Abstract
Objectives
To screen methanolic leaf extracts of 21 timber-yielding plants for antibacterial activity against nine species of uropathogenic bacteria isolated from clinical samples of a hospital (Enterococcus faecalis, Staphylococcus aureus, Acinetobacter baumannii, Citrobacter freundii, Enterobacter aerogenes, Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, and Pseudomonas aeruginosa).
Methods
Bacterial strains were subjected to antibiotic sensitivity tests by the Kirby–Bauer's disc diffusion method. The antibacterial potentiality of leaf extracts was monitored by the agar-well diffusion method with multidrug-resistant (MDR) strains of nine uropathogens.
Results
Two Gram-positive isolates, E. faecalis and S. aureus, were resistant to 14 of the 18 antibiotics used. Gram-negative isolates A. baumannii, C. freundii, E. aerogenes, E. coli, K. pneumoniae, P. mirabilis, and P. aeruginosa were resistant to 10, 12, 9, 11, 11, 10, and 11 antibiotics, respectively, of the 14 antibiotics used. Methanolic leaf extracts of Anogeissus acuminata had the maximum zone of inhibition size—29 mm against S. aureus and 28 mm against E. faecalis and P. aeruginosa. Cassia tora had 29 mm as the zone of inhibition size for E. faecalis, E. aerogenes, and P. aeruginosa. Based on the minimum inhibitory concentration and minimum bactericidal concentration values, the most effective 10 plants against uropathogens could be arranged in decreasing order as follows: C. tora > A. acuminata > Schleichera oleosa > Pterocarpus santalinus > Eugenia jambolana > Bridelia retusa > Mimusops elengi > Stereospermum kunthianum > Tectona grandis > Anthocephalus cadamba. The following eight plants had moderate control capacity: Artocarpus heterophyllus, Azadirachta indica, Dalbergia latifolia, Eucalyptus citriodora, Gmelina arborea, Pongamia pinnata, Pterocarpus marsupium, and Shorea robusta. E. coli, followed by A. baumannii, C. freundii, E. aerogenes, P. mirabilis, and P. aeruginosa were controlled by higher amounts/levels of leaf extracts. Phytochemicals of all plants were qualitatively estimated.
Conclusions
A majority of timber-yielding plants studied had in vitro control capacity against MDR uropathogenic bacteria.
Keywords: antibiograms, Gram-negative bacteria, Gram-positive bacteria, multidrug resistance, timber-yielding plants, uropathogens
1. Introduction
A physician empirically treats acute infection ailment before prognostic evidence, such as antibiograms of causative bacteria, are available. However, a failure in the empiric therapy, because of the unknown mismatch of the prescribed antibiotic from the attack of multidrug-resistant (MDR) bacteria, would create a clinical mis-management that results in the progress of the ailment. If the infecting MDR bacterium is a balanced parasite, such as Pseudomonas or Staphylococcus or a few others, the bacterial progeny subtly migrates to various internal organs, following which pathogenicity progresses and the patient suffers from several comorbidities that could lead to septicemia/bacteremia. Further, the pathogen may spread in the community or in hospital settings during the progress of the disease, if it goes unchecked. Thus, the proper control of MDR pathogenic bacteria by an antimicrobial stewardship program remains an obvious uphill task. Whenever an MDR bacterial infection at innards of a patient even of a younger age remains intractable, it slowly results in a terminal complicated illness. Subsequently, the patient may be transferred from the intensive care unit (ICU) to a hospice. By contrast, if the invasive MDR bacterium is a destructive parasite, such as Vibrio or Salmonella or Diplococcus, an acute life-threatening situation would occur rapidly. To treat patients in both types of situations, a combination therapy with ongoing antibiotics is often followed, which however, has its intrinsic limitations of mild to acute side effects, as exemplified by tubercle bacillus chemotherapy [1]. Consequently, the physician has a dilemma as to whether to use a higher generation antibiotic or not.
Originally inhabiting the nasal nares and other soft tissues of the body, the commensal organism Staphylococcus aureus has become resistant to the β-lactam group of antibiotics. The subsequently emerged strain—methicillin-resistant S. aureus (MRSA) has become even more MDR, with 95% resistance to antibiotics in use nowadays [2]. Those with further resistance to the most preferred antibiotic vancomycin (vancomycin-resistant S. aureus or VRSA) could now be called MDR–MRSA–VRSA strains, which are regarded as the superbugs of the health domain [2]. In addition, MDR bacterial strains originate independently everywhere as complex nexuses and occur far and wide from the place of origin, including its insidious spread in communities. In parallel, among Gram negatives (GNs), Acinetobacter baumannii, Klebsiella pneumoniae, and Pseudomonas aeruginosa have developed strains that are resistant to almost all antibiotics available today and are informally called pandrug-resistant bacteria. Most GN bacteria, with a remarkable capacity of survival and dissemination, dispel the “clean” idea of the ICU of a hospital [3]. Their MDR strains are normally intractable in any infection episode, including urinary tract infections (UTIs), suppurating wounds, or bloodstream infections, etc., thereby causing a fear of the onset of various ailments in people of all age groups. For example, a metallo-β-lactamase strain of K. pneumoniae was responsible for the death of a neonate from acute septicemia in our hospital recently [4]. Not surprisingly, among infectious diseases, UTI is the second most common ailment, next to infections of the chest/lungs, in causing mortality [5].
Further, the precipitation of numerous public health episodes is linked to one or other MDR bacterial strain at each geographical zone or country [6–8]. For example, the Gram-positive (GP) enteropathogen Clostridium difficile with unmatchable notoriety had precipitated two public health episodes in North India alone [9,10]. Similarly, in rural Odisha, MDR Vibrio cholerae precipitated several sporadic episodes of short durations in Aborigine societies in the last decade, causing mortality in men, women, and children [11]. Further, our school has pursued studies on surveillance of common pathogenic bacteria, such as P. aeruginosa [3], S. aureus [2], Escherichia coli [12], as well as the less common strains of Staphylococci and Enterococci from community and hospital sectors [13], the results of which indicated the high levels of multidrug resistance in each pathogen. In addition, studies on systematic surveillance of enteropathogens [11] and uropathogens [14,15] gave pictures of the rapid infection dynamics of two classes of pathogens recently, in addition to explaining the distress experienced by aged, critical, and immunocompromised patients.
Indeed, any or several species of the nine bacteria causing UTIs more frequently infect females (i.e., more than 50% of the population), as reported in our earlier surveillance of hospital sectors [15]. UTIs can cause cystitis, pyelonephritis, and prostatitis, leading to bacteremia/septicemia at an organ, depending on the invasive nature of the pathogen, which enters the bloodstream through the kidneys; such infections often cause terminal illness with multiple comorbidities, subsequently leading to fatality [16]. Obviously, a noncommittal attitude on the issue of diseases caused by multiple infections with MDR bacteria by the clinician, due to the lack of a suitable control agent, would be a medical infraction. Thus, these peripatetic pathogens need be controlled. Thus, complementary, supplementary or alternative therapies are also sought. Plants always remain a tangible source of nonmicrobial antimicrobials [17].
In the crusade against MDR pathogens, a herbaceous terrestrial fern (Lygodium flexuosum) was reported to have promising control effects on both MDR enteropathogens and uropathogens [18]. Being encouraged by the novelty in the use of crude phytoextracts, several higher plants were also used for monitoring the in vitro control efficacy against MDR pathogenic bacteria by our school [19–21]. Indeed, higher plant extracts with an array of phytocompounds were never overcome by any pathogenic bacteria, no matter how MDR they were [17]. Continuing this line of work, several important mostly nonedible timber-yielding plants were chosen from our subtropical forest, to evaluate their antimicrobial efficacy against MDR UTI-causing bacteria, which are documented here. In general, timber-yielding plants live long and have huge stem growth with darkened heart wood in the central zone, which is laden with an array of secondary metabolites, rendering the wood stronger and termite resistant; in addition, these metabolites are present in considerable quantities in barks and leaves as well [22]. Obviously, the characteristic feature of timber-yielding plants is their more unique secondary metabolites, capable of evading the wood-destroying pests which is superior to that of herbs or trees that do not yield quality timber [23]. Moreover, a noncommittal attitude on phytocompounds is nowadays regarded as the pejorative disposition of the natural wealth of drugs, in an era in which plant products for use in complementary and alternative medicine (CAM) are gaining in popularity, even in developed countries [24–26], with the obvious tenet of comparing their success against controls.
Previously reported bacterial uropathogens isolated from clinical samples [15] were used in this study for monitoring the in vitro antimicrobial activity with methanol extracts of 21 timber-yielding plants. We chose to extract, isolate, and characterize the pure antimicrobials only from the leaves of the chosen plants, as choosing to extract the antimicrobials from the bark/heartwood results in heavy damage to plants, especially when large amounts of these alternative sources are used for developing CAM in the future. For the control of the uropathogens, there is a logistic need to prepare a formulation of coalesced effective phytochemicals as CAM along with mainstream medicine in empiric therapy. Because these formulations are obtained eukaryotic sources, the bacterial armamentaria would be ineffective to cause any resistance against these CAMs. Obviously, the host toxicity testing of phytodrugs is an essential corollary in drug development. The great number of phytocompounds stands is a potential unending source of drugs, promising a potential business of plant-based drugs for general well-being and control of many diseases [26]. It is anticipated that the areas of pharmacognosy, pharmacology, and pharmaceutics could take up the work on isolation and characterization of pure phytocompounds of effective plants and their standardization as antimicrobials, because bacterial genomes are changing rapidly or rather dramatically to win over newer antibiotics, which create the fear of precipitation of future public health episodes.
2. Materials and methods
2.1. Collection of plants and preparation of extracts
The plants used in this study were collected from the Kandha tribe, who inhabit the hills in the Eastern range of mountains of India, in the district of Kalahandi (Odisha, India), in February 2013. A total of 70 respondents of 25 hamlets were interviewed in a forest patch and the recorded information was documented (Table 1). The snowball method of survey and sampling was used, as previously followed [27]. The collected dried leaf samples were crushed to powder form; 10 g of powder from a sample was dissolved in an aliquot of 100 mL of methanol and incubated at 4 °C for 72 hours and the suspension was filtered. The methanolic filtrate was concentrated in a rotary evaporator at 40 °C, until a sticky mass was obtained that was weighed and dissolved in an aliquot of 1.0 mL of 10% vol/vol dimethyl sulfoxide (DMSO). The mass was stored at 4 °C until further use.
Table 1.
Ethnomedicinal report of 21 timber-yielding plants.
| Serial No. | Plant name | Family | Local name | Parts used | Ethnomedicinal uses |
|---|---|---|---|---|---|
| 1 | Albizia lebbeck (L.) Benth. | Fabaceae | Sirisha | Leaf/bark | Used to treat cough, boil, stomach problems. Bark is used to treat inflammations. |
| 2 | Alstonia scholaris L.R.Br | Apocynaceae | Chhatiana | Bark | Bark is used for malaria, diarrhea, snake bite, urinary tract, and skin problems. |
| 3 | Anogeissus acuminata (Roxb. ex DC.) Wall. ex Guill. & Perr | Combretaceae | Phasi | Leaf/bark | Its leaf has wound-healing activity, and is used in inflammation, and skin diseases. Its bark is used to treat diabetes. |
| 4 | Anthocephalus cadamba (Roxb.) Miq. | Rubiaceae | Kadamba | Leaf/bark | Its bark is used for urinary infections. Leaf is used to cure diarrhea, fever, inflammation, cough, vomiting, and wounds. |
| 5a | Artocarpus heterophyllus Lam. | Moraceae | Panasa | Leaf | The leaves are useful in fever, boils, wounds, skin diseases. The leaf ash, with corn and coconut shells, is used alone or mixed with coconut oil to heal ulcers. |
| 6 | Azadirachta indica L. Adelb. | Meliaceae | Nimb | Leaf | Used as an antiseptic as it has antibacterial and antiviral action (chicken pox). It is used for the treatment of acne. |
| 7a | Bridelia retusa (L.) Spreng. | Euphorbiaceae | Kasi | Bark | The bark is used against urinary tract problems. |
| 8 | Cassia tora L. | Leguminosae | Chakunda | Leaf, seed | The leaves and seeds are useful in treating leprosy, ringworm, constipation, cough, bronchitis, heart problems. |
| 9 | Dalbergia latifolia Roxb. | Fabaceae | Sisu | Bark | Bark is used for diarrhea, leprosy, and worms. |
| 10 | Eucalyptus citriodora Hook. | Myrtaceae | Nilagiri | Leaf | Leaf is used to cure fever, cold, wounds, skin ailments, and diabetes. |
| 11a | Eugenia jambolana Lam. | Myrtaceae | Jamu | Seed | Seed is used for treatment of diabetes. It is also used for ulcers. |
| 12 | Gmelina arborea Roxb. | Verbenaceae | Gambhari | Root | Root is used for burning sensations, fever, piles, and urinary discharges. |
| 13 | Melia azedarach L. | Meliaceae | Mahanimb | Leaf | Fresh leaf extract is used to cure burns, inflammation of the gum, pyrexia. |
| 14a | Mimusops elengi L. | Sapotaceae | Baula | Leaf/bark | Its bark and leaf extracts are used for urinary tract infections, diarrhea, wound, headache, dental problems, and constipation. |
| 15 | Pongamia pinnata L. | Leguminosae | Karanja | Leaf/root | Leaf juice aids in treatment of leprosy, gonorrhea, diarrhea, coughs, and cold. Root is used as a toothbrush and for killing worms. |
| 16 | Pterocarpus marsupium Roxb. | Fabaceae | Piasala | Bark | Paste of the bark of the plant with the barks of Mangifera indica, Shorea robusta, and Spondias pinnata is used to treat loose motion. |
| 17 | Pterocarpus santalinus Linn.f. | Fabaceae | Rakta-chandan | Leaf/bark | Used as an antiseptic, wound-healing agent, and in antiacne treatment. |
| 18 | Schleichera oleosa (Lour.) Oken | Sapindaceae | Kusuma | Seed | Seed oil is used for massage in rheumatism. |
| 19 | Shorea robusta Roth. | Dipterocarpaceae | Sala | Leaf | Used for wound healing and diarrhea; leaf powder with honey will help in improving blood purification. |
| 20 | Stereospermum kunthianum Cham. | Bignoniaceae | Padala | Leaf/bark | Leaf is used to treat sexual diseases and leprosy. Stem bark is used to cure loose motion, bronchitis, cough, fever, and arthritis. |
| 21 | Tectona grandis L. | Lamiaceae | Saguan | Bark | Used as an antiseptic, wound-healing agent, and in antiacne treatment. |
Artocarpus heterophyllus, Bridelia retusa, Eugenia jambolana, and Mimusops elengi yield edible fruits.
2.2. Isolation, identification of bacterial strains, and antibiotic sensitivity test
Two GP bacteria, S. aureus and Enterococcus faecalis, and seven GN bacteria, A. baumannii, Citrobacter freundii, Enterobacter aerogenes, E. coli, K. pneumoniae, Proteus mirabilis, and P. aeruginosa, were used in the study. All these bacteria were directly collected from clinical samples of Sum Hospital, Bhubaneswar (Odisha, India) using an appropriate medium, specific for the respective bacterium [15]. All bacterial strains were subjected to antibiotic sensitivity tests by the Kirby–Bauer's disc diffusion method, using a 4-mm thick Mueller–Hinton (MH) agar (HiMedia, Mumbai, India) medium, following the standard antibiotic susceptibility test chart of the Clinical Laboratory Standard Institute guidelines, as described previously [3,15].
2.3. Antibacterial test of plant extracts
The agar-well diffusion method was used for monitoring the antibacterial potentiality of plant extracts with gentamicin 30 mg/mL as the standard, and one strain from each bacterial species showing resistance to a maximum number of antibiotics was used for analysis [18,20]. Antibacterial activities were evaluated by measuring the diameter values of zones of inhibition; the monitoring experiment of each solvent extract was conducted three times and results of the third repetition are presented. It was confirmed that 10% DMSO had no inhibitory effect on any bacterium.
2.4. Determinations of minimum inhibitory concentration and minimum bactericidal concentration of plant extracts
Minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of the active plant extracts were determined. Original stock solutions of leaf extracts were prepared with methanol, using 100 mg plant extract/mL of 10% DMSO solution, and distilled with water. Each stock solution was diluted to obtain final concentrations of 0, 1.562, 3.125, 6.25, 12.5, 25, 50, and 100 mg/mL with the DMSO solution. A separate experiment was conducted for each plant extract. An aliquot of 80 μL of each dilution of a plant extract was released into a well on a 96-well (12 × 8) microtiter plate, along with an aliquot of 100-μL MH broth, an aliquot of 20-μL bacterial inocula (109 colony forming units/mL), and a 5-μL aliquot of 0.5% 2,3,5-triphenyltetrazolium chloride (TTC). After pouring all the aforementioned ingredients into a well, the microplate was incubated at 37 °C for 18 hours. The development of pink coloration due to TTC indicated bacterial growth, whereas the absence of the color indicated inhibition of bacterial growth. The first well of the microplate was the control without any plant extract. The MIC value was noted at the well where no color appeared. Further, bacteria from each well of the microplate were subcultured on a nutrient agar plate; the level of dilution, where no bacterial growth on the nutrient agar plate was observed, was noted as the MBC value [20].
2.5. Qualitative phytochemical analyses
The following tests were performed for the plant species used: reducing sugars, anthraquinones, saponins, flavonoids, steroids/terpenes, tannins, alkaloids, resins, and glycosides, as detailed previously [18,19,21].
3. Results
The ethnomedicinal information on 21 timber-yielding plants is documented along with details of modalities of crude extracts used as medicine for many ailments by local ethnic Aborigine groups (Table 1). It is discernible from the recorded information that most plants are in use for cough, diarrhea, diabetes, bronchitis, fever/ pyrexia, killing worms, constipation, heart problems; thus, the majority of plant parts are edible as medicines. In addition, the fruits of Artocarpus heterophyllus, Bridelia retusa, Eugenia jambolana, and Mimusops elengi are also edible.
The antibiotic profile of each pathogenic bacterium was determined using specified antibiotic discs (Table 2). The GP isolates, E. faecalis and S. aureus, were resistant to 14 of the 18 antibiotics used. Among the seven GN isolates, A. baumannii, C. freundii, E. aerogenes, E. coli, K. pneumoniae, P. mirabilis, and P. aeruginosa were resistant to 10, 12, 9, 11, 11, 10, and 11 antibiotics, respectively, of the total 14 antibiotics used. The antibiotic susceptibility results of the isolated bacteria are provided in Table 2. Thus, all isolated bacterial strains were MDR.
Table 2.
Antibiotic susceptibility results of the isolated bacteria.
| Bacterium | Susceptibility to prescribed antibiotics |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aminoglycoside |
β-Lactams |
Cephalosporin |
Fluoroquinolones |
Glycopeptides |
Lincosamides |
Sulfonamides |
Stand alones |
|||||||||||
| Ac | Ge | Ak | Am | Ox | Pt | Ce | Cf | Of | Le | Nx | Gt | Tei | Va | Cd | Cot | Ch | Lz | |
| Enterococcus faecalis | R | R | R | R | R | R | R | R | MS | R | MS | R | R | R | S | R | MS | R |
| Staphylococcus aureus | R | R | R | R | R | R | R | R | R | R | R | R | MS | MS | MS | R | R | S |
| Acinetobacter baumannii | R | R | R | MS | ND | R | R | R | R | R | MS | S | ND | ND | ND | R | R | S |
| Citrobacter freundii | R | R | R | R | ND | R | R | R | R | R | R | MS | ND | ND | ND | R | R | S |
| Enterobacter aerogenes | R | R | R | R | ND | R | R | R | MS | R | R | MS | ND | ND | ND | S | MS | S |
| Escherichia coli | R | R | R | R | ND | S | S | R | R | R | R | R | ND | ND | ND | R | R | S |
| Klebsiella pneumoniae | R | R | R | S | ND | R | R | R | R | R | R | S | ND | ND | ND | R | R | S |
| Proteus mirabilis | R | R | R | S | ND | R | R | R | S | R | S | MS | ND | ND | ND | R | R | R |
| Pseudomonas aeruginosa | R | R | MS | R | ND | R | R | R | R | R | R | MS | ND | ND | ND | R | R | S |
Antibiotics used: μg/disc.
Ac = amikacin 30; Ak = amoxiclav 30; Am = ampicillin 10; Cd = clindamycin 2; Ce = ceftriaxone 30; Cf = cefpodoxime 10; Ch = chloramphenicol 30; Cot = co-trimoxazole 25; Ge = gentamicin 10; Gt = gatifloxacin 5; Le = levofloxacin 5; Lz = linezolid 30; MS = moderately sensitive; ND = not done; Nx = norfloxacin 10; Of = ofloxacin 5; Ox = oxacillin 1; Pt = piperacillin/tazobactam 100/10; R = resistant; S = sensitive; Tei = teicoplanin 5; Va = vancomycin 30.
Methanolic extracts of 21 plants were tested against nine MDR bacterial species for antibacterial properties by the agar-well diffusion method and the values of zone of inhibition were recorded (Table 3). The methanolic extracts of Anogeissus acuminata had a zone of inhibition size of 29 mm against S. aureus and 28 mm against both E. faecalis and P. aeruginosa (Figure 1). Thus, the most effective 12 plants at least causing a zone of inhibition 25–29 mm (for both GP and GN MDR strains used) were A. acuminata, Anthocephalus cadamba, Azadirachta indica, B. retusa, Cassia tora, Eucalyptus citriodora, E. jambolana, Pterocarpus santalinus, Schleichera oleosa, Shorea robusta, Stereospermum kunthianum, and Tectona grandis. The unique 10 plants (from 21 plants) that controlled all the pathogens were A. acuminata, A. cadamba, B. retusa, C. tora, E. jambolana, M. elengi, P. santalinus, S. oleosa, S. kunthianum, and T. grandis, and some of these were without the highest value of 29 mm as the zone of inhibition size. Moderate control capacity was exhibited by the following eight plants (from 21 plants): A. heterophyllus, A. indica, Dalbergia latifolia, E. citriodora, Gmelina arborea, Pongamia pinnata, Pterocarpus marsupium, and S. robusta. The remaining three plants (from 21 plants), Albizia lebbeck, Alstonia scholaris, and Melia azedarach, had the least control over all these MDR bacteria. Detailed data regarding the zones of inhibition by the 21 methanolic plant extracts were recorded (Table 3).
Table 3.
Screening of antibacterial activity of selected timber-yielding plant by the agar-well diffusion method.
| Bacteria | Zone of inhibition by plant (numbers 1–21) extracts (mm) |
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | |
| Enterococcus faecalis | 20 | 12 | 28 | 26 | 19 | 23 | 26 | 29 | 19 | 25 | 23 | 22 | 19 | 22 | 18 | 22 | 26 | 25 | 20 | 27 | 24 |
| Staphylococcus aureus | 19 | 11 | 29 | 26 | 21 | 26 | 27 | 27 | 23 | 26 | 24 | 24 | 23 | 21 | 20 | 18 | 25 | 26 | 21 | 25 | 26 |
| Acinetobacter baumannii | 12 | 11 | 23 | 24 | 22 | 26 | 26 | 26 | 18 | 26 | 23 | 19 | 19 | 19 | 12 | 22 | 25 | 19 | 16 | 24 | 26 |
| Citrobacter freundii | — | 13 | 26 | 24 | 22 | 27 | 23 | 27 | 17 | 25 | 21 | 19 | 12 | 13 | 12 | 19 | 21 | 21 | 17 | 22 | 24 |
| Enterobacter aerogenes | 13 | — | 27 | 25 | 19 | 26 | 28 | 29 | 19 | 28 | 26 | — | — | 19 | 18 | 19 | 22 | 24 | — | 22 | 19 |
| Escherichia coli | — | — | 22 | 19 | — | 17 | 21 | 24 | 19 | 21 | 22 | 19 | — | 19 | — | 19 | 26 | 28 | 12 | 21 | 23 |
| Klebsiella pneumoniae | 12 | 12 | 25 | 20 | 21 | 18 | 22 | 26 | — | — | 26 | 20 | — | 16 | — | 13 | 27 | 26 | 18 | 19 | 22 |
| Proteus mirabilis | 11 | 12 | 25 | 24 | — | — | 21 | 28 | 22 | 23 | 27 | — | 18 | 16 | 19 | — | 24 | 23 | 19 | 21 | 20 |
| Pseudomonas aeruginosa | 13 | 11 | 28 | 22 | 23 | 19 | 25 | 29 | 21 | 26 | 23 | 15 | 15 | 20 | 19 | 17 | 26 | 26 | 25 | 20 | 25 |
Numbers 1–21 are serial numbers of plants given in Table 1; values are measurements of zone of inhibition due to methanol extracts. The “—” sign denotes no activity.
Figure 1.
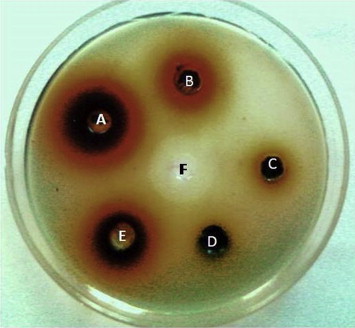
A lawn of Staphylococcus aureus in agar cups with phytoextracts. A = Anogeissus acuminata; B = Schleichera oleosa; C = Bridelia retusa; D = Alstonia scholaris; E = Eugenia jambolana; F = gentamicin 30 mg/mL.
The MDR strains of E. faecalis, S. aureus, A. baumannii, and P. aeruginosa were controlled by extracts of all plants. C. freundii was not controlled by only A. lebbeck; E. aerogenes was not controlled by A. scholaris, G. arborea, M. azedarach, and S. robusta; E. coli was not controlled by A. lebbeck, A. scholaris, A. heterophyllus, M. azedarach, and P. pinnata; K. pneumoniae was not controlled by D. latifolia, E. citriodora, M. azedarach, P. pinnata; and P. mirabilis was not controlled by A. heterophyllus, A. indica, G. arborea, and P. marsupium.
The MIC and MBC values of methanolic extracts of timber-yielding plants were evaluated. A. acuminata had 0.67 mg/mL as the lowest MIC value and 1.51 mg/mL as the lowest MBC value against E. faecalis and S. aureus, but had the highest MIC value of 9.63 mg/mL and the highest MBC value of 21.67 mg/mL for E. coli. A lower MIC/MBC value signifies that a minimum amount (lower level) of plant extract was used, whereas a higher value signifies using a larger amount of plant extract for the control of any bacterium. Based on MIC and MBC values, effective plants could be arranged in the following order (decreasing): C. tora > A. acuminata > S. oleosa > P. santalinus > E. jambolana > B. retusa > M. elengi > S. kunthianum > T. grandis > A. cadamba. E. coli, followed by A. baumannii, C. freundii, E. aerogenes, P. mirabilis, and P. aeruginosa were controlled by higher amounts/levels of leaf extracts, as evident from the MIC and MBC values. The MIC and MBC values of all bacteria are presented in Tables 4–6.
Table 4.
MIC and MBC values of selected timber-yielding plants.
| Bacteria | MIC and MBC values by plant (numbers 1–7) extracts (mg/mL) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
2 |
3 |
4 |
5 |
6 |
7 |
||||||||
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| Enterococcus faecalis | 9.63 | 21.67 | — | — | 0.67 | 1.51 | 3.41 | 4.27 | 9.63 | 21.67 | 9.63 | 21.67 | 1.51 | 3.41 |
| Staphylococcus aureus | 9.63 | 21.67 | — | — | 0.67 | 1.51 | 1.51 | 3.41 | 9.63 | 21.67 | 4.27 | 9.63 | 0.67 | 1.51 |
| Acinetobacter baumannii | — | — | — | — | 4.27 | 9.63 | 4.27 | 9.63 | 9.63 | 21.67 | 4.27 | 9.63 | 3.41 | 4.27 |
| Citrobacter freundii | — | — | — | — | 3.41 | 4.27 | 4.27 | 9.63 | 9.63 | 21.67 | 3.41 | 4.27 | 9.63 | 21.67 |
| Enterobacter aerogenes | — | — | — | — | 1.51 | 3.41 | 4.27 | 9.63 | 9.63 | 21.67 | 3.41 | 4.27 | 1.51 | 3.41 |
| Escherichia coli | — | — | — | — | 9.63 | 21.67 | 9.63 | 21.67 | — | — | 9.63 | 21.67 | 9.63 | 21.67 |
| Klebsiella pneumoniae | — | — | — | — | 4.27 | 9.63 | 9.63 | 21.67 | 9.63 | 21.67 | 9.63 | 21.67 | 9.63 | 21.67 |
| Proteus mirabilis | — | — | — | — | 4.27 | 9.63 | 4.27 | 9.63 | — | — | — | — | 9.63 | 21.67 |
| Pseudomonas aeruginosa | — | — | — | — | 1.51 | 3.41 | 9.63 | 21.67 | 9.63 | 21.67 | 9.63 | 21.67 | 4.27 | 9.63 |
Numbers 1–7 are serial numbers of plants given in Table 1; values are measurements of MIC and MBC due to methanolic extracts. The “—” sign denotes no activity. MIC = minimum inhibitory concentration; MBC = minimum bactericidal concentration.
Table 5.
MIC and MBC values of selected timber-yielding plants.
| Bacteria | MIC and MBC values by plant (numbers 8–14) extracts (mg/mL) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 8 |
9 |
10 |
11 |
12 |
13 |
14 |
||||||||
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| Enterococcus faecalis | 0.67 | 1.51 | 9.63 | 21.67 | 1.51 | 3.41 | 3.41 | 4.27 | 9.63 | 21.67 | 9.63 | 21.67 | 4.27 | 9.63 |
| Staphylococcus aureus | 1.51 | 3.41 | 3.41 | 4.27 | 1.51 | 3.41 | 3.41 | 4.27 | 3.41 | 4.27 | 3.41 | 21.67 | 4.27 | 9.63 |
| Acinetobacter baumannii | 1.51 | 3.41 | 9.63 | 21.67 | 3.41 | 4.27 | 4.27 | 9.63 | 9.63 | 21.67 | 9.63 | 21.67 | 9.63 | 21.67 |
| Citrobacter freundii | 3.41 | 4.27 | 9.63 | 21.67 | 3.41 | 4.27 | 9.63 | 21.67 | 9.63 | 21.67 | — | — | — | — |
| Enterobacter aerogenes | 1.51 | 3.41 | 9.63 | 21.67 | 0.67 | 1.51 | 1.51 | 3.41 | — | — | — | — | 9.63 | 21.67 |
| Escherichia coli | 4.27 | 9.63 | 9.63 | 21.67 | 9.63 | 21.67 | 9.63 | 21.67 | 9.63 | 21.67 | — | — | 9.63 | 21.67 |
| Klebsiella pneumoniae | 3.41 | 4.27 | — | — | — | — | 1.51 | 3.41 | 9.63 | 21.67 | — | — | 9.63 | 21.67 |
| Proteus mirabilis | 3.41 | 4.27 | 4.27 | 9.63 | 4.27 | 9.63 | 1.51 | 3.41 | — | — | 9.63 | 21.67 | 9.63 | 21.67 |
| Pseudomonas aeruginosa | 3.41 | 4.27 | 4.27 | 9.63 | 3.41 | 4.27 | 4.27 | 9.63 | 9.63 | 21.67 | 9.63 | 21.67 | 9.63 | 21.67 |
Numbers 8–14 are serial numbers of plants given in Table 1; values are measurements of MIC and MBC due to methanolic extracts. The “—” sign denotes no activity. MIC = minimum inhibitory concentration; MBC = minimum bactericidal concentration.
Table 6.
MIC and MBC values of selected timber-yielding plants.
| Bacteria | MIC and MBC values by plant (numbers 15–21) extracts (mg/mL) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 15 |
16 |
17 |
18 |
19 |
20 |
21 |
||||||||
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| Enterococcus faecalis | 9.63 | 21.67 | 4.27 | 9.63 | 1.51 | 3.41 | 1.51 | 3.41 | 9.63 | 21.67 | 0.67 | 1.51 | 1.51 | 3.41 |
| Staphylococcus aureus | 4.27 | 9.63 | 4.27 | 9.63 | 1.51 | 3.41 | 0.67 | 1.51 | 4.27 | 9.63 | 0.67 | 1.51 | 0.67 | 1.51 |
| Acinetobacter baumannii | — | — | 9.63 | 21.67 | 3.41 | 4.27 | 4.27 | 9.63 | 9.63 | 21.67 | 3.41 | 4.27 | 1.51 | 3.41 |
| Citrobacter freundii | — | — | 9.63 | 21.67 | 9.63 | 21.67 | 3.41 | 4.27 | 9.63 | 21.67 | 4.27 | 9.63 | 4.27 | 9.63 |
| Enterobacter aerogenes | 9.63 | 21.67 | 9.63 | 21.67 | 9.63 | 21.67 | 9.63 | 21.67 | — | — | 4.27 | 9.63 | 9.63 | 21.67 |
| Escherichia coli | — | — | 9.63 | 21.67 | 3.41 | 4.27 | 0.67 | 1.51 | — | — | 4.27 | 9.63 | 9.63 | 21.67 |
| Klebsiella pneumoniae | — | — | — | — | 3.41 | 4.27 | 0.67 | 1.51 | 9.63 | 21.67 | 9.63 | 21.67 | 9.63 | 21.67 |
| Proteus mirabilis | 9.63 | 21.67 | — | — | 4.27 | 9.63 | 3.41 | 4.27 | 9.63 | 21.67 | 4.27 | 9.63 | 9.63 | 21.67 |
| Pseudomonas aeruginosa | 9.63 | 21.67 | 9.63 | 21.67 | 3.41 | 4.27 | 1.51 | 3.41 | 3.41 | 4.27 | 9.63 | 21.67 | 3.41 | 4.27 |
Numbers 15–21 are serial numbers of plants given in Table 1; values are measurements of MIC and MBC due to methanolic extracts. The “—” sign denotes no activity. MIC = minimum inhibitory concentration; MBC = minimum bactericidal concentration.
Qualitative phytochemical analysis was performed for these plants. A. acuminata, B. retusa, C. tora, E. citriodora, E. jambolana, P. santalinus, S. kunthianum, S. oleosa contained the many phytochemicals, including alkaloids, flavonoids, carbohydrates, terpenoids, steroids, tannins, resins, saponins, and anthraquinones, which explains the recorded significant antibacterial activities. Plants of less control capacity, namely, A. lebbeck, A. scholaris, and M. azedarach, as well as the moderately effective ones, D. latifolia, P. pinnata, and P. marsupium, contained limited phytochemicals. The result of phytochemical analysis of all these plants was recorded (Table 7).
Table 7.
Qualitative phytochemical analysis of methanolic extracts of timber-yielding plants.
| Serial No. | Plants | Alkaloids | Resins | Glycosides | Terpenoids | Carbohydrates | Saponins | Tannins | Flavonoids | Steroids | Anthraquinones |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Albizia lebbeck | — | + | + | — | + | — | + | — | + | — |
| 2 | Alstonia scholaris | + | + | — | — | + | — | — | — | + | + |
| 3 | Anogeissus acuminata | + | — | + | + | + | + | + | + | + | + |
| 4 | Anthocephalus cadamba | + | + | + | — | + | + | + | + | + | — |
| 5 | Artocarpus heterophyllus | — | + | — | + | + | + | + | + | + | + |
| 6 | Azadirachta indica | — | + | + | + | + | + | — | — | + | — |
| 7 | Bridelia retusa | — | + | + | — | + | + | + | + | + | + |
| 8 | Cassia tora | — | + | + | — | + | — | — | — | + | — |
| 9 | Dalbergia latifolia | — | + | — | + | + | — | + | — | + | — |
| 10 | Eucalyptus citriodora | — | — | — | + | — | + | + | + | — | + |
| 11 | Eugenia jambolana | + | + | + | — | + | + | + | + | + | + |
| 12 | Gmelina arborea | — | — | + | — | + | + | + | — | + | — |
| 13 | Melia azedarach | + | + | + | — | + | + | + | — | + | — |
| 14 | Mimusops elengi | + | + | + | + | + | + | + | — | + | + |
| 15 | Pongamia pinnata | + | + | — | — | + | — | — | — | + | — |
| 16 | Pterocarpus marsupium | + | + | — | — | — | + | — | + | — | + |
| 17 | Pterocarpus santalinus | — | + | + | + | + | + | + | — | + | + |
| 18 | Schleichera oleosa | + | + | — | — | + | + | + | + | — | + |
| 19 | Shorea robusta | — | + | + | + | + | + | — | — | + | — |
| 20 | Stereospermum kunthianum | + | — | — | — | + | + | + | + | — | — |
| 21 | Tectona grandis | — | — | + | + | + | + | + | — | + | — |
The “+” sign denotes presence, and the “—“ sign denotes absence of the compound in a plant.
4. Discussion
This work demonstrated that methanolic extracts of 10 plants, C. tora, A. acuminata, S. oleosa, P. santalinus, E. jambolana, B. retusa, M. elengi, S. kunthianum, T. grandis, and A. cadamba in the order of effectivity as stated, were the most effective against the nine isolated MDR uropathogens.
The MDR bacteria could be considered as the return of an enemy with extra strength (i.e., with multiple antibiotic resistance), after being partially injured by an antibiotic that would eliminate all sensitive strains; however, one or more drug-resistant cell(s) survived. Further, defenses produced by the host body are sometimes counteracted by the drug resistance of invading pathogens, as successful parasites live to reproduce, and the pathogenesis is the result. Moreover, many obvious factors trigger their emergence: first, a latest generation antibiotic for the control of an infection is empirically used; and the antibiotic used empirically may be changed after the antibiotic sensitivity report of the sample arrives; second, sometime the history of antibiotics used previously by the patient during is unknown or not taken in account; third, irregularity in the intake of antibiotics by the patient in the treatment period; fourth, intake of medicines without a prescription by patients, seen in some countries (e.g., Japan); fifth, based on symptoms of upper respiratory tract infections, a physician might prescribes antibiotics without confirming the causative agent, which may turn out to be a viral infection; and last, the infrequent application of combination therapy with two or more antibiotics at a relatively lower dose of each, to avoid the nontarget host toxicity. These issues individually may appear less prominent or trivial, but their cumulative effects can cause independent emergences of MDR pathogens everywhere [3].
Several timber-yielding plants reported here were used for several ailments by ethnic groups and their medicinal effects were studied by various groups from other parts of India. For example, P. pinnata was used traditionally by ethnic people for the treatment of bronchitis, whooping cough, rheumatic joint pain, and to quench dipsia in diabetics [28]. The watery extract of P. marsupium is known for the treatment of diabetes due to the presence of pterostilbene [29]. Stereospermum personatum had been reported to have antibacterial, antifungal, and hypoglycemic activity against p338 lymphocytic leukemia [30]. Ethyl ether and alcoholic leaf extracts of A. lebbeck had successfully controlled standard strains of S. aureus and E. coli that infected Wistar rats without any acute toxicity up to levels of 200–400 mg/kg of the extract [31]. Ethanolic extracts of A. lebbeck pods are known to possess antiprotozoal, anticancer, and hypoglycemic properties. Species of Acacia and Albizia controlled drug-sensitive strains of E. coli, Klebsiella sp., and P. aeruginosa; however, the extracts from these plants could not control the GP Bacillus subtilis [32]. The ocular pathogenic bacterium Corynebacterium macginleyi was controlled by methanolic extracts from several trees [33]. Methanolic extracts of Terminalia catappa, Terminalia chebula, Rosa indica, A. lebbeck, and Butea monosperma had been reported to have the maximum antibacterial activity; among the 40 plants studied, 90% plants had antibacterial activity [33]. The antibacterial activity of M. azedarach extracts in solvents such as methanol, ethanol, petroleum ether, and water against standard strains of Bacillus cereus, S. aureus, P. aeruginosa, E. coli, and many fungi had been recorded previously [34]. Five organic solvents and water extracts of A. scholaris had been shown to have the control over three GP organisms, including S. aureus and five GN organisms, including E. coli, P. aeruginosa, and P. mirabilis [35]. The MIC values were determined with lipophilic and aqueous leaf extracts against four GP bacteria, namely, B. cereus, Streptococcus mutans, Lactobacillus acidophilus, and S. aureus, two GN bacteria, namely, E. coli and K. pneumoniae and two yeasts, namely, Candida albicans and Cryptococcus neoformans. Moderate to high antimicrobial activity of Sida cuneifolia against several uropathogens were recorded with MIC values ranging from 0.4 to 6.0 mg/mL [36].
The most popular herbal medicines used by Aborigines do not have institutional/scientific/clinical/pharmaceutical validation and host toxicity testing for the direct use as drugs in mainstream medicine. Many of the concoctions of crude phytodrugs among the cited plants are used as preventives for many diseases, but their curative roles are mostly not established. By contrast, several reports of plants have documented host toxicity in humans [37]. The antioxidant and hepatoprotective activity of the timber-yielding plant Soymida febrifuga was recorded previously [38]. The methanolic and aqueous extracts of this plant had been shown to have considerable phenolic contents. Furthermore, crude extracts from S. kunthianum were shown to exert hypotensive and/or hypoglycemic activities on rats [39].
Located at the eastern range of mountains in the state, with a 40% Aborigine population, the Kalahandi district is richer in vegetation in comparison with other hilly patches of the state. These Aborigine people pass their ethnomedicinal knowledge orally in a secretive manner down through the generations. Furthermore, India with tropical and subtropical forest areas is a home to approximately 550 million plants that serve as the source of traditional medicine (TM), derived from the clandestine ethnic information and Ayurveda [40]. In TM, plants are used in several ways and they are popular for several concoctions and specific modalities for many crucial and common diseases, which have facilitated modern drug development and the use of finished herbal medicines as different formulations, in the herbal medicine trade [26]. TM as a field remains as the major accessible and affordable method of treatment for marginalized urban people and Aborigines. Indeed, TM has been in use in several developed Western countries, as an important mode of CAM [41,42]. According to a previous report, 48% of the population in Australia, 70% in Canada, 42% in the United States, 38% in Belgium, and 75% in France use CAM [43]. Such crude phytodrugs are available in market shelves everywhere and many people like to depend on them, for their well-being or health-boosting effects. For example, Artemisia annua has been in use in China for malaria originally and its active ingredient (i.e., artemisinin) is now used along with mefloquine in mainstream medicine as CAM for malaria [44], so as to overcome the problem of drug resistance.
Large proportions of plant drugs are popular for their performance standards, and are in general use. Here two examples are the use of the decoction of internodes of the antidiabetic Tinospora cordifolia in India and Panax ginseng (ginseng) root, which is best known to lower blood sugar and cholesterol levels, protect against stress, enhance strength, and promote relaxation, from the Eastern world with a remarkable popularity in the United States. Many health-boosting formulations, concocted with many herbal products, have been gaining popularity in Brazilian, American, and Chinese societies [45], and that is also occurring in equal or lesser dimensions in many other countries. Several of these products have some planned clinical evaluations of treatment modality, therapy, and in the marketing of patented formulations [46]. Moreover, several pharmaceutical companies are busy worldwide in preparing and marketing several combinations of natural products as medicines for different purposes, parallel to the scientific exactitude of drug preparations and uses, systematically followed in mainstream medicine [45]. Planned commercial medicinal plant growing establishments are slowly developing in several states in India, apart from government-approved collections of plants of interest from forests [27].
It is possible that when other organic solvents could be used alternately with these 21 plants, a more varied picture of the level of control might be obtained. However, methanol is routinely used as a solvent in the protocol of bioassay-guided fractionation of crude phytoextracts, because of its capability to extract polar, moderately polar, and nonpolar compounds from crude extracts holistically—a characteristic which is not present in most other organic solvents, and is a basic requirement for the isolation of active phytocompounds of specific interest.
In conclusion, the data embodied here demonstrate that MDR UTI bacteria could be well-controlled in vitro, using crude methanolic leaf extracts of the 18 timber-yielding plants described. A total of 10 plants were selected in this study and were shown to have effective control against bacteria, and these could be further studied for isolation and purification of pure phytocompounds and their host toxicity. Thus, those could further be incorporated into CAM as empiric therapies, to prevent the untoward devastation which may be caused by MDR bacteria.
Acknowledgments
This work is a part of PhD thesis of M.P.M. in Biotechnology (Siksha ‘O’ Anusandhan University). This work, as well as M.P.M. as a JRF, is supported by the major research project on “Development of standardized herbal extracts against urinary tract bacterial inflection. (Grant No. BT/PR8214/PBD/17/863/2013), from the Department of Biotechnology, Government of India, New Delhi (India). We are thankful to IMS and Sum Hospital for extended facilities and Sri G. Kar, Managing Member, IMS and Sum Hospital for encouragements.
Footnotes
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
References
- 1.Dubey D., Rath S., Sahu M.C. Status of multidrug resistance in tubercle bacillus and phytochemicals for the control. J Public Health. 2013 Feb;21(1):115–119. [Google Scholar]
- 2.Dubey D., Rath S., Sahu M.C. Surveillance of infection status of drug resistant Staphylococcus aureus in an Indian teaching hospital. Asian Pac J Trop Dis. 2013 Apr;3(2):133–142. doi: 10.1016/S2221-1691(13)60040-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sahu M.C., Rath S., Dubey D. Multidrug resistance of Pseudomonas aeruginosa as known from surveillance of nosocomial and community infections in an Indian teaching hospital. J Public Health. 2012 Jan;20(4):413–423. [Google Scholar]
- 4.Dubey D., Sarangi R., Debata N.K., Padhy R.N. Detection of metallo-β-lactamase producing Klebsiella pneumoniae in a neonatal septicemia. J Acute Dis. 2013 Mar;2(1):82–84. [Google Scholar]
- 5.American Thoracic Society; Infectious Diseases Society of America Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005 Feb;171(4):388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- 6.Quintero B., Araque M., van der Gaast-de Jongh C. Epidemiology of Streptococcus pneumoniae and Staphylococcus aureus colonization in healthy Venezuelan children. Eur J Clin Microbiol Infect Dis. 2011 Jan;30(1):7–19. doi: 10.1007/s10096-010-1044-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sengstock D.M., Thyagarajan R., Apalara J. Multidrug-resistant Acinetobacter baumannii: an emerging pathogen among older adults in community hospitals and nursing homes. Clin Infect Dis. 2010 May;50(12):1611–1616. doi: 10.1086/652759. [DOI] [PubMed] [Google Scholar]
- 8.Yu F., Fan S., Fan X. Analysis of characteristics of paratyphoid A in 157 Chinese inpatients between 1998 and 2009. Eur J Clin Microbiol Infect Dis. 2011 Jan;30(1):71–75. doi: 10.1007/s10096-010-1055-3. [DOI] [PubMed] [Google Scholar]
- 9.Rani P., Khullar N. Antimicrobial evaluation of some medicinal plants for their anti-enteric potential against multidrug resistant Salmonella typhi. Phytother Res. 2004 Aug;18(8):670–673. doi: 10.1002/ptr.1522. [DOI] [PubMed] [Google Scholar]
- 10.Vaishnavi C., Singh M. Preliminary investigation of environmental prevalence of Clostridium difficile affecting inpatients in a north Indian hospital. Indian J Med Microbiol. 2012 Jan–Mar;30(1):89–92. doi: 10.4103/0255-0857.93052. [DOI] [PubMed] [Google Scholar]
- 11.Rath S., Padhy R.N. Surveillance of multidrug resistance of 10 enteropathogens in a teaching hospital and in vitro efficacy of 25 ethnomedicinal plants used by an Indian aborigine. Asian Pac J Trop Dis. 2012 Oct;2(1):S336–S346. [Google Scholar]
- 12.Rath S., Dubey D., Sahu M.C. Surveillance of ESBL producing multidrug resistant Escherichia coli in a teaching hospital in India. Asian Pac J Trop Dis. 2014 Apr;4(2):140–149. [Google Scholar]
- 13.Dubey D., Padhy R.N. Surveillance of multidrug resistance of two Gram-positive pathogenic bacteria in a teaching hospital and in vitro efficacy of 30 ethnomedicinal plants used by an aborigine of India. Asian Pac J Trop Dis. 2012 Aug;2(4):273–281. [Google Scholar]
- 14.Rath S., Dubey D., Sahu M.C. Surveillance of multidrug resistance of 6 uropathogens in a teaching hospital and in vitro control by 25 ethnomedicinal plants used by an aborigine of India. Asian Pac J Trop Biomed. 2012 Aug;2(2):S818–S829. [Google Scholar]
- 15.Mishra M.P., Debata N.K., Padhy R.N. Surveillance of multidrug resistant uropathogenic bacteria in hospitalized patients—an Indian study. Asian Pac J Trop Biomed. 2013 Apr;3(4):315–324. doi: 10.1016/S2221-1691(13)60071-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giamarellos-Bourboulis E.J., Papadimitriou E., Galanakis N. Multidrug resistance to antimicrobials as a predominant factor influencing patient survival. Int J Antimicrob Agents. 2006 Jun;27(6):476–481. doi: 10.1016/j.ijantimicag.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 17.Sahu M.C., Dubey D., Rath S. In vitro efficacy of 30 ethnomedicinal plants used by an Indian aborigine against 23 multidrug resistant pathogenic bacteria. J Herb Med. 2013 in press. [Google Scholar]
- 18.Nayak N., Rath S.N., Mishra M.P. Antibacterial activity of the terrestrial fern Lygodium flexuosum (L.) Sw. against multidrug resistant enteric- and uro-pathogenic bacteria. J Acute Dis. 2013 Dec;2(4):270–276. [Google Scholar]
- 19.Dubey D., Padhy R.N. Antibacterial activity of Lantana camara L. against multidrug resistant pathogens from ICU patients of a teaching hospital. J Herb Med. 2013 Jun;3(2):65–75. [Google Scholar]
- 20.Rath S., Padhy R.N. Monitoring in vitro antibacterial efficacy of Terminalia alata Heyne ex. Roth, against MDR enteropathogenic bacteria isolated from clinical samples. J Acute Med. 2013 Sep;3(3):93–102. [Google Scholar]
- 21.Sahu M.C., Padhy R.N. In vitro efficacy of Butea monosperma Lam. against 12 clinically isolated multidrug resistant bacteria. Asian Pac J Trop Dis. 2013 Jun;3(3):217–226. [Google Scholar]
- 22.Nuopponen M.H., Birch G.M., Sykes R.J. Estimation of wood density and chemical composition by means of diffuse reflectance mid-infrared Fourier transform (DRIFT-MIR) spectroscopy. J Agric Food Chem. 2006 Jan;54(1):34–40. doi: 10.1021/jf051066m. [DOI] [PubMed] [Google Scholar]
- 23.Ali M. vol. 1. CBS Publishers and Distributors; New Delhi: 2011. p. 802. (Pharmacognosy (pharmacognosy and phytochemistry)). [Google Scholar]
- 24.WHO . World Health Organization; Geneva: 2005. Global atlas of traditional, complementary and alternative medicine. [Google Scholar]
- 25.MacLennan E., Pendry B.A. The evolution of herbal medicine as an unorthodox branch of British medicine: the role of English legislation from antiquity to 1914. J Herb Med. 2011 Sep;1(1):2–14. [Google Scholar]
- 26.Dubey D., Rath S., Sahu M.C. Antimicrobials of plant origin against multi-drug resistant bacteria including the TB bacterium and economics of plant-drugs—Introspection. Indian J Tradit Knowl. 2012 Apr;11(2):225–233. [Google Scholar]
- 27.Mallik B.K., Panda T., Padhy R.N. Traditional herbal practices by the ethnic people of Kalahandi district of Odisha, India. Asian Pac J Trop Biomed. 2012;2:S988–S994. [Google Scholar]
- 28.Kirtikar K.R., Basu B.D. 2nd ed. vol. 1. International Book Distributors; Dehradun: 1995. (Indian medicinal plants). [Google Scholar]
- 29.Subbarao A.V. Andhra Pradesh Academy of Sciences; Visakhapatnam: 1975. The chemistry of natural products—three decades in Andhra Pradesh, India; p. 214. [Google Scholar]
- 30.Bakuni D.S., Dhar M.L., Dhar M.M. Screening of Indian plants for biological activity: III. Indian J Exp Biol. 1971 Jan;9(1):91–102. [PubMed] [Google Scholar]
- 31.Chulet R., Jhajharia M., Pradhan P., Sharma S. Analgesic and antipyretic activity of Albizzia lebbeck. Pharmacology online. 2010;3:737–749. [Google Scholar]
- 32.Mahmood A., Mahmood A., Qureshi R.A. Antimicrobial activities of three species of family mimosaceae. Pak J Pharm Sci. 2012 Jan;25(1):203–206. [PubMed] [Google Scholar]
- 33.Koday N.K., Rangaiah G.S., Bobbarala V. Bactericidal activities of different medicinal plants extracts against ocular pathogen viz Corynebacterium macginleyi. Drug Invent Today. 2010 Jan;2(1):5. [Google Scholar]
- 34.Sen A., Batra A. Evaluation of antimicrobial activity of different solvent extracts of medicinal plant: Melia azedarach L. Int J Curr Pharm Res. 2012 Mar;4(2):67–73. [Google Scholar]
- 35.James J., Veettil A.K.T., Kumar P. Phytochemical investigation and antibacterial activity of the fruits of Alstonia scholaris. Int J Phytopharmacol. 2012;3(1):74–77. [Google Scholar]
- 36.van Vuuren S.F., Viljoen A.M. The in vitro antimicrobial activity of toothbrush sticks used in Ethiopia. S Afr J Bot. 2006 Nov;72(4):646–648. [Google Scholar]
- 37.Bussmann R.W., Malca G., Glenn A. Toxicity of medicinal plants used in traditional medicine in Northern Peru. J Ethnopharmacol. 2011 Sep;137(1):121–140. doi: 10.1016/j.jep.2011.04.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reddy B.S., Reddy R.K., Reddy B.P. Potential in vitro antioxidant and protective effects of Soymida febrifuga on ethanol induced oxidative damage in HepG2 cells. Food Chem Toxicol. 2008 Nov;46(11):3429–3442. doi: 10.1016/j.fct.2008.08.034. [DOI] [PubMed] [Google Scholar]
- 39.Kodjo K.M., Contesse V., Do Rego J.L. In vitro effects of crude extracts of Parkia biglobosa (Mimosaceae), Stereospermum kunthianum (Bignoniaceae) and Biophytum petersianum (Oxalidaceae) on corticosteroid secretion in rat. J Steroid Biochem Mol Biol. 2006 Aug;100(4–5):202–208. doi: 10.1016/j.jsbmb.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 40.Desilva T., Bahorun T., Sahu M., Le Mai H. Daya Publishing House; New Delhi: 2009. Traditional and alternative medicines, research and policy perspectives. [Google Scholar]
- 41.Lee J., Bielory L. Complementary and alternative interventions in atopic dermatitis. Immunol Allergy Clin North Am. 2010 Aug;30(3):411–424. doi: 10.1016/j.iac.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 42.Pineda M.J., Singh D.K. What is integrative oncology and can it help my patients? Obstet Gynecol Clin North Am. 2012 Jun;39(2):285–312. doi: 10.1016/j.ogc.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 43.WHO . World Health Organization; Geneva: 2011. World Health Organization traditional medicine strategy 2011 [Internet] pp. 1–14.http://apps.who.int/medicinedocs/documents Available from, [accessed 15.10.13] [Google Scholar]
- 44.Bloland P.B., Ettling M., Meek S. Combination therapy for malaria in Africa: hype or hope? Bull World Health Organ. 2000;78(12):1378–1388. [PMC free article] [PubMed] [Google Scholar]
- 45.Lam K.S. New aspects of natural products in drug discovery. Trends Microbiol. 2007 Jun;15(6):279–289. doi: 10.1016/j.tim.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 46.Gautam R., Saklani A., Jachak S.M. Indian medicinal plants as a source of antimycobacterial agents. J Ethnopharmacol. 2007 Mar;110(2):200–234. doi: 10.1016/j.jep.2006.12.031. [DOI] [PubMed] [Google Scholar]


