Abstract
In the past few years, research in targeted mutation therapies has experienced significant advances, especially in the field of rare diseases. In particular, the efficacy of antisense therapy for suppression of normal, pathogenic, or cryptic splice sites has been demonstrated in cellular and animal models and has already reached the clinical trials phase for Duchenne muscular dystrophy. In different inherited metabolic diseases, splice switching oligonucleotides (SSOs) have been used with success in patients' cells to force pseudoexon skipping or to block cryptic splice sites, in both cases recovering normal transcript and protein and correcting the enzyme deficiency. However, future in vivo studies require individual approaches for delivery depending on the gene defect involved, given the different patterns of tissue and organ expression. Herein we review the state of the art of antisense therapy targeting RNA splicing in metabolic diseases, grouped according to their expression patterns—multisystemic, hepatic, or in central nervous system (CNS)—and summarize the recent progress achieved in the field of in vivo delivery of oligonucleotides to each organ or system. Successful body-wide distribution of SSOs and preferential distribution in the liver after systemic administration have been reported in murine models for different diseases, while for CNS limited data are available, although promising results with intratechal injections have been achieved.
Introduction
In the field of RNA therapeutics, splice switching oligonucleotides (SSOs) that act by sterically blocking the access of the spliceosomal components to targeted pre-messenger (m)RNA regions are a promising tool for the treatment of genetic disorders (Kole et al., 2012; Spitali et al., 2012). To this end, different chemical modifications of the nucleotide backbone have been developed and delivery methods suited to specific disease gene expression explored. Modified oligonucleotides that do not induce RNAse H cleavage when bound to pre-messenger (m)RNA targets and used for splice switching applications have either charged backbones, such as 2′-O-methyl (2′-OMe) and 2′-O-methoxyethyl (2′-O-MOE) nucleotides with phosphorothioate linkages, locked nucleic acids (LNAs) or peptide nucleic acids (PNAs), or charge neutral backbones including PNAs and phosphorodiamidate morpholinos (KURRECK, 2003; Saleh et al., 2012). A fine balance between specificity, binding strength, and stability/protection against nucleases is required. This has been extensively explored for the different chemistries mentioned, as well as with chimeric formulations (e.g., part LNA or part 2′-OMe) (Brenner et al., 2003; Shaked et al., 2009), conjugates, or complexes with cell penetrating peptides, dendrimeric nanoparticles, and small molecules such as cholesterol (Saleh et al., 2012; WINKLER, 2013). Comparing animal and cell culture data, it has become increasingly clear that functional effect in vivo is not readily predictable based on cell data (Saleh et al., 2012). Studies have also shown biological effects unrelated to antisense properties of SSOs, based on aptamer mechanism of action (Sussman et al., 2012).
In the field of inherited metabolic diseases (IMDs) different splice mutations have been found to be amenable to antisense mediated therapy, although research has been limited to date to cellular models of disease. IMDs, which can be collectively described as genetic deficiencies of an enzyme, receptor, or transport protein involved in a metabolic pathway, belong to the group of rare diseases due to their low individual prevalence, although collectively they affect approximately 1 out of 3,000 newborns (Lindner et al., 2011). Most of them are inherited in autosomal recessive fashion and represent good candidates for novel therapeutic strategies aimed at recovering partial enzyme function, as small levels of enzymatic activity have been shown to be associated with improved outcome and milder phenotypes (Perez-Cerda et al., 2000). In addition, most of them exhibit great morbidity and mortality and lack an effective treatment. Even though metabolic control may be adequate, neurological impairment persists raising the need of implementing alternative or supplementary therapies.
Molecular analysis of the genes involved in IMDs have characterized the mutational spectrum in each disease, identifying splicing defects as the second most frequent type of mutations, after the missense type. Among splicing mutations, experimental evidence obtained from transcript analysis in patients' cells has demonstrated the persistent presence in different gene defects of deep intronic mutations that provoke the activation of cryptic exons also known as pseudoexons. These pseudoexons, many of which are derived from transposable elements of the SINE and LINE classes (VORECHOVSKY, 2010), structurally resemble natural exons, with potential 3′ and 5′ splice sites, but are not normally spliced into mRNA, probably due to the presence of splicing silencers or formation of inhibiting RNA structures (Dhir et al., 2010). Pseudoexon insertion as a consequence of a point mutation that activates or creates 3′ or 5′ splicing signals or splicing enhancers, generally results in a nonfunctional out-of-frame transcript causing disease (Fig. 1A). Reported frequencies for such events range between 2%–7% of the total mutant alleles (Gurvich et al., 2008; Perez et al., 2009; Pros et al., 2009). However, it is assumed that this disease mechanism has probably been underreported mostly due to technical reasons (i.e., lack of sequencing analysis of the full intronic sequences, difficulties in recognizing splicing motifs in them, infrequent diagnostic mRNA analysis, and/or nonsense mediated decay mechanism eliminating the aberrant transcript). However, the situation may change with the increasing implementation of next-generation sequencing technologies for diagnostic purposes, allowing the identification of mutations deep in introns affecting the splicing process (Webb et al., 2012; Chandrasekharappa et al., 2013; Flanagan et al., 2013).
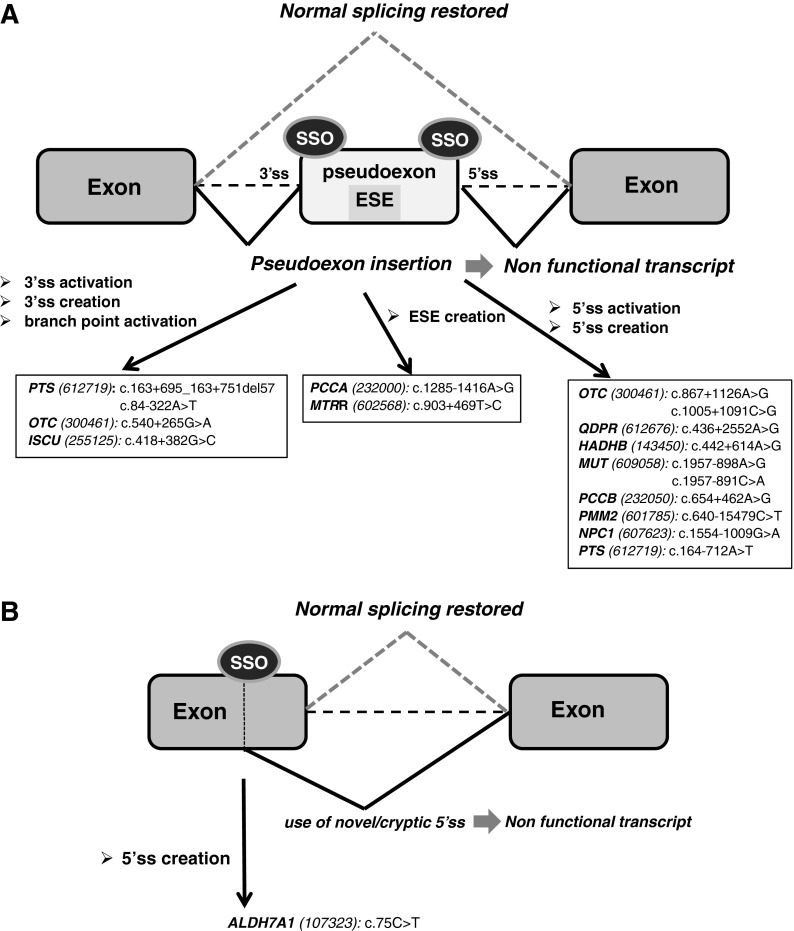
FIG. 1.
Use of splice-switching oligonucleotides (SSOs) to correct RNA splicing defects in inherited metabolic diseases (IMDs). The figure shows the schematics of the mechanisms of exonic and intronic mutations identified in patients causing splicing defects and their correction with antisense therapy. (A) SSO-induced pseudoexon skipping. (B) Use of SSOs to block mutation-created exonic splice sites or intronic cryptic splice sites. The arrows indicate the mutations and corresponding genes (MIM reference number in brackets) targeted by SSOs (as referenced in Desviat et al., 2006; Perez et al., 2010; Brasil et al., 2011; Sanaker et al., 2012; Perez et al., 2013). ESE, exonic splicing enhancer; 3′ss, 3′ splice site; 5′ss, 5′ splice site.
From a therapeutic point of view, pseudoexons represent an excellent target for antisense mediated “exon skipping” therapy, as natural splice sites of the surrounding exons are intact and by forcing pseudoexon skipping, normal splicing is resumed and wild-type transcript and protein produced (Fig. 1A). In line with this, antisense treatment in cells from IMD patients carrying intronic pseudoexon activating mutations in different gene defects responsible for organic acidemias (OAs) (PCCA, MIM 232000; PCCB, MIM 232050; and MUT, MIM 609058), monoamine neurotransmitter deficiencies (PTS, MIM 616719), glycosylation defects (PMM2, MIM 601785), and lysosomal disorders (NPC1, MIM 607623) among others (Fig. 1A), has resulted in complete recovery of protein and activity up to wild-type levels (Perez et al., 2010).
Other type of splicing mutations—exonic or intronic and resulting in the creation of novel splice sites or in the use of cryptic ones—have also been described profusely in metabolic diseases and the proof of concept of the splicing correction with SSOs established in patients' fibroblasts with pyridoxine dependent epilepsy (ALDH7A1 gene, MIM 107323) (Perez et al., 2013) (Fig. 1B). The use of cryptic splice sites and exon skipping are the two most frequent consequences of splicing mutations (Divina et al., 2009) and thus constitutes a potentially abundant source of candidates for SSO therapy, provided that the cryptic sites are located at a convenient distance from the natural splice sites, which should be accessible to the spliceosomal components. However, in contrast to Duchenne muscular dystrophy (DMD, MIM 310200), nonsense mutations or exon deletions, which are also frequent in some metabolic diseases, cannot be functionally corrected with this approach. In DMD, SSOs are designed to skip a mutated exon and/or to recover the open reading frame in deletion mutants (Arechavala-Gomeza et al., 2012), which is suitable only for structural proteins with repetitive stretches. This approach is theoretically applicable to about 85% of DMD patients in whom exon skipping should convert a severe lethal phenotype to a milder form of the disease. This is indeed the case for Becker muscular dystrophy (MIM 300376), where naturally occurring deletions in the dystrophin gene preserve the reading frame, allowing the production of a partially functional dystrophin (Muntoni et al., 2003). In metabolic disease genes, small in-frame deletions or insertions result in severe functional enzyme deficiencies due to disturbances of the three-dimensional conformation of the enzyme or to interference with the correct folding process, rendering the enzyme unstable, so exon skipping is predictably always a pathologically severe process.
The research in exon skipping approaches for DMD has rapidly progressed since more than a decade ago when the proof of concept was demonstrated in muscle cells from patients and in the mdx mouse model (Wilton et al., 1999; Lu et al., 2003; van Deutekom et al., 2007). Both 2′-O-methyl phosphorothioate and phosphordiamidate morpholino oligomers were tested and entered into clinical trials as a treatment for DMD, with promising results to date (Kinali et al., 2009; Cirak et al., 2011; Goemans et al., 2011; Koo et al., 2013; Mendell et al., 2013). In contrast, in the field of metabolic diseases there are to date no published in vivo reports. This is due to a lack of naturally occurring animal models with specific splice defects amenable to antisense treatment and for the expenses and complications in developing them, especially for deep intronic mutations, given that intronic sequences are not well conserved between different species. Other issues to address are the selection of the mutation in the generation of animal models, as in each gene defect there are no prevalent candidates (most of them being private mutations present only in one family) and the potential embryonic/neonatal lethality of the resulting functionally null alleles, which has already been documented for knock-out murine models (Miyazaki et al., 2001; Peters et al., 2003; Thiel et al., 2006). In line with this, patients with the most severe phenotypes such as those caused by splicing defects also generally exhibit potentially lethal neonatal presentations and should ideally be treated soon after birth. This contrasts with the progressive nature of DMD, where the availability of different animal models and the fact that skipping of a specific exon is applicable for many patients (13% of the patients for exon 51 skipping and 6% for exon 44 skipping) has accelerated preclinical and clinical studies (Arechavala-Gomeza et al., 2012). In any case, the development of adequate animal models to optimize antisense protocols for IMD treatment is necessary to pave the way for setting up therapeutic trials. Regarding these, IMDs share common difficulties with other rare diseases in which antisense therapy has been proposed (Osorio et al., 2011), including cost and regulatory hurdles for the development and testing of each SSO that may be used for one or just a few patients. Therefore, continued research in different rare diseases may benefit the achievement of common strategies for clinical application of antisense treatment.
In enzyme deficiencies involved in IMDs, predictably less SSO may be required for therapeutic splicing correction compared with the situation in DMD, where muscle represents up to 40% of body mass (Janssen et al., 2000). However, transcript and enzyme half-lives may be shorter, rendering repeated injections necessary. In DMD, the leaky nature of the membrane of dystrophic muscle fibers facilitates entry of naked oligo (Lu et al., 2011). The ease of uptake in the target tissue will clearly influence the effect observed. In most situations, a delivery agent such as dendrimeric particles (Li et al., 2008) or cationic peptides (Du et al., 2011) may be necessary for efficient cellular uptake, which may, in turn, increase the risk of secondary effects or of raising an immune response in long-term treatments.
The therapeutic potential of SSO treatment for IMDs will also depend on the tissues and organs involved, as each poses different challenges for targeted delivery. Considering the metabolic disorders with splicing defects that have been successfully targeted with SSOs using cellular models, we can identify enzyme deficiencies with hepatic, systemic, and/or central nervous system (CNS) expression. Each pattern of expression represents unique characteristics and challenges for delivery as reviewed below.
Liver as target for antisense therapy
Many enzymes responsible for amino acid and urea cycle disorders (UCDs) are exclusively expressed in liver although they lead to other organ (e.g., brain) damage. UCDs comprise a series of metabolic pathways aimed at converting ammonia to excretory nontoxic compounds. The common feature is hyperammonemia that is highly toxic to the development of the central nervous system (Lanpher et al., 2003). In the most frequent urea cycle defect, ornithine transcarbamylase (OTC) deficiency (MIM 311250), analysis of hepatic biopsies identified up to three different pseudoexon insertions caused by point mutations creating novel 3′ or 5′ splice sites (Ogino et al., 2007; Engel et al., 2008). This approach improved the mutation detection rate, which is currently estimated to be around 80% after standard gene sequencing and multiplex ligation-dependent probe amplification (MLPA) or microarray analysis (Shchelochkov et al., 2009). Predictably, several yet unidentified alleles may harbor pseudoexon activating mutations.
Concerning amino acid disorders, in the well-studied and paradigmatic metabolic disease phenylketonuria (PKU; MIM261600), a deficiency of hepatic phenylalanine hydroxylase (PAH) leads to an increase of l-phenylalanine in body fluids (hyperphenylalaninemia) that enters the brain resulting in severe mental retardation unless adequate treatment is implemented (Blau et al., 2010). Recently, splicing analysis in minigenes identified a pseudoexon potentially pathogenic if activated by mutations (Heintz et al., 2012). In each population, there is a small fraction of PKU patients (1%–3%) with incomplete genotype (only one identified mutation) after standard genomic DNA sequencing and deletion analysis using MLPA. It can be hypothesized that these patients may harbor deep intronic mutations affecting the splicing process. In a recent study using next-generation sequencing of full genomic sequence of the PAH gene, deep intronic changes potentially affecting splicing signals were identified in a patient with only one characterized allele, although functional validation studies are necessary to confirm a splicing alteration (Trujillano et al., 2013).
In other IMDs, the enzyme defect is expressed at systemic level, but the major producer of toxic metabolites is the liver. This is the case for OAs, characterized by the excretion of organic acids in urine and generally caused by an enzyme deficiency in the catabolic pathway of lysine or branched-chain amino acids (Seashore, 2010). The pathophysiology mainly results from the toxic accumulation of precursors and of their derivatives in brain, liver, kidney and other organs. Clinically, they share many clinical similarities usually presenting as toxic encephalopathies, with vomiting, poor feeding, seizures, and other neurologic symptoms and lethargy progressing to coma (Seashore, 2010). In OA, ubiquitous expression of the enzymes involved facilitates mRNA analysis, which has led to the identification of different pseudoexon insertions (Tsuruta et al., 1998; Rincon et al., 2007; Perez et al., 2009).
In OA, liver is considered the target organ for therapy, as a major contributor to accumulating toxic metabolites and due to its function of “metabolic sink” eliminating them from circulation. In this sense, liver or hepatocyte transplantation has been successfully performed for the management of different OAs, improving significantly the outcome and quality of life of patients (MCKIERNAN, 2013). Although liver transplantation only partially replaces the defective enzyme activity, the functional correction appears to be more complete, resulting in some cases in normal metabolite homeostasis in peripheral tissues (Fagiuoli et al., 2013; MCKIERNAN, 2013). Thus, delivering SSOs to the liver could have a positive impact on the overall outcome of patients with splicing mutations in organic academia genes. The difference between the oligonucleotide location and the observed effect in other tissues has also been revealed by studies of inflammatory reactions in primates (Evron et al., 2005). Although oral or intravenously delivered antisense oligonucleotide targeting acetylcholinesterase stress-induced variant (AChE-R) does not penetrate the blood–brain barrier (BBB), both peripheral suppression of AChE-R overproduction under stress as well as specific responses in spinal cord neurons were documented due to an indirect mechanism (Evron et al., 2005).
For in vivo studies of liver associated diseases such as OA, UCD, or amino acid disorders as described above, the most adequate models would be mice carrying deep intronic mutations which are not available to date. However, the well characterized murine model of OTC deficiency, the spf/ash mouse, carries a point mutation in the last nucleotide of exon 4 of the Otc gene (c.386G>A) which was reported to affect splicing by the use of an intronic cryptic splice site at intronic position c.386+48 (Hodges et al., 1989). Antisense mediated splicing suppression at this cryptic splice site could represent a valuable in vivo model to study the use of SSO for a liver enzyme.
Concerning liver targeted antisense therapy, substantial data have been produced, especially regarding apolipoprotein B-100 (apoB-100) synthesis inhibition for the management of hypercholesterolemia (Visser et al., 2012). Mipomersen, a chimeric 2′-MOE phosphorothioate oligonucleotide with a central domain of 10 contiguous 2′-deoxyribose residues supporting RNase H–mediated degradation of the target human apoB mRNA was recently approved by the Food and Drug Administration for the treatment of familial hypercholesterolemia. The drug developed by ISIS Pharmaceuticals was evaluated in animal models and clinical trials, showing similar pharmacokinetics across species and resulting in a reduction in plasma apoB levels and consequently reduced low-density lipoprotein cholesterol (Raal et al., 2010). After subcutaneous injection, the antisense oligonucleotide accumulates in the liver, which helps explain the success in reducing hepatic synthesis of ApoB (Visser et al., 2012).
In line with this, SSOs of different chemistries injected systemically have also been shown to end up in the liver and kidney, which are considered the organs most efficiently targeted by antisense reagents (Sazani et al., 2002; White et al., 2009; Wu et al., 2009; Parra et al., 2011). In the EGFP-654 transgenic mouse, enhanced green fluorescent reporter protein is expressed after SSO-mediated splice suppression at an aberrant intronic sequence, representing an adequate model to study biodistribution and pharmacokinetics of the oligonucleotides. In this model, quantitative analysis of the reverse transcription–polymerase chain reaction (RT-PCR) data indicated that splice correction levels reached 40% in liver and kidney for the most effective oligonucleotide chemistries (PNAs coupled to four lysines) (Sazani et al., 2002). In another study using EGFP-654 mice, LNA oligomers achieved 85% splice correction in liver, exhibiting high potency and persistence up to 22 days after injection (Roberts et al., 2006). These promising results warrant successful application in vivo for antisense-mediated splice modulation therapy in liver associated metabolic diseases.
Enzyme defects with multisystemic phenotypes
The most representative example of metabolic enzyme defects affecting every organ and tissue is the group of congenital disorders of glycosylation (CDG), a rapidly expanding group of IMDs with multisystemic phenotypes, most of them exhibiting severe neurological impairment (FREEZE, 2013). Protein glycosylation is a complex posttranslational modification process necessary for correct trafficking and biological activity of a vast number of proteins involved in numerous cellular pathways. Defects in one of the enzymes involved in the glycosylation machinery in cytosol, endoplasmic reticulum, or Golgi apparatus lead to loss or altered oligosaccharide moieties linked to glycoproteins. The most frequent CDG is phosphomannomutase (PMM2) deficiency (MIM 212065) (Jaeken et al., 1997). This enzyme converts mannose-6-phosphate to mannose 1-phosphate and is a key enzyme in the generation of N-linked glycans. An intronic mutation in the PMM2 gene was shown to provoke the exonization of 123 bp of intronic sequence, which was successfully reverted with SSO (Vega et al., 2009). Other pseudoexons activated by mutations have also been described in different glycosylation defects such as TMEM165 deficiency (MIM 614727), affecting a protein located mainly in the Golgi compartment and involved in calcium and pH homeostasis necessary for correct processing of glycan chains (Foulquier et al., 2012).
Systemic delivery with different SSO chemistries and subsequent body-wide distribution has been explored in mouse models of disease and could represent a starting point for its therapeutic development in CDG. In the EGFP-654 mouse described above, detailed quantification of splice switching after systemic injection showed best results for liver, colon, and intestine, while other tissues such as heart, lung, and skeletal muscle were less efficiently targeted, and no effect was observed in brain tissue (Sazani et al., 2002; Roberts et al., 2006). Other authors have shown similar results, with differences that may be explained by the different routes of delivery (intravenous or intraperitoneal), oligonucleotide chemistries, dosing regime and experimental model used (Parra et al., 2011). In DMD, one of the main caveats is the need of efficient delivery of the SSO body-wide and many present studies are focused on that aim. Evidence is accumulating that conjugation to short cationic peptides or encapsulation in polymeric nanoparticles greatly enhances delivery and body-wide cellular uptake of the SSO (Wood et al., 2010). These ongoing studies will favor the establishment of protocols and methods for efficient systemic distribution applicable to CDG and other systemic metabolic defects.
CNS as target for antisense therapy
Synthesis of neurotransmitters dopamine and serotonin takes place in defined brain regions and is dependent on the activity of tyrosine hydroxylase (TH) and tryptophan hydroxylase, respectively. These two enzymes use tetrahydrobiopterin (BH4) as cofactor. BH4 is also the cofactor of hepatic phenylalanine hydroxylase, nitric oxide synthases, and glycerol ether monooxygenase (Werner et al., 2011). Intracellular BH4 synthesis involves three subsequent reactions starting from GTP, the second one catalyzed by 6-pyruvoyltetrahydropterin synthase (PTPS), encoded by the PTS gene. Autosomal recessive PTPS deficiency (MIM 612719) is the most common form of BH4 deficiency. Patients present with abnormal pterin levels, hyperphenylalaninemia, and monoamine neurotransmitter deficiency in the CNS (Thony et al., 2006). They exhibit progressive mental and physical retardation, seizures, temperature instability, hypersalivation, lethargy, hypo/hypertonia, and other neurological symptoms (Kurian et al., 2011). Oral administration of BH4 is effective to control hyperphenylalaninemia but l-Dopa and 5-hydroxytryptophan also have to be administered due to the inability of BH4 to cross the BBB. Early diagnosis and treatment can be effective in most cases but alternative strategies are needed to avoid lifelong dependency from BH4 and monoamine neurotransmitter precursors. Recently, we showed the proof of concept of the use of pseudoexon-exclusion therapy with antisense oligonucleotides in cells from three patients with different intronic mutations activating one of two different pseudoexons (Brasil et al., 2011). For in vivo application of this genetic therapy, brain as well as peripheral tissues should be targeted.
Another disease that should be included in this category is Niemann-Pick type C (NPC; MIM 257220). It is a rare autosomal recessive metabolic, neurodegenerative disease caused by mutations in the NPC1 or NPC2 genes that code for two non-enzyme proteins involved in cholesterol trafficking. It is characterized by the accumulation of cholesterol and glycosphingolipids that leads to hepatic disease and progressive neurological impairment. The majority of NPC patients die in their teen years due to the progressive neurodegenerative process; however, their liver disease is also significant (Garver et al., 2007).
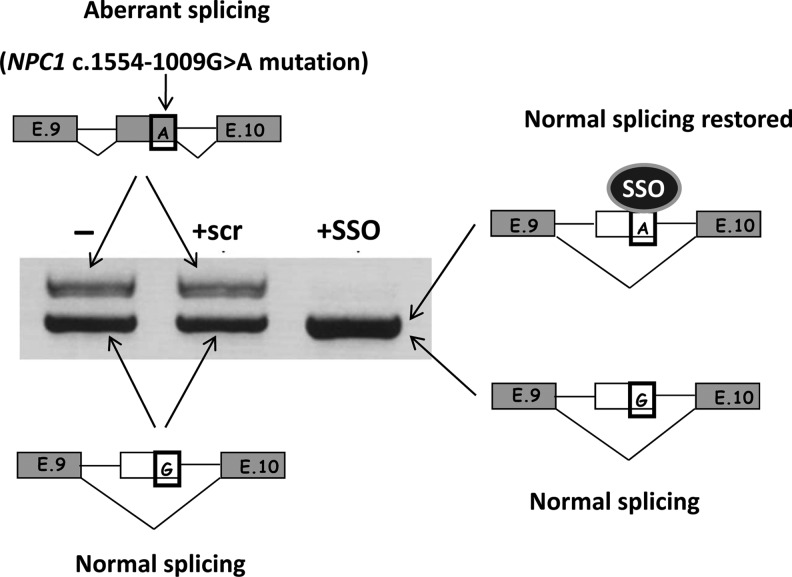
Recently, we identified a pseudoexon-generating mutation located 1009 bp upstream of the 3′ end of intron 9 in the NPC1 gene. We showed that the use of a specific SSO targeted to the cryptic splice site activated by the mutation restored normal splicing in fibroblasts of the patient (Fig. 2) (Rodriguez-Pascau et al., 2009). This novel mutation (c.1554-1009G>A) was recently identified in another Spanish patient (Macias-Vidal et al., 2011) and in two twins from the United States (Chris Hempel, the Addi and Cassi Fund, personal communication), indicating that it is not a private mutation. For in vivo studies, a knock-in mouse model bearing human intron 9 (instead of mouse intron 9) with the deep intronic mutation described above has been generated. This could be a valuable model to explore antisense technology in Niemann-Pick disease.
FIG. 2.
SSO treatment in fibroblasts from an Niemann-Pick type C (NPC) patient with a heterozygous intronic mutation in the NPC1 gene that generates pseudoexon inclusion. Reverse transcription–polymerase chain reaction of part of the gene shows two bands: the upper one corresponds to the allele bearing the pseudoexon, and the lower one to the normally spliced allele. Treatment with a specific SSO targeting the pseudoexon splice site abolishes its inclusion (upper band), while the use of a scrambled oligonucleotide (scr) had no effect. The normal-size band after the treatment was shown to correspond to both alleles through the sequencing of a polymorphism for which the patient was heterozygous. Exons included in the mRNA are indicated in grey. The nucleotide corresponding to the G>A mutation is boxed.
As mentioned above, several authors have reported that in mice, after systemic delivery, SSOs mainly accumulate in the liver, which would be an advantage for the NPC liver disease treatment. However, for CNS targets, direct CNS delivery is preferable. Typical oligonucleotides do not cross the BBB and although some have been shown to do it, 100-fold higher doses must be delivered systemically to achieve concentrations similar to those achieved by intra-CNS delivery, increasing the risk of toxicity (Southwell et al., 2012; Spitali et al., 2012). In addition to animal models, a range of in vitro models of the BBB has been developed for different mammalian species including mouse and human (Deli et al., 2005). These models, although not replicating exactly all aspects of the in vivo brain endothelium, could have a value for assessing BBB permeability to SSO complexes for CNS delivery of antisense therapy.
In animal models, intravenous or intraperitoneal administration of oligonucleotides in rodents has shown a very low uptake in brain. Strategies to improve brain uptake after peripheral delivery are being assayed, including increasing the permeability of the BBB (Emerich et al., 2001), complexing to cell-penetrating peptides, encapsulation in liposomes conjugated to monoclonal antibodies, or use of exosomes (Douglas et al., 2013). However, local injections in the desired brain region or injections in the cerebrospinal fluid seem to be a better option (Zalachoras et al., 2011). It has been shown that oligonucleotides delivered to cerebrospinal fluid distribute throughout the entire CNS (Smith et al., 2006). In rodents, oligonucleotides are generally delivered into cerebral ventricles due to the technical difficulties associated with intrathecal administration in small animals; however, in larger animals intratechal delivery has shown to result in therapeutic doses of oligonucleotides throughout the CNS (Southwell et al., 2012).
A disease in which important progress has been made regarding antisense oligonucleotide therapy is spinal muscular atrophy (SMA, MIM 253300). In this pathology, the correction of the splicing process in the SMN2 gene could compensate the lack of SMN1 functional copies (as recently reviewed in Porensky et al., 2013). Injection of an SSO derivative to promote splice modification into the cerebral lateral ventricles of a severe mice model of SMA was efficacious and improved muscle physiology motor function and survival of mice (Passini et al., 2011). In another study, the effects of systemic versus CNS restoration in a severe mouse model of the disease were compared, obtaining long-lasting survival after intracerebroventricular (ICV) administration of the SSO (Hua et al., 2011). Surprisingly, systemic administration in neonates resulted to be more effective than ICV administration, showing a relevant role for the liver and indicating that SSOs may cross the immature BBB of neonates. In general, a variety of cis-acting RNA sequences of the SMN2 gene have been targeted by different oligonucleotide chemistries, such as 2′-OMe, 2′-O-MOE or morpholino. In particular, the discovery of the intronic splicing silencer N1 (ISS-N1) was an important breakthrough and many reports selected this sequence as the target for SSO treatment (Seo et al., 2013). As previous steps to apply SSO therapy to cure SMA in humans, intrathecal infusion of the SSO was assayed in cynomolgus monkeys, showing this strategy provided therapeutic levels of SSO to all regions of the spinal cord (Passini et al., 2011). Regarding clinical trials, ISIS Pharmaceuticals has recently concluded a phase 1 trial in which the safety, tolerability, and pharmacokinetics of escalating doses of the ISIS-SMNRx drug, an ISS-N1–targeting MOE SSO, administered into the spinal fluid as a single injection in patients with SMA (NCT01494701) has been evaluated (Southwell et al., 2012). Moreover, a phase 2 clinical trial has been initiated in patients with infantile-onset spinal muscular atrophy (NCT01839656) (Seo et al., 2013).
Conclusions
IMDs represent an expanding area of research in the field of splice switching antisense therapy, as more splicing mutations, both intronic and exonic, are being identified resulting in splicing defects which could be reverted with this type of treatment, recovering a normally spliced functional transcript. However, with some exceptions, most are private mutations affecting one or few patients, which poses many difficulties for clinical application of SSO therapy. In addition, the range of gene expression patterns and resulting phenotypes is large and thus each disease or group of diseases should be considered separately according to specific delivery issues to the tissues and organs involved. Liver associated diseases, which include many amino acid disorders and OAs, are potentially easy to address by systemic delivery based on the experience with SSO injections in different animal models.
Regarding diseases with CNS involvement, SSOs have several favorable characteristics such us good delivery and CNS-wide distribution capabilities that makes them particularly promising for treating these kind of disorders. However, there is a need to work on strategies to increase BBB permeability, to optimize direct intra-brain injections and/or noninvasive delivery protocols for in vivo application of antisense therapy.
Acknowledgments
This work was supported by grants SAF2010-17272 and SAF2011-25431 from Ministerio de Ciencia e Innovación (to L.R.D and D.G., respectively) and PI10/00455 from Instituto de Salud Carlos III (to B.P.). The authors also acknowledge the institutional grant from Fundación Ramón Areces to the Centro de Biología Molecular Severo Ochoa.
Author Disclosure Statement
No competing financial interests exist.
References
- ARECHAVALA-GOMEZA V., ANTHONY K., MORGAN J., and MUNTONI F. (2012). Antisense oligonucleotide-mediated exon skipping for Duchenne muscular dystrophy: progress and challenges. Curr. Gene Ther. 12,152–160 [DOI] [PubMed] [Google Scholar]
- BLAU N., VAN SPRONSEN F.J., and LEVY H.L. (2010). Phenylketonuria. Lancet 376,1417–1427 [DOI] [PubMed] [Google Scholar]
- BRASIL S., VIECELLI H.M., MEILI D., RASSI A., DESVIAT L.R., PEREZ B., UGARTE M., and THONY B. (2011). Pseudoexon exclusion by antisense therapy in 6-pyruvoyl-tetrahydropterin synthase deficiency. Hum. Mutat. 32,1019–1027 [DOI] [PubMed] [Google Scholar]
- BRENNER T., HAMRA-AMITAY Y., EVRON T., BONEVA N., SEIDMAN S., and SOREQ H. (2003). The role of readthrough acetylcholinesterase in the pathophysiology of myasthenia gravis. FASEB J., 17,214–222 [DOI] [PubMed] [Google Scholar]
- CHANDRASEKHARAPPA S.C., LACH F.P., KIMBLE D.C., KAMAT A., TEER J.K., DONOVAN F.X., FLYNN E., SEN S. K., THONGTHIP S., SANBORN E., et al. (2013). Massively parallel sequencing, aCGH, and RNA-Seq technologies provide a comprehensive molecular diagnosis of Fanconi anemia. Blood 121,e138–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- CIRAK S., ARECHAVALA-GOMEZA V., GUGLIERI M., FENG L., TORELLI S., ANTHONY K., ABBS S., GARRALDA M.E., BOURKE J., WELLS D.J., et al. Exon skipping and dystrophin restoration in patients with Duchenne muscular dystrophy after systemic phosphorodiamidate morpholino oligomer treatment: an open-label, phase 2, dose-escalation study. Lancet 378,595–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DELI M.A., ABRAHAM C.S., KATAOKA Y., and NIWA M. (2005). Permeability studies on in vitro blood-brain barrier models: physiology, pathology, and pharmacology. Cell. Mol. Neurobiol. 25,59–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DESVIAT L.R., CLAVERO S., PEREZ-CERDA C., NAVARRETE R., UGARTE M., and PEREZ B. (2006). New splicing mutations in propionic acidemia. J. Hum. Genet. 51,992–997 [DOI] [PubMed] [Google Scholar]
- DHIR A., and BURATTI E. (2010). Alternative splicing: role of pseudoexons in human disease and potential therapeutic strategies. FEBS J. 277,841–855 [DOI] [PubMed] [Google Scholar]
- DIVINA P., KVITKOVICOVA A., BURATTI E., and VORECHOVSKY I. (2009). Ab initio prediction of mutation-induced cryptic splice-site activation and exon skipping. Eur. J. Hum. Genet. 17,759–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGLAS A.G., and WOOD M.J. (2013). Splicing therapy for neuromuscular disease. Mol. Cell. Neurosci. 56,169–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DU L., KAYALI R., BERTONI C., FIKE F., HU H., IVERSEN P.L., and GATTI R.A. (2011). Arginine-rich cell-penetrating peptide dramatically enhances AMO-mediated ATM aberrant splicing correction and enables delivery to brain and cerebellum. Hum. Mol. Genet. 20,3151–3160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EMERICH D.F., DEAN R.L., OSBORN C., and BARTUS R.T. (2001). The development of the bradykinin agonist labradimil as a means to increase the permeability of the blood-brain barrier: from concept to clinical evaluation. Clin. Pharmacokinet. 40,105–123 [DOI] [PubMed] [Google Scholar]
- ENGEL K., NUOFFER J.M., MUHLHAUSEN C., KLAUS V., LARGIADER C.R., TSIAKAS K., SANTER R., WERMUTH B., and HABERLE J. (2008). Analysis of mRNA transcripts improves the success rate of molecular genetic testing in OTC deficiency. Mol. Genet. Metab. 94,292–297 [DOI] [PubMed] [Google Scholar]
- EVRON T., MOYAL-SEGAL L.B., LAMM N., GEFFEN A., and SOREQ H. (2005). RNA-targeted suppression of stress-induced allostasis in primate spinal cord neurons. Neurodegener. Dis. 2,16–27 [DOI] [PubMed] [Google Scholar]
- FAGIUOLI S., DAINA E., D'ANTIGA L., COLLEDAN M., and REMUZZI G. (2013). Monogenic diseases that can be cured by liver transplantation. J. Hepatol. 59,595–612 [DOI] [PubMed] [Google Scholar]
- FLANAGAN S.E., XIE W., CASWELL R., DAMHUIS A., VIANEY-SABAN C., AKCAY T., DARENDELILER F., BAS F., GUVEN A., SIKLAR Z., et al. (2013). Next-generation sequencing reveals deep intronic cryptic ABCC8 and HADH splicing founder mutations causing hyperinsulinism by pseudoexon activation. Am. J. Hum. Genet. 92,131–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOULQUIER F., AMYERE M., JAEKEN J., ZEEVAERT R., SCHOLLEN E., RACE V., BAMMENS R., MORELLE W., ROSNOBLET C., LEGRAND D., et al. (2012). TMEM165 deficiency causes a congenital disorder of glycosylation. Am. J. Hum. Genet. 91,15–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREEZE H.H. (2013). Understanding human glycosylation disorders: biochemistry leads the charge. J. Biol. Chem. 288,6936–6945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARVER W.S., FRANCIS G.A., JELINEK D., SHEPHERD G., FLYNN J., CASTRO G., WALSH VOCKLEY C., COPPOCK D. L., PETTIT K.M., HEIDENREICH R.A., and MEANEY F. J. (2007). The National Niemann-Pick C1 disease database: report of clinical features and health problems. Am. J. Med. Genet. A. 143A,1204–1211 [DOI] [PubMed] [Google Scholar]
- GOEMANS N.M., TULINIUS M., VAN DEN AKKER J.T., BURM B.E., EKHART P.F., HEUVELMANS N., HOLLING T., JANSON A.A., PLATENBURG G.J., SIPKENS J.A., et al. (2011). Systemic administration of PRO051 in Duchenne's muscular dystrophy. N. Engl. J. Med. 364,1513–1522 [DOI] [PubMed] [Google Scholar]
- GURVICH O.L., TUOHY T.M., HOWARD M.T., FINKEL R. S., MEDNE L., ANDERSON C.B., WEISS R.B., WILTON S. D., and FLANIGAN K.M. (2008). DMD pseudoexon mutations: splicing efficiency, phenotype, and potential therapy. Ann. Neurol. 63,81–89 [DOI] [PubMed] [Google Scholar]
- HEINTZ C., DOBROWOLSKI S.F., ANDERSEN H.S., DEMIRKOL M., BLAU N., and ANDRESEN B.S. (2012). Splicing of phenylalanine hydroxylase (PAH) exon 11 is vulnerable: molecular pathology of mutations in PAH exon 11. Mol. Genet. Metab. 106),403–411 [DOI] [PubMed] [Google Scholar]
- HODGES P.E., and ROSENBERG L.E. (1989). The spfash mouse: a missense mutation in the ornithine transcarbamylase gene also causes aberrant mRNA splicing. Proc. Natl. Acad. Sci. U. S. A. 86,4142–4146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUA Y., SAHASHI K., RIGO F., HUNG G., HOREV G., BENNETT C.F., and KRAINER A.R. (2011). Peripheral SMN restoration is essential for long-term rescue of a severe spinal muscular atrophy mouse model. Nature 478,123–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- JAEKEN J., ARTIGAS J., BARONE R., FIUMARA A., DE KONING T.J., POLL-THE B.T., DE RIJK-VAN ANDEL J.F., HOFFMANN G.F., ASSMANN B., MAYATEPEK E., et al. (1997). Phosphomannomutase deficiency is the main cause of carbohydrate-deficient glycoprotein syndrome with type I isoelectrofocusing pattern of serum sialotransferrins. J. Inherit. Metab. Dis. 20,447–449 [DOI] [PubMed] [Google Scholar]
- JANSSEN I., HEYMSFIELD S.B., WANG Z.M., and ROSS R. (2000). Skeletal muscle mass and distribution in 468 men and women aged 18–88 yr. J. Appl. Physiol. 89,81–88 [DOI] [PubMed] [Google Scholar]
- KINALI M., ARECHAVALA-GOMEZA V., FENG L., CIRAK S., HUNT D., ADKIN C., GUGLIERI M., ASHTON E., ABBS S., NIHOYANNOPOULOS P., et al. (2009). Local restoration of dystrophin expression with the morpholino oligomer AVI-4658 in Duchenne muscular dystrophy: a single-blind, placebo-controlled, dose-escalation, proof-of-concept study. Lancet Neurol. 8,918–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOLE R., KRAINER A.R., and ALTMAN S. (2012). RNA therapeutics: beyond RNA interference and antisense oligonucleotides. Nature Rev. 11,125–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOO T., and WOOD M.J. (2013). Clinical trials using antisense oligonucleotides in duchenne muscular dystrophy. Hum. Gene Ther. 24,479–488 [DOI] [PubMed] [Google Scholar]
- KURIAN M.A., GISSEN P., SMITH M., HEALES S., JR., and CLAYTON P.T. (2011). The monoamine neurotransmitter disorders: an expanding range of neurological syndromes. Lancet Neurol. 10,721–733 [DOI] [PubMed] [Google Scholar]
- KURRECK J. (2003). Antisense technologies. Improvement through novel chemical modifications. Eur. J. Biochem. 270,1628–1644 [DOI] [PubMed] [Google Scholar]
- LANPHER B.C., GROPMAN A., CHAPMAN K.A., LICHTER-KONECKI U., and SUMMAR M.L. (2003). Urea Cycle Disorders Overview. In: GeneReviews™ [Internet], Pagon R.A., Adam M.P., Bird T.D., Dolan C.R., Fong C.T., Stephens K., eds. (University of Washington, Seattle, WA: ) [PubMed] [Google Scholar]
- LI Y.F., and MORCOS P.A. (2008). Design and synthesis of dendritic molecular transporter that achieves efficient in vivo delivery of morpholino antisense oligo. Bioconj. Chem. 19,1464–1470 [DOI] [PubMed] [Google Scholar]
- LINDNER M., GRAMER G., HAEGE G., FANG-HOFFMANN J., SCHWAB K.O., TACKE U., TREFZ F.K., MENGEL E., WENDEL U., LEICHSENRING M., et al. (2011). Efficacy and outcome of expanded newborn screening for metabolic diseases—report of 10 years from South-West Germany. Orphanet. J. Rare Dis. 6, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LU Q.L., MANN C.J., LOU F., BOU-GHARIOS G., MORRIS G.E., XUE S.A., FLETCHER S., PARTRIDGE T.A., and WILTON S.D. (2003). Functional amounts of dystrophin produced by skipping the mutated exon in the mdx dystrophic mouse. Nat. Med. 9,1009–1014 [DOI] [PubMed] [Google Scholar]
- LU Q.L., YOKOTA T., TAKEDA S., GARCIA L., MUNTONI F., and PARTRIDGE T. (2011). The status of exon skipping as a therapeutic approach to Duchenne muscular dystrophy. Mol. Ther. 19,9–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACIAS-VIDAL J., RODRIGUEZ-PASCAU L., SANCHEZ-OLLE G., LLUCH M., VILAGELIU L., GRINBERG D., and COLL M. J. (2011). Molecular analysis of 30 Niemann-Pick type C patients from Spain. Clin. Genet. 80,39–49 [DOI] [PubMed] [Google Scholar]
- MCKIERNAN P. (2013). Liver transplantation and cell therapies for inborn errors of metabolism. J. Inherit. Metab. Dis. 36,675–680 [DOI] [PubMed] [Google Scholar]
- MENDELL J.R., RODINO-KLAPAC L.R., SAHENK Z., ROUSH K., BIRD L., LOWES L.P., ALFANO L., GOMEZ A. M., LEWIS S., KOTA J., et al. (2013). Eteplirsen for the treatment of Duchenne muscular dystrophy. Ann. Neurol. 74,637–647 [DOI] [PubMed] [Google Scholar]
- MIYAZAKI T., OHURA T., KOBAYASHI M., SHIGEMATSU Y., YAMAGUCHI S., SUZUKI Y., HATA I., AOKI Y., YANG X., MINJARES , et al. (2001). Fatal propionic acidemia in mice lacking propionyl-CoA carboxylase and its rescue by postnatal, liver-specific supplementation via a transgene. J. Biol. Chem. 276,35995–35999 [DOI] [PubMed] [Google Scholar]
- MUNTONI F., TORELLI S., and FERLINI A. (2003). Dystrophin and mutations: one gene, several proteins, multiple phenotypes. Lancet Neurol. 2,731–740 [DOI] [PubMed] [Google Scholar]
- OGINO W., TAKESHIMA Y., NISHIYAMA A., OKIZUKA Y., YAGI M., TSUNEISHI S., SAIKI K., KUGO M., and MATSUO M. (2007). Mutation analysis of the ornithine transcarbamylase (OTC) gene in five Japanese OTC deficiency patients revealed two known and three novel mutations including a deep intronic mutation. Kobe J. Med. Sci., 53,229–240 [PubMed] [Google Scholar]
- OSORIO F.G., NAVARRO C.L., CADINANOS J., LOPEZ-MEJIA I.C., QUIROS P.M., BARTOLI C., RIVERA J., TAZI J., GUZMAN G., VARELA I., et al. (2011). Splicing-directed therapy in a new mouse model of human accelerated aging. Sci. Transl. Med. 3, 106ra107. [DOI] [PubMed] [Google Scholar]
- PARRA M.K., GEE S., MOHANDAS N., and CONBOY J.G. (2011). Efficient in vivo manipulation of alternative pre-mRNA splicing events using antisense morpholinos in mice. J. Biol. Chem. 286,6033–6039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- PASSINI M.A., BU J., RICHARDS A.M., KINNECOM C., SARDI S.P., STANEK L.M., HUA Y., RIGO F., MATSON J., HUNG G., et al. (2011). Antisense oligonucleotides delivered to the mouse CNS ameliorate symptoms of severe spinal muscular atrophy. Sci. Transl. Med. 3, 72ra18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PEREZ-CERDA C., MERINERO B., RODRIGUEZ-POMBO P., PEREZ B., DESVIAT L. R., MURO S., RICHARD E., GARCIA M.J., GANGOITI J., RUIZ SALA P., et al. (2000). Potential relationship between genotype and clinical outcome in propionic acidaemia patients. Eur. J. Hum. Genet. 8,187–194 [DOI] [PubMed] [Google Scholar]
- PEREZ B., GUTIERREZ-SOLANA L.G., VERDU A., MERINERO B., YUSTE-CHECA P., RUIZ-SALA P., CALVO R., JALAN A., MARIN L.L., CAMPOS O., et al. (2013). Clinical, biochemical, and molecular studies in pyridoxine-dependent epilepsy. Antisense therapy as possible new therapeutic option. Epilepsia 54,239–248 [DOI] [PubMed] [Google Scholar]
- PEREZ B., RINCON A., JORGE-FINNIGAN A., RICHARD E., MERINERO B., UGARTE M., and DESVIAT L.R. (2009). Pseudoexon exclusion by antisense therapy in methylmalonic aciduria (MMAuria). Hum. Mutat. 30,1676–1682 [DOI] [PubMed] [Google Scholar]
- PEREZ B., RODRIGUEZ-PASCAU L., VILAGELIU L., GRINBERG D., UGARTE M., and DESVIAT L.R. (2010). Present and future of antisense therapy for splicing modulation in inherited metabolic disease. J. Inherit. Metab. Dis. 33,397–403 [DOI] [PubMed] [Google Scholar]
- PETERS H., NEFEDOV M., SARSERO J., PITT J., FOWLER K. J., GAZEAS S., KAHLER S.G., and LOANNOU P.A. (2003). A knock-out mouse model for methylmalonic aciduria resulting in neonatal lethality. J. Biol. Chem. 278,52909–52913 [DOI] [PubMed] [Google Scholar]
- PORENSKY P.N., and BURGHES A.H. (2013). Antisense oligonucleotides for the treatment of spinal muscular atrophy. Hum. Gene Ther. 24,489–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- PROS E., FERNANDEZ-RODRIGUEZ J., CANET B., BENITO L., SANCHEZ A., BENAVIDES A., RAMOS F. J., LOPEZ-ARIZTEGUI M.A., CAPELLA G., et al. (2009). Antisense therapeutics for neurofibromatosis type 1 caused by deep intronic mutations. Hum. Mutat. 30,454–462 [DOI] [PubMed] [Google Scholar]
- RAAL F.J., SANTOS R.D., BLOM D.J., MARAIS A.D., CHARNG M.J., CROMWELL W.C., LACHMANN R.H., GAUDET D., TAN J.L., CHASAN-TABER S., et al. (2010). Mipomersen, an apolipoprotein B synthesis inhibitor, for lowering of LDL cholesterol concentrations in patients with homozygous familial hypercholesterolaemia: a randomised, double-blind, placebo-controlled trial. Lancet 375,998–1006 [DOI] [PubMed] [Google Scholar]
- RINCON A., AGUADO C., DESVIAT L.R., SANCHEZ-ALCUDIA R., UGARTE M., and PEREZ B. (2007). Propionic and Methylmalonic Acidemia: Antisense Therapeutics for Intronic Variations Causing Aberrantly Spliced Messenger RNA. Am. J. Hum. Genet. 81,1262–1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBERTS J., PALMA E., SAZANI P., ORUM H., CHO M., and KOLE R. (2006). Efficient and persistent splice switching by systemically delivered LNA oligonucleotides in mice. Mol. Ther. 14,471–475 [DOI] [PubMed] [Google Scholar]
- RODRIGUEZ-PASCAU L., COLL M. J., VILAGELIU L., and GRINBERG D. (2009). Antisense oligonucleotide treatment for a pseudoexon-generating mutation in the NPC1 gene causing Niemann-Pick type C diseaseb. Hum. Mutat. 30,E993–E1001 [DOI] [PubMed] [Google Scholar]
- SALEH A.F., ARZUMANOV A.A., and GAIT M.J. (2012). Overview of alternative oligonucleotide chemistries for exon skipping. Methods Mol. Biol. 867,365–378 [DOI] [PubMed] [Google Scholar]
- SANAKER P.S., TOOMPUU M., MCCLOREY G., and BINDOFF L.A. (2012). Antisense oligonucleotide corrects splice abnormality in hereditary myopathy with lactic acidosis. Gene 494,231–236 [DOI] [PubMed] [Google Scholar]
- SAZANI P., GEMIGNANI F., KANG S.H., MAIER M.A., MANOHARAN M., PERSMARK M., BORTNER D., and KOLE R. (2002). Systemically delivered antisense oligomers upregulate gene expression in mouse tissues. Nat. Biotechnol. 20,1228–1233 [DOI] [PubMed] [Google Scholar]
- SEASHORE M.R. (2010). The Organic Acidemias: An Overview. In: GeneReviews™ [Internet]. Pagon R.A., Adam M.P., Bird T.D., Dolan C.R., Fong C.T., Stephens K., eds. (University of Washington, Seattle, WA: ) [Google Scholar]
- SEO J., HOWELL M.D., SINGH N.N., and SINGH R.N. (2013). Spinal muscular atrophy: An update on therapeutic progress. Biochim. Biophys. Acta 1832,2180–2190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHAKED I., MEERSON A., WOLF Y., AVNI R., GREENBERG D., GILBOA-GEFFEN A., and SOREQ H. (2009). MicroRNA-132 potentiates cholinergic anti-inflammatory signaling by targeting acetylcholinesterase. Immunity 31,965–973 [DOI] [PubMed] [Google Scholar]
- SHCHELOCHKOV O.A., LI F.Y., GERAGHTY M.T., GALLAGHER R.C., VAN HOVE J.L., LICHTER-KONECKI U., FERNHOFF P.M., COPELAND S., REIMSCHISEL T., CEDERBAUM S., et al. (2009). High-frequency detection of deletions and variable rearrangements at the ornithine transcarbamylase (OTC) locus by oligonucleotide array CGH. Mol. Genet. Metab. 96,97–105 [DOI] [PubMed] [Google Scholar]
- SMITH R.A., MILLER T.M., YAMANAKA K., MONIA B.P., CONDON T.P., HUNG G., LOBSIGER C.S., WARD C.M., MCALONIS-DOWNES M., WEI H., et al. (2006). Antisense oligonucleotide therapy for neurodegenerative disease. J. Clin. Invest. 116,2290–2296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOUTHWELL A.L., SKOTTE N.H., BENNETT C.F., and HAYDEN M.R. (2012). Antisense oligonucleotide therapeutics for inherited neurodegenerative diseases. Trends Mol. Med. 18,634–643 [DOI] [PubMed] [Google Scholar]
- SPITALI P., and AARTSMA-RUS A. (2012). Splice modulating therapies for human disease. Cell 148,1085–1088 [DOI] [PubMed] [Google Scholar]
- SUSSMAN J., ARGOV Z., WIRGUIN Y., APOLSKI S., MILIC-RASIC V., and SOREQ H. (2012). Further developments with antisense treatment for myasthenia gravis. Ann. N. Y. Acad. Sci. 1275,13–16 [DOI] [PubMed] [Google Scholar]
- THIEL C., LUBKE T., MATTHIJS G., VON FIGURA K., and KORNER C. (2006). Targeted disruption of the mouse phosphomannomutase 2 gene causes early embryonic lethality. Mol. Cell Biol. 26,5615–5620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- THONY B., and BLAU N. (2006). Mutations in the BH4-metabolizing genes GTP cyclohydrolase I, 6-pyruvoyl-tetrahydropterin synthase, sepiapterin reductase, carbinolamine-4a-dehydratase, and dihydropteridine reductase. Hum. Mutat. 27,870–878 [DOI] [PubMed] [Google Scholar]
- TRUJILLANO D., PEREZ B., GONZALEZ J., TORNADOR C., NAVARRETE R., ESCARAMIS G., OSSOWSKI S., ARMENGOL L., CORNEJO V., DESVIAT L. R., et al. (2013). Accurate molecular diagnosis of phenylketonuria and tetrahydrobiopterin-deficient hyperphenylalaninemias using high-throughput targeted sequencing. Eur. J. Hum. Genet. 10.1038/ejhg.2013.175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- TSURUTA M., MITSUBUCHI H., MARDY S., MIURA Y., HAYASHIDA Y., KINUGASA A., ISHITSU T., MATSUDA I., and INDO Y. (1998). Molecular basis of intermittent maple syrup urine disease: novel mutations in the E2 gene of the branched-chain alpha-keto acid dehydrogenase complex. J. Hum. Genet. 43,91–100 [DOI] [PubMed] [Google Scholar]
- VAN DEUTEKOM J.C., JANSON A.A., GINJAAR I.B., FRANKHUIZEN W.S., AARTSMA-RUS A., BREMMER-BOUT M., DEN DUNNEN J.T., KOOP K., VAN DER KOOI A.J., GOEMANS N.M.,. (2007). Local dystrophin restoration with antisense oligonucleotide PRO051. N. Engl. J. Med. 357,2677–2686 [DOI] [PubMed] [Google Scholar]
- VEGA A.I., PEREZ-CERDA C., DESVIAT L.R., MATTHIJS G., UGARTE M., and PEREZ B. (2009). Functional analysis of three splicing mutations identified in the PMM2 gene: toward a new therapy for congenital disorder of glycosylation type Ia. Hum. Mutat. 30,795–803 [DOI] [PubMed] [Google Scholar]
- VISSER M.E., WAGENER G., BAKER B.F., GEARY R.S., DONOVAN J.M., BEUERS U.H., NEDERVEEN A.J., VERHEIJ J., TRIP M.D., BASART D.C., et al. (2012). Mipomersen, an apolipoprotein B synthesis inhibitor, lowers low-density lipoprotein cholesterol in high-risk statin-intolerant patients: a randomized, double-blind, placebo-controlled trial. Eur. Heart J. 33,1142–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- VORECHOVSKY I. (2010). Transposable elements in disease-associated cryptic exons. Hum. Genet. 127,135–154 [DOI] [PubMed] [Google Scholar]
- WEBB T.R., PARFITT D.A., GARDNER J.C., MARTINEZ A., BEVILACQUA D., DAVIDSON A.E., ZITO I., THISELTON D. L., RESSA J.H., APERGI M., et al. (2012). Deep intronic mutation in OFD1, identified by targeted genomic next-generation sequencing, causes a severe form of X-linked retinitis pigmentosa (RP23). Hum. Mol. Genet. 21,3647–3654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WERNER E.R., BLAU N., and THONY B. (2011). Tetrahydrobiopterin: biochemistry and pathophysiology. Biochem. J. 438,397–414 [DOI] [PubMed] [Google Scholar]
- WHITE P.J., ANASTASOPOULOS F., POUTON C.W., and BOYD B.J. (2009). Overcoming biological barriers to in vivo efficacy of antisense oligonucleotides. Expert Rev. Mol. Med. 11, e10. [DOI] [PubMed] [Google Scholar]
- WILTON S.D., LLOYD F., CARVILLE K., FLETCHER S., HONEYMAN K., AGRAWAL S., and KOLE R. (1999). Specific removal of the nonsense mutation from the mdx dystrophin mRNA using antisense oligonucleotides. Neuromuscul. Disord. 9,330–338 [DOI] [PubMed] [Google Scholar]
- WINKLER J. (2013). Oligonucleotide conjugates for therapeutic applications. Ther. Deliv. 4,791–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOOD M.J., GAIT M.J., and YIN H. (2010). RNA-targeted splice-correction therapy for neuromuscular disease. Brain 133,957–972 [DOI] [PubMed] [Google Scholar]
- WU B., LI Y., MORCOS P.A., DORAN T.J., LU P., and LU Q.L. (2009). Octa-guanidine morpholino restores dystrophin expression in cardiac and skeletal muscles and ameliorates pathology in dystrophic mdx mice. Mol. Ther. 17,864–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZALACHORAS I., EVERS M.M., VAN ROON-MOM W.M., AARTSMA-RUS A.M., and MEIJER O.C. (2011). Antisense-mediated RNA targeting: versatile and expedient genetic manipulation in the brain. Front. Mol. Neurosci. 4, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]