Abstract
Background
A barrier to statin therapy is myopathy associated with elevated systemic drug exposure. Our objective was to examine the association between clinical and pharmacogenetic variables and statin concentrations in patients.
Methods and Results
In total, 299 patients taking atorvastatin or rosuvastatin were prospectively recruited at an outpatient referral center. The contribution of clinical variables and transporter gene polymorphisms to statin concentration was assessed using multiple linear regression. We observed 45-fold variation in statin concentration among patients taking the same dose. After adjustment for gender, age, body mass index, ethnicity, dose, and time from last dose, SLCO1B1 c.521T>C (p < 0.001) and ABCG2 c.421C>A (p < 0.01) were important to rosuvastatin concentration (adjusted R2 = 0.56 for the final model). Atorvastatin concentration was associated with SLCO1B1 c.388A>G (p < 0.01) and c.521T>C (p < 0.05), and 4β-hydroxycholesterol, a CYP3A activity marker (adjusted R2 = 0.47). A second cohort of 579 patients from primary and specialty care databases were retrospectively genotyped. In this cohort, genotypes associated with statin concentration were not differently distributed among dosing groups, implying providers had not yet optimized each patient's risk-benefit ratio. Nearly 50% of patients in routine practice taking the highest doses were predicted to have statin concentrations greater than the 90th percentile.
Conclusions
Interindividual variability in statin exposure in patients is associated with uptake and efflux transporter polymorphisms. An algorithm incorporating genomic and clinical variables to avoid high atorvastatin and rosuvastatin levels is described; further study will determine if this approach reduces incidence of statin-myopathy.
Keywords: statin therapy, pharmacogenetics, pharmacokinetics, drug transporters
Introduction
The 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors, or statins, are commonly prescribed and proven to be effective in reducing cardiovascular event risk by lowering plasma concentration of low-density lipoprotein cholesterol (LDL-C)1. A recent report indicates that 25% of Americans over age 45 takes a statin, and it is predicted the number will grow as the populations of Westernized countries continue to age and maintain unhealthy lifestyles2-3. A significant barrier to statin therapy is skeletal muscle toxicity associated with elevated systemic drug exposure4. Up to 10% of statin-treated individuals will experience muscle pain or weakness, and in rare cases, life-threatening rhabdomyolysis occurs5-7. Currently, we do not fully understand the drug exposure necessary for optimal statin therapy, making it difficult to predict an individual's dose requirement to maximize LDL-C lowering, while minimizing the risk for muscle injury.
Remarkably few data are readily available regarding interpatient variability in plasma statin level, especially considering the number of large multicenter clinical trials of cardiovascular outcomes with statins performed to date. Until recently, drug metabolizing enzymes such as cytochrome P450 enzymes (CYPs) were considered to be the major determinants of statin disposition. However, studies from our laboratory and others suggest that statins, particularly the pharmacologically active acid forms of statins, are highly dependent on drug transporter proteins for their disposition and efficacy8-9.
Our objective was to characterize the relationship between drug transporter polymorphisms and interindividual variability in plasma statin concentration, which, in the clinical situation, is not well understood. We measured 4β-hydroxycholesterol concentration as a marker of CYP3A metabolic activity in vivo, and lathosterol concentration to assess the efficacy of statin-mediated inhibition of endogenous cholesterol synthesis, as well as its relationship to statin concentration. Taken together, these data describe the relative contribution of transport genetics and metabolism to interindividual variability in statin pharmacokinetics and response.
Methods
Study population
We prospectively invited adult outpatients at London Health Sciences Center (LHSC, London, Canada) taking atorvastatin or rosuvastatin daily to participate. Patients were excluded if they were taking atorvastatin or rosuvastatin in an alternate day dosing regimen, or if they had not taken their last atorvastatin or rosuvastatin dose within 24 hours of their clinic visit and blood draw. All patients had been taking atorvastatin or rosuvastatin at the same dose for at least six weeks prior to participation, with the exception of one patient who had been switched from 40 mg to 80 mg one week prior to blood sampling to achieve better cholesterol lowering. The study was conducted between August 2009 and May 2011. A detailed medical history was obtained, and the time of the last oral statin dose in relation to plasma level measurement was recorded. Ethnicity was self-reported. LDL-C response was defined by attainment of LDL-C target values according to the 2009 Canadian Lipid Guidelines1 and by the clinical judgment of the treating physician. All subjects provided written informed consent. The study protocol was approved by the Research Ethics Board of the University of Western Ontario (London, Canada).
Sample collection
A single venous 8 mL blood sample was drawn into EDTA-containing tubes, and placed immediately on ice. Samples were centrifuged 2,000 × g for 10 minutes; plasma was collected and stored at −80 °C until further analysis. Genomic DNA was isolated from blood samples using the Gentra Puregene extraction kit (Qiagen, Alameda, CA, USA).
Retrospective statin dosing analysis
We retrospectively examined genotype, clinical variables, and statin dose in a separate cohort comprised of outpatients from LHSC and Vanderbilt University Medical Center (BioVU, Nashville, Tennessee).
BioVU
BioVU at Vanderbilt University is a large collection of DNA samples linked to a comprehensive EMR10. BioVU is a dynamic clinical practice-based cohort, nested within an even larger database containing a secure, de-identified copy of the entire EMR utilized by Vanderbilt University Medical Center. Referred to as the Synthetic Derivative (SD), this practice-derived database incorporates clinical information from multiple sources, including diagnostic and procedural codes, as well as provider progress notes, hospital admissions, discharge summaries, clinical laboratory data, and medication data.
Determination of plasma statin concentration
All chemical and deuterated standards were obtained from Toronto Research Chemicals (North York, Canada). Plasma aliquots of 100 μL were precipitated in 300 μL acetonitrile containing internal standard d5-atorvastatin or d6-rosuvastatin, and centrifuged at 14,000 rpm for 20 minutes at 4 °C. The supernatant was diluted 1:1 in 0.05% formic acid. Analytes were separated using mobile phases 0.05% formic acid in water and 0.05% formic acid in acetonitrile, starting at a ratio of 70:30, with a gradient to ratio of 10:90. Concentrations of rosuvastatin and atorvastatin were measured by liquid chromatography-mass spectrometry (LCMS) instrumentation and transitions as previously described 11.
Determination of lathosterol and 4β-hydroxycholesterol concentrations
Sterol concentrations were measured according to published methods for LCMS 12-13. Lathosterol, 4β-hydroxycholesterol, and 4β-hydroxycholesterol-d7 were obtained from Avanti Polar Lipids (Alabaster, Alabama), and lathosterol-d4 was obtained from CDN Isotopes (Pointe-Claire, Canada). All other chemicals were obtained from Sigma-Aldrich (St. Louis, MO). Standard curves ranging from 0-50 μg/mL lathosterol were prepared in 1% fatty acid-free bovine serum albumin in phosphate-buffered saline. Aliquots of 50 μL of plasma or standard curve were saponified in 1 mL of 1M KOH in ethanol for 1 hour at 37 °C. The samples were extracted twice, in 750 μL of hexanes each time. After evaporation at 80 °C to dryness, a mixture of the following derivatization reagents was added to each sample: 15 mg 2-methyl-6-nitrobenzoic anhydride, 4.5 mg 4-dimethylaminopyridine, 12 mg picolinic acid, 225 μL pyridine, and 30 μL triethylamine. Samples were incubated with the derivatization reagents at 80 °C for 1 hour, extracted in 1 mL of hexanes, and evaporated at 80 °C to dryness. Samples were reconstituted in 20 μL 0.9% NaCl and 80 μL water; 20 μL of sample was injected on an Eclipse Plus C18 column (1.8 μm pore size; 2.1 × 100 mm; Agilent Technologies, Mississauga, Canada) attached to an Agilent 1290 Infinity ultra high pressure liquid chromatography system (Agilent Technologies) coupled with a TSQ Quantum triple-quadrupole mass spectrometer (Thermo Scientific). Analytes were separated and eluted with a gradient from 80% to 98% methanol:acetonitrile (1:1). The transition used for lathosterol was m/z 555.3 to 513.8. The transition used for 4β-hydroxycholesterol was m/z 635.4 to 146.5. Interday variability was less than 25% for lathosterol and less than 30% for 4β-hydroxycholesterol, at relevant concentrations.
Determination of total cholesterol
Total cholesterol was measured by the enzymatic colorimetric method, using the Cholesterol E kit from Wako (Richmond, VA). Samples were measured in triplicate using the microplate procedure, according to manufacturer's directions.
Genotyping
We identified single nucleotide polymorphisms (SNPs) with a minor allelic frequency greater than 10% in genes encoding drug transporters for which statins are known substrates, and genotyped SNPs that have been demonstrated to have a functional effect on one or more substrates in vivo. Genotype was determined by TaqMan assay (Applied Biosystems, Foster City, CA) for uptake transporter polymorphisms SLCO1B1 c.388A>G (rs2306283); SLCO1B1 c.521T>C (rs4149056); SLCO1B3 c.699G>A (rs7311358); SLCO2B1 c.935G>A (rs12422149), and efflux transporter polymorphisms ABCB1 c.3435C>T (rs1045642); ABCC2 c.1249G>A (rs2273697); and ABCG2 c.421C>A (rs2231142). For the atorvastatin group, polymorphisms in the drug metabolizing enzymes CYP3A4 (rs35599367) and CYP3A5 (rs776746) were also assessed. Patients in the rosuvastatin group were also genotyped for CYP2C9 *2 (rs1799853) and CYP2C9 *3 (rs1057910). The SNPs assessed in the present study are summarized in Supplementary Table 1. Missing genotypes ranged from 0% to 0.7%, depending on the polymorphism. We repeated genotyping of 10% of the samples; 100% of replicated genotypes were concordant. Haplotypes were determined using the haplo.stats library in R using an indirect design matrix and linear regression was conducted by comparing alternative haplotypes to the reference haplotype SLCO1B1 c.388A-c521T.
Hardy-Weinberg equilibrium was tested using the Chi-square method of the genetics package of R. All genotypes tested were in Hardy-Weinberg equilibrium with the exception of ABCB1 c.3435C>T (p = 0.010) and SLCO1B1 c.521T>C (p = 0.041). Genotypes associated with statin concentration were not differently distributed between Caucasians and other ethnicities in our patient cohort, by Chi-square test.
Statistical analysis
Statistical analysis was performed using the statistical software R14 and GraphPad Prism 5 (La Jolla, CA). Differences in statin concentration with respect to each dose group were assessed by Tukey's multiple comparisons tests. We defined the explainable variability as the variability attributed to characteristics other than dose and time from last dose. We calculated this by totaling the sum of squares for each final model and assessing the proportion contributed by the genetic variables.
For log-transformed rosuvastatin concentration, the effect sizes detectable with a power of 0.80 or higher are 0.141, 0.145 and 0.187 for SLCO1B1 c.521T>C, SLCO1B1 c.388A>G, and ABCG2 c.421C>A, respectively. For log-transformed atorvastatin concentration, the effect sizes detectable with a power of 0.80 or higher are 0.274, 0.223 and 0.324 for SLCO1B1 c.521T>C, SLCO1B1 c.388A>G, and ABCG2 c.421C>A, respectively.
Multiple linear regression analysis
Statin concentration was log-transformed to adjust for right-skew. Only those patients with blood sampling times after the tmax of the statin were included (1.5 hours and 4.0 hours for atorvastatin and rosuvastatin, respectively11). Different genetic models–dominant, co-dominant, recessive, and additive models–were considered for each transporter polymorphism and the model that best described the fit with log-transformed statin concentration or lathosterol concentration was chosen. Each polymorphism was assessed for association with log statin concentration with a cut-off p-value of 0.20 for further inclusion in the multiple linear regression model. SLCO1B1 c.521T>C and c.388A>G, and ABCG2 c.421C>A, were included in the model as additive models. In the additive model, homozygous wildtype genotypes were coded as 0, heterozygous genotypes were coded as 1, and homozygous variant genotypes were coded as 2. Regression analysis was performed by a step-wise search. All models were adjusted for the demographic and dosing variables age, gender, body mass index (BMI), ethnicity, statin dose, and hours from last dose. Of these variables, age, dose, and time from last dose were statistically significant. Next, the number of concomitant medications or presence of the specific medications ezetimibe, niacin, and fibrate were assessed for their contribution to the model and retained if p < 0.20. 4β-hydroxycholesterol values and transporter and drug metabolizing enzyme genotypes were similarly introduced into the model. In the final model, only those variables with p < 0.05 were retained, with the exception of the demographic and dosing variables listed above.
Dosing algorithm
Maximum doses predicted to result in statin concentrations less than the 90th percentile were calculated based on our linear regression models. The 90th percentile was determined by adjusting the atorvastatin or rosuvastatin concentrations measured in our population to the concentration predicted at the average time of the blood sampling across the population (11.5 hours for atorvastatin and 12.9 hours for rosuvastatin). For covariates that were not significant in the model, we substituted the average population value: predicted concentration was calculated for a hypothetical Caucasian patient of our average population height and weight (body mass index of 29.0 kg/m2 for atorvastatin and 30.1 kg/m2 rosuvastatin), and for atorvastatin, average 4β-hydroxycholesterol concentration (22 ng/ml). Age was rounded to the nearest 5-year interval.
Results
Patient characteristics
The patients’ baseline characteristics are summarized in Table 1. In total, 299 patients were enrolled in the study, with 134 taking atorvastatin and 165 patients on rosuvastatin therapy. Of these patients, 3 taking rosuvastatin and 6 taking atorvastatin had undetectable statin levels, and were excluded from further analysis. Two patients taking rosuvastatin were excluded from lathosterol-related analysis, due to inability to measure lathosterol or total cholesterol. A list of the concomitant medications observed in our population is provided in Supplemental Table 2.
Table 1.
Population characteristics of prospective cohort of atorvastatin- and rosuvastatin-treated patients
| Atorvastatin | Rosuvastatin | |
|---|---|---|
| Number of patients | 134 | 165 |
| Male | 83 (61.9%) | 115 (69.7%) |
| Age at enrolment (years) | 59.5 (24-86) | 59 (18-80) |
| Caucasian | 113 (83.7%) | 143 (86.7%) |
| Body mass index (kg/m2) | 29.0 (5.2) | 30.1 (6.8) |
| Number of concomitant medications | 4.9 (3.1) | 4.7 (3.1) |
| Statin dose (mg/kg) | 0.45 (0.31) | 0.22 (0.15) |
| 5 mg | -- | 24 (14.5%) |
| 10 mg | 22 (16.4%) | 52 (31.5%) |
| 20 mg | 30 (22.4%) | 47 (28.4%) |
| 40 mg | 58 (43.2%) | 38 (23.0%) |
| 80 mg | 23 (17.1%) | -- |
| Other | 1 (0.7%) | 4 (2.4%) |
| Hours from last dose | 12.9 (5.0) | 11.5 (5.3) |
| 4β-hydroxycholesterol (ng/mL) | 22.0 (14.1) | 18.7 (11.9) |
| Lathosterol (μg/mL) | 3.9 (2.1) | 3.4 (2.2) |
| Minor allelic frequency | ||
| ABCG2 c.421A | 25/268 (9.3%) | 36/330 (10.9%) |
| SLCO1B1 c.388G | 119/268 (44.4%) | 145/330 (43.9%) |
| SLCO1B1 c.521C | 30/268 (11.2%) | 61/330 (18.5%) |
Data are presented as number (%), mean (S.D.), or median (range).
Rosuvastatin concentration
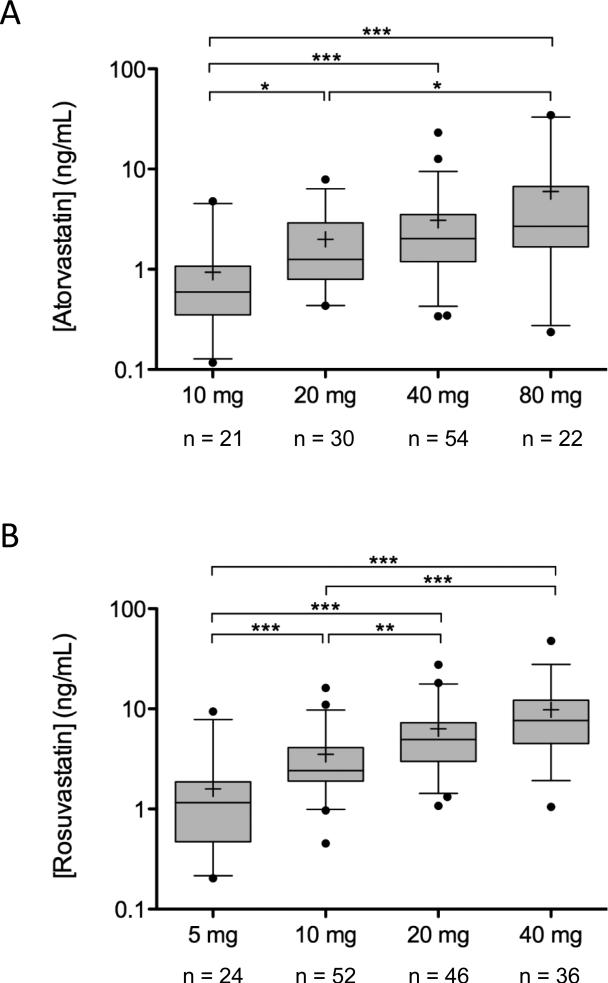
We observed up to 45-fold variability in plasma rosuvastatin concentration among individuals on the same dose (Figure 1A). In patients taking 5, 10, 20, or 40 mg rosuvastatin daily, mean plasma concentration of rosuvastatin was 1.6 ng/mL (SD 1.8), 3.5 ng/mL (2.9), 6.3 ng/mL (5.3), and 9.8 ng/mL (8.6), respectively. There was a significant difference in plasma rosuvastatin concentration between those taking 5 mg vs. 10 mg, 20 mg or 40 mg (p < 0.001 for all) and for those taking 10 mg vs. 20 mg or 40 mg (p < 0.01 and p < 0.001, respectively; Figure 1A).
Figure 1.
Prospective analysis of atorvastatin (A) plasma concentrations in patients taking 10, 20, 40, or 80 mg daily, and rosuvastatin (B) plasma concentrations in patients taking 5, 10, 20, or 40 mg daily. Rosuvastatin concentrations were collected within 0 to 24 hours of the last oral dose. Levels are presented on a log-scale axis as box and whisker plots with the whiskers depicting 5th and 95th percentile; means are depicted by +. Significance of the mean difference between two groups is depicted by *p < 0.05; ** p < 0.01; *** p < 0.001.
In order to assess the association of clinical and pharmacogenetic variables with the rosuvastatin levels observed, we performed multiple linear regression analysis. Only those patients with blood drawn at least four hours after their last oral dose were included in this analysis (n = 130). Multiple linear regression analysis indicated that plasma rosuvastatin concentration was higher in individuals with the reduced function hepatic uptake transporter allele SLCO1B1 c.521C (p < 0.0001), and the reduced function efflux transporter polymorphism ABCG2 c.421A (p < 0.05). Age also contributed to plasma rosuvastatin level (p < 0.01) (Table 2). The adjusted R2 value of the model was 0.56. Similar results were obtained using SLCO1B1 haplotypes (Supplemental Table 5). Polymorphisms in transporter genes SLCO1B1 and ABCG2 contributed to 88% of the explainable variability, after adjusting for dose and time from last dose. The variables gender, ethnicity, BMI, and SLCO1B1 c.388, SLCO1B3, SLCO2B1, ABCB1, ABCC2, and CYP2C9 genotype were not significantly associated with rosuvastatin concentration.
Table 2.
Plasma statin concentration linear regression model coefficients from prospective cohort
| Variable | Effect (B) | p value | |
|---|---|---|---|
| Atorvastatin-treated patients (n = 128) | |||
| 1 | Age (yr) | 0.018 | 0.002 |
| 2 | 4β-hydroxycholesterol | −0.015 | 0.006 |
| 3 | SLCO1B1 c.521T>C | 0.339 | 0.020 |
| 4 | SLCO1B1 c.388A>G | −0.278 | 0.009 |
| Rosuvastatin-treated patients (n = 130) | |||
| 1 | Age (yr) | 0.012 | 0.005 |
| 2 | SLCO1B1 c.521T>C | 0.413 | <0.001 |
| 3 | ABCG2 c.421C>A | 0.310 | 0.020 |
Adjusted for gender, ethnicity, BMI, dose and time from last dose. Dose and time from last dose were significant in both models (p < 0.001 for atorvastatin and rosuvastatin). Coefficients for all variables for atorvastatin and rosuvastatin are described in Supplemental Tables 3 and 4, respectively.
Atorvastatin concentration
Similar to rosuvastatin, we observed 45-fold or higher variability between patients on the same daily atorvastatin dose (Figure 1B). In patients taking 10, 20, 40, or 80 mg atorvastatin daily, mean plasma concentration of atorvastatin was 0.9 ng/mL (SD 1.0), 2.0 ng/mL (1.7), 3.0 ng/mL (3.5), and 6.0 ng/mL (8.2), respectively. There was a significant difference in plasma atorvastatin concentration between those taking 20 mg, 40 mg, or 80 mg vs. 10 mg (p < 0.05, p < 0.001, and p < 0.001, respectively) and between those taking 20 mg vs 80 mg (p < 0.05; Figure 1B).
Multiple linear regression analysis indicated that plasma atorvastatin concentration was higher in individuals with the SLCO1B1 c.521C allele (p < 0.05) but lower in those patients with the SLCO1B1 c.388G allele (p < 0.01). 4β-hydroxycholesterol also contributed significantly to the variability observed (p < 0.01). In addition, age was a significant predictor of atorvastatin level (p < 0.01) (Table 2). The adjusted R2 value of the model was 0.47. SLCO1B1 haplotype-based analysis produced similar results (Supplemental Table 5). In contrast to rosuvastatin, the genetic component of the model contributed only 38% of the explainable variability observed. Metabolism, as measured by 4β-hydroxycholesterol concentration, accounted for an additional 30% of the explainable variability in atorvastatin concentration. A list of CYP3A inhibitors and inducers prescribed to patients taking atorvastatin is provided in Supplemental Table 6. Similar results were obtained when 4β-hydroxycholesterol levels were normalized by total cholesterol. The following variables were not significantly associated with atorvastatin concentration: gender, ethnicity, BMI, and SLCO1B3, SLCO2B1, ABCB1, ABCC2, ABCG2, CYP3A4 and CYP3A5 genotype.
Lathosterol concentration
The mean lathosterol concentration in patients taking atorvastatin was 3.9 μg/mL (SD 2.1) and rosuvastatin was 3.4 μg/mL (2.2). In patients taking atorvastatin, lathosterol concentration was lower in patients taking a higher dose of atorvastatin (p < 0.01), however, there was no significant association between rosuvastatin or atorvastatin concentrations and lathosterol concentration detected in this population. In both groups, lathosterol was associated with total cholesterol, and was higher in patients taking ezetimibe (Table 3). The adjusted R2 value of the model of lathosterol concentration in atorvastatin-treated patients was 0.08; for rosuvastatin-treated patients the adjusted R2 value was 0.39.
Table 3.
Lathosterol plasma concentration linear regression model coefficients from prospective cohort
| Variable | Effect (B) | p value | |
|---|---|---|---|
| Atorvastatin-treated patients (n=128) | |||
| 1 | Atorvastatin dose (mg) | −0.02 | 0.009 |
| 2 | Total cholesterol (mmol/L) | 0.23 | 0.032 |
| 3 | Ezetimibe use | 0.96 | 0.012 |
| Rosuvastatin-treated patients (n=128) | |||
| 1 | Total cholesterol (mmol/L) | 0.54 | < 0.001 |
| 2 | Ezetimibe use | 1.70 | < 0.001 |
Adjusted for gender, age, ethnicity, and BMI
LDL-C lowering response to rosuvastatin
Despite the lack of association between lathosterol level and statin level, some insight can be gained from this rare opportunity to examine lipid-lowering response in combination with plasma statin concentration. We examined rosuvastatin acid concentrations in patients taking 40 mg rosuvastatin daily, for whom no higher dose or more potent statin is available, and included only those with blood taken 9 to 24 hours post-dose, to be within the linear range of statin elimination and minimize the variability associated with the peak statin absorption. Patients who were not at target LDL-C (n = 12) had a mean plasma rosuvastatin concentration of 9.183 ng/mL (SD 1.6; 13.6 hours post dose) compared with a mean plasma concentration of 7.497 ng/mL (SD 1.8; 13.7 hours post dose) for those who were at target (n = 13); the difference between the two groups was not significant (p = 0.45). There was also a trend toward lower lathosterol level in those individuals at target compared with those not at target (3.4 μg/mL (SD 0.49) vs. 4.7 μg/mL (0.45), p = 0.065). Notably, there is a higher proportion of SLCO1B1 c.521T>C variants in the non-responders (8 of 12 patients are SLCO1B1 c.521CT heterozygotes) vs. responders (3 of 13 heterozygotes; p = 0.047, Fisher's exact test).
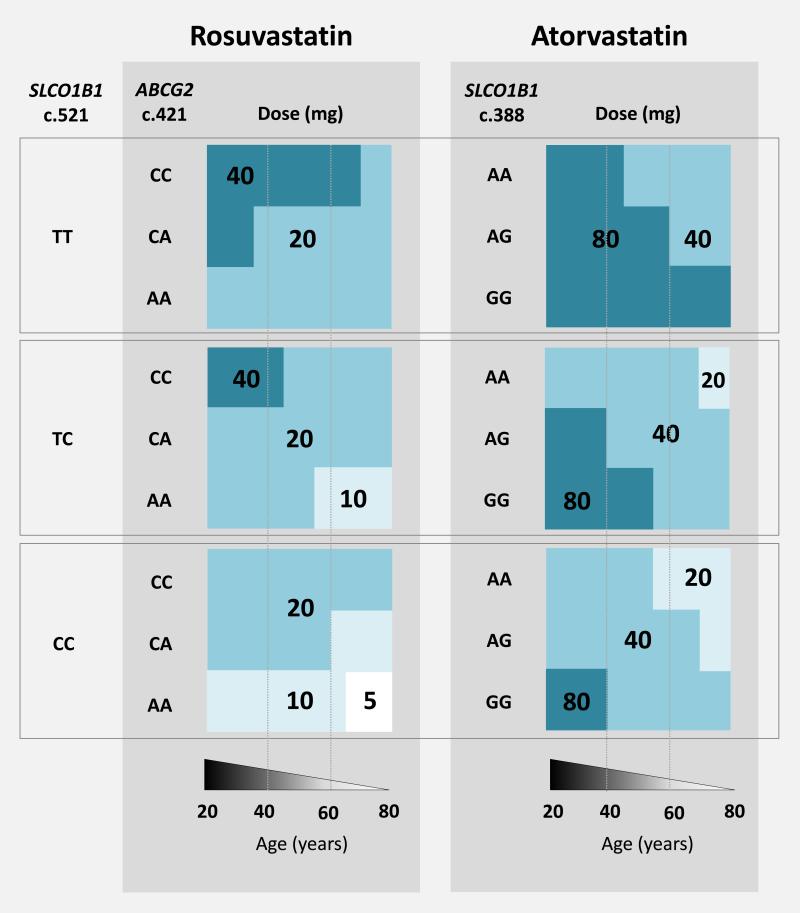
Statin dosing algorithm
In Figure 2, we summarize recommendations for maximum atorvastatin and rosuvastatin doses, based on a patient's age and transporter genotype, and the linear regression analysis described above. These doses are predicted to result in plasma concentrations that remain lower than the 90th percentile, a value chosen to reflect the fact that 10% of individuals will experience statin-related muscle complaints.
Figure 2.
Rosuvastatin and atorvastatin dosing decision support algorithm. Doses are the maximum doses that result in a predicted rosuvastatin or atorvastatin concentration that is less than the 90th percentile. In patients taking atorvastatin, dose should be lowered if the patient is taking a CYP3A4 inhibitor, including an antifungal, macrolide antibiotic, or HIV protease inhibitor. The OATP inhibitors cyclosporine and gemfibrozil have also been associated with risk for statin-induced muscle toxicity; a dose reduction should be considered for both atorvastatin and rosuvastatin if cyclosporine or gemfibrozil are also prescribed. It should be noted that this algorithm is based on data collected from a predominantly Caucasian population, and may not apply to other ethnicities, particularly Asians, who demonstrate increased sensitivity to statins.
Retrospective analysis of statin dosing
We further examined the impact of genotype and clinical covariates on statin dose, retrospectively in two clinical populations (n = 579). The first cohort contained 224 patients taking atorvastatin and 37 patients taking rosuvastatin in the context of routine clinical care at a large academic center in the U.S.; the second cohort contained 121 patients taking atorvastatin and 198 patients taking rosuvastatin treated in a lipid clinic at a large academic center in Canada. Thus, we were able to assess the potential clinical utility of our model in the context of both primary and specialty care. Population characteristics of each cohort are described in Table 4.
Table 4.
Population characteristics of retrospective atorvastatin and rosuvastatin dosing cohort
| Atorvastatin | Rosuvastatin | |
|---|---|---|
| Number of patients | 345 | 234 |
| Male | 203 (58.8%) | 141 (60.2%) |
| Age at enrolment (years) | 54 (14) | 57 (13) |
| Statin dose | ||
| 5 mg | 9 (2.6%) | 25 (10.7%) |
| 10 mg | 131 (38.0%) | 74 (31.6%) |
| 20 mg | 106 (30.7%) | 98 (41.9%) |
| 40 mg | 69 (20.0%) | 37 (15.8%) |
| 80 mg | 30 (8.7%) | 0 |
| Minor allelic frequency | ||
| SLCO1B1 c.388G | 336/690 (48.7%) | Not determined |
| SLCO1B1 c.521C | 95/690 (13.8%) | 86/468 (18.4%) |
| ABCG2 c.421A | Not determined | 50/468 (10.7%) |
Data are presented as number (%), mean (S.D.).
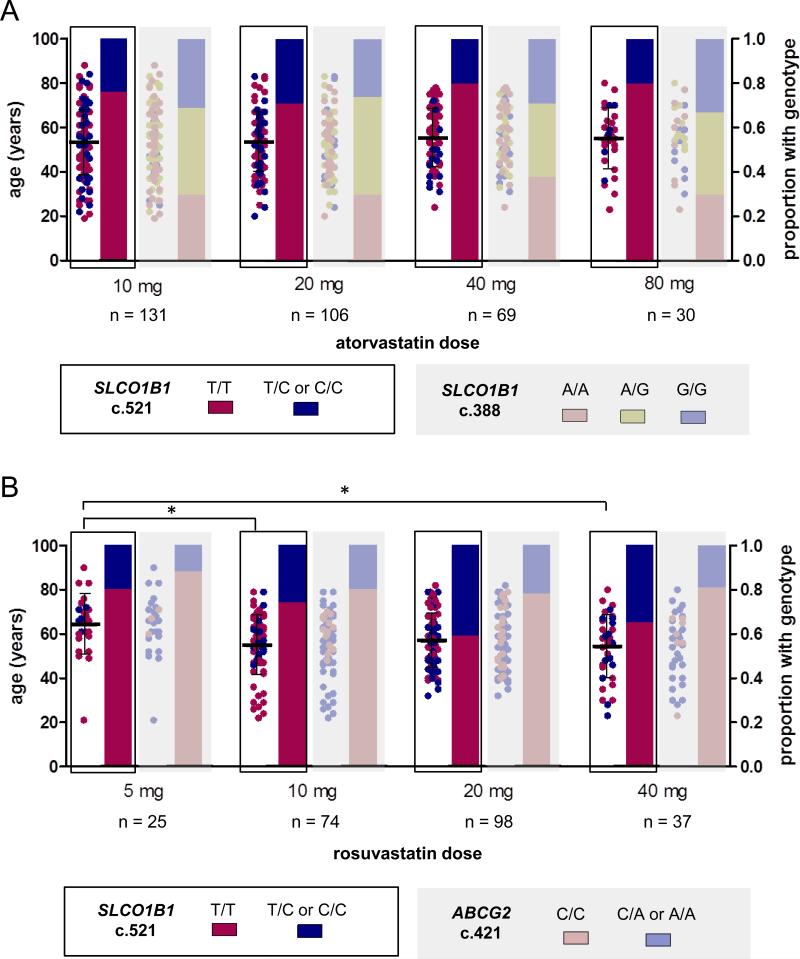
For these clinical practice-based cohorts, the relationship between genotype, age, and most recent statin dose has been summarized in Figure 3. We observed that patients taking 5 mg rosuvastatin were older than those taking 10 mg and 40 mg (p < 0.05). The average ages are 64 years (S.D. 13.7) for the group taking 5 mg daily vs 55 (13.3) and 54 (14.4) for groups taking 10 mg and 40 mg, respectively. The transporter genotypes associated with statin concentration were not differently distributed among statin dose, implying that physicians may not yet have dosed each respective patient to their optimal serum statin level. Utilizing the genotypes and ages of these subjects to determine the dose recommended by our model indicates that only those patients at the highest doses exceeded the recommended dose (Table 5).
Figure 3.
Distribution of transporter genotypes and age among atorvastatin (A) doses of 10, 20, 40, or 80 mg daily and rosuvastatin (B) doses of 5, 10, 20, or 40 mg daily in retrospective analysis of statin-treated patients. Age is depicted on the left y-axis with points colored according to transporter genotype. The proportion of patients on any given dose with a particular genotype is depicted by the bars, colored according to transporter genotype and associated with the right y-axis. * indicates the age difference between two groups is significant (64 years (S.D. 13.7) for the group taking 5 mg daily vs 55 (13.3) and 54 (14.4) for groups taking 10 mg and 40 mg, respectively p < 0.05).
Table 5.
Accuracy of dose prediction in retrospective analysis of atorvastatin and rosuvastatin dosing
| At or below dose recommended by algorithm | Exceeds dose recommended by algorithm | |
|---|---|---|
| Atorvastatin dose | ||
| 5 mg | 9 (100%) | 0 |
| 10 mg | 131 (100%) | 0 |
| 20 mg | 106 (100%) | 0 |
| 40 mg | 69 (100%) | 0 |
| 80 mg | 15 (50%) | 15 (50%) |
| Rosuvastatin dose | ||
| 5 mg | 25 (100%) | 0 |
| 10 mg | 74 (100%) | 0 |
| 20 mg | 98 (100%) | 0 |
| 40 mg | 21 (56.8%) | 16 (43.2%) |
Among 67 patients on high-dose rosuvastatin (40 mg) or atorvastatin (80 mg), nearly 50% exceeded the maximum dose recommended by our model, suggesting that many were at risk of developing intolerance. Of the 16 patients taking high-dose atorvastatin (80 mg) within our cohort derived from an EMR-linked biobank, 9 exceeded the maximum recommended dose, and only 7 of these patients were still on 80 mg atorvastatin one year later. Conversely, all (7 of 7) subjects predicted by our algorithm to tolerate 80 mg were still on high-dose atorvastatin one year later, however, this result was not statistically significant (p = 0.48, Fisher's exact test). Collectively, these observations suggest that clinicians may benefit from the use of this model, when weighing the risks and benefits of implementing a high dose prospectively.
Discussion
This study investigated the relationship between common drug transporter polymorphisms and plasma concentrations of atorvastatin and rosuvastatin in a real world population. We found a marked, 45-fold interpatient variability in observed plasma level, especially at the higher doses. In our clinical situation, where statin dose has been titrated to effect, statin transporter polymorphisms are associated with a detectable change in statin level. Indeed, nearly 90% of the explainable variability in rosuvastatin concentration can be accounted for by two reduced function transporter polymorphisms, in the uptake transporter SLCO1B1 and the efflux transporter ABCG2. In contrast, explainable variability in atorvastatin level is almost equally divided between two polymorphisms in SLCO1B1, and the activity of CYP3A as measured by 4β-hydroxycholesterol concentration. Taking our findings together, we propose a dosing algorithm for atorvastatin and rosuvastatin that, based on our data regarding the association between transporter genotype, age, and statin concentration, would minimize risk for high plasma statin exposure.
Indeed, genetic polymorphisms in transport proteins contribute to interindividual variation in exposure to a number of drugs, including the statins15-17. The SLCO1B1 gene encodes organic anion-transporting polypeptide 1B1 (OATP1B1; previously known as OATP-C or OATP2); in 2001, our group was the first to identify functionally relevant single nucleotide polymorphisms (SNPs) in this transporter18. Healthy subjects harboring certain SLCO1B1 SNPs had higher plasma concentrations of such statins as atorvastatin, rosuvastatin, simvastatin, pravastatin, and pitavastatin19-22. Importantly, a genome-wide analysis revealed an association between susceptibility to biochemical myopathy on high-dose simvastatin and a common reduced-function variant in SLCO1B1, namely c.521T>C (rs4149056)4. SLCO1B1 c.521T>C has also been associated with reduced LDL-C lowering response to rosuvastatin therapy23. The other SLCO1B1 polymorphism genotyped, c.388A>G (rs2306283), appears, in vitro, to have activity equivalent to the reference sequence18, and has been shown in some, but not all, healthy volunteer studies to be associated with a trend towards lower plasma atorvastatin level22, 24. Interestingly, the SEARCH study showed a link between this SNP and reduced risk for simvastatin-associated myopathy4. In our population, the SLCO1B1 c.521T>C variant was not in Hardy-Weinberg equilibrium; it is possible that individuals homozygous for this variant are less likely to tolerate and remain on statin therapy. The ABCB1 polymorphism c.3435 T>C was also not in equilibrium in our population, however, the reason for this result is unclear.
Polymorphisms in the ATP-binding cassette (ABC) efflux transporter ABCG2 have been associated with higher rosuvastatin concentration in healthy volunteers25 and recently, with improved lipid lowering response in Korean subjects26 and Caucasians23, 27. The effect of reduced activity ABCG2 polymorphism on rosuvastatin concentration suggests increased statin exposure is the mechanism resulting in the augmented lipid lowering response observed by other studies.
It is important to note that this study was conducted in a predominantly Caucasian population, and that caution may be warranted in extrapolating these results to other ethnicities. In particular, ethnicity-dependent differences have been observed in studies comparing statin pharmacokinetics in healthy volunteers of Asian and Caucasian ethnicity 24. Moreover, in Asian countries such as Japan, the maximum approved dose of rosuvastatin is 20 mg/day compared with 40 mg/day in North American and European countries. Since the increase in rosuvastatin exposure is not strictly related to environment 24, physicians in North America and Europe treating patients of Asian descent should be particularly aware that the maximum recommended dose of 40 mg/day may not be appropriate.
It has long been recognized that there is significant interindividual variation in CYP3A activity; however, the genetic basis for this variability has remained elusive. 4β-hydroxycholesterol is produced by CYP3A enzymes from cholesterol, and has been proposed to be a marker of CYP3A activity in vivo28-29. In our population, low 4β-hydroxycholesterol level was associated with higher atorvastatin but not rosuvastatin concentration. Rosuvastatin concentration was also not associated with CYP2C9 *2 and CYP2C9 *3 genotypes, consistent with previous studies which have indicated that rosuvastatin is predominantly eliminated unchanged30. Previous reports have associated reduced CYP3A function with higher creatine kinase levels in patients taking atorvastatin, suggesting these individuals are prone to more severe myopathy31-32. Numerous drug interaction studies have described increased risk of adverse events resulting from the concomitant use of CYP3A inhibitors and statins metabolized by CYP3A, particularly atorvastatin and simvastatin. The US Food and Drug Administration (US FDA) recommendations advocate for a reduced dose of these statins if moderate CYP3A inhibitors are prescribed, and for some potent CYP3A inhibitors, contraindicate their use entirely33.
Finally, our study identified age as a significant factor in predicting the concentrations of atorvastatin and rosuvastatin in patients. Age has been recognized as a clinical risk factor for statin-induced muscle toxicity5, 34. In early pharmacokinetic studies, age was associated with increased exposure to atorvastatin35, but not rosuvastatin36. Rosuvastatin clearance, however, is partially mediated by tubular secretion in the kidney, thus the reduced renal function associated with advanced age may account for this effect30. Older patients are also more likely to take more medications, though the number of co-medications was not a significant predictor of atorvastatin or rosuvastatin concentrations in our population.
Lathosterol is a late intermediate in cholesterol synthesis that can be used to measure the efficacy of statin-mediated HMG-CoA reductase inhibition37-38. In our population, plasma concentrations of atorvastatin and rosuvastatin did not correlate with lathosterol levels. This suggests statin concentration in the liver, not the plasma, is the most important factor in determining the inhibition of HMG-CoA reductase. Ezetimibe is a cholesterol absorption inhibitor that has been previously associated with lathosterol level39; here we observed that lathosterol level is increased in patients taking ezetimibe even when they are concurrently taking statins, which limit lathosterol synthesis by inhibiting HMG-CoA reductase.
Our analysis of statin dosing patterns indicates SLCO1B1 and ABCG2 variant carriers were distributed throughout the dosing groups (Figure 3), indicating physicians did not adjust dose based on the altered pharmacokinetics profile caused by these variants. This suggests that, based on clinical presentation alone, it is not possible for clinicians to detect those that are driven by pharmacokinetics-related mechanisms linked to polymorphisms in SLCO1B1 and ABCG2, and thus preventable by lowering the dose.
At the highest available dose, nearly 50% of patients taking atorvastatin or rosuvastatin exceeded the maximum genotype-based dose recommended by our algorithm. At lower doses, no patient in our cohort exceeded the maximum dose recommended by our algorithm, though it is important to note that rare individuals, not represented in our cohort, may in fact exceed their recommended dose even at lower doses. Taken together, genetic testing may be most useful when a patient is starting the highest dose of atorvastatin or rosuvastatin, bearing in mind that SLCO1B1 c.521 T>C variant carriers in particular are more likely to require higher doses as a result of reduced hepatic uptake.
Here we present the range of atorvastatin and rosuvastatin concentrations in a patient population, providing a framework by which to assess normal variability in statin concentration, and identify the relationship between statin exposure and common statin transporter polymorphisms. In the clinical review of rosuvastatin originally submitted to the US FDA, all patients with serious adverse events for whom drug levels were available (n = 6) had high rosuvastatin concentrations (> 50 ng/mL; http://www.accessdata.fda.gov/drugsatfda_docs/nda/2003/21-366_Crestor_Medr_P4.pdf). The FDA recently updated advice on statin risk to include memory loss and diabetes (http://www.fda.gov/ForConsumers/ConsumerUpdates/ucm293330.htm), indicating that we do not yet fully understand the consequences of statin exposure systemically. While several groups have called for transporter genetics-guided statin dosing4, 8, 26-27, 40 to our knowledge, this study is the first to propose guidelines based on interindividual differences in statin concentration. These guidelines provide a maximum starting dose in order to reduce the risk for high plasma statin concentration. Controlled, randomized trials are required to determine whether statin myopathy is reduced if statins are prescribed using this approach. In summary, this initial report of prospectively assessed plasma statin level and transporter genotypes in a patient care setting creates a framework for individualized statin selection and dosing.
Supplementary Material
Acknowledgments
The authors wish to thank Brooke Kennedy and Julie Lorenzin for help with blood sampling, and Cameron Ross, Adam McIntyre, and Matthew Ban for technical assistance.
Funding Sources: This work was supported by grants from the Canadian Institutes of Health Research (MOP-89753), the Drug Safety and Effectiveness Network (DESN-PREVENT) and Wolfe Medical Research Chair in Pharmacogenomics to Richard Kim, and Academic Medical Organization of Southwestern Ontario Alternate Funding Plan Innovation Fund to Richard Kim, George Dresser and Kathryn Myers. BioVU is supported by the National Institutes of Health (UL1RR024975). Marianne DeGorter is the recipient of a Vanier Canada Graduate Scholarship from the Canadian Institutes of Health Research.
Footnotes
Conflict of Interest Disclosures: Dr Robert Hegele serves on the advisory board and speaker's bureau of Abbott, Aegerion, Amgen, AstraZeneca, Merck, Pfizer, Roche, Sunovion, and Valeant. The other authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Genest J, McPherson R, Frohlich J, Anderson T, Campbell N, Carpentier A, et al. 2009 Canadian Cardiovascular Society/Canadian guidelines for the diagnosis and treatment of dyslipidemia and prevention of cardiovascular disease in the adult - 2009 recommendations. Can J Cardiol. 2009;25:567–579. doi: 10.1016/s0828-282x(09)70715-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mann D, Reynolds K, Smith D, Muntner P. Trends in statin use and low-density lipoprotein cholesterol levels among US adults: Impact of the 2001 national cholesterol education program guidelines. Ann Pharmacother. 2008;42:1208–1215. doi: 10.1345/aph.1L181. [DOI] [PubMed] [Google Scholar]
- 3.US Department of Health and Human Services Health, United States, 2010, with special feature on death and dying. 2011 [PubMed] [Google Scholar]
- 4.Link E, Parish S, Armitage J, Bowman L, Heath S, Matsuda F, et al. SLCO1B1 variants and statin-induced myopathy--a genomewide study. N Engl J Med. 2008;359:789–799. doi: 10.1056/NEJMoa0801936. [DOI] [PubMed] [Google Scholar]
- 5.Joy TR, Hegele RA. Narrative review: Statin-related myopathy. Ann Intern Med. 2009;150:858–868. doi: 10.7326/0003-4819-150-12-200906160-00009. [DOI] [PubMed] [Google Scholar]
- 6.Thompson PD, Clarkson PM, Rosenson RS. An assessment of statin safety by muscle experts. Am J Cardiol. 2006;97:69C–76C. doi: 10.1016/j.amjcard.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 7.Bruckert E, Hayem G, Dejager S, Yau C, Begaud B. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients--the PRIMO study. Cardiovasc Drugs Ther. 2005;19:403–414. doi: 10.1007/s10557-005-5686-z. [DOI] [PubMed] [Google Scholar]
- 8.Niemi M. Transporter pharmacogenetics and statin toxicity. Clin Pharmacol Ther. 2010;87:130–133. doi: 10.1038/clpt.2009.197. [DOI] [PubMed] [Google Scholar]
- 9.DeGorter MK, Xia CQ, Yang JJ, Kim RB. Drug transporters in drug efficacy and toxicity. Annu Rev Pharmacol Toxicol. 2012;52:249–273. doi: 10.1146/annurev-pharmtox-010611-134529. [DOI] [PubMed] [Google Scholar]
- 10.Ritchie MD, Denny JC, Crawford DC, Ramirez AH, Weiner JB, Pulley JM, et al. Robust replication of genotype-phenotype associations across multiple diseases in an electronic medical record. Am J Hum Genet. 2010;86:560–572. doi: 10.1016/j.ajhg.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeGorter MK, Urquhart BL, Gradhand U, Tirona RG, Kim RB. Disposition of atorvastatin, rosuvastatin, and simvastatin in Oatp1b2−/− mice and intraindividual variability in human subjects. J Clin Pharmacol. 2012;9:986–95. doi: 10.1177/0091270011422815. [DOI] [PubMed] [Google Scholar]
- 12.Honda A, Yamashita K, Hara T, Ikegami T, Miyazaki T, Shirai M, et al. Highly sensitive quantification of key regulatory oxysterols in biological samples by LC-ESI-MS/MS. J Lipid Res. 2009;50:350–357. doi: 10.1194/jlr.D800040-JLR200. [DOI] [PubMed] [Google Scholar]
- 13.Honda A, Yamashita K, Miyazaki H, Shirai M, Ikegami T, Xu G, et al. Highly sensitive analysis of sterol profiles in human serum by LC-ESI-MS/MS. J Lipid Res. 2008;49:2063–2073. doi: 10.1194/jlr.D800017-JLR200. [DOI] [PubMed] [Google Scholar]
- 14.R Development Core Team . R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2012. http://www.R-project.org. [Google Scholar]
- 15.Giacomini KM, Huang SM, Tweedie DJ, Benet LZ, Brouwer KL, Chu X, et al. Membrane transporters in drug development. Nat Rev Drug Discov. 2010;9:215–236. doi: 10.1038/nrd3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gradhand U, Kim RB. Pharmacogenomics of MRP transporters (ABCC1-5) and BCRP (ABCG2). Drug Metab Rev. 2008;40:317–354. doi: 10.1080/03602530801952617. [DOI] [PubMed] [Google Scholar]
- 17.Zair ZM, Eloranta JJ, Stieger B, Kullak-Ublick GA. Pharmacogenetics of OATP (SLC21/SLCO), OAT and OCT (SLC22) and PEPT (SLC15) transporters in the intestine, liver and kidney. Pharmacogenomics. 2008;9:597–624. doi: 10.2217/14622416.9.5.597. [DOI] [PubMed] [Google Scholar]
- 18.Tirona RG, Leake BF, Merino G, Kim RB. Polymorphisms in OATP-C: Identification of multiple allelic variants associated with altered transport activity among European- and African-Americans. J Biol Chem. 2001;276:35669–35675. doi: 10.1074/jbc.M103792200. [DOI] [PubMed] [Google Scholar]
- 19.Pasanen MK, Fredrikson H, Neuvonen PJ, Niemi M. Different effects of SLCO1B1 polymorphism on the pharmacokinetics of atorvastatin and rosuvastatin. Clin Pharmacol Ther. 2007;82:726–733. doi: 10.1038/sj.clpt.6100220. [DOI] [PubMed] [Google Scholar]
- 20.Pasanen MK, Neuvonen M, Neuvonen PJ, Niemi M. SLCO1B1 polymorphism markedly affects the pharmacokinetics of simvastatin acid. Pharmacogenet Genomics. 2006;16:873–879. doi: 10.1097/01.fpc.0000230416.82349.90. [DOI] [PubMed] [Google Scholar]
- 21.Ho RH, Choi L, Lee W, Mayo G, Schwarz UI, Tirona RG, et al. Effect of drug transporter genotypes on pravastatin disposition in European- and African-American participants. Pharmacogenet Genomics. 2007;17:647–656. doi: 10.1097/FPC.0b013e3280ef698f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chung JY, Cho JY, Yu KS, Kim JR, Oh DS, Jung HR, et al. Effect of OATP1B1 (SLCO1B1) variant alleles on the pharmacokinetics of pitavastatin in healthy volunteers. Clin Pharmacol Ther. 2005;78:342–350. doi: 10.1016/j.clpt.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 23.Chasman DI, Giulianini F, MacFadyen J, Barratt BJ, Nyberg F, Ridker PM. Genetic determinants of statin-induced low-density lipoprotein cholesterol reduction: The justification for the use of statins in prevention: An intervention trial evaluating rosuvastatin (JUPITER) trial. Circ Cardiovasc Genet. 2012;5:257–264. doi: 10.1161/CIRCGENETICS.111.961144. [DOI] [PubMed] [Google Scholar]
- 24.Lee E, Ryan S, Birmingham B, Zalikowski J, March R, Ambrose H, et al. Rosuvastatin pharmacokinetics and pharmacogenetics in white and Asian subjects residing in the same environment. Clin Pharmacol Ther. 2005;78:330–341. doi: 10.1016/j.clpt.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 25.Keskitalo JE, Zolk O, Fromm MF, Kurkinen KJ, Neuvonen PJ, Niemi M. ABCG2 polymorphism markedly affects the pharmacokinetics of atorvastatin and rosuvastatin. Clin Pharmacol Ther. 2009;86:197–203. doi: 10.1038/clpt.2009.79. [DOI] [PubMed] [Google Scholar]
- 26.Tomlinson B, Hu M, Lee VW, Lui SS, Chu TT, Poon EW, et al. ABCG2 polymorphism is associated with the low-density lipoprotein cholesterol response to rosuvastatin. Clin Pharmacol Ther. 2010;87:558–562. doi: 10.1038/clpt.2009.232. [DOI] [PubMed] [Google Scholar]
- 27.Bailey KM, Romaine SP, Jackson BM, Farrin AJ, Efthymiou M, Barth JH, et al. Hepatic metabolism and transporter gene variants enhance response to rosuvastatin in patients with acute myocardial infarction: The GEOSTAT-1 study. Circ Cardiovasc Genet. 2010;3:276–285. doi: 10.1161/CIRCGENETICS.109.898502. [DOI] [PubMed] [Google Scholar]
- 28.Bodin K, Bretillon L, Aden Y, Bertilsson L, Broome U, Einarsson C, et al. Antiepileptic drugs increase plasma levels of 4beta-hydroxycholesterol in humans: Evidence for involvement of cytochrome P450 3A4. J Biol Chem. 2001;276:38685–38689. doi: 10.1074/jbc.M105127200. [DOI] [PubMed] [Google Scholar]
- 29.Diczfalusy U, Nylen H, Elander P, Bertilsson L. 4beta-hydroxycholesterol, an endogenous marker of CYP3A4/5 activity in humans. Br J Clin Pharmacol. 2011;71:183–189. doi: 10.1111/j.1365-2125.2010.03773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martin PD, Warwick MJ, Dane AL, Brindley C, Short T. Absolute oral bioavailability of rosuvastatin in healthy white adult male volunteers. Clin Ther. 2003;25:2553–2563. doi: 10.1016/s0149-2918(03)80316-8. [DOI] [PubMed] [Google Scholar]
- 31.Wilke RA, Moore JH, Burmester JK. Relative impact of CYP3A genotype and concomitant medication on the severity of atorvastatin-induced muscle damage. Pharmacogenet Genomics. 2005;15:415–421. doi: 10.1097/01213011-200506000-00007. [DOI] [PubMed] [Google Scholar]
- 32.Wilke RA, Reif DM, Moore JH. Combinatorial pharmacogenetics. Nat Rev Drug Discov. 2005;4:911–918. doi: 10.1038/nrd1874. [DOI] [PubMed] [Google Scholar]
- 33.Egan A, Colman E. Weighing the benefits of high-dose simvastatin against the risk of myopathy. N Engl J Med. 2011;365:285–287. doi: 10.1056/NEJMp1106689. [DOI] [PubMed] [Google Scholar]
- 34.Thompson PD, Clarkson P, Karas RH. Statin-associated myopathy. JAMA. 2003;289:1681–1690. doi: 10.1001/jama.289.13.1681. [DOI] [PubMed] [Google Scholar]
- 35.Gibson DM, Bron NJ, Richens A, Hounslow NJ, Sedman AJ, Whitfield LR. Effect of age and gender on pharmacokinetics of atorvastatin in humans. J Clin Pharmacol. 1996;36:242–246. doi: 10.1002/j.1552-4604.1996.tb04194.x. [DOI] [PubMed] [Google Scholar]
- 36.Martin PD, Dane AL, Nwose OM, Schneck DW, Warwick MJ. No effect of age or gender on the pharmacokinetics of rosuvastatin: A new HMG-Coa reductase inhibitor. J Clin Pharmacol. 2002;42:1116–1121. doi: 10.1177/009127002401382722. [DOI] [PubMed] [Google Scholar]
- 37.Kempen HJ, Glatz JF, Gevers Leuven JA, van der Voort HA, Katan MB. Serum lathosterol concentration is an indicator of whole-body cholesterol synthesis in humans. J Lipid Res. 1988;29:1149–1155. [PubMed] [Google Scholar]
- 38.De Cuyper I, Wolthers BG, van Doormaal JJ, Wijnandts PN. Determination of changes in serum lathosterol during treatment with simvastatin to evaluate the role of lathosterol as a parameter for whole body cholesterol synthesis. Clin Chim Acta. 1993;219:123–130. doi: 10.1016/0009-8981(93)90203-g. [DOI] [PubMed] [Google Scholar]
- 39.Sudhop T, Lutjohann D, Kodal A, Igel M, Tribble DL, Shah S, et al. Inhibition of intestinal cholesterol absorption by ezetimibe in humans. Circulation. 2002;106:1943–1948. doi: 10.1161/01.cir.0000034044.95911.dc. [DOI] [PubMed] [Google Scholar]
- 40.Wilke RA, Ramsey LB, Johnson SG, Maxwell WD, McLeod HL, Voora D, et al. The clinical pharmacogenomics implementation consortium: CPIC guideline for SLCO1B1 and simvastatin-induced myopathy. Clin Pharmacol Ther. 2012;92:112–117. doi: 10.1038/clpt.2012.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.