 days post-blast (Table 2). When all of the optokinetics data from the various time-points post-blast was combined, the visual acuity thresholds were 0.37±0.11 and 0.27±0.13c/d in contralateral and blast-exposed eyes, respectively (p=0.01). One eye in particular showed a dramatic loss of visual acuity despite having a clear cornea and lens (Figure 4).
On average, the IOPs were unchanged after blast-exposure at all time-points tested (Figure 5 C). Four mice (31%) had a decrease in IOP of greater than 5 mmHg. The average overall decrease in IOP in these four mice was 8.6±2.0 mmHg. In the mice that were followed longitudinally, a statistically significant decrease in IOP was only detected at 7 days post-blast (F= 4.9, p<0.05, df= 26). No abnormalities were detected in the retina by OCT imaging (Figure 6 D).
4. Discussion
We have demonstrated the successful development, optimization, and calibration of a novel mouse model of primary blast injury to the eye. This system reproducibly exposes the eye to an open wave-form primary blast of known pressures that can be controlled by altering the input pressure and/or increasing the distance between the barrel and the eye. The rest of the body was protected from blast, so all reported injuries were a direct result of the exposure of the eye to the pressurized air blast. The contralateral eye was mostly unaffected.
Although exposure to the blast wave was limited to the eye of the mouse, there was an unanticipated effect on body weight and survival even at the lowest pressure tested. The drop in body weight was temporary and was mitigated but not entirely prevented by provision of gel food and acetaminophen. It appears that the exposure of just the eye to a blast wave induces distress resulting in loss of body weight for the initial three days post-blast. The mortality rate correlated to the pressure level, increasing from approximately 24% after a 23.6 or 26.4psi blast to 46% after a 30.4psi blast. In some mice the mortality was associated with morbidity of the eye. In other cases the cause of death is unknown. In future studies we will perform histological examination of the brains of these mice to determine if the mortality was due to acute brain damage from the propagation of the blast wave through the head.
The incidence of cataracts and corneal edema was very low despite the large lens in the mouse eye. In fact, the incidence was comparable to that in the patient population. Cockerham et al., 2011 reported cataracts in 6% of blast-exposed patients, and corneal scars in 12% of eyes. In this study, cataracts were detected in 7% of eyes exposed to a 30psi blast, and corneal scars were detected in about 13% of eyes exposed to a 23 or 26psi blast, likely as a result of unresolved corneal edema. A subset of mice developed delayed corneal edema, which may have been due to undetected lid edema. In future studies we will characterize the gross pathology on a histological level. These data support the hypothesis that despite the different architecture of mouse and human eyes, a mouse model of ocular blast injury can be relevant and accurate.
The measured decrease in IOP by applanation tonometry in the mice after blast exposure is most consistent with the presence of corneal edema (for review see Chihara, 2008). While we did not see corneal edema in some of the mice with decreased IOP, subclinical edema may be evident upon histological examination, which will follow. Sustained decreases in IOP after resolution of corneal edema (as seen in three of the blast-exposed eyes) may be indicative of damage to the iris or ciliary body resulting in increased outflow or decreased production of aqueous humor.
Damage to the retina, retinal pigment epithelium, and choroid was only detected in one eye from the 26psi exposure group corresponding to 9% of eyes at the 28 day time-point. This is comparable to the percentage of eyes (11%) in which choroid rupture or retinal tears, holes, or detachments were detected in blast-exposed Veterans (Cockerham et al., 2011). It is not possible to image the entire mouse retina by OCT, so it is possible that areas of retinal thinning were present in other eyes as well but were not detected. In addition, those eyes with anterior pathologies could not be imaged. In fact, we expect that retinal damage is present in the eyes from the 30psi group based on the visual acuity deficit detected in those eyes. Future histological analysis will provide a more detailed analysis of effects on the posterior pole.
The divergent response of eyes to the 30psi blast was surprising. One possibility is that the eye was not placed flush with the hole in the PVC pipe in a subset of mice, and therefore the pressure wave caused rotation of the globe resulting in torn extraocular muscles and optic nerve avulsion. In future studies, we will further optimize the mouse housing to minimize this effect.
Future studies will also assess blast-induced changes in the retina on the molecular and cellular level at different time-points post-blast and characterize morphological changes in ocular structures.
5. Conclusion
The injuries to the eye seen in this new ocular blast injury research platform are similar to those experienced by service members exposed to blasts from improvised explosive devices and by blunt trauma (Thach et al., 2008; Cockerham et al., 2011; Hilber et al., 2011). For example, both the mice in this study and the patient population exhibit a combination of the following conditions: corneal edema, photoreceptor cell loss, physical damage to the RPE and/or choroid, and optic nerve avulsion. These findings support the accumulating data that primary blast exposure alone is sufficient to induce severe and permanent damage to the eye and the retina. Not surprisingly, we detected an increase in severity of injury as the output pressure was increased. The type of damage also changed with time post-blast, for example, deficits in visual acuity increased over time.
This model will be very useful for studying the molecular mechanisms underlying blast injury and will serve as an excellent platform for identifying and testing potential therapeutics.
Highlights.
A novel mouse model of ocular blast injury
Accurately models damage in closed eye injury patients
Useful model for screening potential therapeutic agents
Acknowledgments
Unrestricted grant from Research to Prevent Blindness; NIH (5P30EY13080); U.S. Army Medical Research and Materiel Command and the Telemedicine & Advanced Technology Research Center W81XWH-10-1-0528; Research to Prevent Blindness Career Development Award; Glaucoma Research Foundation; UTHSC Neuroscience Institute; the Plough Foundation, Memphis, TN, and Roche Foundation for Anemia Research.
The sponsor or funding organizations had no role in the design or conduct of this research.
Footnotes
Department of Defense Non-endorsement Disclaimer: The views, opinions and/or findings contained in this research presentation are those of the authors and do not necessarily reflect the views of the Department of Defense and should not be construed as an official DoD/Army position, policy or decision unless so designated by other documentation. No official endorsement should be made.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Blanch RJ, Scott RAH. Military Ocular Injury: Presentation, Assessment and Management. J R Army Med Corps. 2009;155:279–84. doi: 10.1136/jramc-155-04-08. [DOI] [PubMed] [Google Scholar]
- Cernak I, Merkle AC, Koliatsos VE, Bilik JM, Luong QT, Mahota TM, Xu L, Slack N, Windle D, Ahmed FA. The pathobiology of blast injuries and blast-induced neurotrauma as identified using a new experimental model of injury in mice. Neurobiol Dis. 2011;41:538–51. doi: 10.1016/j.nbd.2010.10.025. [DOI] [PubMed] [Google Scholar]
- Chalioulias K, Sim KT, Scott R. Retinal sequelae of primary ocular blast injuries. J R Army Med Corps. 2007;153:124–5. doi: 10.1136/jramc-153-02-11. [DOI] [PubMed] [Google Scholar]
- Chihara E. Assessment of true intraocular pressure: The gap between theory and practical data. Surv Ophthalmol. 2008;53:203–18. doi: 10.1016/j.survophthal.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Cockerham CG, Rice TA, Hewes EH, Cockerham KP, Lemke S, Wang G, Lin RC, Glynn-Milley C. Closed-eye ocular injuries in the Iraq and Afghanistan wars. New Engl J Med. 2011;364:2172–3. doi: 10.1056/NEJMc1010683. [DOI] [PubMed] [Google Scholar]
- Douglas RM, Alam NM, Silver BD, McGill TJ, Tschetter WW, Prusky GT. Independent visual threshold measurements in the two eyes of freely moving rats and mice using a virtual-reality optokinetic system. Vis Neurosci. 2005;22:677–684. doi: 10.1017/S0952523805225166. [DOI] [PubMed] [Google Scholar]
- Heier JS, Enzenauer RW, Wintermeyer SF. Ocular injuries and diseases at a combat support hospital in support of operations Desert Shield and Desert Storm. Arch Ophthalmol. 1993;111:795–8. doi: 10.1001/archopht.1993.01090060083028. [DOI] [PubMed] [Google Scholar]
- Hilber DJ. Eye Injuries, active component, U.S. Armed Forces, 2000-2010. MSMR. 2011;18:2–7. [PubMed] [Google Scholar]
- Koliatsos VE, Cernak I, Xu L, Song Y, Savonenko A, Crain BJ, Eberhart CG, Frangakis CE, Melnikova T, Kim H, Lee D. A mouse model of blast injury to brain: initial pathological, neuropathological, and behavioral characterization. J Neuropathol Exp Neurol. 2011;70:399–416. doi: 10.1097/NEN.0b013e3182189f06. [DOI] [PubMed] [Google Scholar]
- Long JB, Bentley TL, Wessner KA, Cerone C, Sweeney S, Bauman RA. Blast overpressure in rats: Recreating a battlefield injury in the laboratory. J Neurotrauma. 2009;26:827–40. doi: 10.1089/neu.2008.0748. [DOI] [PubMed] [Google Scholar]
- Mader TH, Carroll RD, Slade CS, George RK, Ritchey JP, Neville SP. Ocular war injuries of the Iraqi insurgency January-September 2004. Ophthalmol. 2006;113:97–104. doi: 10.1016/j.ophtha.2005.07.018. [DOI] [PubMed] [Google Scholar]
- Petras J, Bauman R, Elsayed N. Visual system degeneration induced by blast overpressure. Toxicol. 1997;121:41–9. doi: 10.1016/s0300-483x(97)03654-8. [DOI] [PubMed] [Google Scholar]
- Prusky GT, Alam NM, Beekman S, Douglas RM. Rapid quantification of adult and developing mouse spatial vision using a virtual optomotor system. Invest Ophthalmol Vis Sci. 2004;45:4611–4616. doi: 10.1167/iovs.04-0541. [DOI] [PubMed] [Google Scholar]
- Sponsel WE, Gray W, Groth SL, Stern AR, Walker JD. Paintball trauma and mechanisms of optic nerve injury: rotational avulsion and rebound evulsion. Invest Ophthalmol Vis Sci. 2011 doi: 10.1167/iovs.11-8472. Epub. [DOI] [PubMed] [Google Scholar]
- Thach AB, Johnson AJ, Carroll RB, Huchun A, Ainbinder DJ, Stutzman RD, Blaydon SM, Demartelaere SL, Mader TH, Slade CS, George RK, Ritchey JP, Barnes SD, Fannin LA. Severe eye injuries in the war in Iraq, 2003-2005. Ophthalmol. 2008;115:377–82. doi: 10.1016/j.ophtha.2007.04.032. [DOI] [PubMed] [Google Scholar]
- Thomas R, McManus JG, Johnson A, Mayer P, Wade C, Holcomb JB. Ocular injury reduction from ocular protection use in current combat operations. J Trauma. 2009;66:S99–103. doi: 10.1097/TA.0b013e31819d8695. [DOI] [PubMed] [Google Scholar]
- Umino Y, Solessio E, Barlow RB. Speed, spatial, and temporal tuning of rod and cone vision in mouse. J Neurosci. 2008;28:189–98. doi: 10.1523/JNEUROSCI.3551-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weichel E, Colyer M. Combat ocular trauma and systemic injury. Curr Opin Ophthalmol. 2008;19:519–25. doi: 10.1097/ICU.0b013e3283140e98. [DOI] [PubMed] [Google Scholar]


