Abstract
Objectives
Our previous longitudinal multicenter-based carriage study showed that the average carriage rate of Streptococcus pneumoniae was 16.8% in 582 healthy children attending kindergarten or elementary school in Seoul, Korea. We assessed serotype-specific prevalence and antimicrobial resistance among colonizing pneumococcal isolates from young children in the era of low use of the seven-valent pneumococcal conjugate vaccine (PCV7).
Methods
Serotypes were determined by an agglutination test with specific antisera or by a multiplex polymerase chain reaction (PCR) assay. An antimicrobial susceptibility test was performed with broth microdilution in Korean 96-well panels from Dade-MicroScan (Sacramento, CA, USA).
Results
Pneumococcal colonization patterns were dynamic and longterm persistent carriage was rare, which indicated a sequential turnover of pneumococcal strains. Of the 369 pneumococci (except for 23 killed isolates), 129 (34.9%) isolates were PCV7 vaccine serotypes (VTs); 213 (57.8%) isolates were nonvaccine serotypes (NVTs); and the remaining 27 (7.2%) isolates were nontypable (NT). The highest rates of multidrug resistance (MDR) were observed in VTs (86.0%; 111/129 isolates) and NVTs (70.0%; 149/213 isolates).
Conclusion
This study overall showed the frequent carriage of VTs and NVTs with MDR in healthy children attending kindergarten or elementary school. Efforts should be directed toward reducing the extensive prescription of antibiotics and using new broader vaccines to reduce the expansion of MDR strains of NVTs in our community.
Keywords: multidrug-resistant strains, nasal carriage, nonvaccine serotypes, Streptococcus pneumoniae
1. Introduction
Asymptomatic pneumococcal colonization is very prevalent in the upper respiratory tracts of young children, and ranges 20–70% with levels falling with increasing age [1]. This colonization is a source of transmission to other individuals and a prerequisite for the development of respiratory infections [2]. Pneumococcal colonization rarely progresses to local or systemic disease; however, pediatric nasal carriage is a reservoir of disease-causing pneumococci circulating within a community [3].
A pneumococcal capsule is a key factor that determines disease outcomes. More than 92 serotypes have been identified on the basis of antigenic differences in the capsular polysaccharide [4,5]. Many epidemiological studies have shown that serotype distribution differs with respect to invasive pneumococcal disease (IPD) and carriage, and certain serotypes have much higher potential for invasiveness [5–7].
The seven-valent pneumococcal conjugate vaccine (PCV7), which targets seven serotypes that are highly relevant to IPD in children, is efficacious in preventing IPD in vaccinated young children and in unvaccinated persons [8,9]. After the implementation of the PCV7 in the National Immunization Program (NIP), the prevalence of pneumococcal serotypes has undergone a significant shift with a reduction in PCV7 vaccine serotypes (VTs) and an increase in nonvaccine serotypes (NVTs) in pneumococcal disease and carriage in many communities [8–10]. These changes create a need for a novel expanded-coverage vaccine for pneumococcal diseases [11]. However, pneumococcal conjugate vaccines with limited serotypes do not cover all pneumococcal serotypes that cause disease.
Since November 2003, the PCV7 has been voluntarily adopted by the private sector in Korea. This vaccine is still not included in the NIP in our community; therefore, PCV7 coverage may be smaller in our community than in countries with a routine PCV7 NIP. The evidence of PCV7 impact on the distribution of pneumococcal serotypes is not fully clear in our community. Several observational studies indicate that the serotype-specific distribution of IPD and respiratory disease have changed in response to even low use of PCV7 in Korea [12,13].
Our previous longitudinal carriage study described the nasal carriage and co-colonization patterns of four potential respiratory pathogens in young Korean children through four consecutive examinations over the course of 1 year; the average carriage rate of Streptococcus pneumoniae was 16.8% in 582 healthy children aged 3–10 years [14]. Because the true burden of IPD has not been defined in Korea, the distribution of serotypes in pneumococcal carriage can provide an additional useful insight into the impact of partial vaccine pressure on pneumococcal disease. In this study, we assessed serotype-specific prevalence and antimicrobial resistance among longitudinally colonizing pneumococcal isolates from young children attending kindergarten and/or elementary-school in Seoul, the capital of Korea, in the era of low use of the PCV7.
2. Materials and Methods
2.1. Bacterial isolates
At four sampling times (June 2006, September 2006, December 2006, and February 2007), a total of 392 S. pneumoniae strains were isolated from 2328 nasal aspirates of 582 healthy child volunteers attending three kindergartens (165 children with a mean age of 5.6 ± 1.2 years) or attending the first 2 years of one elementary school (417 children with a mean age of 8.4 ± 0.6 years). This procedure was based on our previous longitudinal multicenter-based carriage study [14]. All isolates were examined for α-hemolytic colonies with typical pneumococcal morphology and were identified by conventional procedures [13].
2.2. Serotyping
Pneumococcal serogroups and serotypes were primarily identified by using a chessboard with a commercial Pneumotest-Latex agglutination kit (Statens Serum Institut, Copenhagen, Denmark) and further analyzed by the capsular swelling (Quellung reaction) test with type/group-specific antisera (Staten Serum Institut, Copenhagen, Denmark). When the serotype of an isolate could not be determined by the agglutination test with pooled or specific antisera, the serotype was determined by a multiplex polymerase chain reaction (PCR) assay, as described at www.cdc.gov/ncidod/biotech/strep/pcr.htm. Because of the recently recognized serological cross-reactivity of serotype 6C with serotype 6A, all apparent serotype 6A strains were differentiated by a serotype 6C-specific PCR [15].
2.3. Antimicrobial susceptibility test
The minimum inhibitory concentrations of amoxicillin/clavulanate, azithromycin, cefaclor, cefotaxime, chloramphenicol, clindamycin, erythromycin, levofloxacin, penicillin, tetracycline, and trimethoprim/sulfamethoxazole were determined by broth microdilution in Korean microbroth 96-well panels manufactured by Dade-MicroScan (Sacramento, CA, USA); the Clinical and Laboratory Standards Institute (CLSI) guidelines were followed [16]. Minimum inhibitory concentration interpretive criteria followed the most recent CLSI guidelines by using oral breakpoints for penicillin (i.e., susceptible, ≤ 0.06 μg/mL; intermediate, 0.12–1 μg/mL; resistant, ≥ 2 μg/mL). Multidrug resistance (MDR) was defined as nonsusceptibility to three or more antimicrobial classes such as penicillin (amoxicillin/clavulanate); macrolides (erythromycin and/or azithromycin); clindamycin; cephalosporins (cefaclor and/or cefotaxime); chloramphenicol; trimethoprim/sulfamethoxazole; and tetracycline.
2.4. Statistical analysis
The statistical analysis was performed with a two-sided Fisher’s exact test by using SAS software version 9.2 (SAS Institute, Cary, NC, USA) to compare kindergarten and elementary school children in carriage prevalence, serotype distribution, and the resistance rate between VTs and NVTs and the MDR rate. We considered a p < 0.05 as statistically significant.
3. Results
3.1. Divergent pneumococcal carriage status
Among the 582 healthy child volunteers without respiratory symptoms, 45.4% (264) children were colonized at least once with S. pneumoniae during the 1-year longitudinal carriage study (Table 1). In the four sampling times, occurring at approximately 3-month intervals, each child had divergent patterns of pneumococcal carriage. In total, 264 carriers were occasionally carrying one colonizing S. pneumoniae strain [167 (28.7%) children] or two S. pneumoniae strains [69 (11.9%) children] at the four sampling points. In this study, persistent carriers who were positive at all four time points were very rare (0.5%). The frequency of pneumococcal carriage decreased with age. For the elementary school children, the proportion was significantly higher for the noncarriers (62.8%) than for the kindergarten children (33.9%; p < 0.0001). Elementary school children relatively rarely exhibit repeated colonization by pneumococcus.
Table 1.
Carriage status of Streptococcus pneumoniae among 582 children during a 1-year longitudinal nasal carriage study
| Carriage Frequency |
No. (%) of Children Colonized |
|||||||
|---|---|---|---|---|---|---|---|---|
| 1st | 2nd | 3rd | 4th | No. of children | Total (n = 582) | Kindergarten (n = 165) | Elementary school (n = 417) | p |
| ● | ● | ● | ● | 3 | 3 (0.5) | 3 (1.8) | 0 (0.0) | 0.0225 |
| ● | ● | ● | – | 6 | 25 (4.3) | 17 (10.3) | 8 (1.9) | <0.0001 |
| ● | ● | – | ● | 7 | ||||
| ● | – | ● | ● | 2 | ||||
| – | ● | ● | ● | 10 | ||||
| ● | ● | – | – | 13 | 69 (11.9) | 38 (23.0) | 31 (7.4) | <0.0001 |
| ● | – | ● | – | 12 | ||||
| ● | – | – | ● | 10 | ||||
| – | ● | ● | – | 15 | ||||
| – | ● | – | ● | 7 | ||||
| – | – | ● | ● | 12 | ||||
| ● | – | – | – | 47 | 167 (28.7) | 51 (30.9) | 116 (27.8) | 0.4774 |
| – | ● | – | – | 31 | ||||
| – | – | ● | – | 55 | ||||
| – | – | – | ● | 34 | ||||
| – | – | – | – | 318 | 318 (54.6) | 56 (33.9) | 262 (62.8) | <0.0001 |
Data are presented as n (%).
“●” = Streptococcus pneumoniae isolated; “–“ = Streptococcus pneumoniae not isolated.
3.2. Prevalent serotype distribution
Table 2 shows the prevalent serotypes of the carriage isolates. These 19 serotypes accounted for 81.3% of 369 isolates (excluding 23 killed isolates). The proportion of VTs and NVTs was 34.9% and 57.8%, respectively. The remaining 7.2% were nontypable (NT). Among the VTs, the most predominant were 6B (9.2%), 19F (7.3%), and 23F (7.3%). Among all 35 NVTs, the most frequent was 35B (6.0%), 6A (5.1%), 15A (4.9%), 37 (4.9%), 19A (4.6%), and 10A (4.3%). In the two age groups, the proportion of children who carried VTs and NVTs did not exhibit a statistically significant difference (p = 0.5038). However, certain serotypes showed different patterns by age group: serotypes 15A and 23A were relatively abundant among children attending kindergarten (p = 0.004 and p = 0.033, respectively), and serotypes 18C, 34, and 37 were frequent in the elementary school children (p = 0.020, p = 0.020, and p = 0.001, respectively).
Table 2.
Prevalence of PCV7 serotypes and NVTs in 369 Streptococcus pneumoniae isolates (except for 23 killed isolates) from 582 children attending kindergarten or elementary school during a 1-year longitudinal nasal carriage study
| Serotypes | % |
||
|---|---|---|---|
| Total (n = 369) | Kindergarten (n = 180) | Elementary school (n = 189) | |
| PCV7 | |||
| 4 | 1.1 | 0.6 | 1.6 |
| 6B | 9.2 | 8.3 | 10.1 |
| 9V | 3.8 | 3.3 | 4.2 |
| 14 | 3.5 | 5.6 | 1.6 |
| 18C | 2.7 | 0.6 | 4.8 |
| 19F | 7.3 | 6.7 | 7.9 |
| 23F | 7.3 | 7.8 | 6.9 |
| Vaccine coverage | 35.0 | 32.8 | 37.0 |
| Non-PCV7 | |||
| 3 | 3.0 | 1.1 | 4.8 |
| 6A | 5.1 | 6.7 | 3.7 |
| 7F | 3.0 | 1.7 | 4.2 |
| 10A | 4.3 | 3.3 | 5.3 |
| 11A | 2.2 | 2.2 | 2.1 |
| 15A | 4.9 | 8.9 | 1.1 |
| 19A | 4.6 | 5.0 | 4.2 |
| 23A | 2.2 | 3.9 | 0.5 |
| 34 | 2.7 | 0.6 | 4.8 |
| 35A | 3.5 | 4.4 | 2.6 |
| 35B | 6.0 | 7.8 | 4.2 |
| 37 | 4.9 | 1.1 | 8.5 |
| Othersa | 11.4 | 12.1 | 10.6 |
| Nonvaccine coverage | 57.7 | 58.9 | 56.6 |
| Nontypable | 7.3 | 8.3 | 6.4 |
NVTs = nonvaccine serotype; PCV7 = seven-valent pneumococcal conjugate vaccine.
Others include serotypes 1, 7C, 8, 9N, 11C, 12F, 13, 15B, 15C, 15F, 16F, 17F, 22F, 23B, 24F, 25A, 29, 33F, 35F, and 38.
3.3. Antimicrobial resistance
An in vitro antimicrobial susceptibility test was performed on 364 S. pneumoniae isolates (28 isolates were excluded because of viability loss during storage). Figure 1 summarizes the findings. The prevalence of MDR was 76.9% in the pneumococcal carriage isolates. The highest resistance was evident for penicillin (78.6%), erythromycin (75.8%), azithromycin (75.5%), cefaclor (76.6%), and tetracycline (79.9%), whereas 14.0% and 13.7% of all isolates were nonsusceptible to amoxicillin/clavulanate and cefotaxime, respectively. All isolates were susceptible to levofloxacin.
Figure 1.
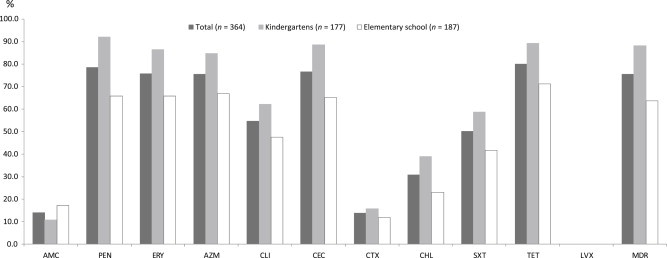
The proportion of antimicrobial nonsusceptibility of pneumococcal carriage strains, according to age (kindergarten or elementary school). AMC = amoxicillin/clavulanate; AZM = azithromycin; CEC = cefaclor; CHL = chloramphenicol; CLI = clindamycin; CTX = cefotaxime; ERY = erythromycin; MDR = multidrug resistance; PEN = penicillin G; SXT = trimethoprim/sulfamethoxazole; TET = tetracycline. LVX = levofloxacin.
Resistance to seven tested antimicrobial agents was higher in the carriage isolates from the kindergarten children than from the elementary school children, as follows: penicillin (92.1% vs. 65.8%; p < 0.0001); erythromycin (86.4% vs. 65.8%; p < 0.0001); azithromycin (84.7% vs. 66.8%; p < 0.0001); tetracycline (89.3% vs. 71.1%; p < 0.0001); cefaclor (88.7% vs. 65.2%; p < 0.0001); clindamycin (62.1% vs. 47.6%; p = 0.0062); chloramphenicol (39.0% vs. 23.0%; p = 0.0010); and trimethoprim-sulfamethoxazole (58.8% vs. 41.7%; p = 0.0016). However, the percent of strains that were nonsusceptible to amoxicillin/clavulanate and cefotaxime did not significantly differ with age. The rate of MDR was significantly higher among younger children (89.3% in kindergarten children vs. 65.3% in elementary school children; p < 0.0001).
3.4. High prevalence of MDR among VTs and NVTs
Figure 2 presents the proportions of MDR in individual serotypes covered or not covered by the PCV7. Most VTs tended to have high rates of MDR (≥ 75.0%)—except for VT 18C, which had a low MDR level (30.0%). The most prevalent NVTs (6A, 15A, 19A, 35A, and 35B) had also the highest rates of MDR (≥ 75.0%). The number of isolates of a given serotype was relatively small, although the NVTs 11A, 15B, 16F, and 23A exhibited highly prevalent MDR (≥ 75.0%). The highest rates of MDR were observed in VTs and NVTs. However, MDR was significantly lower among the NVTs (70.0%; 149/213 isolates) than among the VTs (86.0%; 111/129 isolates; p = 0.0015).
Figure 2.
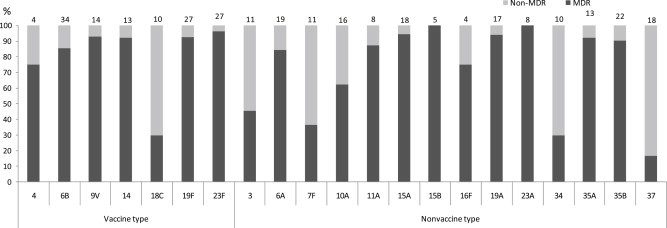
The proportion of serotype-specific MDR rates in Streptococcus pneumoniae nasal carriage isolates. The number on top of each bar indicates the total number of serotype-specific strains. MDR = multidrug-resistant.
4. Discussion
A pneumococcal carriage study could be informative in regard to the serotype distributions of S. pneumoniae that is circulating within a country because of different local factors such as the introduction of PCVs and socio-economic conditions. To our knowledge, alterations in serotype-specific carriage in children are more likely to affect the pneumococcal disease potential in a community. The outcomes of routine PCV7 vaccination, a key selective pressure, have resulted in serotype replacement because of a reduction in VTs and an increase in certain NVTs in IPD and carriage in many communities [1,3,10,11].
Our previous longitudinal multicenter-based carriage study described average pneumococcal carriage rates of 28.6% and 12.2% in healthy preschool children and elementary-school children, respectively, based on measurements obtained at four time points [14]. At the time of our study in 2006–2007, the PCV7 had been available in the private sector since 2003. The uptake of PCV7 was approximately 20–25% in Korean children who were younger than 2 years of age in 2004–2006 [12]. However, most children recruited in this study were born during the period of 1996–2003, which was prior to the introduction of the PCV7, and parents did not know the PCV7 vaccination status for their children. This study did not include the status of PCV7 vaccination for each child. We nevertheless hypothesized that many of the recruited children had not been vaccinated with PCV7. Because of the low coverage rate of the PCV7, we assessed the serotype prevalence and antimicrobial resistance in pneumococci carried by healthy children attending three kindergartens or the first 2 years of one elementary school in Seoul, Korea.
For the longitudinal carriage study, the serotype-specific carriage status of S. pneumoniae was very dynamic—for any pair of two consecutive S. pneumoniae isolates from the same children, only a few were successively colonized by the same serotype (16.7%; 15 of 90 consecutive pairs; data not shown). Among the persistent carriers colonized by pneumococci for more than three periods, the carriage isolates with the same serotype usually persisted for short periods (less than two periods), indicating the rapid elimination of the initial strain and the sequential acquisition of a new pneumococcal strain with another serotype.
Compared to available serotype-specific carriage data from healthy children prior to the introduction of the PCV7 (1997–1998) [17], we observed a high prevalence (57.8%) of NVTs in S. pneumoniae isolates in this study. The proportion of VTs decreased, although the VTs 6B, 19F, and 23F remained dominant. This finding supports a few direct effects of the PCV7 on the children recruited in this study. These data could also be consistent with the findings of our previous report that indicated that the PCV7 serotypes 19F, 23F, 9V, 6B, and 14 remain prevalent in IPD, despite a declining trend of VTs from 69.2% in the pre-PCV7 era (1996–1999) to 50.0% in the post-PCV7 era (2007–2008) in Korean children younger than 5 years of age [18].
In this study, the frequent colonizing NVTs were 35B, 6A, 15A, 37, 19A, 10A, 35A, 3, and 7F. This finding was consistent with the replacement serotypes that were reported in many recent epidemiological studies or that were predicted to have improved fitness for pneumococcal carriage [19–22]. There was an interesting and notably high prevalence of two serogroups—serotype 35 (35A and 35B; 9.5%) and serotype 15 (15A, 15B, 15C, and 15F; 6.8%)—and two immunologically PCV7-related serotypes 6A (5.1%) and 19A (4.6%). Many countries describe an increase in serotype 19A and a decrease in serotype 6A with the increased uptake of the PCV7 [19]. By contrast, our study showed that serotype 6A remained prevalent among pneumococcal carriage isolates. The cross-protection of the PCV7 does not fully contribute to a decrease in serotype 6A. A similar phenomenon occurred in our IPD study during the period from 1996–2008 [18].
The most frequent serotypes generally carried in children tend to demonstrate resistance because of the greater likelihood of antibiotic exposure [20]. Prior to the introduction of the PCV7, the highest rates of penicillin resistance tended to occur in the common colonizing VTs 6B, 9V, 14, 19F, and 23F. In an American study and other studies, the use of PCV7 caused an unexpected increase in antimicrobial resistance among NVTs and particularly in serotypes 6A, 19A, and 35B [20,21]. In this study, even more remarkable was the high prevalence of MDR in most NVTs (6A, 11A, 15A, 15B, 16F, 19A, 23A, 35A, and 35B). We believe that the frequent prescribing of antimicrobial drugs may drive the emergence of MDR strains of NVTs after PCV7 introduction or even without vaccination in Korea. Such a high MDR rate for NVTs is similar to the rates for the pneumococcal isolates reported in a previous IPD study [23].
The recent switch from the PCV7 to the newly approved vaccines PCV10 and PCV13 is likely to be substantially offset by an increase in eventual non-PCV10 or PCV13 serotypes. We anticipate the greatest impact of the PCV13 will be a reduction in the carriage and disease attributable to 6A and 19A with MDR. However, partially covered novel vaccines such as PCV10 and PCV13 will alter the pneumococcal population structure in IPD and carriage in our community and replace nonvaccine-related serotypes. This phenomenon continues to be a clinical problem in this region.
The design of pneumococcal vaccines has been based on the serotype distribution of IPD in a community. Many public health experts recognize that elevated NVTs in pneumococcal disease and/or carriage with the use of the PCV7 is a growing problem. The progression of pneumococcal disease is complex; however, certain NVTs commonly colonize the nasopharynx, thereby having a greater potential opportunity to cause IPD. In Korean children younger than 5 years of age, the overall incidence of IPD remains very rare. Evaluation of ongoing changes in the ecology of pneumococcus carriage necessitates an estimation of the potential impacts of pneumococcal vaccines and ultimate full control of pneumococcal disease in our community. To reduce the rapid expansion of MDR strains of NVTs, further efforts should be directed toward using more expanded vaccines and reducing the extensive prescription of antibiotics in Korea.
Acknowledgments
This study was supported by a grant from the Korea National Institute of Health (Osong, Korea; Grant No. 4851-300-210-13). The authors thank all participants who collaborated in this study: directors, staffs, children, and the parents of the children in three kindergarten programs and one elementary school.
Footnotes
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
References
- 1.García-Rodríguez J.Á., Frensnadillo Martínez M.J. Dynamics of nasopharyngeal colonization by potential respiratory pathogens. J Antimicrob Chemother. 2002 Dec;50(Suppl. S2):59–73. doi: 10.1093/jac/dkf506. [DOI] [PubMed] [Google Scholar]
- 2.Murphy T.F., Bakaletz L.O., Smeesters P.R. Microbial interactions in the respiratory tract. Pediatr Infect Dis J. 2009 Oct;28(10 Suppl.):S121–S126. doi: 10.1097/INF.0b013e3181b6d7ec. [DOI] [PubMed] [Google Scholar]
- 3.Bogaert D., De Groot R., Hermans P.W. Streptococcus pneumoniae colonization: the key to pneumococcal disease. Lancet Infect Dis. 2004 Mar;4(3):144–154. doi: 10.1016/S1473-3099(04)00938-7. [DOI] [PubMed] [Google Scholar]
- 4.Henrichsen J. Six newly recognized types of Streptococcus pneumoniae. J Clin Microbiol. 1995 Oct;33(10):2759–2762. doi: 10.1128/jcm.33.10.2759-2762.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calix J.J., Nahm M.H. A new pneumococcal serotype, 11E, has a variably inactivated wcjE gene. J Infect Dis. 2010 Jul;202(1):29–38. doi: 10.1086/653123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hausdorff W.P., Feikin D.R., Klugman K.P. Epidemiological differences among pneumococcal serotypes. Lancet Infect Dis. 2005 Feb;5(2):83–93. doi: 10.1016/S1473-3099(05)01280-6. [DOI] [PubMed] [Google Scholar]
- 7.Klugman K.P., Welsh K.J. Nasopharyngeal carriage of pneumococcal pediatric serotypes; a risk for acute and recurrent otitis media in children and for invasive disease in susceptible adults. J Infect Dis. 2005 Jun;191(11):1790–1792. doi: 10.1086/429924. [DOI] [PubMed] [Google Scholar]
- 8.Whitney C.G., Farley M.M., Hadler J. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N Engl J Med. 2003 May;348(18):1737–1746. doi: 10.1056/NEJMoa022823. [DOI] [PubMed] [Google Scholar]
- 9.Talbot T.R., Poehling K.A., Hartert T.V. Reduction in high rates of antibiotic-nonsusceptible invasive pneumococcal disease in Tennessee after introduction of the pneumococcal conjugate vaccine. Clin Infect Dis. 2004 Sep;39(4):614–618. doi: 10.1086/422653. [DOI] [PubMed] [Google Scholar]
- 10.Sá-Leão R., Nunes S., Brito-Avô A. Changes in pneumococcal serotypes and antibiotypes carried by vaccinated and unvaccinated day-care centre attendees in Portugal, a country with widespread use of the seven-valent pneumococcal conjugate vaccine. Clin Microbiol Infect. 2009 Nov;15(11):1002–1007. doi: 10.1111/j.1469-0691.2009.02775.x. [DOI] [PubMed] [Google Scholar]
- 11.Flasche S., Hoek A.J.V., Sheasby E. Effect of pneumococcal conjugate vaccination on serotype-specific carriage and invasive disease in England: a cross-sectional study. PLoS Medicine. 2011 Apr;8(4):e1001017. doi: 10.1371/journal.pmed.1001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi E.H., Kim S.H., Eun B.W. Streptococcus pneumoniae serotype 19A in children, South Korea. Emerg Infect Dis. 2008 Feb;14(2):275–281. doi: 10.3201/eid1402.070807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee S.K., Lee K.J., Kang Y.H. Prevalence of serotype and multidrug-resistance of Streptococcus pneumoniae respiratory tract isolates in 265 adults and 36 children in Korea, 2002–2005. Microb Drug Resist. 2010 Jun;16(2):135–142. doi: 10.1089/mdr.2009.0114. [DOI] [PubMed] [Google Scholar]
- 14.Bae S., Yu J., Lee K. Nasal colonization of four potential respiratory bacteria in healthy children attending kindergarten or elementary school in Seoul, Korea. J Med Microbiol. 2012 May;61(Pt 5):678–685. doi: 10.1099/jmm.0.040584-0. [DOI] [PubMed] [Google Scholar]
- 15.Park I.H., Park S., Holingshead S.K. Genetic basis for the new pneumococcal serotype, 6C. Infect Immun. 2007 Sep;75(9):4482–4489. doi: 10.1128/IAI.00510-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Institute CaLS . Clinical and Laboratory Standards Institute (CLSI); Wayne, PA: 2009. Performance Standards for Antimicrobial Susceptibility Testing; Fifteenth Informational Supplement. Approved Standard, M100–S19. [Google Scholar]
- 17.Lee J.A., Kim N.H., Kim D.H. Serotypes and penicillin susceptibility of Streptococcus pneumoniae isolated from clinical specimens and healthy carriers of Korean children. J Korean Pediatr Soc. 2003 Sep;46(9):846–853. Korean. [Google Scholar]
- 18.Lee S., Bae S., Lee K.J. Changes in serotype prevalence and antimicrobial resistance among invasive Streptococcus pneumoniae isolates in Korea, 1996-2008. J Med Microbiol. 2013 Aug;62(Pt 8):1204–1210. doi: 10.1099/jmm.0.058164-0. [DOI] [PubMed] [Google Scholar]
- 19.Song J.H., Dagan R., Klugman K.P. The relationship between pneumococcal serotypes and antibiotic resistance. Vaccine. 2012 Apr;30(17):2728–2737. doi: 10.1016/j.vaccine.2012.01.091. [DOI] [PubMed] [Google Scholar]
- 20.Huang S.S., Hinrichsen V.L., Stevenson A.E. Continued impact of pneumococcal conjugate vaccine on carriage in young children. Pediatrics. 2009 Jul;124(1):e1–11. doi: 10.1542/peds.2008-3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spikerman J., van Gils E.J., Veenhoven R.H. Carriage of Streptococcus pneumoniae 3 years after start of vaccination program, The Netherlands. Emerg Infect Dis. 2011 Apr;17(4):584–591. doi: 10.3201/eid1704101115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weinberger D.M., Trzcinski K., Lu Y.J. Pneumococcal capsular polysaccharide structure predicts serotype prevalence. PLoS Pathog. 2009 Jun;5(6):e1000476. doi: 10.1371/journal.ppat.1000476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanage W.P., Bishop C.K., Huang S.S. Carried pneumococci in Massachusetts children; the contribution of clonal expansion and serotype switching. Pediatr Infect Dis J. 2011 April;30(4):302–308. doi: 10.1097/INF.0b013e318201a154. [DOI] [PMC free article] [PubMed] [Google Scholar]


