Abstract
BACKGROUND
Young breast cancer survivors often need to deal with adverse effects of treatments on fertility and complex reproductive decisions. In this systematic review, we highlight what is known about childbearing and parenthood attitudes and decisions of young breast cancer survivors from their own perspective.
METHODS
We conducted manual and electronic searches on Pubmed, PsychInf and CINAHL databases for articles, published in English between 1 January 1990 and 31 October 2012, that assessed childbearing, pregnancy and parenthood attitudes/decisions of female breast cancer survivors (premenopausal and/or <50 years old). Eligible articles were classified into quantitative studies, qualitative studies and mixed methods studies. Data from each study were individually extracted by all the authors, and standardized tables were created and discussed to ensure congruence of the information extracted.
RESULTS
Of the 493 publications identified in PubMed (results are presented for PubMed searches as the other databases did not yield any new relevant papers), 8 met the inclusion criteria, in addition to 2 publications retrieved manually. A total of 10 studies provided information on pregnancy and parenthood attitudes and decisions, in addition to risks and benefits of childbearing after breast cancer. Survivors had mixed attitudes towards the issue. Fear associated with future pregnancy was reported, namely the risk of cancer recurrence. However, for many survivors, pregnancy and parenthood can represent normalcy, happiness and life fulfilment.
CONCLUSIONS
Childbearing after breast cancer is an important issue for survivors. Future larger and prospective studies should be implemented to increase certainty of conclusions of current research. Clinicians may benefit from a deeper understanding of the importance of pregnancy and parenthood to survivors in order to provide the needed educational and psychosocial support services, overcome misinformation and better assist women with their fertility-related decisions.
Keywords: pregnancy, parenthood, young survivors, breast cancer, systematic review
Introduction
The potential impact of breast cancer on the reproductive health of young women has become a frontline research topic in oncology and a matter of utmost importance in clinical settings. This is broadly motivated by four primary factors. First, breast cancer is the most common type of malignancy in reproductive aged women. It accounts for ∼45% of all female cancers in women aged 25–49 years (Cancer Research UK, 2012). Breast cancer in younger women tends to be more aggressive and results in poorer prognostic outcomes (Hillner et al., 1996; Pronzato et al., 2011). Given this scenario, adjuvant chemotherapy is commonly part of the treatment plan. Although it enhances survival, the toxic effect of chemotherapy often impairs fertility, temporary or permanently (Tschudin and Bitzer, 2009), or causes premature ovarian failure due to massive destruction of the ovarian reserve (Maltaris et al., 2006). Additionally, women are commonly advised to delay pregnancy for at least 2 years after the diagnosis, given that the majority of recurrences occur within this time range (Lawrenz et al., 2011). Furthermore, women undergoing endocrine therapy, which commonly continues for at least 5 years and during which time a pregnancy is not recommended, may face a decrease in fertility due to age-related factors (Hickey et al., 2009). Therefore, young women with a diagnosis of breast cancer face the uncertainty of whether they have the ability to become pregnant after cancer treatments. Current evidence supports that childbearing after breast cancer is not contra-indicated (Hickey et al., 2009; de Bree et al., 2010; Pagani et al., 2011). However, individualized counselling is warranted, regarding prognosis and risk of relapse based on age and cancer pathological features, before patients can make informed reproductive decisions (Banks and Reeves, 2007; Hickey et al., 2009). Second, as a result of the improvement of screening procedures and advances in treatment, there is a growing number of survivors at childbearing age who have not yet begun or completed their families. Consequently, the issue of pregnancy and parenthood is of utmost importance. In clinical practice, gynaecologists and oncologists are frequently faced with the issue of educating women about childbearing options after breast cancer. However, some studies suggest that these professionals often feel discomfort and a lack of knowledge about how best to educate women with cancer-related fertility matters (Duffy and Allen, 2009), leaving women's fertility concerns poorly addressed (Partridge et al., 2004). One reason is that providers may perceive the importance of fertility as low compared with treating the cancer (Vadaparampil et al., 2008). Additionally, younger and premenopausal survivors often report higher levels of distress and lower quality of life (QOL) than older survivors (Wenzel et al., 1999; Ganz et al., 2003; Kornblith et al., 2007; Rosen et al., 2009). Younger women face unique physical and psychological challenges with breast cancer, including but not limited to reproductive difficulties and concerns (Mor et al., 1994; Dunn and Steginga, 2000; Avis et al., 2004; Partridge et al., 2004; Schover, 2005; Connell et al., 2006; Partridge et al., 2008). For some young survivors, the threat to their childbearing plans has major psychosocial and developmental consequences (Schover, 2005; Camp-Sorrell, 2009). Thus, as treatments improve and mortality declines, the ability to retain reproductive potential is becoming a major factor that women may experience during survivorship and a determinant QOL factor in an increasing number of survivors (Dunn and Steginga, 2000; Maltaris et al., 2006; Camp-Sorrell, 2009; Hickey et al., 2009). Finally, the advent of advanced assisted reproductive technology within the oncology field has made fertility preservation an option for women, prior to the initiation of treatment.
Literature and clinical practice demonstrate that some women remain fertile and have become pregnant after a history of cancer. Additionally, there are an increasing number of options to resolve fertility complications (Dow and Kuhn, 2004; Quinn et al., 2010; Quinn and Vadaparampil, 2013). However, these methods are not always affordable due to time constrictions or personal finances, and success is not guaranteed (Tschudin and Bitzer, 2009). Other options are available to women who could not preserve fertility prior to treatment or for whom these efforts were unsuccessful, such as adoption and third-party reproduction for those who are unable to have biological children. However, a history of cancer may preclude some women from qualifying for adoption and third-party reproduction may be unaffordable or unacceptable to some women (Rosen et al., 2009). Reproductive decisions are complex and become more complicated with a breast cancer diagnosis, given the level of uncertainty associated with this disease (Knobf, 2006). Decisions encompass a combination of several factors that go beyond medical and physical aspects. Among those, there is the interplay of personal and psychosocial factors (Bekker et al., 1999). In this context, it is paramount to understand how the experience of breast cancer shapes and influences (or not) the desire of survivors to have children. Insight into childbearing attitudes and desire for parenthood among this population will improve our ability to provide the needed educational and psychosocial support services and will aid clinicians to better assist women with their fertility-related decisions.
The purpose of this review is to highlight what is known to date about childbearing and parenthood attitudes and decisions of young breast cancer survivors from their own perspective.
Methods
Search strategy and study selection
A systematic review was conducted taking into consideration the Preferred Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines (Moher et al., 2009). Peer-reviewed journals were searched in PubMed from 1 January 1990 to 31 October 2012, limiting the search to human females. The search was conducted using combinations of these phrases or keywords: ‘breast cancer survivors OR breast cancer survivorship’ AND ‘fertility OR reproductive issues OR childbearing OR pregnancy OR parenthood’. An identical search was replicated using PsychInf and CINAHL databases. The following selection criteria were then applied: studies conducted exclusively with young female breast cancer survivors; outcome variables being childbearing, fertility, pregnancy and parenthood attitudes/decisions after breast cancer from the women's perspective (studies focusing on biological or medical outcomes were excluded); studies published in the English language and primary research (review articles, conference abstracts, editorials, commentaries, correspondence or case reports were excluded). The definition of ‘young’ women is not consistent in the literature (Northouse, 1994; Peate et al., 2009; Beadle et al., 2011). In this review, young refers to women who were premenopausal or younger than 50 years of age. The definition of ‘survivors’ is also not consistent. Some research defines a woman as a survivor from the day of diagnosis (American Cancer Society, 2010), while others refer to a survivor as one who has completed treatment (Hewitt et al., 2005) and still others denote survivors as those who are 5 years post-treatment with no evidence of disease (Leigh, 1996). For the purpose of this study, we refer to women who, at the time of the study analysis, were not receiving treatment (except endocrine therapy) and were disease free. Mixed methods, and exclusively quantitative and qualitative studies are included in this review. Studies were screened and selected for inclusion in the review according to the inclusion criteria. Articles were initially screened based on title and type of article. Abstracts of articles potentially meeting the inclusion criteria were screened for eligibility. When abstracts appeared to meet the criteria, full articles were read. Those that met all the inclusion criteria were included in the review. The search was complemented by manually searching the reference lists of studies identified in these electronic databases. Reference lists of review articles focusing on fertility issues on this targeted population were also examined (Peate et al., 2009; Tschudin and Bitzer, 2009; Adams et al., 2011).
Data synthesis
Eligible studies were classified into three categories: quantitative studies, qualitative studies and studies using mixed methods. Data extracted from each eligible study were recorded in three standardized tables. Key information was collected on the study origin, aims, inclusion criteria, sample, study design, relevant measures/data collection and relevant findings. The three authors (V.G., I.S. and G.Q.) individually extracted data from the papers into a template containing key information for each study. The data were then discussed to ensure congruence of information extracted.
Results
Search results
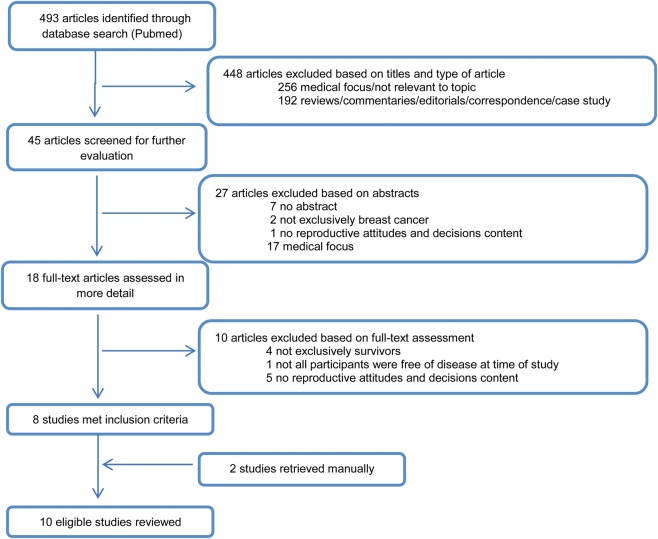
The initial search using PubMed resulted in 493 articles, 8 of which met all the inclusion criteria and were included in the review. Searches were also carried out within PsychInfo and CINAHL databases to ensure all potential papers were reviewed. These searches did not yield any new relevant papers. Therefore, the summary of the search strategy is only presented for searches conducted in Pubmed (Fig. 1). Additionally, two articles were obtained by manually searching the reference lists of studies identified in the electronic databases. A total of 10 articles were included in the final review (Fig. 1). Among those, there were three quantitative studies, five qualitative studies and two used mixed methods.
Figure 1.
Flow diagram illustrating the search strategy used to include studies in the review.
Overview of the included studies
Quantitative studies
Three studies provided women's views, attitudes and decisions towards pregnancy and parenthood after breast cancer in a quantitative format (Table I) (Ganz et al., 2003; Gorman et al., 2010; Ruddy et al., 2011). However, none of these studies had attitudes and/or decisions towards pregnancy and parenthood after breast cancer as the main outcomes. All the studies were multicentre studies, which were conducted in the USA. In total, 728 young survivors participated. The sample size exceeded 100 participants in two studies (Ganz et al., 2003; Gorman et al., 2010). All the studies assessed early-stage young survivors; however, participants in the Ruddy et al. (2011) study were on average older. Ganz et al. (2003) also presented data for four different age groups (25–34; 35–39; 40–44 and 45–51). Gorman et al. (2010) failed to provide information on participants' age range. Only one study compared a sample of survivors with healthy controls, matched on age and gravidity (Ruddy et al., 2011). The length of time since diagnosis varied among the studies. In the Ganz et al. (2003) study, survivors were on average 6 years post-diagnosis, while survivors in the Gorman et al. (2010) study were on average 12 years post-diagnosis. Ruddy et al. (2011) failed to display the range of length of time since diagnosis. These authors only stated that 55% of their sample was ≥3 years post-diagnosis, failing to provide this variable range (Ruddy et al., 2011).
Table I.
Quantitative studies included in the review.
| Study, origin | Aims | Inclusion criteria | Sample | Study design | Relevant measures | Relevant findings |
|---|---|---|---|---|---|---|
| Ruddy et al. (2011), USA | Assessment of menopausal symptoms and infertility concerns | BC survivors: (i) ≤40 years diagnosis; (ii) BC ≥ 1 from diagnosis; (iii) history of chemotherapy treatment for stage I–IIIa BC; (iv) premenopausal; (v) no history of infertility or infertility treatment at diagnosis; (vi) not receiving gonadotrophin-releasing hormone-agonist therapy; (vii) no evidence of recurrence at time of survey Controls: (i) <43 years; (ii) reported menses at least every 2 months; (iii) no current use of hormonal contraception; (iv) absence of comorbidity likely to affect fertility; (v) no history of invasive cancer, previous cytotoxic chemotherapy, tamoxifen use or known infertility or infertility treatment |
n = 40 (20 survivors; 20 controls) Mean age : 36.8 years (survivors + controls) (range 31–42 years) (SD not stated) 55% survivors ≥3 years from diagnosis 30% took steps to minimise infertility 80% survivors chemotherapy interrupted their menses Race/ethnicity: Survivors group 16 (80%) White 1 (5%) African American/black 0 (0%) Hispanic 2 (10%) Asian 0 (0%) Other (Latina) 1 (5%) Other (West Indian) Control group: 10 (50%) White 4 (20%) African American/black 4 (20%) Hispanic 1 (5%) Asian 1 (5%) Other (Latina) 0 (0%) Other (West Indian) |
Cross sectional Survivors compared with healthy age- and gravidity matched controls |
Fertility Issues Survey | 16 survivors and 10 controls desired a future child. This desire was associated with greater fertility concerns in both survivors and controls (trend statistically significant in survivors (P = 0.01) Among women in both groups who desire a future child, survivors had more fertility concerns than controls (P = 0.005). |
| Gorman et al. (2010), USA | Investigate whether the level of reproductive concerns after treatment is associated with long-term depressive symptoms | (i) ≤40 years diagnosis, (ii) had treatment for early-stage (I-IIIa) BC within previous 4 years; (iii) completed treatment with no evidence of recurrent disease; (iv) no other cancer within 10 years of study enrolment Participants recruited from the WHEL Study for the WHEL Survivorship Study |
n = 131 Mean age at diagnosis: 36.7 years (SD not stated) Participants completed the Survivorship Study 11.9 years (SD = not stated) post-diagnosis at 49.1 years (SD = not stated) Race/ethnicity: 115 (87.8%)White 16 (12.2%) Other |
Follow-up study | CES-Dsf Depressive symptoms measured at baseline, 1, 2, 3, 4, 6 years of WHEL study and Survivorship Study enrolment Reproductive Concerns Scale Assessed at Survivorship Study enrolment |
27.1% (36/131) wanted a child post-diagnosis 48.1% possibly wanted a (another) child pre-diagnosis 18% wanted a child after and before diagnosis but were nulliparous at the time of Survivorship study 52.7% (69/131) did not avoid pregnancy after treatment. Of those, 22% (n = 15) were post-menopausal at WHEL Study entry, which may have influenced their reports. 7% (9/131) attempting pregnancy after treatment High reproductive concerns group were more likely to choose treatment based on fertility preservation (P = 0.05). Larger proportion of those in high concerns group wanted children pre- (P < 0.0001) and post-diagnosis (P = 0.01). Greater reproductive concerns predicted higher levels of depressive symptoms (SE = 0.02, P = 0.04) Depressive symptoms not significantly associated with having child after cancer |
| Ganz et al. (2003), USA | Evaluate QOL and reproductive health outcomes in younger female BC survivors CAMS: main findings of the survey phase |
(i) Diagnosis with first invasive or non-invasive BC (ductal carcinoma in situ) at 50 years of age or younger; (ii) alive and disease free without a recurrence; (iii) no cancer before BC; (iv) stages 0, I or II disease; (v) living in the USA; (vi) ability to read and understand English Recruited 2–10 years after diagnosis |
n = 577 (56% response rate) Mean age at diagnosis: 43.6 years (range: 25–51) (SD not stated) Mean age of study: 49.5 years (range: 30–62) (SD not stated) Mean time since diagnosis: 5.9 years (SD = 1.5) Race/ethnicity: 405 (70.2%) White 67 (11.6%) African American 42 (7.3%) Hispanic 49 (8.5%) Asian 14 (2.4%) Other |
Cross-sectional study | Several questions on reproductive history | 65% (373/577) had at least one live birth 5% (17/373) reported pregnancy and live birth after BC 11% (n = 61) considered getting pregnant since BC. From this:
15% still considering pregnancy but undecided |
BC, breast cancer; SD, standard deviation; SE, standard estimate; WHEL, Women's Healthy Eating and Living; CES-Dsf, Centre for Epidemiologic Studies Depression Scale; QOL, quality of life; CAMS, Cancer and Menopause Study.
The criteria used to methodologically appraise the studies were based on those suggested in the literature on quantitative research (Sanderson et al., 2007; National Collaborating Centre for Methods and Tools, 2008; Jack et al., 2010). The study design was identified and appropriately applied in each study evaluated. Two studies used a cross-sectional design (Ganz et al., 2003; Ruddy et al., 2011) and one study was a follow-up study (Gorman et al., 2010). Low response rates may potentially lead to selection bias and may compromise the representativeness of the sample. Particularly, in the Ganz et al. (2003) and Gorman et al. (2010) studies, there were significant differences between respondents and non-respondents with regard to some characteristics. Ruddy et al. (2011) did not provide details regarding the recruitment of participants for the study. The authors simply suggested that possibly respondents and non-respondents may vary regarding fertility concerns and menopausal symptoms. In addition, in this study, survivors and controls differed significantly on marital status and education and, at a trend level, on menstrual frequency. The measures used were valid and reliable in the three studies; however, none employed a measure specifically devised to assess attitudes and decisions in this group of survivors.
Qualitative studies
Five studies provided data on young survivors' pregnancy and parenthood attitudes and decisions after breast cancer (Table II) (Dow, 1994; Siegel et al., 1997; Connell et al., 2006; Gorman et al., 2011; Lee et al., 2011). Only three studies reported experiences and perceptions related to pregnancy and having children after breast cancer (Dow, 1994; Siegel et al., 1997; Connell et al., 2006), while the other two studies focused mainly on how health services offered had impacted women's experiences with breast cancer (Lee et al., 2011) and the role of fertility in their cancer treatment decisions (Gorman et al., 2011). Three studies were completed in the USA (Dow, 1994; Siegel et al., 1997; Gorman et al., 2011), while the other two were conducted in Australia and the UK, respectively (Connell et al., 2006; Lee et al., 2011). Four studies used cross-sectional designs (Dow, 1994; Siegel et al., 1997; Gorman et al., 2011; Lee et al., 2011) and one study was best described as longitudinal (Connell et al., 2006). Survivors were recruited from varied contexts, including organizations (Siegel et al., 1997; Gorman et al., 2011), cancer centres (Dow, 1994; Siegel et al., 1997; Lee et al., 2011), fertility services (Lee et al., 2011), breast cancer support groups (Connell et al., 2006), breast cancer events (Connell et al., 2006) and other studies, such as the Women's Healthy Eating and Living (WHEL) study (Gorman et al., 2011). In the study by Connell et al. (2006) participants were selected from the 35 women who participated in the baseline interview on the basis that their greatest unmet need was related to support. Participants in the Dow (1994) study were restricted to women who became pregnant after breast-conserving surgery and radiation therapy and Siegel et al. (1997) only included women who were deciding whether to attempt pregnancy (or had actively considered it and had made a decision within the previous 3 years) and believed that they still had the capacity to become pregnant at the time of the study. Gorman et al. (2011) included participants who had at least one biological child after treatment (50% of the sample) in order to ensure the inclusion of women interested in fertility. The other researchers did not consider pregnancy status as a condition to include women in their studies. In total, 178 survivors participated in these studies. Their ages at diagnosis in the five studies varied from 22 to 44 years. The length of time since diagnosis among the four studies ranged from 5 months to 13 years (Siegel et al., 1997; Connell et al., 2006; Gorman et al., 2011; Lee et al., 2011). The length of time since diagnosis was not provided in the Dow (1994) study.
Table II.
Qualitative studies included in the review.
| Study, origin | Aims | Inclusion criteria | Sample | Study design, data collection | Relevant findings |
|---|---|---|---|---|---|
| Gorman et al. (2011), USA | To explore how young women make cancer treatment decisions and role of fertility concerns in that process | (i) Early-stage BC survivors (stage I or II); (ii) ≤40 years at diagnosis |
n = 20 Age range: 26–38 years (means, SD, medians not stated) 7 women <30 years 10 women 30–34 3 women ≥35 Length time since diagnosis: range 1–13 years (means, SD, medians not stated) Participants recruited from the WHEL study and YSC To ensure the inclusion of women interested in fertility, half of those in the sample had at least 1 biological child after treatment Race/ethnicity: 17 (85%) White 3 (15%) Hispanic |
Cross sectional In dept telephone semi-structured interviews Cross-case analysis |
Fertility largely viewed as secondary to the importance of survival/preventing future recurrence Most women interested in possibility of having children post-treatment At the time of interview: 2 unable to have children; 1 trying pregnancy; 4 open to a possible pregnancy; 3 decided against having children due to older age, life circumstances, concerns about recurrence, the baby's health and passing on genetic risk; 2 considering adoption Themes: ‘I was young, I wanted to do everything possible to move forward with my life and not have the cancer come back’, ‘fertility concerns are different for every woman’, ‘my oncologist was great … a huge part of my survivorship’, ‘they did not tell me about my options, and I didn't think about fertility until it was too late’ |
| Lee et al. (2011), UK | Women's reactions to finding out that cancer treatment could affect fertility and how interactions with health services impacted on their ability to deal with this effectively | (i) History of early-stage BC; (ii) ≤40 years at diagnosis; (iii) fluency in English |
n = 24 Median age: 32 years (range: 23–39) Median length time since diagnosis: 32 months (range 7–72 months) No women of ethnic minorities recruited (although researchers approached some) |
Cross sectional 3 focus groups using flexible interview structure Thematic analysis |
Fertility was important; however; survival was priority over having children With the exception of 3 women, not having children would be better than the detrimental effects of a child not having a mother The majority of women thought fertility choices affected their risk of recurrence Themes: ‘survival a priority’, ‘perceived risks to survival’, ‘advice from health professionals’, ‘denial of choice’, ‘women with children neglected’, ‘role of services’, ‘written information provision’, and ‘timing of discussions’ |
|
Connell et al. (2006), Australia |
Explore young women's issues and concerns over time Focus on changing views of reproductive issues over a period of 12–18 months |
(i) Adult female with BC; (ii) ≤40 years at diagnosis; (iii) English speaking; (iv) diagnosis ≤4 years ago; (v) not suffering extreme distress or not in palliative care or not all of these |
Phase I (baseline): n = 35 FU women: n = 13 Median age diagnosis (FU) = 37 years (range 29–40 years) Median time since diagnosis = 26 months (range 5–37 months) Ethnicity not specifically recorded. Authors stated that participants were representative of mainstream Australia (i.e. 80% of Australian population's ancestry is Australian, English and Irish) |
Longitudinal study (data collected 3 times over a 12–18 months period) Home or telephone one-to-one semi-structured interviews (∼6 months apart) Analytical analysis |
Women's perspectives on fertility change over time Mixed desire about pregnancy/wanting children Some women expressed fear of recurrence after pregnancy |
| Siegel et al. (1997), USA | Women's perceptions of the possible risks and benefits of having a baby after breast cancer Gain insights into factors that influenced women's decision-making concerning pregnancy |
(i) Completed treatment for BC ≥ 6 months before study; (ii) <46 years; (iii) black or white non-Hispanic; (iv) be currently deciding whether to attempt pregnancy (or had actively consider it and had made a decision within the previous 3 years); (v) believe that they still have capacity to become pregnant |
n = 50 Median age: 33.4 years at diagnosis (range: 22–44 years) Median length of time since diagnosis: 33 months (range: 8 months-8 years) Race/ethnicity: 42 (84%) White non-Hispanic 8 (16%) Black |
Cross sectional In-depth focused interview, most interview questions open-ended Content analysis |
7 women pregnant since diagnosis (10 pregnancies in total) Most of sample contemplating pregnancy after BC, but not yet attempted to conceive Longer length time since treatment, more women were willing to get pregnant Deterrents for pregnancy (risks/dangers): ‘Fear their disease would recur’, ‘Fear the child would have birth defect because of the cancer treatment’, ‘Fear their child would be born with a greater susceptibility to cancer’; ‘Concerns that caring for a child would be too stressful’ Incentives for pregnancy: ‘Having a baby is a cherished part of a life plan’; ‘Having a baby is life affirming’; ‘Having a child will promote a feeling of normalcy’, ‘Having a baby would give her husband something that would make him very happy’; ‘Having a child would improve their quality of life’ |
|
Dow (1994), USA |
Identify reasons why young women with BC decide to become pregnant Describe concerns about subsequent pregnancy Describe helpful behaviours in decision-making Explore the meaning of having children after BC |
(i) Early-stage BC; (ii) pregnancy after breast-conserving surgery and radiation therapy |
n = 16 Mean age at diagnosis: 29.6 years at diagnosis (SD not stated) (range: 25–35 years) Mean age at interview: 38.8 years at diagnosis (SD not stated) (range: 32–45 years) Race/ethnicity: 15 (94%) White 1 (6%) Greek |
Cross sectional Semi-structured interview Content analysis |
Reasons for pregnancy related to women's developmental age Concerns related to disease recurrence, breast self-examination and mammography during pregnancy and about surviving to see their children grow up 3 themes related to future children: ‘anchoring’, ‘getting well again’, ‘feeling complete’ |
BC, breast cancer; SD, standard deviation; RCT, randomized control study; SPIRIT, Sisters Peer Counselling in Reproductive Issues after Treatment; WHEL, Women's Healthy Eating and Living; YSC, Young Survival Coalition; FU, follow-up.
There is less agreement on the most appropriate method for appraising qualitative research (Walsh and Downe, 2006). The rigour of the articles included in this review was critically evaluated using criteria for reporting qualitative research (Côté and Turgeon, 2005; Tong et al., 2007; Kuper et al., 2008). The five studies included had clearly described aims and objectives and their proposed research questions were suitable to qualitative methods. Details about sampling, data collection and analysis procedures were clearly provided in the most recent studies (Connell et al., 2006; Gorman et al., 2011; Lee et al., 2011). Data were collected by focus groups (Lee et al., 2011) or individual in-depth interviews, using semi-structured interview guides or open-ended questions (Dow, 1994; Siegel et al., 1997; Connell et al., 2006; Gorman et al., 2011). Two studies used content analysis (Dow, 1994; Siegel et al., 1997), while the other three used analytical analysis, cross-case analysis and thematic analysis, respectively (Connell et al., 2006; Gorman et al., 2011; Lee et al., 2011). In each of the five studies, the main findings were clearly presented. Details about reliability and validity of the results (member-checking, etc.) were provided solely in two studies (Connell et al., 2006; Lee et al., 2011). Four studies provided conclusions representing synthesis of the results, in which study limitations and avenues for further research and implications for practice were identified (Dow, 1994; Connell et al., 2006; Gorman et al., 2011; Lee et al., 2011). None of the studies addressed the researchers' reflexive practice, a suggested tool for improving the validity of the reported results.
Studies using mixed methods
Two studies addressed women's views, attitudes and decisions towards pregnancy and parenthood after breast cancer using mixed methods (Table III) (Braun et al., 2005; Lewis et al., 2012). Braun et al. (2005) conducted a multicentre cross-sectional study in Israel. They compared a sample of 30 survivors and their husbands (n = 13) on positive and negative motivations towards childbirth with a sample of 29 healthy controls and their husbands (n = 15). The mean age of survivors in the study was 35 years (age range not reported) and the controls' mean age was 31.7 years (age range not reported). The length of time since cancer treatment ranged from 2 to 5 years. Besides other inclusion criteria (Table III), the sample was limited to women without children or with no more than two children (less than the Israeli average). There was a potential selection bias due to significant differences among survivors and controls, with respect to women's age and religiosity. Data were collected by means of a self-report questionnaire containing a qualitative and quantitative section. The measures included in the quantitative portion had established validity and reliability. The authors reported using content analysis to analyse the qualitative data. Lewis et al. (2012) provided cross-sectional data on survivors' psychosocial concerns from the randomized control study SPIRIT (Sisters Peer Counselling in Reproductive Issues after Treatment) counselling programme (Schover et al., 2011). This study included 33 African American survivors with a mean age of 37.39 years at diagnosis (range 25–45 years). The length of time since diagnosis was not reported. The authors suggested that study participants' were likely to be more educated, more liberal and more informed about breast cancer than the average African American survivors. Data were collected by conducting semi-structured telephone interviews that included questions regarding fertility issues. Qualitative analysis included content analysis, while quantitative analysis encompassed descriptive statistics of the interview data. The authors did not report on any psychometric properties of the questions/items within the interview guide (which would be a suggested practice for quantifying qualitative data). Both studies provided information on triangulation of the data, but failed to address researchers' reflexive practice.
Table III.
Studies using mixed methods included in the review.
| Study, origin | Aims | Inclusion criteria | Sample | Study design, data collection |
Related findings |
|---|---|---|---|---|---|
| Lewis et al. (2012), USA | To understand psychosocial concerns of young African American BC survivors | (i) Self-identification as African American; (ii) ≥1 year post-diagnosis; (iii) absence of active treatment other than hormonal therapy; (iv) adequate English skills; (v) ≤45 years at diagnosis |
n = 33 Mean age at diagnosis: 37.39 (SD = 6.00) (range 25–45 years) Mean age at interview: not stated Length time since diagnosis: not stated Race/ethnicity: 33 (100%) African American |
Cross-sectional data from the RCT SPIRIT counselling programme Semi-structured telephone interview after participants completed the 1-year follow-up assessments at SPIRIT |
55% had at least 1 child at cancer diagnosis 45% wanted a child at cancer diagnosis (retrospectively) 48% did not recall discussing infertility with medical team 14% (3/22 who had chemotherapy) were offered option to preserve fertility 4 became pregnant after cancer treatment 1 sought infertility treatment, at the time of interview was trying IVF 2 adopted children after cancer |
| Braun et al. (2005), Israel | Positive and negative motivations towards childbirth of breast cancer survivors and their husbands | Survivors (study group): (i) BC at Stages I–III, without metastasis; (ii) treatment completed at least 2 years prior to the study; (iii) in remission, since then or with only local recurrence; (iv) without other physical or mental illness; (v) ≤40 years old; (vi) premenopausal or no indication of being menopausal; (vii) without children or ≤2 (less than the Israeli average); (viii) Jewish, Hebrew speaking and living in Israel more than 10 years Controls: (i) without other physical or mental illness; (ii) ≤40 years old; (iii) premenopausal or no indication of being menopausal; (iv) without children or ≤2; (v) Jewish, Hebrew speaking and living in Israel more than 10 years |
Study group: recruitment in 3 hospitals Control group (healthy women), convenience sample recruited from the community, hospitals and university n = 30 survivors + 13 survivors' husbands Survivors group: 35 years mean age (SD = 3.1) Survivors group (husbands): mean age of 38.2 years (SD = 2.9) Treatment completed: range 2–5 years before study n = 29 controls + 15 controls' husbands Control group: mean age of 31.7 years (SD = 3.9) Control group (husbands): mean age of 35.2 years (SD = 4.9) Details on race/ethnicity not reported |
Cross-sectional study BC survivors and husbands compared with healthy women and their husbands Data collection: (1) Qualitative component: participants list 3 reasons against and in favour of having children (2) Quantitative component : PMQ-R EMS BSI IES MAC |
Quantitative data: No differences between groups about negative and positive motivations No differences between groups in the desire for children Survivors desired less number of children than controls (F(1,49) = 4.86, P < 0.05) Survivors' husbands desired less number of children than controls (F(1,25) = 16.67, P < 0.001) Qualitative data: Positive motivations BC survivors: Immortality, big family, siblings for their existing children, happiness and giving meaning to life BC survivors' unique positive motivations compared with controls: desire to have siblings for their existing children and strong desire to have a big family. Happiness was less reported compared with controls Negative motivations BC survivors: Mother's health, child's health, financial concerns, personal restrictions and world view. BC survivors unique negative motivations compared with controls: concerns over mother's health (most reported motivation). Concerns over child's health were more reported and pessimistic world view, personal restrictions and immaturity were less reported than in controls Positive motivations not correlated with mental distress. Negative motivations correlated with mental distress only in husbands of BC survivors (r = 0.66, P < 0.05) |
| BC did not impede overall positive motivations, nor increased negative motivations towards childbirth |
BC, breast cancer; SD, standard deviation; PMQ-R, parenthood motivation questionnaire revised; EMS, ENRICH Marital Satisfaction Scale; BSI, Brief Symptom Inventory; IES, Impact of Event Scale; MAC, Mental Adjustment to Cancer Scale.
Young survivors' attitudes and decisions towards pregnancy and having children after breast cancer
Collectively, the studies reviewed documented that childbearing is an important issue for many young survivors. However, in general, there were mixed views and perceptions about this issue.
Positive attitudes
Some findings demonstrated that survivors viewed pregnancy and parenthood positively (Siegel et al., 1997; Braun et al., 2005; Connell et al., 2006; Gorman et al., 2011; Ruddy et al., 2011; Lewis et al., 2012). Two studies compared survivors with healthy controls (Braun et al., 2005; Ruddy et al., 2011). In the Ruddy et al. (2011) study, 16 survivors (n = 20) and 10 age- and gravidity-matched controls (n = 20) desired a future child. The desire to have a child in the future was associated with more fertility concerns in both survivors and controls, though this trend was only significant for survivors. The study conducted by Braun et al. (2005) did not find differences between survivors and their husbands in either the survivor or control group regarding their desire for children. However, survivors and their husbands desired significantly fewer children than controls (Braun et al., 2005). Some qualitative studies suggested that most of women in their samples were interested in having children after treatment (Siegel et al., 1997; Gorman et al., 2011) and were delighted when pregnancies occurred (Connell et al., 2006). In the Gorman et al. (2011) study, all women who were childless (n = 10) reported possibly (n = 9) or definitely (n = 1) wanting a child after treatment. At the time of the study, two women were not able to have a child, four were still welcoming that possibility, one was trying to become pregnant, three decided not to have children and two were considering adoption (but none had adopted a child). Lewis et al. (2012) reported that almost half of their sample desired children at diagnosis; however, few women became pregnant post-treatment or pursued means to become a parent (adoption or fertility preservation techniques). This was mostly attributed to cultural stigma related to African Americans for whom the importance of fertility was often dismissed by health professionals (Lewis et al., 2012).
Negative attitudes
Two qualitative studies showed that the issue of pregnancy and parenthood after breast cancer may also evoke negative views and attitudes in some survivors (Connell et al., 2006; Lee et al., 2011). In the Connell et al. (2006) study, some participants who became pregnant during the study considered terminating the pregnancy. In another study, with the exception of three women, participants felt that not having a child would be better than the negative effects of a child not having a mother. These women's thoughts about childbirth were influenced by their perceptions about survival risks, which appeared to be increased by conflicting views and advice given to them by different health professionals. Women who were childless were more likely to prioritize fertility over survival (Lee et al., 2011). Following this line, quantitative findings showed that although some survivors reported having children or hoping to have children after treatments, several decided against becoming pregnant. In the Gorman et al. study (Gorman et al., 2010), 27% of participants (36/131) wanted a child (or more children) after diagnosis, while a larger proportion (48%) stated wanting a child (or more children) before diagnosis. Additionally, 53% (69/131) did not avoid pregnancy after treatment; however, as researchers noted, 22% (15/131) of those were post-menopausal at the study entry, which might have introduced bias in this report. Only 7% of women in this study (9/131) reported trying to become pregnant after treatment. Another study investigated QOL and health outcomes, with a specific focus on the reproductive and late health effects of treatment, in a large cohort of 577 survivors (Ganz et al., 2003). Reproductive details of participants revealed that 373 women (65%) reported at least one live birth but only 5% of these (17/373) had became pregnant and had a child after breast cancer. Of all of the survivors, another 20% reported that they were planning or hoping to have children before diagnosis, whereas only 11% (n = 61) had considered pregnancy since the diagnosis. Among these 61 survivors, some were not planning to become pregnant due to physicians' recommendations (19%), worries about risks (17%) and other reasons mainly related to age and/or personal relationship circumstances (29%, these categories were not mutually exclusive). Only 7% of these women were attempting to become pregnant; 17% had became pregnant and 12% had specific plans or fertility treatments underway (these categories were not mutually exclusive) (Ganz et al., 2003).
Uncertainty about future pregnancy and desiring a child
Mixed perceptions about desiring a child were expressed by some survivors. These were fuelled by thoughts related to their possible shorter lifespan and feelings of guilt and selfishness. Remaining fertile after cancer also triggered anxiety in some women, raising concerns for safe and reliable contraception. Women with a shorter length of time since diagnosis were more likely to be uncertain about their reproductive plans at the time of the study, but wanted to have the option open for the future, while women with a longer time since their diagnosis were more likely to have already made their reproductive choices (Connell et al., 2006). Although fertility and parenthood were important for many survivors, qualitative data showed survival was the main priority when making treatment-related decisions (Connell et al., 2006; Gorman et al., 2011; Lee et al., 2011). Uncertainty regarding whether to have a child or not was captured by Ganz et al. (2003), who reported that 15% of survivors in their study were still considering having a child but had not yet decided.
Pregnancy and parenthood after breast cancer: perceived risks and benefits
Risks
The studies documented some concerns or fears that women held towards becoming pregnant and having children after breast cancer (Dow, 1994; Siegel et al., 1997; Ganz et al., 2003; Braun et al., 2005; Connell et al., 2006; Gorman et al., 2011; Lee et al., 2011; Ruddy et al., 2011). These fears or concerns were the main reason many women decided against having children. One of the most commonly cited fears was that pregnancy may enhance cancer recurrence (Dow, 1994; Siegel et al., 1997; Braun et al., 2005; Connell et al., 2006; Gorman et al., 2011; Lee et al., 2011; Ruddy et al., 2011). Specifically, women were afraid that hormonal changes brought on by the pregnancy could stimulate a cancer recurrence. Fears of recurrence were expressed in relation to women's mortality (Dow, 1994; Siegel et al., 1997; Connell et al., 2006; Gorman et al., 2011) and in the reduction of their survival chances (Lee et al., 2011). Given this concern, women wondered if it was morally right (Siegel et al., 1997) or if they were being selfish by having a child when their mother could experience a premature death (Siegel et al., 1997; Connell et al., 2006). Some participants expressed that even if they did not die after recurrence, it would be a significant emotional burden for a young child to have a mother so seriously ill (Siegel et al., 1997). Furthermore, some women with children felt that they could not jeopardize their current children's QOL by putting their own health at risk (Siegel et al., 1997; Lee et al., 2011). A specific concern expressed by participants was the inability to detect any significant breast changes during pregnancy (Dow, 1994; Connell et al., 2006) and not being able to have mammograms during pregnancy. The only study that compared motivations towards childbirth of survivors and their husbands and controls found that concerns over the mother's health was a negative motivation confined uniquely to the survivors group. This also corresponded to their most frequently reported negative motivation towards childbirth (Braun et al., 2005).
Fears related to the health of a child born after breast cancer were also common (Dow, 1994; Siegel et al., 1997; Braun et al., 2005; Gorman et al., 2011). Women worried that the child might be born with a birth defect because of the chemotherapeutic agents that they received or their previous exposure to toxins and radiation (Dow, 1994; Siegel et al., 1997). Furthermore, women expressed fears that the child would have a greater susceptibility to being diagnosed with childhood cancer or breast cancer as an adult. Several women worried about genetically passing on a general susceptibility to cancer to their offspring. These fears were associated with concerns about whether it would be selfish or morally right to have a child at risk for cancer (Siegel et al., 1997). Dow (1994) found that women who had children after breast cancer became hypervigilant towards their child's health. In contrast to Siegel et al. (1997), these participants did not focus greatly on their children's potential risk of developing cancer. Dow (1994) had advocated that one of the possible reasons for this was poor knowledge about genetic risks of breast cancer at the time of the study. Survivors reported more negative motivations towards childbirth related to concerns over the child's health compared with controls (Braun et al., 2005). Additional concerns included not having a normal pregnancy (Dow, 1994), lacking the energy to provide the level of care and involvement the survivor would desire to offer to her children (Siegel et al., 1997), life circumstances (Gorman et al., 2011), older age (Gorman et al., 2011), financial concerns (Braun et al., 2005), pessimistic world view (Braun et al., 2005) and personal restrictions (Braun et al., 2005). However, when compared with healthy women, survivors expressed less negative motivations due to pessimistic world views, personal restrictions and feelings of immaturity (Braun et al., 2005).
Benefits
Young survivors also perceived important benefits that could be achieved by becoming pregnant and having a child after breast cancer (Dow, 1994; Siegel et al., 1997; Braun et al., 2005; Connell et al., 2006). Many women stated that one of the reasons for desiring a child was that having a baby was a cherished goal of a life plan (Dow, 1994; Siegel et al., 1997). They had a longstanding desire to have a child or family, which was interrupted but not altered by the illness. For those women, this represented an important life goal that they wanted to pursue.
Another potential benefit to having children after breast cancer was that raising a child would be a powerful motivator to stay alive and healthy. Some women expressed that a child would give them more hope and optimism about the future (Dow, 1994; Siegel et al., 1997; Connell et al., 2006). A related benefit would be a desire for a sense of normalcy in their lives achieved through childbirth. Besides giving them the opportunity to reconnect with their peers who were having or raising children (Dow, 1994; Siegel et al., 1997), bearing a child would restore their sense of normal femininity and sexuality, as well as increasing their sense of self-worth (Siegel et al., 1997).
Other motivating factors included the desire to make a husband happy (Siegel et al., 1997), the improvements in survivors' QOL (Siegel et al., 1997), the absence of a compelling reason for not having children (Dow, 1994), symbolic immortality, desire to have a big family, desire to have siblings for existing children, achieving happiness and giving meaning to life (Braun et al., 2005). The desire to have siblings for their existing children was a positive motivation limited uniquely to survivors when compared with controls (Braun et al., 2005). Survivors expressed more positive motivations towards having a big family than controls. In addition, survivors reported the desire to feel happiness less often than healthy women. Survivors were less likely to report the relationship with their husbands as a positive motivation to have children compared with healthy women who did report this desire. Positive motivations towards childbirth were not affected by mental distress (Braun et al., 2005).
Meaning of parenthood after breast cancer
One of the first qualitative studies conducted with a population of breast cancer survivors attempted to collect data on the meaning of having children for young survivors (Dow, 1994). Three themes emerged: anchoring, getting well again and feeling complete. The results showed women perceived having a child would help anchor their lives in a very positive way and would help them focus on their practical daily activities. Further, they perceived a child would help them to stay well, feel complete and look forward to the future, which is the opposite of what survivors reported a cancer diagnosis might mean for them (concerns about dying in the future).
Discussion
This review sought to improve our understanding about pregnancy and parenthood attitudes, views and decisions of young breast cancer survivors by examining quantitative and qualitative findings. Although data on attitudes and intentions do not always reflect actual behaviour, it provides important insights into the thoughts, views and needs of those women. From the studies reviewed, it is apparent that childbearing after breast cancer is an important and sensitive matter for young survivors. Collectively, the study findings reflected survivors' mixed attitudes and perceptions towards this subject. While on the one hand some women welcomed the idea of becoming pregnant and having (more) children, some women were against this idea after experiencing cancer. Other women were unsure and could not make these decisions yet. Fuelling these attitudes and decisions were often women's perceptions about the risks that a pregnancy would carry to cancer recurrence and, ultimately, to their survival. Fear that a possible pregnancy would increase the chance of recurrence was reported even among women who held positive attitudes towards childbirth (Dow, 1994; Connell et al., 2006). However, while some studies reported that pregnancy may mean fear, risks and eventually premature death, other studies supported the view that pregnancy may also mean happiness, normalcy and a fulfilling life. Therefore, pregnancy and parenthood seem to represent, in many respects, the opposite of a cancer diagnosis for several survivors. In fact, the ability to have children after treatment is a concern for many women, even for those who were against having children (Lee et al., 2011).
It has been nearly 20 years since Dow (1994) published one of the first attempts to understand why young women decided to have children after breast cancer. Since then, unfortunately, a limited number of studies have explored this subject. The majority of studies reviewed provided details on pregnancy and parenthood attitudes and decisions, but their main focus was on other health outcomes. This was particularly true for quantitative studies. Most of the data pertaining to attitudes, views and decisions in this review were obtained through qualitative methods. Qualitative methods provide valuable insight and the ability to capture in-depth information about a subject. Given that the literature has now provided a good sense of the survivors' range of attitudes and perceptions on this topic, there is a clear need to expand the literature with quantitative methods using validated and reliable assessments of attitudes in large representative samples of participants. In this review, we were unable to find a study that used a valid and reliable measure to assess pregnancy and parenthood attitudes specifically after breast cancer. Thus, the development of such an instrument would be a highly useful tool for clinicians.
There are additional other relevant methodological limitations to the quantitative studies reviewed. With the exception of the Ganz et al. (2003) study, samples were generally small. In addition, these samples were heterogeneous regarding the length of time since diagnosis or treatment and pregnancy status, which prevented us drawing firm conclusions from their findings. It is recommended that future studies compare groups of survivors by disease stage at diagnosis, age or pregnancy status. Few studies included samples of controls, and the few who did reported problems in matching their samples, which may lead to selection bias. Methodological limitations of the qualitative studies included a lack of information about data trustworthiness and none of the researchers informed readers about their use of reflexive practice. Only one of the studies in this review examined women's perceptions at more than one point in time and it is likely attitudes may fluctuate over the cancer trajectory. Therefore, we suggest that it would be of utmost importance to capture this variability in a prospective longitudinal study. Additionally, most of the literature has been produced in the USA and it is highly likely there are cultural differences in attitudes. More studies are needed with populations of women from other countries. Specifically, as minorities are underreported in existing research, future efforts should be made to include these minority populations and under-served women in their samples. Further, all the studies in this review were retrospective. While it can be ethically challenging to potentially burden women with surveys and requests for interviews at the time of diagnosis and throughout treatment, it is very important to capture the prospective attitudes of women who are newly diagnosed or undergoing treatment. Such data will improve clinicians' ability to provide guidance and education on fertility preservation or other assisted reproductive technologies.
Given the increasing interest and importance surrounding fertility issues in the context of cancer care, conducting a systematic review about childbearing attitudes and decisions among breast cancer survivors is timely and relevant. Our review provides a valuable overview about this topic; however, it has some limitations. First, despite the efforts made during the search process, it is probable that some studies were not identified. Furthermore, only peer-reviewed papers were screened, which excluded unpublished studies and those studies published in non-peer-reviewed journals. Second, we only included studies published in the English language and it is possible important contributions to this field have been published in other languages. Finally, the small number of studies identified for the review and the dominance of studies conducted in the USA are also a limitation of the current body of research. Despite these limitations, our review offers the first systematic compilation of data specifically on this topic, opening avenues to strengthen future research and clinical practice. Consideration of our findings will be essential to develop and improve educational tools and psychosocial support services appropriated to survivors' needs.
Conclusions and implications for clinical practice and future research
Our review highlights the importance of addressing childbearing and parenthood attitudes in young breast cancer survivors. It supports the need for frank discussions among health professionals and women, especially since many attitudes and views may be based on misinformation about pregnancy-associated risks. Women need and want fertility information in a timely manner (Partridge et al., 2004), which may help to ameliorate their fertility concerns (Braun et al., 2005). This may constitute a valuable aid when making informed decisions. Literature advocates that one of the most important factors in the complex process of decision-making in cancer management is the patient preference (Duffy and Allen, 2009; Zafar et al., 2009). Following this line, a true understanding or effort to understand patient's attitudes towards pregnancy and parenthood is a matter of clinical urgency when dealing with fertility issues. This provides the opportunity to deliver the care that fits patients' needs and preferences, their system of belief (Zafar et al., 2009) and ultimately, improve their QOL. Given the limited current body of research, more studies need to be conducted in order to understand, with more depth and precision, women's perceptions on fertility and parenthood after the experience of breast cancer. Knowledge about this topic will remain low unless a framework or theory that attends to the different variables that possibly influence survivors' attitudes and decisions is provided.
The growing interest in research and clinical practice on the application of the use of fertility preservation techniques in oncology patients should take into consideration the value that these patients place on the ability to have and parent a child after cancer (Schover et al., 1999; Zebrack et al., 2004). The value of adoption and third-party reproduction for these survivors has not been thoroughly investigated, although preliminary studies in this field suggest survivors prefer biological children when possible (Schover et al., 1999; Zebrack et al., 2004). The options for having children after breast cancer using adoption, fertility preservation or other types of assistive reproductive technologies involve complex and difficult decisions, in which personal values and cultural and religious preferences play a pivotal role. With regard to fertility preservation techniques, decisions often have to be made quickly, such as whether to preserve oocytes or embryos, and dealing with these complex decisions in the midst of a cancer diagnosis can be overwhelming (Quinn and Vadaparampil, 2013). Decisions about using fertility preservation necessitate a woman be fully informed about potential fertility risks due to treatment. Further, fertility preservation options yield the best results when undertaken prior to cancer treatment but in some cases may require a delay in treatment. In addition, health-care providers may need to inform patients considering fertility preservation about future choices, based on their current decisions such as the fate of unused cryopreserved oocytes or embryos in the event of the survivors death (Quinn and Vadaparampil, 2013).
To finalize, we recommend future larger and more rigorous studies that not only overcome methodological flaws identified in the existing research but also bring new dynamics to this topic, such as for example investigating the need and efficacy for psychosocial interventions that could target women who have positive attitudes towards fertility and parenthood but are unable to conceive. Fertility for breast cancer survivors is a relevant matter. For some women, infertility due to cancer treatments can be a devastating psychological challenge. The importance of identifying women at high risk for distress is a mandatory duty for those involved in their clinical care.
Authors' roles
V.G. conducted the scientific search, data collection and critical appraisal and wrote the report. G.Q. and I.S. assisted with the scientific search, data extraction and manuscript revision. All authors approved the final version of the manuscript.
Funding
This work was supported by funding from the European Union Seventh Framework Programme (FP7/2007-2013) under grant agreement no. PCOFUND-GA-2009-246542 and from the Foundation for Science and Technology of Portugal.
Conflict of interest
The authors state that there are no conflicts of interest.
References
- Adams E, McCann L, Armes J, Richardson A, Stark D, Watson E, Hubbard G. The experiences, needs and concerns of younger women with breast cancer: a meta-ethnography. Psychooncology. 2011;20:851–861. doi: 10.1002/pon.1792. doi:10.1002/pon.1792. [DOI] [PubMed] [Google Scholar]
- American Cancer Society. ACS definition of survivor and caregiver. 2010. http://relay.acsevents.org/site/DocServer/ACS_definition_of_Survivor___Caregiver.pdf?docID=163900 .
- Avis NE, Crawford S, Manuel J. Psychosocial problems among younger women with breast cancer. Psychooncology. 2004;13:295–308. doi: 10.1002/pon.744. doi:10.1002/pon.744. [DOI] [PubMed] [Google Scholar]
- Banks E, Reeves G. Pregnancy in women with a history of breast cancer. BMJ. 2007;334:166–167. doi: 10.1136/bmj.39098.376181.BE. doi:10.1136/bmj.39098.376181.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beadle BM, Woodward WA, Buchholz TA. The impact of age on outcome in early-stage breast cancer. Semin Radiat Oncol. 2011;21:26–34. doi: 10.1016/j.semradonc.2010.09.001. doi:10.1016/j.semradonc.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekker H, Thornton JG, Airey CM, Connelly JB, Robinson MB, Lilleyman J, MacIntosh M, Maule AJ, Michie S, Pearman AD. Informed decision making: an annotated bibliography and systematic review. Health Technol Assess. 1999;3:1–156. [PubMed] [Google Scholar]
- Braun M, Hasson-Ohayon I, Perry S, Kaufman B, Uziely B. Motivation for giving birth after breast cancer. Psychooncology. 2005;14:282–296. doi: 10.1002/pon.844. doi:10.1002/pon.844. [DOI] [PubMed] [Google Scholar]
- Camp-Sorrell D. Cancer and its treatment effect on young breast cancer survivors. Semin Oncol Nurs. 2009;25:251–258. doi: 10.1016/j.soncn.2009.08.002. doi:10.1016/j.soncn.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Cancer Research UK. UK cancer incidence statistics by age. 2012,. 2006–2008 http://info.cancerresearchuk.org/cancerstats/incidence/age/ (30 January date last accessed)
- Connell S, Patterson C, Newman B. A qualitative analysis of reproductive issues raised by young australian women with breast cancer. Health Care Women Int. 2006;27:94–110. doi: 10.1080/07399330500377580. doi:10.1080/07399330500377580. [DOI] [PubMed] [Google Scholar]
- Côté L, Turgeon J. Appraising qualitative research articles in medicine and medical education. Med Teach. 2005;27:71–75. doi: 10.1080/01421590400016308. doi:10.1080/01421590400016308. [DOI] [PubMed] [Google Scholar]
- de Bree E, Makrigiannakis A, Askoxylakis J, Melissas J, Tsiftsis DD. Pregnancy after breast cancer. A comprehensive review. J Surg Oncol. 2010;101:534–542. doi: 10.1002/jso.21514. doi:10.1002/jso.21514. [DOI] [PubMed] [Google Scholar]
- Dow KH. Having children after breast cancer. Cancer Pract. 1994;2:407–413. [PubMed] [Google Scholar]
- Dow KH, Kuhn D. Fertility options in young breast cancer survivors: a review of the literature. Oncol Nurs Forum. 2004;31:E46–E53. doi: 10.1188/04.ONF.E46-E53. doi:10.1188/04.ONF.E46-E53. [DOI] [PubMed] [Google Scholar]
- Duffy C, Allen S. Medical and psychological aspects of fertility after cancer. Cancer J. 2009;15:27–33. doi: 10.1097/PPO.0b013e3181976602. doi:10.1097/PPO.0b013e3181976602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn J, Steginga SK. Young women's experience of breast cancer: defining young and identifying concerns. Psychooncology. 2000;9:137–146. doi: 10.1002/(sici)1099-1611(200003/04)9:2<137::aid-pon442>3.0.co;2-0. doi:10.1002/(SICI)1099-1611(200003/04)9:2<137::AID-PON442>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Ganz PA, Greendale GA, Petersen L, Kahn B, Bower JE. Breast cancer in younger women: reproductive and late health effects of treatment. J Clin Oncol. 2003;21:4184–4193. doi: 10.1200/JCO.2003.04.196. doi:10.1200/JCO.2003.04.196. [DOI] [PubMed] [Google Scholar]
- Gorman JR, Malcarne VL, Roesch SC, Madlensky L, Pierce JP. Depressive symptoms among young breast cancer survivors: the importance of reproductive concerns. Breast Cancer Res Treat. 2010;123:477–485. doi: 10.1007/s10549-010-0768-4. doi:10.1007/s10549-010-0768-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman JR, Usita PM, Madlensky L, Pierce JP. Young breast cancer survivors: their perspectives on treatment decisions and fertility concerns. Cancer Nurs. 2011;34:32–40. doi: 10.1097/NCC.0b013e3181e4528d. doi:10.1097/NCC.0b013e3181e4528d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey M, Peate M, Saunders CM, Friedlander M. Breast cancer in young women and its impact on reproductive function. Hum Reprod Update. 2009;15:323–339. doi: 10.1093/humupd/dmn064. doi:10.1093/humupd/dmn064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillner BE, Penberthy L, Desch CE, McDonald MK, Smith TJ, Retchin SM. Variation in staging and treatment of local and regional breast cancer in the elderly. Breast Cancer Res Treat. 1996;40:75–86. doi: 10.1007/BF01806004. doi:10.1007/BF01806004. [DOI] [PubMed] [Google Scholar]
- Hewitt M, Greenfield S, Stovall E. Committee on Cancer Survivorship: Improving Care and Quality of Life, Institute of Medicine and National Research Council. From Cancer Patient to Cancer Survivor: Lost in Translation. Washington, DC: The National Academies Press; 2005. [Google Scholar]
- Jack L, Jr, Hayes SC, Scharalda JG, Stetson B, Jones-Jack NH, Valliere M, Kirchain WR, LeBlanc C. Appraising quantitative research in health education: guidelines for public health educators. Health Promot Pract. 2010;11:161–165. doi: 10.1177/1524839909353023. doi:10.1177/1524839909353023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knobf MT. The influence of endocrine effects of adjuvant therapy on quality of life outcomes in younger breast cancer survivors. Oncologist. 2006;11:96–110. doi: 10.1634/theoncologist.11-2-96. doi:10.1634/theoncologist.11-2-96. [DOI] [PubMed] [Google Scholar]
- Kornblith AB, Powell M, Regan MM, Bennett S, Krasner C, Moy B, Younger J, Goodman A, Berkowitz R, Winer E. Long-term psychosocial adjustment of older vs younger survivors of breast and endometrial cancer. Psychooncology. 2007;16:895–903. doi: 10.1002/pon.1146. doi:10.1002/pon.1146. [DOI] [PubMed] [Google Scholar]
- Kuper A, Lorelei L, Levinson W. Critically appraising qualitative research. BMJ. 2008;337:a1035. doi: 10.1136/bmj.a1035. doi:10.1136/bmj.a1035. [DOI] [PubMed] [Google Scholar]
- Lawrenz B, Banys M, Henes M, Neunhoeffer E, Grischke EM, Fehm T. Pregnancy after breast cancer: case report and review of the literature. Arch Gynecol Obstet. 2011;283:837–843. doi: 10.1007/s00404-010-1829-y. doi:10.1007/s00404-010-1829-y. [DOI] [PubMed] [Google Scholar]
- Lee RJ, Wakefield A, Foy S, Howell SJ, Wardley AM, Armstrong AC. Facilitating reproductive choices: the impact of health services on the experiences of young women with breast cancer. Psychooncology. 2011;20:1044–1052. doi: 10.1002/pon.1826. doi:10.1002/pon.1826. [DOI] [PubMed] [Google Scholar]
- Leigh SA. Defining our destiny. In: Hoffman B, editor. A Cancer Survivor's Almanac: Charting the Journey. Minneapolis, MN: Chronimed Publishing; 1996. pp. 261–271. [Google Scholar]
- Lewis PE, Sheng M, Rhodes MM, Jackson KE, Schover LR. Psychosocial concerns of young African American breast cancer survivors. J Psychosoc Oncol. 2012;30:168–184. doi: 10.1080/07347332.2011.651259. doi:10.1080/07347332.2011.651259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltaris T, Boehm D, Dittrich R, Seufert R, Koelbl H. Reproduction beyond cancer: a message of hope for young women. Gynecol Oncol. 2006;103:1109–1121. doi: 10.1016/j.ygyno.2006.08.003. doi:10.1016/j.ygyno.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. doi:10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mor V, Allen S, Malin M. The psychological impact of cancer on older versus younger patients and their families. Cancer. 1994;74:2118–2127. doi: 10.1002/1097-0142(19941001)74:7+<2118::aid-cncr2820741720>3.0.co;2-n. doi:10.1002/1097-0142(19941001)74:7+<2118::AID-CNCR2820741720>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- National Collaborating Centre for Methods and Tools. Hamilton, ON: McMaster University; 2008. Quality assessment tool for quantitative studies method. http://www.nccmt.ca/registry/view/eng/15.html. (4 December 2012, date last accessed) [Google Scholar]
- Northouse LL. Breast cancer in younger women: effects on interpersonal and family relationships. J. Nat Cancer Inst Monogr. 1994;16:183–190. [PubMed] [Google Scholar]
- Pagani O, Partridge A, Korde L, Badve S, Bartlett J, Albain K, Gelber R, Goldhirsch A Breast International Group; North American Breast Cancer Group Endocrine Working Group. Pregnancy after breast cancer: if you wish, ma'am. Breast Cancer Res Treat. 2011;129:309–317. doi: 10.1007/s10549-011-1643-7. doi:10.1007/s10549-011-1643-7. [DOI] [PubMed] [Google Scholar]
- Partridge AH, Gelber S, Peppercorn J, Sampson E, Knudsen K, Laufer M, Rosenberg R, Przypyszny M, Rein A, Winer EP. Web-based survey of fertility issues in young women with breast cancer. J Clin Oncol. 2004;22:4174–4183. doi: 10.1200/JCO.2004.01.159. doi:10.1200/JCO.2004.01.159. [DOI] [PubMed] [Google Scholar]
- Partridge AH, Gelber S, Peppercorn J, Ginsburg E, Sampson E, Rosenberg R, Przypyszny M, Winder EP. Fertility and menopausal outcomes in young breast cancer survivors. Clin Breast Cancer. 2008;8:65–69. doi: 10.3816/CBC.2008.n.004. doi:10.3816/CBC.2008.n.004. [DOI] [PubMed] [Google Scholar]
- Peate M, Meiser B, Hickey M, Friedlander M. The fertility-related concerns, needs and preferences of younger women with breast cancer: a systematic review. Breast Cancer Res Treat. 2009;116:215–223. doi: 10.1007/s10549-009-0401-6. doi:10.1007/s10549-009-0401-6. [DOI] [PubMed] [Google Scholar]
- Pronzato P, Mustacchi G, De Matteis A, Di Constanzo F, Rulli E, Floriani I, Cazzaniga ME. Biological characteristics and medical treatment of breast cancer in young women-a featured population: results from the NORA study. Int J Breast Cancer. 2011;2011:534256. doi: 10.4061/2011/534256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn GP, Vadaparampil ST, Jacobsen PB, Knapp C, Keefe DL, Bell GE Moffitt Fertility Preservation Group. Frozen hope: fertility preservation for women with cancer. J Midwifery Womens Health. 2010;55:175–180. doi: 10.1016/j.jmwh.2009.07.009. doi:10.1016/j.jmwh.2009.07.009. [DOI] [PubMed] [Google Scholar]
- Quinn GP, Vadaparampil ST More research, more responsibility. the expansion of duty to warn in cancer patients considering fertility preservation. Am J Obstet Gynecol. 2013 doi: 10.1016/j.ajog.2013.02.031. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Rosen A, Rodriguez-Wallberg KA, Rosenzweig L. Psychosocial distress in young cancer survivors. Semin Oncol Nurs. 2009;25:268–277. doi: 10.1016/j.soncn.2009.08.004. doi:10.1016/j.soncn.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Ruddy KJ, Gelber S, Ginsburg ES, Schapira L, Abusief ME, Meyer ME, Partridge AH. Menopausal symptoms and fertility concerns in premenopausal breast cancer survivors: a comparison to age- and gravidity-matched controls. Menopause. 2011;18:105–108. doi: 10.1097/gme.0b013e3181ef39f8. doi:10.1097/gme.0b013e3181ef39f8. [DOI] [PubMed] [Google Scholar]
- Sanderson S, Tatt ID, Higgins JP. Tools for assessing quality and susceptibility to bias in observational studies in epidemiology: a systematic review and annotated bibliography. Int J Epidemiol. 2007;36:666–676. doi: 10.1093/ije/dym018. doi:10.1093/ije/dym018. [DOI] [PubMed] [Google Scholar]
- Siegel K, Gorey E, Gluhosk V. Pregnancy decision making among women previously treated for breast cancer. J Psychosoc Oncol. 1997;15:27–42. doi:10.1300/J077v15n01_03. [Google Scholar]
- Schover L. Motivations for parenthood after cancer: a review. J Natl Cancer Inst Monogr. 2005;34:2–5. doi: 10.1093/jncimonographs/lgi010. doi:10.1093/jncimonographs/lgi010. [DOI] [PubMed] [Google Scholar]
- Schover LR, Rybicki LA, Martin BA, Bringelsen KA. Having children after cancer: a pilot survey of survivors’ attitudes and experiences. Cancer. 1999;86:697–707. doi: 10.1002/(sici)1097-0142(19990815)86:4<697::aid-cncr20>3.0.co;2-j. doi:10.1002/(SICI)1097-0142(19990815)86:4<697::AID-CNCR20>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Schover L, Rhodes M, Baum G, Adams JH, Jenkins R, Lewis P, Jackson K. Sisters Peer Counseling in Reproductive Issues After Treatment (SPIRIT): a peer counseling program to improve reproductive health among African American breast cancer survivors. Cancer. 2011;117:4983–4992. doi: 10.1002/cncr.26139. doi:10.1002/cncr.26139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care. 2007;19:349–357. doi: 10.1093/intqhc/mzm042. doi:10.1093/intqhc/mzm042. [DOI] [PubMed] [Google Scholar]
- Tschudin S, Bitzer J. Psychological aspects of fertility preservation in men and women affected by cancer and other life-threatening diseases. Hum Reprod Update. 2009;15:587–597. doi: 10.1093/humupd/dmp015. doi:10.1093/humupd/dmp015. [DOI] [PubMed] [Google Scholar]
- Vadaparampil S, Quinn G, King L, Wilson C, Nieder M. Barriers to fertility preservation among pediatric oncologists. Patient Educ Couns. 2008;72:402–410. doi: 10.1016/j.pec.2008.05.013. doi:10.1016/j.pec.2008.05.013. [DOI] [PubMed] [Google Scholar]
- Walsh D, Downe S. Appraising the quality of qualitative research. Midwifery. 2006;22:108–119. doi: 10.1016/j.midw.2005.05.004. doi:10.1016/j.midw.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Wenzel LB, Fairclough DL, Brady MJ, Cella D, Garrett KM, Kluhsman BC, Crane LA, Marcus AC. Age-related differences in the quality of life of breast carcinoma patients after treatment. Cancer. 1999;86:1768–1774. doi:10.1002/(SICI)1097-0142(19991101)86:9<1768::AID-CNCR19>3.0.CO;2-O. [PubMed] [Google Scholar]
- Zafar SY, Alexander SC, Weinfurt KP, Schulman KA, Abernethy AP. Decision making and quality of life in the treatment of cancer: a review. Support Care Cancer. 2009;17:117–127. doi: 10.1007/s00520-008-0505-2. doi:10.1007/s00520-008-0505-2. [DOI] [PubMed] [Google Scholar]
- Zebrack BJ, Casillas J, Nohr L, Adams H, Zeltzer LK. Fertility issues for young adult survivors of childhood cancer. Psychooncology. 2004;13:689–699. doi: 10.1002/pon.784. doi:10.1002/pon.784. [DOI] [PubMed] [Google Scholar]