Abstract
Background
It is well established that tumors are dependent on angiogenesis for their growth and survival. Although uterine fibroids are known to be benign tumors with reduced vascularization, recent work demonstrates that the vasculature of fibroids is grossly and microscopically abnormal. Accumulating evidence suggests that angiogenic growth factor dysregulation may be implicated in these vascular and other features of fibroid pathophysiology.
Methods
Literature searches were performed in PubMed and Google Scholar for articles with content related to angiogenic growth factors and myometrium/leiomyoma. The findings are hereby reviewed and discussed.
Results
Multiple growth factors involved in angiogenesis are differentially expressed in leiomyoma compared with myometrium. These include epidermal growth factor (EGF), heparin-binding-EGF, vascular endothelial growth factor, basic fibroblast growth factor, platelet-derived growth factor, transforming growth factor-β and adrenomedullin. An important paradox is that although leiomyoma tissues are hypoxic, leiomyoma feature down-regulation of key molecular regulators of the hypoxia response. Furthermore, the hypoxic milieu of leiomyoma may contribute to fibroid development and growth. Notably, common treatments for fibroids such as GnRH agonists and uterine artery embolization (UAE) are shown to work at least partly via anti-angiogenic mechanisms.
Conclusions
Angiogenic growth factors play an important role in mechanisms of fibroid pathophysiology, including abnormal vasculature and fibroid growth and survival. Moreover, the fibroid's abnormal vasculature together with its aberrant hypoxic and angiogenic response may make it especially vulnerable to disruption of its vascular supply, a feature which could be exploited for treatment. Further experimental studies are required in order to gain a better understanding of the growth factors that are involved in normal and pathological myometrial angiogenesis, and to assess the potential of anti-angiogenic treatment strategies for uterine fibroids.
Keywords: uterine leiomyoma, angiogenesis, hypoxia, vasculature, anti-angiogenesis
Introduction
Uterine leiomyomas (fibroids or myomas) are the most common tumor of the female reproductive tract, found in up to 70% of Caucasians and 80% of African-American women by the age of 50 (Baird et al., 2003). They are monoclonal tumors, arising from a single smooth-muscle cell (SMC; Bowden et al., 2009). Although the majority of leiomyomas are asymptomatic, up to 20% cause symptoms, which include excessive and irregular bleeding and anemia, pelvic pain, bowel and bladder dysfunction and pain during intercourse (Marsh and Bulun, 2006). Moreover, leiomyomas have been associated with infertility and recurrent miscarriage (Farhi et al., 1995; Eldar-Geva et al., 1998; Surrey et al., 2001). Leiomyomas are a major public health-care burden, being the most common indication for hysterectomy, and were estimated to add 34 billion dollars annually to US health-care expenditures (Farquhar and Steiner, 2002; Cardozo et al., 2012). Despite this, the etiology of these common tumors is still largely unknown and effective treatment strategies are limited by the narrow understanding of the pathogenesis of this disease. Growth of leiomyomas is dependent on gonadal steroids; they are diagnosed only after menarche and they regress after menopause (Okolo, 2008). It is known that there are increased numbers of gonadal steroid receptors in fibroids compared with normal myometrium (Wilson et al., 1980; Tamaya et al., 1985). In addition, black race, age, nulliparity and obesity are risk factors for leiomyoma development (Okolo, 2008).
A genetic predisposition to leiomyomas appears to be present as a familial association has been shown (Vikhlyaeva et al., 1995) and monozygotic twins' concordance for uterine leiomyoma diagnosis is almost twice that of dizygotic twins (Treloar et al., 1998). In addition, rare genetic conditions such as hereditary leiomyomatosis and renal cell cancer (HLRCC) and Alport syndrome development of feature leiomyoma (Launonen et al., 2001; Uliana et al., 2011). Moreover, two recent genome-wide association studies identified several single nucleotide polymorphisms that are significantly associated with uterine leiomyoma diagnosis (Cha et al., 2011; Eggert et al., 2012). Leiomyomas show a very limited malignant potential with transformation to malignancy occurring in <1% of tumors. Cytogenetically, the majority of uterine fibroids (60%) are chromosomally normal and the remainder share similar tumor-specific chromosomal rearrangements that are associated with tumor growth (Levy et al., 2012).
Histologically, leiomyomas are well-encapsulated tissues that consist of SMCs and connective tissue fibroblasts. Recent molecular evidence supports a major role for genes involved in fibrosis and extracellular matrix (ECM) production in the pathophysiology of this disease, as these account for 30% of the altered gene expression between leiomyomas and normal myometrium (Leppert et al., 2006). Although fibroids are clonal in origin, considerable heterogeneity exists and fibroids vary greatly in size, location and appearance even within the same uterus. In addition, fibroids from the same woman grow at very different rates with some regressing, despite a uniform hormonal milieu (Peddada et al., 2008). Moreover, fibroid characteristics such as size, location and ultrasonographic blood flow do not correlate with fibroid growth, uterine bleeding or other symptomatology (Peddada et al., 2008; Tsiligiannis et al., 2013), and further research is needed to identify other biological or molecular factors that determine fibroid behavior.
In recent years, angiogenesis and vascularization have been regarded as crucial factors controlling the growth of tumors (Folkman, 2006). Classical studies suggest that fibroids have an abnormal vasculature and are generally much less vascular than the surrounding myometrium (Sampson, 1912; Faulkner, 1944; Farrer-Brown et al., 1970). This decreased vasculature likely accounts for the severe hypoxia noted in fibroid tissue (Mayer et al., 2008). Moreover, accumulating molecular evidence suggests that expression of multiple angiogenic factors is dysregulated in fibroid tissue. Understanding this vascular dysregulation may help to delineate the pathophysiology of this important disorder, and may lead to innovative targeted treatments.
This article will discuss normal angiogenesis in the uterus as a basis for the molecular understanding of the pathophysiology of leiomyomas. This will be followed by a review of the abnormal vasculature and angiogenic factor dysregulation which characterize leiomyomas when compared with normal myometrium. After reviewing the aberrant hypoxic response that underlies leiomyoma pathophysiology, and how this may be linked to leiomyoma growth and vascularization, we discuss possible therapeutic implications in light of the understanding of leiomyoma vascular biology.
Methods
A comprehensive literature search was performed using PubMed and Google Scholar to identify relevant studies on angiogenic growth factors and myometrium/leiomyoma. The searches included combinations of the following key words: angiogenesis, vasculature, angiogenic growth factors (EGF, HB-EGF, VEGF, bFGF, PDGF, TGF-β and adrenomedullin), myometrium, leiomyoma, leiomyosarcoma, HLRCC, hypoxia, hypoxia-inducible factor-1α, PI3/AKT/mTOR pathway, GnRH agonist, progesterone receptor modulator, uterine artery embolization (UAE), laparoscopic uterine artery occlusion and anti-angiogenic. Bibliographies were cross-referenced to identify additional studies. All relevant articles and additional articles cited in primary references are included.
Angiogenesis in the uterus
Angiogenesis is the formation of new blood vessels from pre-existing ones, and is an essential process for tissue growth and development. Angiogenesis in the adult is usually associated with pathological conditions such as wound healing, retinopathy, ischemia and tumor formation (Torry and Rongish, 1992). The female reproductive tract (i.e. ovary, uterus and placenta) is unique since, in the adult, these tissues normally undergo physiological angiogenesis. These tissues undergo cyclic changes in angiogenesis necessary to supply the nutrients and hormone precursors essential for the establishment and maintenance of pregnancy (Fraser and Lunn, 2000; Smith, 1998). In order to support their rapid periodic growth and regression, equally dynamic microvascular growth and development occur, accompanied by dramatic changes in blood flow to these tissues (Jaffe, 2000; Reynolds et al., 2002).
New blood vessel growth can occur by four different mechanisms in the adult: sprouting, intussusception, vessel elongation and vasculogenesis (Gargett and Rogers, 2001). Classical angiogenesis or sprouting begins with capillary formation and culminates in formation of a new mature microcirculatory bed composed of arterioles, capillaries and venules (Carmeliet, 2003; Fig. 1). The process involves an interaction between the blood vessels themselves and their surrounding ECM. The sequence of events that occurs in sprouting is: (i) basement membrane degradation and ECM remodeling, (ii) endothelial cell migration, (iii) endothelial cell proliferation and (iv) capillary tube formation and stabilization (Torry and Rongish, 1992). Basement membrane degradation and ECM remodeling occur by the action of proteinases, including plasminogen activator, matrix metalloproteinase, tissue inhibitor of metalloproteinase, heparinase, chymase, tryptase and cathepsin (Luttun et al., 2000; Pepper, 2001; Jackson, 2002). Subsequently, endothelial cell proliferation and migration are mediated mainly through vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF) and platelet-derived growth factor (PDGF; Carmeliet, 2003). Once the initial capillary structure is formed, stabilizing factors such as PDGF, transforming growth factor-β (TGF-β) and angiopoietin-1 lead to SMC recruitment, differentiation and vessel stabilization (Carmeliet, 2003; Jain, 2003). In contrast, angiopoietin-2 and other inhibitors of angiogenesis lead to vessel destabilization and regression, and ultimately, it is the interplay between these factors that determines the neovessel's fate (Carmeliet, 2003). Intussusception is the process in which the vessel's lumen is divided into two internally by the inward migration of proliferating endothelial cells, producing a network of interlocking vessels (Risau, 1997; Fig. 1). Elongation occurs in growing tissues as existing vessels constantly restructure in response to the metabolic demands of the surrounding cells, a process also known as remodeling or pruning (Risau, 1997; Fig. 1). Vasculogenesis, the process of de novo formation of new blood vessels from endothelial cell precursors, has been shown to occur in the post-natal period (Asahara et al., 1999). More recently, it was demonstrated that circulating endothelial progenitor cells incorporate into growing vessels, thus contributing to uterine vasculogenesis (Masuda et al., 2007; Fig. 1).
Figure 1.
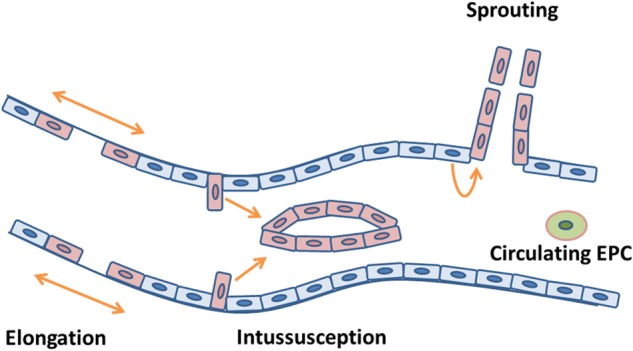
Mechanisms of blood vessel formation. Schematic representation of the four mechanisms of blood vessel formation: angiogenesis by sprouting, intussusception and elongation and incorporation of circulating endothelial progenitor cells (EPC) by vasculogenesis. Quiescent endothelial cells are depicted in blue, proliferating endothelial cells are depicted in pink and endothelial progenitor cells are depicted in green.
The sex steroids estrogen and progesterone appear to be the primary regulators of uterine angiogenesis in all mammalian species (Hyder and Stancel, 2000; Reynolds and Redmer, 2001; Fraser and Duncan, 2009). To date, the growth factors with angiogenic activity (see Fig. 1) known to be expressed in the myometrium are: epidermal growth factor (EGF) (Yeh et al., 1991; Dixon et al., 2000), heparin-binding EGF (HB-EGF) (Mangrulkar et al., 1995; Nowak, 2000), VEGF (Harrison-Woolrych et al., 1995; Dixon et al., 2000; Sanci et al., 2011), basic FGF (bFGF) (Pekonen et al., 1993; Mangrulkar et al., 1995; Anania et al., 1997; Dixon et al., 2000), PDGF (Boehm et al., 1990; Mangrulkar et al., 1995), TGF-β (Chegini et al., 1994; Tang et al., 1997) and adrenomedullin (ADM) (Hague et al., 2000; Xu et al., 2006).
Fibroid vasculature: an angiogenic perspective
The human uterus has an anastomotic blood supply arising from the uterine, ovarian and vaginal arteries (Ramsey, 1994). Bilateral uterine arteries penetrate the myometrium and give rise to the circumferential arcuate arteries. These in turn branch to form the radial arteries, which transverse the myometrium and perforate the endometrium as small vessels, known as spiral arterioles (Ramsey, 1994). In his original work a century ago, Sampson described vascular abnormalities within fibroids and proposed that these may be associated with clinical symptoms of fibroids, such as abnormal uterine bleeding (Sampson, 1912). Since Sampson's early structural study, there has been a consensus that small fibroids are significantly less vascular than the surrounding myometrium, but structural studies regarding large fibroids have reported conflicting data with some describing increased blood vessel density (Sampson, 1912; Faulkner, 1944), while others observed the opposite (Farrer-Brown et al., 1970). Similarly, blood flow studies performed in leiomyomas have reported conflicting results. Forssman et al. (1976a, b) employing local intra-arterial injection of 133Xe, demonstrated significantly lower blood flow in fibroids compared with the surrounding myometrium. However, more recent studies using transvaginal Doppler ultrasound have reported increased blood velocity and a lower resistance index and pulsatility index (PI) in uterine and fibroid arteries of leiomyomatous uteri when compared with uterine arteries of normal uteri (Kurjak et al., 1992; Alatas et al., 1997). Several groups have reported a positive correlation between blood flow and fibroid size (Huang et al., 1996; Sosic et al., 1996; Alatas et al., 1997). It has also been recognized that different regions exist within fibroids in terms of blood perfusion (Forssman, 1976a; Kurjak et al., 1992). This differential blood flow according to the fibroid size as well as the intratumor region may account for the variability in reported results.
More recent studies employing various advanced quantitative techniques have helped shed light on the issue of fibroid vessel density. Casey et al. (2000) used immunohistochemical staining with the endothelial cell markers CD31, CD34, factor VIII and lectin, demonstrating that the vascular density of fibroids is decreased compared with the surrounding normal myometrium. Using a similar technique, Poncelet et al. (2002) found comparable findings. However, using CD31 immunostaining to determine vascular density, Hauge et al. (2000) observed no differences between fibroid and normal myometrium. Methodological differences may account for the discrepant findings between these studies. While Casey et al. (2000) and Poncelet et al. (2002) examined regions of high vascular density using a digital image analyzer, Hauge et al. (2000) used the Chalkley counting technique in a non-random fashion, which may not reflect the true fibroid vascular density. Consistent with the findings by Casey et al. (2000) and Poncelet et al. (2002) and using different approaches, other investigators confirmed the decreased vascularity of fibroid tissue compared with the surrounding myometrium (Walocha et al., 2003; Aitken et al., 2006). Walocha et al. (2003) used a corrosion casting technique combined with scanning electron microscopy to study fibroid vasculature. This technique is thought to be one of the best currently available for morphological examination of vascular networks (Lametschwandtner et al., 1990). Walocha et al. found the smallest myomas (1–3 mm) to be almost avascular, being surrounded by a relatively dense myometrial vascular network composed mostly of capillaries. Larger fibroids (>1 cm) contained a chaotic network of blood vessels, mostly capillaries, arterioles and venules, with generally lower vascular density than unaffected myometrium. Again, larger fibroids were surrounded by a dense ‘vascular capsule’ (Walocha et al., 2003). Using stereological analysis, Aitken et al. (2006) demonstrated fibroids to be relatively avascular in contrast to the vascularized peri-fibroid tissue. The authors suggested that this vascular capsule region likely corresponds to the peripheral rim of well-vascularized vessels seen on ultrasound and used as the plane of tissue dissection in myomectomy. In agreement with these studies, it was recently reported that the peri-fibroid region has an increased blood flow, as measured by Doppler ultrasound, compared with the fibroid (Tsiligiannis et al., 2013).
The unique vascular architecture of fibroids consisting of an avascular core surrounded by a well-vascularized capsule is most likely due to an angiogenic imbalance. While most studies have reported that fibroids seem to have increased expression of pro-angiogenic factors, it is ultimately the balance between angiogenic promoters and inhibitors that determines vessel development (Carmeliet, 2003). There are several possible explanations for the diminished fibroid vasculature despite an increased presence of angiogenic factors, including an aberrant hypoxia response, lack of response to angiogenic promoters and overexpression of angiogenic inhibitors. One of the most potent stimuli for angiogenesis in tumors is hypoxia and, as may be expected, the avascular fibroid tissue is very hypoxic (Mayer et al., 2008). However, hypoxia-inducible factor-1α (HIF-1α) expression, which is usually up-regulated in hypoxia, driving expression of multiple angiogenic factors and orchestrating the tissue adaptations to hypoxia (Vincent et al., 2002), was not identified in fibroid tissue, in contrast to being up-regulated in leiomyosarcoma (Mayer et al., 2010). This suggests an aberrant response of fibroids to tissue hypoxia. This is a very important finding and could lead to an inadequate angiogenic response and resultant diminished vasculature in fibroids. Consistent with this hypothesis, it was shown that HIF-1α disruption prevents the formation of large blood vessels and impairs vascular function, resulting in a hypoxic microenvironment within the tumor mass (Carmeliet et al., 1998). Remarkably, the authors reported that the growth of HIF-1α deficient tumors was not retarded but accelerated, owing to decreased hypoxia-induced apoptosis and increased stress-induced proliferation (Carmeliet et al., 1998). Taken together, HIF-1α down-regulation may explain several features of fibroid biology, including the diminished vasculature and their continuous growth despite it. Another explanation for the diminished vascularization within fibroids may be an inadequate response to angiogenic factor stimuli. This could be due to various genetic aberrations as up to 40% of fibroids show cytogenetic anomalies (Levy et al., 2012), and fibroids were demonstrated to have a globally abnormal DNA methylation status compared with myometrium (Yamagata et al., 2009). Alternatively, overproduction of angiogenic inhibitors could counteract the action of angiogenic promoters, resulting in the reduced microvascularity of fibroids. Weston et al. have shown that collagen 4A2, the precursor for the angiogenic inhibitor canstatin (Kamphaus et al., 2000), is overexpressed in fibroid tissue compared with myometrium (Weston et al., 2003). To date, the expression of other angiogenesis inhibitors such as angiopoietin-2 (Maisonpierre et al., 1997), angiostatin (O'Reilly et al., 1994) and endostatin (O'Reilly et al., 1997) has not been investigated in fibroids and would be an interesting avenue to explore.
It has been suggested that the vascularized capsule surrounding the avascular fibroid may be due to the stimulatory effects of angiogenic factor secretion by the tumor on the surrounding normal myometrium (Hague et al., 2000; Walocha et al., 2003). In support of this hypothesis, Wei et al. (2006) described a VEGF concentration gradient in the fibroid, with a steady increase in VEGF expression from the central zone to the periphery of the fibroid. In addition, Hague et al. (2000) showed increased expression of the hypoxia-induced angiogenic factors ADM, VEGF and bFGF in the myometrium of leiomyoma-bearing uteri compared with myometrium of normal uteri. Moreover, these authors demonstrated that ADM expression correlated with the vascular density of the leiomyoma and myometrium in fibroid-bearing uteri, suggesting that this angiogenic factor may have an important role in the development of the vascular fibroid capsule (Hague et al., 2000). The apparent increase in angiogenesis and vascular density in the normal myometrium surrounding the fibroids may account for the symptoms of menorrhagia observed in women with fibroids and their tendency to bleed during myomectomy.
Other vascular abnormalities recently described in fibroids are a lack of vascular SMCs and arterial spiraling, as opposed to normal myometrium (Aitken et al., 2006), suggesting a vascular maturation defect. This may be explained by the failure of SMC precursors to migrate into the tumor or differentiate. Alternatively, the angiogenic environment within the tumor may be suppressive for vessel maturation. One system that is known to be crucial in determining blood vessel maturation and regression is that of the angiopoietins and their Tie-1 and Tie-2 receptors (Maisonpierre et al., 1997; Carmeliet, 2003). However, no studies to date have examined the expression of these critical factors in fibroids or myometrium. Future molecular studies should clarify whether this system and others are involved in the defective vascular maturation exhibited by fibroids. Nonetheless, the striking difference in vascularization seen in small fibroids makes it likely that the angiogenic stimulus or anti-angiogenic profile is altered within fibroids relative to that occurring in normal tissue development, with a corresponding lack of muscularization and spiralization.
Evidence for an abnormal hypoxia response of fibroids: implications for growth and vascularization
Tumor hypoxia is a crucial driving force in malignant progression promoting local invasion, intravasation and metastatic spread, and has been shown to correlate closely with poor patient prognosis (Harris, 2002). Hypoxia-induced changes in gene expression are coordinated mainly by the hypoxia-inducible transcription factor HIF-1 (Semenza, 2010). Downstream effects of HIF-1 activation, which promote malignancy, include modulation of glucose metabolism through increased cellular glucose uptake via glucose transporter-1 and enhanced glycolysis by up-regulation of key glycolytic enzymes such as carbonic anhydrase IX, increased angiogenesis by overexpression of VEGF and activation of the c-MET/HGF system characterized by cell proliferation, migration and apoptosis protection (Semenza, 2003). Consistent with these effects, the majority of primary human cancers and their metastases overexpress HIF-1α (Semenza, 2010).
HIF-1 is a master regulator of the cellular response to low oxygen tension and is central to maintenance of oxygen homeostasis. It is a heterodimeric transcription factor consisting of two subunits, α and β. While HIF-1β is constitutively active, HIF-1α is oxygen-sensitive, being rapidly inactivated and degraded in normoxia. Under normal oxygen tension, the interaction between the tumor suppressor Von-Hippel–Lindau (VHL) and HIF-1α is enabled by two prolyl hydroxylations within the HIF-1α degradation domain, at residues P402 and P564 by prolyl hydroxylases (PHDs; Bruick and McKnight, 2001; Epstein et al., 2001; Jaakkola et al., 2001; Masson et al., 2001). VHL functions as the recognition component of an E3 ubiquitin ligase that targets HIF-1α for ubiquitination and proteasomal degradation. In addition, asparaginyl hydroxylation at residue N803 within the C-transactivation domain of HIF-1α by the action of the asparaginyl hydroxylase factor-inhibiting HIF-1 inhibits the interaction of HIF-1α with various co-activators such as p300/CBP, resulting in its inactivation (Mahon et al., 2001; Lando et al., 2002). During hypoxia, the activity of the prolyl and asparaginyl hydroxylases, which is oxygen-dependent, is markedly reduced. The accumulating HIF-1α therefore heterodimerizes with HIF-1β and the resulting complex binds to the hypoxia responsive element of various target genes, activating their transcription.
The origins of leiomyoma are still unknown but accumulating evidence suggests that hypoxia is likely implicated in early cellular events which lead to myometrial SMC transformation into leiomyoma (Fig. 2). Uterine leiomyomas are known to be clonal in origin and only 40% of them harbor cytogenetic abnormalities (Walker and Stewart, 2005). The most common is a chromosomal translocation between chromosomes 12 and 14, found in ∼15% of leiomyomas (Gross and Morton, 2001). The affected gene encodes a high-mobility group AT-hook 2 protein (HMGA2), a non-histone component of chromatin that acts as a transcriptional modulator (Gross and Morton, 2001). Although this chromosomal rearrangement is found in only a subset of leiomyomas, it has been recently shown that even leiomyomas that do not carry this cytogenetic aberration overexpress the HMGA2 gene compared with myometrium (Klemke et al., 2009), suggesting that HMGA2 may play an important role in leiomyoma development even in those fibroids that do not carry the specific t(12;14) rearrangement. In support of this notion, a dinucleotide repeat within the HMGA2 gene has been shown to be highly associated with HMGA2 overexpression and leiomyoma development (Hodge et al., 2009). Moreover, the occurrence of gene mutations in leiomyomas has been recently reported to be much higher than that of cytogenetic abnormalities.
Figure 2.
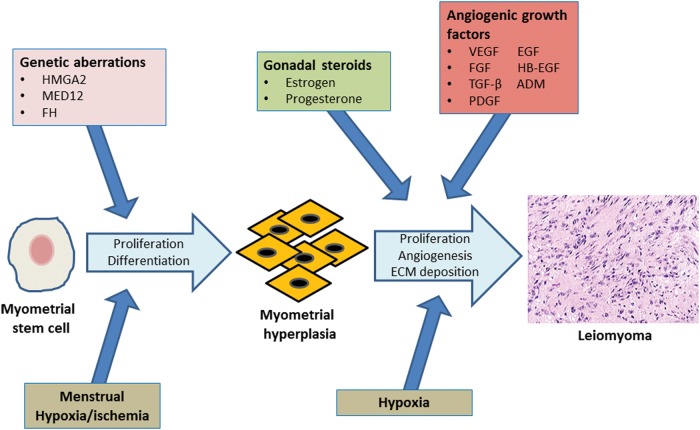
Leiomyoma development. Genetic aberrations involving genes such as HMGA2, MED12 and FH may initiate unregulated cell proliferation of myometrial stem cells. Cyclic menstrual contractions of the myometrium result in periodic hypoxia/ischemia, which may lead to differentiation of myometrial stem cells into SMCs. Continued uncontrolled proliferation of mutated stem cell derived SMCs would result in foci of MMH. The effects of gonadal steroids in combination with the chronic hypoxia associated with the rapidly expanding MMH cell mass would stimulate local angiogenic growth factor expression. These, in turn, would promote continued cell proliferation, and ECM deposition, and provide a vascular support to the growing myometrial cell mass, resulting in leiomyoma formation. SMCs, smooth-muscle cells; HMGA2, high-mobility group AT-hook 2 protein; MED12, mediator subunit complex 12; FH, fumarate hydratase; MMH, myometrial hyperplasia; ECM, extracellular matrix; ADM, adrenomedullin.
The tumor suppressor gene, mediator subunit complex 12 (MED12), has been found to be mutated in ∼70% of leiomyomas but not in myometrium in several studies on populations of different ethnic backgrounds (Makinen et al., 2011a, b; McGuire et al., 2012). Genetic aberrations involving HMGA2, MED12 and other genes have been suggested to be the initial insult leading to unregulated cell growth. Genetic mutations are considered to play a critical role in the pathogenesis of hyperplastic lesions, which are known as the precursors of various cancers, including breast, endometrial and cervical carcinomas (Tavassoli and Devilee, 2003).
Recently, Cramer et al. have reported that myometrial hyperplasia (MMH), zones of increased myometrial cellularity, are extremely common in the myometrium. The authors demonstrated that most leiomyomas arise within these MMH zones and suggested that MMH likely plays a similar precursor role in leiomyoma development (Cramer et al., 2009). MMH is responsible for rapid uterine growth during pregnancy (Shynlova et al., 2006). In the pregnant rat, MMH has been shown to result in myometrial hypoxia and overexpression of HIF-1α, suggesting that hypoxia is a key feature of MMH (Shynlova et al., 2010). Moreover, HIF-1α expression is absent in normal endometrial tissue but its expression gradually rises from endometrial hyperplasia to adenocarcinoma, indicating that hypoxia may be central in promoting tumorigenesis of precursor hyperplastic lesions (Horree et al., 2007).
Further supportive evidence for an important role for hypoxia in SMC transformation into a leiomyoma derives from studies on stem cells. Recently, a side population of myometrial cells with phenotypic and functional characteristics of stem cells has been characterized in the uterus and shown to account for 1% of the total myometrial cell population (Ono et al., 2007). Upon implantation into immunodeficient mice, these myometrial stem cells were shown to form large leiomyoma tumors with higher proliferative activity than regular myometrial cells which formed much smaller tumors (Ono et al., 2012), suggesting that myometrial stem cells may be critical for leiomyoma development. Interestingly, these myometrial stem cells were shown to differentiate into mature myometrial cells only under hypoxic conditions in vitro, suggesting that hypoxia may be the driving force behind their growth and transformation into leiomyoma cells (Ono et al., 2007). Since the menstrual cycle is associated with periodic ischemic/hypoxic stress due to myometrial contractions, which lead to cessation of bleeding (Fujii et al., 1999), these hypoxic events may be the stimuli for myometrial stem cell differentiation in vivo. Taking these data together, it may be hypothesized that specific genetic aberrations may lead a myometrial stem cell to begin uncontrolled proliferation, and differentiation to an SMC driven by cyclic menstrual hypoxic events, resulting in a precursor MMH lesion. The chronic hypoxia associated with rapidly expanding MMH cell mass, in combination with further genetic mutations and perhaps the influence of gonadal steroids, may then culminate in the formation of a leiomyoma (Fig. 2).
Similar to other tumors, leiomyomas have been shown to be very hypoxic. However, Mayer et al. have shown that despite this extreme hypoxia, leiomyomas do not express HIF-1α, HIF-2α and other hypoxia-related genes, indicating a lack of response to tissue hypoxia (Mayer et al., 2008, 2010). These authors have also reported that in contrast to leiomyoma tumors, leiomyosarcomas, their malignant counterparts, show abundant expression of HIF-related markers and have suggested that this may be mechanistically linked to their transformed phenotype (Mayer et al., 2010). The aberrant hypoxia response and lack of HIF-1α expression in fibroids may be implicated in several features of their pathophysiology, including their diminished vascularization, limited growth potential and lack of tendency for invasion or metastasis. Several pieces of evidence are in line with this tenet. Carmeliet et al. have examined tumors derived from embryonic stem cells and demonstrated that loss of HIF-1α expression in these tumors resulted in a lack of formation of large blood vessels, impaired vascular function and down-regulation of hypoxia-induced expression of VEGF. In addition, loss of HIF-1α in these tumors resulted in decreased hypoxia-induced apoptosis (Carmeliet et al., 1998). Similar to these HIF-1α-deficient tumors, leiomyomas also characteristically show a diminished vasculature (Hong et al., 2001) and reduced apoptosis when compared with leiomyosarcomas, which overexpress HIF-1α (Mayer et al., 2008).
HLRCC is a genetic syndrome that is characterized by multiple leiomyomas of the uterus and skin, and an increased susceptibility to type II papillary and collecting duct renal cell carcinoma (Grubb et al., 2007). HLRCC results from an inactivating germline mutation in the fumarate hydratase (FH) gene, which encodes the Krebs cycle enzyme which acts as a classical tumor suppressor (Tomlinson et al., 2002; Alam et al., 2003). Lack of FH activity has been shown to result in accumulation of fumarate leading to inhibition of HIF prolyl hydroxylase and consequent HIF-1α stabilization and activation, with a subsequent release of hypoxia-inducible factors such as VEGF (Isaacs et al., 2005; Pollard et al., 2005b; Selak et al., 2005). Interestingly, the uterine leiomyomas in HLRCC are significantly more vascularized than sporadic leiomyomas, and show activation of components of the hypoxic pathway such as overexpression of HIF-1α and VEGF, in contrast to sporadic leiomyomas (Pollard et al., 2005a). This observation supports the notion that the lack of HIF-1α expression and consequent lack of up-regulation of its downstream effectors may be responsible for the relatively avascular phenotype of sporadic leiomyomas. Clinically, when compared with their sporadic counterparts, the HLRCC uterine leiomyomas tend to develop at a younger age, grow more aggressively and reach a larger size, frequently requiring a hysterectomy at an earlier age, and, furthermore, have a greater predisposition to become malignant (Toro et al., 2003; Lehtonen et al., 2006; Stewart et al., 2008; Garg et al., 2011; Sanz-Ortega et al., 2013). Thus, HLRCC leiomyoma may be conceptualized as an intermediate stage between the HIF-1α-deficient, slow-growing and relatively avascular sporadic leiomyoma and the HIF-1α-overexpressing vascularized malignant leiomyosarcoma. With this conceptual construct (see Fig. 3), HIF-1α overexpression may be a necessary step in tumorigenesis of leiomyomas, promoting the expression of hypoxia-induced genes, leading to various tumor adaptations including increased vascularization. Additional steps, however, would be required for malignant transformation to leiomyosarcoma. In support of this hypothesis, renal and fibroblast cells lacking the FH gene and overexpressing HIF-1α are protected from apoptosis via HIF-1α-independent mechanisms (Costa et al., 2010). Moreover, renal cyst formation occurs in FH-deficient mice but not in PHD-deficient mice despite identical HIF-1α overexpression (Adam et al., 2011), suggesting that additional mechanisms independent of HIF-1α are required for malignant transformation.
Figure 3.
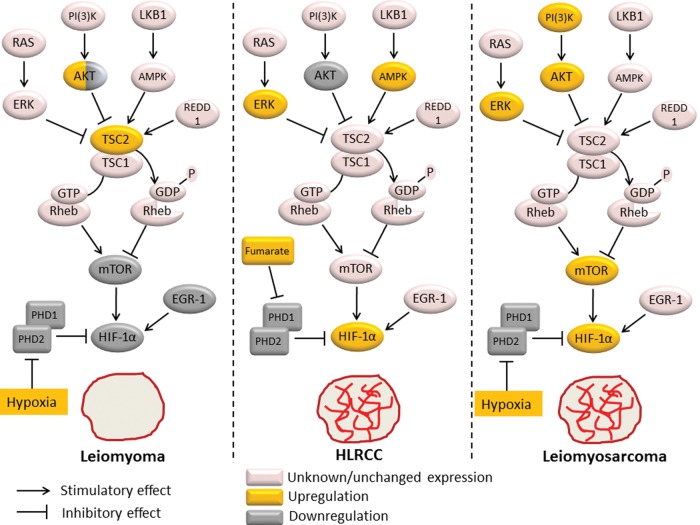
Differential expression of mTOR and HIF-1α pathways between leiomyoma, HLRCC and leiomyosarcoma. Hypoxia up-regulates HIF-1α expression through inhibition of the prolyl hydroxylases PHD1 and PHD2. In addition, hypoxia activates the TSC1/TSC2 (hamartin/tuberin) complex via sensing proteins such as AMPK or REDD1. The TSC1/TSC2 complex functions as a GTPase activator for Rheb, promoting Rheb inactivation. As mTOR activity is dependent on stimulation by Rheb, this leads to mTOR inactivation and resultant down-regulation of HIF-1α protein expression. Thus, the mTOR pathway provides a negative feedback mechanism of HIF-1α activation under hypoxic conditions. mTOR and EGR-1 are potent inducers of HIF-1α activation, and are both down-regulated in leiomyoma tissue, explaining HIF-1α down-regulation despite tumor hypoxia in leiomyomas. Lack of HIF-1α expression is likely responsible for the reduced vascularity of these fibroid tumors. In HLRCC fibroids, accumulation of fumarate due to FH mutation leads to PHD1 and PHD2 inactivation and resultant HIF-1α overexpression, which is likely responsible for the increased vascularity of these tumors. AMPK up-regulation and AKT down-regulation in HLRCC fibroids suggests that mTOR activity is reduced in these tumors. In leiomyosarcoma, up-regulation of AKT and ERK inhibit the TSC1/TSC2 complex, leading to mTOR activation, contributing to tumor progression and metastases. In addition to mTOR activation, hypoxia likely leads to the observed HIF-1α overexpression in leiomyosarcomas, which in turn contributes to the increased vascularity of these tumors. PHD1, prolyl hydroxylase 1; PHD2, prolyl hydroxylase 2; AMPK, AMP-activated protein kinase; REDD1, regulated in development and DNA damage response 1; Rheb, RAS homologue enriched in brain; PI3K, phosphatidylinositol-3-kinase; ERK, extracellular signal-regulated kinase; RAS, rat sarcoma; mTOR, mammalian target of rapamycin; EGR-1, early growth response-1; HIF-1α, hypoxia-inducible factor-1α; FH, fumarate hydratase; HLRCC, hereditary leiomyomatosis.
The mechanism responsible for the lack of HIF-1α up-regulation, which underlies the aberrant hypoxic response in leiomyomas, is currently unclear. Mayer et al. (2010) have shown that expression of the HIF PHDs is unchanged in leiomyomas and thus is not responsible for down-regulation of the HIF system in these tumors. The same authors suggested that the serine/threonine kinase mammalian target of rapamycin (mTOR) may play a role in HIF-1α down-regulation in leiomyomas. The mTOR pathway is involved in cell signaling pathways that promote tumorigenesis through coordinated phosphorylation of proteins that directly regulates protein synthesis, cell-cycle progression, cell growth and proliferation (for a review, see Wullschleger et al., 2006). Hypoxia activates the TSC1/TSC2 (hamartin/tuberin) complex via sensing proteins such as AMP-activated protein kinase (AMPK; Liu et al., 2006) or regulated in development and DNA damage response (REDD1; Brugarolas et al., 2004). The TSC1/TSC2 complex functions as a GTPase activator for the RAS homologue enriched in the brain (Rheb), promoting Rheb inactivation (Pouyssegur et al., 2006). As mTOR activity is dependent on stimulation by Rheb, hypoxia may down-regulate its activity via this pathway. HIF-1α expression is dependent on mTOR activity and inhibition of mTOR has been shown to result in direct down-regulation of HIF-1α protein expression (Hudson et al., 2002; Toschi et al., 2008). In the pregnant rat, myometrial hypoxia ensues once the uterus reaches a certain size and this is associated with rapid mTOR down-regulation (Jaffer et al., 2009; Shynlova et al., 2010). Thus, the mTOR pathway may be seen as a regulatory mechanism induced by hypoxia to control HIF-1α levels. Dhingra et al. have shown that in leiomyomas phosphorylated AKT (pAKT) and activated mTOR levels are very low and comparable with normal myometrium, when compared with leiomyosarcomas, which demonstrate significantly increased levels of pAKT and activated mTOR (Dhingra et al., 2011), consistent with a role for the mTOR pathway in HIF-1α down-regulation in leiomyomas. In line with this hypothesis, Cui et al. (2011) recently reported that expression of tuberin, the protein product of TSC2, was increased in leiomyomas compared with normal myometrium with consequent inhibition of mTOR activity. However, several studies have reported conflicting findings. Crabtree et al. demonstrated that in comparison to normal myometrium, both human and rat leiomyomas demonstrate increased mRNA expression of mTOR pathway genes suggestive of increased mTOR activation. However, the authors failed to confirm their gene pathway analysis at the protein level (Crabtree et al., 2009). In addition, others have reported increased levels of pAKT in leiomyomas compared with normal myometrial cells (Kovacs et al., 2007; Hoekstra et al., 2009). Recently, Varghese et al. (2013) showed that G protein-coupled receptor 10, which activates the PI3 K/AKT–mTOR pathway, is overexpressed in uterine leiomyomas correlating with increased pAKT in these tumors. Further studies are warranted to clarify these discrepancies by directly comparing expression and activity of the PI3 K/pAKT/mTOR pathway, as well as its upstream regulators, in normal myometrium, leiomyoma and leiomyosarcoma tissues. With respect to HLRCC leiomyomas, kidney cells with FH deficiency, the same defect observed in HLRCC, demonstrate increased activation of AMPK combined with decreased pAKT levels compared with normal cells, pointing towards decreased mTOR activity in these cells (Bardella et al., 2012). Although this has yet to be confirmed by direct experiments of mTOR activity in HLRCC leiomyomas, these data provide further molecular support to the notion that HLRCC is an intermediate between sporadic leiomyomas and leiomyosarcomas. Furthermore, Hernando et al. (2007) have demonstrated in a mouse model that PI3K-AKT activation due to PTEN deletion in SMCs leads to constitutive mTOR activation and a high incidence of abdominal leiomyosarcomas, suggesting that mTOR activation is a critical step in smooth-muscle transformation to leiomyosarcoma. Taken together, these data suggest that the differential expression of HIF-1α between leiomyomas and leiomyosarcomas may be explained by mTOR activity, and that both mTOR and HIF-1α may play important roles in leiomyoma transformation to malignant leiomyosarcoma (Fig. 3).
Another possible explanation for the decreased HIF-1α expression observed in leiomyomas may be related to the hypoxia-inducible transcription factor early growth response-1 (EGR-1). EGR-1 directly binds the promoter of HIF-1α, activating its transcription in a cancer cell line (Sperandio et al., 2009). Interestingly, EGR-1 is significantly down-regulated in leiomyomas (Pambuccian et al., 2002; Shozu et al., 2004), and this may contribute to the observed lack of HIF-1α expression in these tumors. Similar to HIF-1α, EGR-1 is a transcriptional factor that is induced by hypoxia, leads to up-regulation of angiogenic factors, and is implicated in tumor growth and angiogenesis (Yan et al., 1999; Fahmy et al., 2003; Lucerna et al., 2003). Remarkably, expression of EGR-1 has been shown to be significantly down-regulated in leiomyomas to ∼10% of levels found in normal myometrium (Pambuccian et al., 2002; Shozu et al., 2004), providing further evidence for the aberrant response to hypoxia in fibroid tumors. Ishikawa et al. (2007) showed that ∼15% of genes found to be down-regulated in fibroids contain an EGR-1-binding site within their promoter sequences, including known angiogenic promoters such as PDGF and VEGF, suggesting that reduced EGR-1 expression is likely involved in their down-regulation. Consistent with a role in angiogenic factor down-regulation, decreased EGR-1 expression was shown to result in a lack of up-regulation of angiogenic promoters (FGF-2), which was associated with inhibition of tumor angiogenesis (Fahmy et al., 2003). These data suggest that, similar to HIF-1α, lack of hypoxic EGR-1 up-regulation may contribute to the relatively avascular nature of fibroids. Moreover, it was shown that the growth of myometrial cells is retarded by EGR-1 expression, suggesting a role also for EGR-1 down-regulation in fibroid growth (Shozu et al., 2004). In their study, Shozu et al. (2004) found no DNA mutations in the EGR-1 gene and its function was shown to be intact in leiomyomas, suggesting that its low gene expression may account for its low activity. Further studies would be needed to investigate the expression of EGR-1 in leiomyosarcomas and HLRCC leiomyomas when compared with leiomyomas, and its putative role in their tumorigenesis.
Angiogenic factor dysregulation in fibroid pathophysiology
There have been multiple studies examining the differential gene expression between leiomyoma and normal myometrium using microarray analysis, providing variable results regarding angiogenic factor gene expression (Table I; Tsibris et al., 2002; Ahn et al., 2003; Chegini et al., 2003b; Wang et al., 2003; Weston et al., 2003; Catherino et al., 2004; Hoffman et al., 2004; Quade et al., 2004; Arslan et al., 2005; Ishikawa et al., 2007; Tsiligiannis et al., 2013). These variable findings may be due to inherent fibroid heterogeneity as well as differences between studies in patients' characteristics, laboratory methods and analytical approaches. Several genes with angiogenic activity, however, showed consistent differential expression between leiomyoma and normal myometrium. Namely, TGF-β was consistently up-regulated, while connective tissue growth factor (CTGF) and cysteine-rich angiogenic inducer 61 (CYR61) were consistently down-regulated in leiomyomas when compared with normal myometrium (Table I).
Table I.
Summary of angiogenesis-related genes differentially expressed in leiomyoma relative to myometrium in microarray studies.
| Angiogenic factor | Gene symbol | Tsibris et al. (2002) | Chegini et al. (2003b) | Wang et al. (2003) | Weston et al. (2003) | Ahn et al. (2003) | Catherino et al. (2004) | Quade et al. (2004) | Hoffman et al. (2004) | Arslan et al. (2005) | Ishikawa et al. (2007) | Tsiligiannis et al. (2013) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EGF | EGF | ↑ | ||||||||||
| HB-EGF | HBEGF | ↓ | ||||||||||
| VEGF | VEGF | ↓ | ↓ | ↓ | ||||||||
| aFGF | FGFI | ↑ | ||||||||||
| PDGF | PDGFA | ↑ | ||||||||||
| PDGFB | ↓ | |||||||||||
| PDGFC | ↑ | |||||||||||
| TGF-β | TGFB1 | ↑ | ||||||||||
| TGFB2 | ↑ | |||||||||||
| TGFB3 | ↑ | ↑ | ↑ | ↑ | ||||||||
| CTGF | CTGF | ↓ | ↓ | ↓ | ↓ | ↓ | ||||||
| CYR61 | CYR61 | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ||||
| Col4α2 | COL4A2 | ↑ | ↑ | |||||||||
| HIF-1α | HIF1A | ↓ | ↑ | |||||||||
| EGR-1 | EGR1 | ↓ | ↓ | ↓ |
↑ or ↓denote increased or decreased expression, respectively, in leiomyoma compared with myometrium. EGF, epidermal growth factor; HB-EGF, heparin-binding EGF; VEGF, vascular endothelial growth factor; aFGF, acidic fibroblast growth factor; PDGF, platelet-derived growth factor; TGF-β, transforming growth factor-β; CTGF, connective tissue growth factor; CYR61, cysteine-rich angiogenic inducer 61; Col4α2, collagen 4α2; HIF-1α, hypoxia-inducible factor-1α; EGR-1, early growth response-1.
While gene microarray studies have demonstrated largely variable findings, studies on protein expression of angiogenic factors have been more consistent, providing accumulating evidence that multiple factors involved in angiogenesis are differentially expressed in leiomyomas when compared with normal myometrium; these include EGF, HB-EGF, VEGF, bFGF, PDGF, TGF-β and ADM (Table II and Fig. 4). These differences may be responsible for the abnormalities seen in fibroid vasculature and fibroid-associated abnormal bleeding. In addition, as tumor growth is dependent on angiogenesis (Folkman, 2006), these differences in expression may explain differences observed in the fibroid growth rate. We refer the reader to Table II for a summary of the growth factors, their expression and their angiogenic actions on myometrium/leiomyoma.
Table II.
Angiogenic growth factors in human myometrium and leiomyoma: expression and angiogenic action.
| Growth factors | Receptors | Expression in myometrium and/or leiomyoma | Evidence for angiogenic action on myometrium and/or leiomyoma |
|---|---|---|---|
| EGF | EGF-R (HER1) | Expression of EGF and EGF-R in leiomyoma and myometrial cells (Yeh et al., 1991). Higher EGF expression in leiomyoma than in myometrium (Harrison-Woolrych et al., 1994). No difference in EGF expression between leiomyoma and myometrium (Vollenhoven et al., 1995). Lower EGF expression in leiomyoma than in myometrium (Dixon et al., 2000). Higher EGF-R expression in leiomyoma than in myometrium (Yu et al., 2008) | The decrease in EGF and EGF-R expression in leiomyoma (Wang et al., 2006; Ohara et al., 2007) following SPRM treatment is associated with decreased uterine blood flow (Wilkens et al., 2008) |
| HB-EGF | EGF-R (HER1), HER4 | Expression of HB-EGF in both myometrium and leiomyoma (Mangrulkar et al., 1995; Nowak, 2000). Reduced expression of HB-EGF in leiomyoma compared with myometrium (Mangrulkar et al., 1995). Increased EGF-R expression in leiomyoma compared with myometrium (Yu et al., 2008) | EGF-R expression in leiomyoma cells is decreased following SPRM asoprisnil treatment (Wang et al., 2006; Ohara et al., 2007) |
| VEGF | VEGFR-1 (flt-1), VEGFR-2 (flk-1) | Expression of VEGF in myometrium and leiomyoma (Harrison-Woolrych et al., 1995; Dixon et al., 2000; Sanci et al., 2011). Expression of VEGFR-1 and VEGFR-2 in myometrium and leiomyoma (Brown et al., 1997; Sanci et al., 2011). VEGF expression is greater in leiomyomas than in myometrium (Hague et al., 2000; Gentry et al., 2001; Wei et al., 2006; Lewicka et al., 2010). VEGF, VEGFR-1 and VEGFR-2 expression is stronger in leiomyosarcoma compared with leiomyoma (Hong et al., 2001; Sanci et al., 2011) | Pretreatment of leiomyoma xenografts with VEGF is required for continued growth in vivo (Hassan et al., 2008). GnRHa and SPRM result in decreased leiomyoma vascularization associated with reduced VEGF expression (De Falco et al., 2006; Xu et al., 2006) |
| bFGF | FGFR-1, FGFR-2 | bFGF and its receptors FGFR-1 and FGFR-2 are expressed in normal myometrium and leiomyoma (Pekonen et al., 1993; Anania et al., 1997; Dixon et al., 2000). Expression of bFGF and its receptors in leiomyoma is greater than in myometrium (Mangrulkar et al., 1995; Wolanska and Bankowski, 2006; Yu et al., 2008) | Leiomyoma bFGF expression and vascularity were diminished following treatment with GnRHa (Di Lieto et al., 2003; Di Lieto et al., 2005a) |
| PDGF | PDGFR-α, PDGFR-β | PDGF and PDGFR are expressed in leiomyoma and myometrium (Boehm et al., 1990; Mangrulkar et al., 1995). PDGF-AA, PDGF-BB and PDGF-CC (Liang et al., 2006; Suo et al., 2009) and their receptors (Yu et al., 2008) (Liang et al., 2006) are greater in leiomyoma than in myometrium. Greater PDGF expression in leiomyosarcoma than leiomyoma (Hong et al., 2001) | PDGF increases VEGF production in human myometrial SMCs resulting in angiogenesis stimulation (Taniguchi et al., 2001). Leiomyoma PDGF expression and vascularity were decreased following treatment with GnRHa (Di Lieto et al., 2005a, b) |
| TGF-β | TGF-β receptor type I, II and III | All three TGF-β isoforms and their receptors are expressed in myometrium and leiomyomas (Chegini et al., 1994; Dou et al., 1996; Tang et al., 1997). TGF-β isoforms and their receptors are overexpressed in leiomyoma compared with myometrium (Dou et al., 1996; Arici and Sozen, 2000; Lee and Nowak, 2001; Chegini et al., 2003a, b; De Falco et al., 2006; Norian et al., 2009) | GnRHa and SPRM treatments result in decreased TGF-β and TGF-β receptor expression associated with reduced vascularity in leiomyoma (De Falco et al., 2006; Ohara et al., 2007) |
| ADM | Calcitonin receptor-like receptor | ADM is expressed in myometrium and leiomyoma (Hague et al., 2000; Xu et al., 2006). ADM expression is higher in leiomyoma compared with myometrium (Hague et al., 2000) | ADM induced proliferation and migration of myometrial endothelial cells in vitro (Nikitenko et al., 2006). ADM expression correlates with vascular density of leiomyoma (Hague et al., 2000). SPRM treatment down-regulates expression of ADM and its receptor in leiomyoma cells (Xu et al., 2006) |
EGF, epidermal growth factor; HB-EGF, heparin-binding EGF; VEGF, vascular endothelial growth factor; bFGF, basic fibroblast growth factor; PDGF, platelet-derived growth factor; TGF-β, transforming growth factor; ADM, adrenomedullin; SPRM, selective progesterone receptor modulator; GnRHa, gonadotrophin-releasing hormone agonist; SMCs, smooth-muscle cells.
Figure 4.
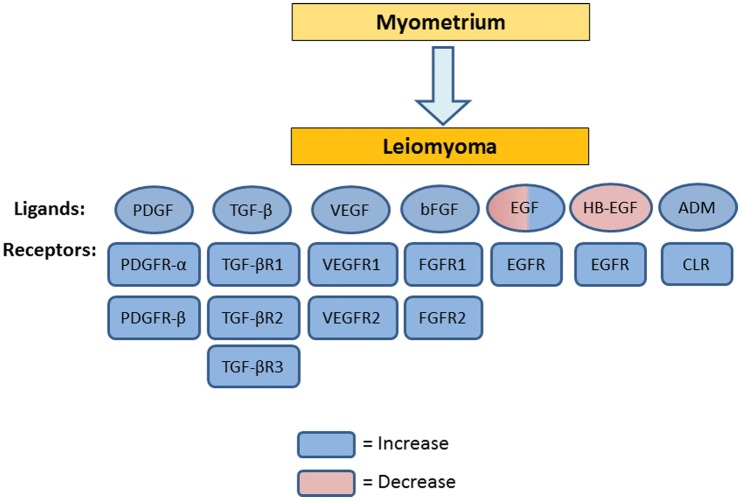
Changes in expression of angiogenic growth factors and their receptors in leiomyoma compared with myometrium. Circles denote angiogenic growth factors, while rectangles denote their receptors. Blue denotes an increase, while pink denotes a decrease in expression. EGF, epidermal growth factor; HB-EGF, heparin-binding EGF; VEGF, vascular endothelial growth factor; bFGF, basic fibroblast growth factor; PDGF, platelet-derived growth factor; TGF-β, transforming growth factor-β; ADM, adrenomedullin.
Epidermal growth factor
EGF is a 6045-kDa protein which signals via its transmembrane EGF-receptor (EGF-R aka ErbB1 or HER1) to regulate key processes of cell biology such as proliferation, survival and differentiation during development, tissue homeostasis and tumorigenesis (Carpenter and Cohen, 1990). EGF promotes angiogenesis in human endothelial cells (Mehta and Besner, 2007; Zhang et al., 2009). In addition, inhibition of the EGF-R has been shown to have an anti-angiogenic effect in many types of cancer (Karashima et al., 2002; van Cruijsen et al., 2005).
EGF and EGF-R mRNA have both been identified in myometrial and leiomyoma cells (Yeh et al., 1991) and immunolocalization of both proteins has also been described in the cytoplasm of SMCs of leiomyomas and matched myometrium (Dixon et al., 2000). The EGF-R protein has been detected in both myometrial and leiomyoma cells (Dixon et al., 2000). Studies comparing EGF expression between leiomyoma and myometrium have reported conflicting results. Harrison-Woolrych et al. (1994) found significantly higher amounts of EGF mRNA in leiomyomata compared with myometrium from a normal uterus in the secretory phase of the cycle, whereas no differences were found in samples taken during the proliferative phase. Vollenhoven et al. (1995) did not find any differences, while Dixon et al. (2000) reported decreased EGF protein immunostaining in leiomyoma compared with myometrium. The different findings are likely due to differences in the myometrial cycle phase between studies as only Harrison-Woolrych et al. used samples from the secretory phase. Dixon et al. (2000) studied uterine samples taken from women in the proliferative phase while Vollenhoven et al. (1995) studied hysterectomy specimens without reference to the specific phase of the cycle. The increased EGF expression observed in leiomyomas during the secretory phase suggests an important role for progesterone in its regulation. Indeed, it has been shown that progesterone up-regulates EGF expression in fibroids (Shimomura et al., 1998), while asoprisnil, a selective progesterone receptor modulator (SPRM) was shown to down-regulate EGF expression in leiomyoma cells, consistent with an important role for progesterone in fibroid EGF up-regulation (Wang et al., 2006). EGF-R has also been found to be increased in leiomyoma compared with myometrium (Yu et al., 2008).
EGF is mitogenic for both cultured myometrium and leiomyoma cells (Fayed et al., 1989; Rossi et al., 1992) and EGF plays a crucial role in promoting leiomyoma growth (Yeh et al., 1991; Rossi et al., 1992). Consistent with this role, EGF-R blockade by tyrosine kinase inhibitors was shown to inhibit the growth of leiomyoma cells in vitro and has been proposed as a future molecular therapy for leiomyomas (Shushan et al., 2004; Shushan et al., 2007). Since EGF-R blockade has been shown to inhibit angiogenesis in other tumor systems (Karashima et al., 2002; van Cruijsen et al., 2005), future studies could test whether anti-angiogenesis may be one of the mechanisms by which EGF-R inhibitors could potentially affect leiomyomas.
Heparin-binding epidermal growth factor
HB-EGF is a 22 kDa protein that was first identified in the macrophage-like cell-conditioned medium (Higashiyama et al., 1991). HB-EGF has an EGF-like domain and thus binds to its cognate receptors, EGF-receptor 1 (HER1) and HER4 (Higashiyama et al., 1991; Elenius et al., 1997). HB-EGF is a potent autocrine/paracrine mitogen for fibroblasts, SMCs, keratinocytes and endometrial stromal cells (Higashiyama et al., 1991; Marikovsky et al., 1993; Chobotova et al., 2002), as well as a chemo-attractant for SMCs (Higashiyama et al., 1993). In fact, HB-EGF has a greater affinity for the EGF-R on smooth-muscle cells than does EGF, and thus is a more potent mitogen (Higashiyama et al., 1991). HB-EGF has also been shown to promote angiogenesis in endothelial cells (Mehta and Besner, 2007) and tumors (Ongusaha et al., 2004).
HB-EGF mRNA and protein expression have been reported in both myometrial and leiomyoma tissues (Mangrulkar et al., 1995; Nowak, 2000). Protein expression of its receptor EGF-R has also been detected in myometrial and leiomyoma cells (Dixon et al., 2000). HB-EGF protein expression has been reported to be decreased in leiomyoma compared with adjacent myometrial tissue (Mangrulkar et al., 1995), while EGF-R has been found to be increased in leiomyoma compared with myometrium (Yu et al., 2008). Treatment of leiomyoma and myometrial cells with HB-EGF has been shown to stimulate proliferation and inhibit apoptosis of these cells in a dose-dependent fashion through augmentation of EGF-R expression. However, the proliferative effects of HB-EGF treatment on myometrial cells occurred at a much lower dose compared with leiomyoma cells (Wang et al., 2005). Taken together, these data suggest that HB-EGF may play a more important role in normal myometrial growth than in leiomyoma growth.
Vascular endothelial growth factor
VEGF is one of the major regulators of angiogenesis and its important role has been demonstrated in developmental, physiological and pathological angiogenesis (Ferrara and Davis-Smyth, 1997). It is a heparin-binding homodimeric protein of 46 kDa that consists of six isoforms (Tischer et al., 1991; Poltorak et al., 1997; Lei et al., 1998). VEGF is a potent mitogen for endothelial cells (Charnock-Jones et al., 1993) and its action is mediated by binding to tyrosine kinase receptors, VEGFR-1 (fms-like tyrosine kinase: flt-1) and VEGFR-2 (kinase domain-containing receptor: KDR/flk-1).
VEGF mRNA and protein expression have been identified in SMCs of both myometrium and leiomyoma (Harrison-Woolrych et al., 1995; Dixon et al., 2000; Sanci et al., 2011). The VEGF receptors, VEGFR-1 and VEGFR-2, are also expressed in myometrium and leiomyoma (Brown et al., 1997; Sanci et al., 2011). Several researchers have reported that VEGF expression is higher in leiomyomas compared with both adjacent myometrium (Hague et al., 2000; Gentry et al., 2001) and normal myometrium of uteri without fibroids (Hague et al., 2000; Wei et al., 2006; Lewicka et al., 2010), suggesting that angiogenesis may be important for fibroid development and growth. In addition, Hassan et al. (2008) described a novel murine model for leiomyoma, achieved by subcutaneous insertion of human leiomyoma xenografts in immunodeficient mice. Pretreatment of xenografts with VEGF and cyclooxygenase-2 was required for the continued growth of leiomyoma tissue in vivo (Hassan et al., 2008), providing further support to the notion that VEGF-induced angiogenesis is essential for fibroid growth. Moreover, studies showed that VEGF, VEGFR-1 and VEGFR-2 expression is stronger in leiomyosarcoma compared with leiomyoma (Hong et al., 2001; Sanci et al., 2011), indicating that the VEGF/VEGFR system is likely involved in uterine smooth-muscle tumorigenesis.
Basic fibroblast growth factor
Basic FGF is an 18 kDa protein that induces mitogenesis and differentiation of a variety of neuroectodermal and mesodermal cells, including fibroblasts, SMCs and endothelial cells (Klagsbrun and Dluz, 1993). bFGF binds to two major receptors: fibroblast growth factor type 1 receptor (FGFR-1) and type 2 receptor (FGFR-2) (Fernig and Gallagher, 1994). It promotes angiogenesis through a number of mechanisms, including the induction of endothelial cell proliferation, chemotaxis and the production of matrix-remodeling enzymes such as collagenase and plasminogen activator (Presta, 1988). bFGF also causes proliferation of vascular SMCs in response to vascular injury (Lindner and Reidy, 1991).
Protein and mRNA expression of both bFGF and its receptors FGFR-1 and FGFR-2 have been identified in normal myometrium as well as leiomyoma cells (Pekonen et al., 1993; Mangrulkar et al., 1995; Anania et al., 1997; Dixon et al., 2000). Several studies have reported greater expression of bFGF in leiomyoma compared with myometrium (Mangrulkar et al., 1995; Wolanska and Bankowski, 2006). In addition, FGFR-1 and FGFR-2 expression have been demonstrated to be increased in leiomyoma compared with adjacent myometrium (Wolanska and Bankowski, 2006; Yu et al., 2008). One of the histological features that distinguishes leiomyomas from normal myometrium is their large quantity of ECM. Of note, the majority of bFGF in leiomyomas is found stored in that ECM component (Mangrulkar et al., 1995; Dixon et al., 2000). The increased bFGF content of the ECM found in leiomyomas may play an important role in angiogenesis within fibroids as it has been previously shown that the growth factor content of the ECM determines endothelial cell angiogenic responses (Dye et al., 2004).
Platelet-derived growth factor
PDGF is a dimeric growth factor which exists in five different isoforms; four are homodimers (PDGF-AA, PDGF-BB, PDGF-CC and PDGF-DD), while the only heterodimer is PDGF-AB (Heldin and Westermark, 1999; Heldin, 2004). PDGF isoforms exert their cellular effects by activating two structurally related cell surface receptor tyrosine kinases (PDGFR-α and PDGFR-β). Specifically, PDGFR-α binds the A-, B- and C-chains with high affinity while PDGFR-β binds only the B- and D-chains (Heldin et al., 2002). Functionally, PDGF acts as a mitogen and chemo-attractant for both SMCs and fibroblasts (Ross et al., 1986). Its role in angiogenesis is well established as it has been shown to lead to proliferation and migration of SMCs during vascular maturation (Hellstrom et al., 1999).
PDGF and PDGF-R mRNA and protein expression have been described in both normal myometrium and leiomyoma tissues (Boehm et al., 1990; Mangrulkar et al., 1995). Hwu et al. (2008) reported that only mRNA expression of PDGF-C is increased in leiomyoma compared with the adjacent myometrium, while they did not find differences in mRNA expression of PDGF-A, -B, -D and their receptors between the tissues. In contrast, several studies examining protein expression have demonstrated greater levels of PDGF-AA, PDGF-BB and PDGF-CC (Liang et al., 2006; Suo et al., 2009) and of their receptors (Liang et al., 2006; Yu et al., 2008) in leiomyoma compared with the adjacent myometrium. In addition, Suo et al. (2009) reported higher PDGF-CC expression in fibroid-derived SMCs compared with myometrial-derived SMCs. Taniguchi et al. (2001) showed that PDGF can stimulate VEGF production in human myometrial SMCs resulting in angiogenesis stimulation, suggesting that the differential expression of PDGF/PDGFR observed between leiomyomas and myometrium may have an important role in the pathological angiogenesis occurring in fibroids. In addition, greater PDGF expression has been reported in leiomyosarcoma compared with leiomyoma, suggesting that PDGF may be involved in leiomyoma tumorigenesis (Hong et al., 2001).
Transforming growth factor-β
TGF-βs are secreted as latent molecules (large latent complex) requiring local activation for receptor binding, which releases a 25 kDa active dimer composed of identical polypeptide chains (Massague, 1990). In humans, there are three TGF-β isoforms (TGF-β1, TGF-β2 and TGF-β3), which are encoded by separate genes (Sozen and Arici, 2002). TGF-βs signal through a heterotetrameric receptor complex that consists of dimers of type I and type II receptors, both of which are required for signal transduction. The TGF-β type II receptor binds the ligand and the TGF-β type I receptor, also named activin-like kinase 5 (ALK5), is a serine/threonine kinase that phosphorylates intracellular secondary messengers Smad2 and Smad3. Their complex ultimately translocates to the nucleus to act as a transcriptional regulator for responsive genes mediating a wide range of TGF-β functions (Attisano and Wrana, 2002; Derynck and Zhang, 2003). TGF-βs exert a diverse range of biological functions regulating cell proliferation (Laiho et al., 1990; Satterwhite and Moses, 1994), apoptosis (Rotello et al., 1991; Hyman et al., 2002), angiogenesis (Yang and Moses, 1990), immune function (Letterio and Roberts, 1998) and synthesis of ECM components (Ignotz et al., 1987; Chin et al., 2001). All three TGF-β isoforms and their receptors are expressed at the mRNA and protein level in human myometrium (Chegini et al., 1994; Tang et al., 1997) and leiomyomas (Dou et al., 1996; Chegini et al., 2003b).
A large body of evidence, both from animal and human studies implicates TGF-β in the pathogenesis of uterine fibroids. TGF-βs, their receptors and downstream signaling mediators, like Smad 2/3 complexes, are overexpressed in leiomyomas compared with normal myometrium (Dou et al., 1996; Arici and Sozen, 2000; Lee and Nowak, 2001; Chegini et al., 2003a; De Falco et al., 2006; Norian et al., 2009). Several in vitro studies have reported that TGF-β isoforms at low concentration induce the proliferation of human myometrial (Tang et al., 1997) and leiomyoma cells (Arici and Sozen, 2000; Lee and Nowak, 2001). TGF-β3 has also been shown to increase the expression of ECM-related genes such as fibronectin, collagen type 1 and versican while decreasing the expression of ECM degradation-related genes in both cultured myometrial and leiomyoma cells (Norian et al., 2009; Joseph et al., 2010; Malik and Catherino, 2012), suggesting that TGF-β3 may stimulate fibroid growth by playing an important role in the excessive ECM deposition and tissue fibrosis that characterizes leiomyomas. Moreover, Malik et al. (2008) demonstrated that treatment of leiomyoma cells with retinoic acid decreased cell proliferation and ECM protein production and was associated with TGF-β3 pathway down-regulation. In addition, in mutant rats with a genetic susceptibility for spontaneous fibroid development, treatment with the TGF-β type I receptor inhibitor SB-525334 resulted in significant reduction in the incidence, number and size of the tumors, suggesting that TGF-β signaling may be essential for the formation and maintenance of fibroids (Laping et al., 2007).
Most studies on the role of TGF-β in fibroid pathophysiology have focused on the contribution of TGF-β to leiomyoma cell proliferation and ECM production as the underlying abnormalities responsible for this disease. In addition, TGF-β is also essential for tumor angiogenesis, being involved in multiple steps of this process including endothelial cell proliferation, migration, ECM metabolism and expression of adhesion molecules (Li et al., 2001); thus, TGF-β may also be related to the pathogenesis of leiomyoma through its effects on fibroid angiogenesis. In line with this hypothesis, Bandyopadhyay et al. (2002, 2005) reported, in xenograft-bearing athymic nude mice, that TGF-β blockade using soluble TGF-β receptor caused significant reduction in the tumor microvessel density and tumor blood volume, associated with tumor growth inhibition of breast cancer and prostate cancer xenografts. Similarly, it was observed that inhibition of TGF-β receptor type I in a chick embryo hepatocellular carcinoma model resulted in significant inhibition of tumor angiogenesis, which was more effective than the anti-angiogenic effect of the VEGF inhibitor bevacizumab (Mazzocca et al., 2009). Future studies of the effects of TGF-β (and its inhibition) on fibroid angiogenesis are of interest.
Adrenomedullin
ADM belongs to the calcitonin superfamily of peptides that includes calcitonin, calcitonin gene-related peptide (CGRP) and amilyn. ADM acts as an angiogenic growth factor (Zhao et al., 1998; Oehler et al., 2002; Ribatti et al., 2003), as an autocrine growth factor in several human cancer cell lines and endometrium (Miller et al., 1996; Nikitenko et al., 2000) and as an inhibitor of apoptosis (Shichiri et al., 1999; Oehler et al., 2001). ADM mediates its action through a G protein-coupled receptor, the calcitonin receptor-like receptor (CLR). Receptor activity-modifying proteins (RAMP) associate with CLR to determine its ligand-binding specificity. CLR association with RAMP2 or RAMP3 promotes adrenomedullin binding, whereas heterodimerization with RAMP1 promotes CGRP binding (McLatchie et al., 1998).
ADM protein expression has been reported in both myometrium and leiomyoma tissues (Hague et al., 2000; Xu et al., 2006). Hague et al. (2000) showed ADM expression to be significantly increased in leiomyoma compared with myometrium and to correlate with vascular density of leiomyoma, suggesting an important role for this factor in uterine leiomyoma angiogenesis. Recently, the progesterone receptor modulator CDB-2914 has been reported to significantly reduce uterine leiomyoma volume in a randomized controlled clinical trial (Levens et al., 2008). Consistent with an important role for ADM in uterine fibroid growth and angiogenesis, Xu et al. (2006) have shown that CDB-2914 treatment down-regulated ADM and VEGF and their receptors in human leiomyoma cells.
GnRH agonist, progesterone receptor modulator and UAE: anti-angiogenesis as a common mechanism of action
Uterine cellular proliferation and differentiation are regulated by gonadal steroid hormones, and fibroids are considered to be steroid-dependent for their growth. Leiomyomas are hyperresponsive to estrogen and exhibit increased levels of estrogen and progesterone receptors (Walker and Stewart, 2005). The mitogenic action of steroids which leads to leiomyoma growth is considered to be mediated by local production of growth factors, many of which have angiogenic properties, acting through paracrine and/or autocrine mechanisms (Ciarmela et al., 2011). A complete review on steroids and their interaction with growth factors in relation to leiomyoma growth has been provided by Ciarmela et al. (2011). Estrogen and/or progesterone have been shown to up-regulate the expression of multiple angiogenic factors in myometrial and leiomyoma tissues, including EGF (Shimomura et al., 1998; Matsuo et al., 1999), HB-EGF (Wang et al., 1994), VEGF (Hyder et al., 2000), bFGF (Rider et al., 1997), PDGF (Barbarisi et al., 2001), TGF-β (Arici and Sozen, 2000) and ADM (Xu et al., 2006). In addition, various progestogens have been shown to induce endometrial angiogenesis following intrauterine exposure (Hague et al., 2002). Therefore, it is plausible that leiomyoma growth is dependent on steroids partly due to their induction of local angiogenic factors for provision of fibroid vasculature. Following the same rationale, current hormonal treatments for fibroids that target these steroid pathways may exert their effects through inhibition of angiogenesis. Peddada et al. (2008) have recently shown that fibroids from the same woman grow at very different rates, with some regressing, despite a uniform hormonal milieu, indicating that factors other than steroids may determine leiomyoma growth. Moreover, many of the shrinking fibroids showed evidence of necrosis, suggesting that hypoxia and vascular events may account for tumor growth variations (Peddada et al., 2008).
GnRHa has been commonly used for the treatment of uterine fibroids. GnRHa therapy aims for the resolution of pain and bleeding symptoms and regression of fibroids by causing a state of hypoestrogenemia. Treatment results are variable and side effects and health risks limit the long-term use of these compounds (Cook and Walker, 2004). Evidence that GnRHa affects fibroid vascular supply and angiogenesis is provided by several studies. Matta et al. (1988) found that GnRHa treatment results in a reduction in uterine and fibroid blood flow as measured by Doppler ultrasound. The group of Di Lieto have demonstrated that GnRHa treatment of women with fibroids results in decreased protein expression of the angiogenesis-related factors bFGF, VEGF, PDGF and TGF-β3 together with the total number of vessels in the leiomyomas, and is associated with a reduction in uterine volume when compared with control patients (Di Lieto et al., 2003b; Di Lieto et al., 2005a, b; De Falco et al., 2006). Similarly, Khan et al. (2010) reported that GnRHa treatment led to a decrease in microvascular density and angiogenesis in myometrial and endometrial tissue of myomatous uteri, associated with a reduction in size of the fibroid tumors. These data suggest that GnRHa may shrink fibroids by reducing angiogenic factor expression and, consequently, the blood supply to the leiomyomas or the surrounding myometrial tissue. It is likely that the anti-angiogenic effects of GnRHa are mediated via estrogen and progesterone down-regulation since these gonadal steroids up-regulate the expression of various angiogenic growth factors, which are considered to be the ultimate effectors of these gonadal steroids, in the myometrium (Ciarmela et al., 2011).
Accumulating data support the concept that progesterone plays a vital role in uterine leiomyoma growth (Rein et al., 1995; Maruo et al., 2004). The action of progesterone on target tissues is known to be mediated through interaction with the progesterone receptor (PR), which belongs to the nuclear receptor family. Several studies have demonstrated that expression of PR is up-regulated in uterine leiomyomas compared with myometrium (Brandon et al., 1993; Viville et al., 1997; Nisolle et al., 1999). Moreover, clinical trials using progesterone antagonist RU486 (Eisinger et al., 2003, 2009) and SPRM asoprisnil (J867) (Chwalisz et al., 2007) have shown that both compounds are effective in shrinking uterine leiomyomas. SPRM asoprisnil and CDB-2914 were shown to inhibit growth and induce apoptosis in cultured leiomyoma cells (Xu et al., 2005; Chen et al., 2006). These effects were shown to be associated with down-regulation of the angiogenic factors VEGF and ADM and their receptors (Xu et al., 2006), as well as TGF-β and EGF and their receptors (Ohara et al., 2007), in a cell-type-specific manner affecting leiomyoma but not normal myometrial cells. In agreement with the anti-angiogenic profile induced by SPRMs in leiomyoma cells, a moderate reduction in uterine artery blood flow was noted following treatment in patients receiving asoprisnil therapy for leiomyoma (Wilkens et al., 2008). These data suggest that SPRMs may induce leiomyoma shrinkage through an anti-angiogenic mechanism.
UAE is a well-known treatment for uterine fibroids, leading to their shrinkage and symptomatic improvement in most patients (Ravina et al., 1995; Walker and Pelage, 2002). It is well established that vascular supply is crucial for fibroid growth and survival since disruption of the uterine vasculature leads to fibroid shrinkage, while sparing the myometrium (deSouza and Williams, 2002). In fact, UAE leads to transient uterine ischemia involving the myometrium and leiomyoma tissues, and while complete reperfusion of myometrial tissue is observed within 48–72 h, ischemic leiomyomas develop irreversible infarction (Scheurig-Muenkler et al., 2010). Several studies have reported that the degree of leiomyoma devascularization following UAE correlates with improvements in the clinical outcome, including better symptomatic relief and fewer reinterventions (Fennessy et al., 2007; Katsumori et al., 2008; Kroencke et al., 2010). Moreover, UAE has been shown to result in a compensatory increase in plasma VEGF levels of patients, and the magnitude of this increase correlates with increased uterine artery blood flow and treatment failure (Takeda et al., 2005), providing further evidence for the strong association between angiogenesis, vascular supply and fibroid growth. There are several reasons that may explain why UAE leads to selective death of fibroids, while normal myometrial tissue survives. First, fibroids may be more sensitive to tissue ischemia than normal myometrium. This could be due to the avascular nature of fibroids, which makes them more vulnerable to ischemia than normal myometrium. In addition, the aberrant hypoxic response in fibroid tissue, evident by the lack of HIF-1α and EGR-1 up-regulation (Shozu et al., 2004; Mayer et al., 2008), would prevent the fibroids from undergoing the necessary cellular adaptations to survive the ischemic insult. Second, differences in the fibrinolytic system between leiomyoma and myometrial tissues have been suggested to predispose fibroids to form thrombi, accounting for this differential response (Burbank, 2004). Radiological evidence based on MR and CT imaging studies suggests that following UAE, thrombi form in the uterine vasculature (Vott et al., 2000; deSouza and Williams, 2002). In leiomyoma tissue, thrombi tend to form earlier and persist, leading to long-term under-perfusion, in contrast with myometrial tissue where thrombi lyse relatively rapidly, allowing re-perfusion (Burn et al., 2000; Jha et al., 2000; deSouza and Williams, 2002). Consistent with these observations, the expression of tissue plasminogen activator and urokinase plasminogen activator is lower, while the expression of plasminogen activator inhibitor type 1 is higher in fibroid tissue when compared with myometrium, indicating diminished fibrinolytic activity in fibroid tissue (Astrup et al., 1965; Cheng et al., 2008). Therefore, thrombi may form more readily in fibroids and may be less susceptible to lysis than in adjacent myometrium, leading to selective demise of leiomyoma tissue. Another possible explanation for the differential tissue effect of UAE on fibroids compared with myometrium may reside in preferential occlusion of the fibroid blood vessels and hence the blood supply of the fibroid. In support of this theory, it has been reported that the majority of embolic spheres collected post-embolization are in medium-sized vessels in the vicinity of the fibroid (Colgan et al., 2003; Weichert et al., 2005), likely corresponding to the previously characterized peri-fibroid vascular capsule (Walocha et al., 2003; Aitken et al., 2006).
Anti-angiogenic therapy: a future treatment modality for fibroids?
Despite the high prevalence and significant morbidity of uterine fibroids, an understanding of their angiogenesis and growth is still limited, and consequently optimal therapeutic options are lacking. Current approaches for treatment of fibroids involve hormonal therapy, uterine artery ligation or embolization, and surgical interventions including myomectomy or hysterectomy. Hysterectomy is the only definitive treatment for women with fibroids. However, this option is associated with substantial surgical complications, and is unsuitable for patients wishing to preserve their fertility (Farquhar et al., 2006). Aside from hysterectomy, the effects of other therapies are usually short-lived and are associated with significant morbidity, while the recurrence leiomyoma and symptoms is common (De Leo et al., 2002; Olufowobi et al., 2004; Sharp, 2006). Therefore, there is an urgent need for alternative therapies that would provide long-lasting inhibition of leiomyoma growth without the unwanted side effects.
Since Judah Folkman's pioneering work on fighting cancer by targeting its blood supply (Folkman, 1971), anti-angiogenic therapy has been proposed as a promising strategy for several angiogenic diseases. Targeting the endothelium offers a number of advantages: (i) endothelial cells are easily accessible; (ii) drugs do not have to penetrate tissues in order to reach their target cells; (iii) endothelial cells are genetically very stable due to their low proliferation rate and so drug resistance is avoided and (iv) angiogenesis in adults occurs only in a limited number of physiological processes, including menstrual cycles in the endometrium, corpus luteum development, early pregnancy and wound healing. Although blood vessels within fibroids are not abundant, it is well established that angiogenesis and vascularization are crucial for fibroid growth and survival, evident by the shrinkage of fibroids in response to uterine artery ligation/embolization, and the decrease in vascular density associated with angiogenic factor down-regulation following hormonal treatments such as GnRHa and SPRMs. There are several aspects of fibroid vascular and cellular biology that may make them especially susceptible for anti-angiogenic therapy. First, the absence of vascular SMCs around fibroid blood vessels and lack of spiraling suggests that fibroid vasculature is immature, in contrast to the adjacent myometrium (Aitken et al., 2006). Since anti-angiogenic treatment targets angiogenic and immature blood vessels and not mature vessels surrounded by pericytes (Benjamin et al., 1999; Nap et al., 2004), it should selectively target the fibroid vasculature and not that of the surrounding myometrium. Secondly, the aberrant hypoxia response in fibroids may prevent fibroids from undergoing the necessary cellular adaptations to blood perfusion disruption, thus making them more vulnerable to tissue ischemia than adjacent normal myometrium. Consistent with this argument, several studies have reported that HIF-1α knockout or suppression leads to significantly greater tissue damage following ischemic insult in experimental animal models of heart and brain ischemia (Baranova et al., 2007; Eckle et al., 2008; Bullock et al., 2009; Sarkar et al., 2012). Moreover, the selective killing effects of UAE on fibroid tissue while sparing myometrium (deSouza and Williams, 2002) provide further support for this hypothesis.
Within the scope of gynecology, most experimental studies and clinical trials investigating the safety and efficacy of various anti-angiogenic compounds have focused upon gynecologic malignancies and endometriosis (Rasila et al., 2005; Fujimoto, 2009; Liu and Matulonis, 2011; Laschke and Menger, 2012). Anti-angiogenic agents can be divided into several categories. Some are angiogenic growth factor inhibitors such as the anti-VEGF monoclonal antibody, bevacizumab (Ferrara et al., 2004), which is FDA-approved for treatment of metastatic colon cancer; some are endogenous angiogenesis inhibitors, proteins or fragments of proteins formed in the body that inhibit the formation of new blood vessels, such as angiostatin and endostatin (Ribatti, 2009); some are analogues of fumagillin (i.e. TNP-470), a naturally occurring antibiotic with anti-angiogenic activity (Ingber et al., 1990); and some others are phytochemical compounds such as curcumin (Zhang et al., 2011). A complete review on various anti-angiogenic drugs, their mode of action, efficacy and side effects, has been provided by Laschke and Menger (2012).
GnRHa and SPRMs appear to exert their action on fibroids, at least partly, via an anti-angiogenic effect. However, these compounds also likely affect fibroids through other mechanisms. Despite the potential applicability of anti-angiogenic therapy for the treatment of fibroids, aside from these hormonal treatments, there are very few studies in the literature that have explored this possibility. Based on previous observations by Hague et al. (2000) that ADM expression was increased in leiomyomas compared with myometrium and correlated with its endothelial proliferation index and vascular density, Nikitenko et al. (2006) investigated the potential of the ADM receptor, CLR, as an anti-angiogenic target. The investigators showed that ADM induced proliferation and migration of myometrial endothelial cells in vitro, and that CLR localized exclusively in the endothelium of both leiomyoma and adjacent myometrium in vivo (Nikitenko et al., 2006). However, CLR levels were found to be lower in leiomyomas compared with the adjacent myometrium, concurrent with the lower vascular density in the leiomyoma tissue. The authors concluded that further evaluation of CLR in tumor and normal endothelium is essential before it may become a viable anti-angiogenic target (Nikitenko et al., 2006). In a different study, Malik et al. examined the effects of curcumin, a nutritional supplement with known anti-neoplastic and anti-angiogenic properties (Gururaj et al., 2002), on leiomyoma cells in vitro. They demonstrated that curcumin had an anti-proliferative effect on leiomyoma cells via regulation of the apoptotic pathway (Malik et al., 2009). It would be interesting to investigate the potential anti-angiogenic effects of this compound, which is currently in clinical trials for a variety of neoplastic conditions (Hatcher et al., 2008), on fibroids.
When considering anti-angiogenic therapy for fibroids, one must take into account potential obstacles for its success. Although malignant neoplasms and leiomyomas are similar in that angiogenesis is crucial for their growth and survival, the safety requirements for an anti-angiogenic treatment completely differ between the two diseases. Unlike cancer, uterine fibroids are not life-threatening. Moreover, many of the affected patients are women of reproductive age who still desire fertility, and several anti-angiogenic agents have been shown to have a detrimental effect on reproductive function in both animal models and patients (Klauber et al., 1997; Pauli et al., 2005). In addition, angiogenesis is essential for placental and embryo-fetal development, and administration of anti-angiogenic inhibitors during pregnancy could result in significant teratogenicity and adverse effects on fetal development (Patyna et al., 2009). It is therefore likely that an anti-angiogenic treatment strategy for fibroids would have to be strictly limited to women who have completed their family planning and/or are beyond their reproductive potential. Furthermore, one of the main challenges of the proposed anti-angiogenic treatment would be to selectively target fibroid vasculature without affecting that of the surrounding myometrium or ovaries. In contrast to metastatic disease, where systemic drug administration is necessary to reach the malignant cells, fibroid disease usually consists of a few well-circumscribed lesions and, therefore, one mode of targeting may be direct intrafibroid administration of the anti-angiogenic agent. This route has been previously reported to result in successful shrinkage of fibroids in a leiomyoma rat model using gene therapy with adenoviruses expressing dominant negative estrogen receptor or Herpes simplex virus 1 thymidine kinase transgenes (Hassan et al., 2009, 2010). Alternatively, gene therapy may be further designed to selectively target the fibroid endothelium through transcriptional targeting or modification of the viral vector tropism. It has been reported that adenoviral gene therapy could efficiently target tumor angiogenic endothelium while sparing quiescent endothelial cells using a modified preproendothelin-1 promoter that encodes an apoptotic receptor (Triozzi and Borden, 2011). In addition, modification of the adenoviral capsid was shown to modify its tropism, targeting the virus to tumor endothelial cells (Shinozaki et al., 2006). Moreover, dual targeting by both transcriptional and tropism control has been suggested to further enhance safety of these treatments (Work et al., 2004). Future research on characterization of the molecular signature of fibroid vasculature may ultimately lead to the design of safe and selective anti-angiogenic therapies. Table III summarizes the potential anti-angiogenic strategies for treatment of leiomyomas.
Table III.
Potential anti-angiogenic strategies for treatment of leiomyoma.
| Substance group | Mechanism of action | Anti-angiogenic agent | Studies | Anti-angiogenic effects on myometrium |
|---|---|---|---|---|
| GnRH agonist | Reduction of estrogen and progesterone concentrations | Leuprolide | De Falco et al. (2006); Di Lieto et al. (2003b; 2005a, b) | Decrease in expression of bFGF, VEGF, PDGF, IGF-1 receptor and TGF-β3 together with the total number of vessels in leiomyomas |
| Khan et al. (2010) | Decrease in microvascular density and angiogenesis in leiomyomas | |||
| SPRM | Selective blockade of progesterone receptors | CDB-2914 | Xu et al. (2006) | Reduction in expression of the angiogenic factors VEGF, adrenomedullin and their receptors in leiomyoma cells but not in myometrial cells |
| Asoprisnil (J867) | Ohara et al. (2007) | Decreased expression of IGF-1, TGF-β, EGF and their receptors in leiomyoma cells but not normal myometrial cells | ||
| Growth factor inhibitors | Inhibition of VEGF binding to its receptor | Anti-VEGF antibody | Kim et al. (1993) | Decreased blood vessel density and growth inhibition of transplanted leiomyosarcoma tumors in nude mice |
| Inhibition of tyrosine kinase activity of VEGF receptor-2 and EGF receptor | Vandetanib | Ren et al. (2008) | Inhibition of leiomyosarcoma xenograft tumor growth and angiogenesis in nude mice | |
| Inhibition of TGF-β binding to its receptor | TGF-beta receptor kinase inhibitor (SB-525334) | Laping et al. (2007) | Reduction of leiomyoma tumor size in Eker rats. Potential anti-angiogenic effects were not investigated | |
| Fumagillin analogues | Suppression of Rac-1 activation; induction of p53, MetAP-2 | TNP-470 | Naganuma et al. (2011) | Suppression of VEGF production, intratumoral microvessel density and tumor growth of uterine sarcoma xenografts in nude mice |
| Phytochemical compounds | Pleiotropic interaction with numerous molecular targets, including transcription factors, growth factors, protein kinases and adhesion molecules | Curcumin | Malik et al. (2009) | Antiproliferative effect on leiomyoma but not in myometrial cells. Potential anti-angiogenic effects were not investigated |
| Zhang et al. (2011) | Reduction in VEGF, microvessel density and size of transplanted endometrium in in rats |
GnRH, gonadotrophin-releasing hormone; SPRM, selective progesterone receptor modulator; EGF, epidermal growth factor; HB-EGF, heparin-binding EGF; VEGF, vascular endothelial growth factor; bFGF, basic fibroblast growth factor; PDGF, platelet-derived growth factor; TGF-β, transforming growth factor; IGF, insulin-like growth factor; ADM, adrenomedullin; SMCs, smooth-muscle cells; MetAP-2, methionine aminopeptidase-2.
Furthermore, it should be considered that the development of new blood vessels in fibroids is most probably mediated by several angiogenic signaling pathways. In support of this is the up-regulation of multiple angiogenic growth factors in these tumors as well as their down-regulation associated with blood vessel regression following GnRHa or SPRM treatment. Thus, inhibition of a specific growth factor may be compensated by up-regulation of other factors. To overcome this, the use of pleiotropic compounds which simultaneously target different mechanisms of blood vessel development, and/or their combination with other established drugs, may be appropriate. In line with this, Laschke et al. (2007) have shown in an animal model that an anti-angiogenic agent inhibiting the three angiogenic growth factors VEGF, bFGF and PDGF was much more effective in suppressing vascularization and growth of endometriosis lesions than blockade of VEGF alone.
Conclusions
There is strong evidence to show that dysregulated angiogenesis plays a critical role in fibroid pathophysiology, perhaps contributing to early events in tumor development. Despite some variability reported between studies, multiple angiogenic growth factors display altered expression in leiomyomas and are likely responsible for the vascular abnormalities seen in this disease. Moreover, the fibroid's aberrant hypoxic and angiogenic response may make it especially vulnerable to disruption of its vascular supply, a feature that could be exploited for treatment purposes. However, experimental evidence for the efficacy of anti-angiogenic treatment strategies in uterine fibroids is limited. Thus, there is a need for in-depth investigations of the growth factors that are involved in normal and pathological myometrial angiogenesis, as well as translation of the accumulating basic science evidence of abnormalities in fibroid vascular biology to possible therapeutic advantage.
Authors' roles
R.T. designed the study, identified the articles, and drafted and revised the manuscript. J.H.S. designed the study, contributed to the discussion of the topic and revised the manuscript. Both authors approved the final version of the manuscript.
Funding
This manuscript was supported, in part, by the intramural research Program of Reproductive and Adult Endocrinology, NICHD, National Institutes of Health, Bethesda, MD, USA.
Conflicts of interest
The authors have nothing to declare.
Acknowledgements
The authors wish to thank Dr Alan DeCherney for his helpful comments and suggestions, and Dr Howard Minkoff for his support. The authors appreciate the suggestions and critical review of the manuscript by Dr Carter Owen.
References
- Adam J, Hatipoglu E, O'Flaherty L, Ternette N, Sahgal N, Lockstone H, Baban D, Nye E, Stamp GW, Wolhuter K, et al. Renal cyst formation in Fh1-deficient mice is independent of the Hif/Phd pathway: roles for fumarate in KEAP1 succination and Nrf2 signaling. Cancer Cell. 2011;20:524–537. doi: 10.1016/j.ccr.2011.09.006. doi:10.1016/j.ccr.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn WS, Kim KW, Bae SM, Yoon JH, Lee JM, Namkoong SE, Kim JH, Kim CK, Lee YJ, Kim YW. Targeted cellular process profiling approach for uterine leiomyoma using cDNA microarray, proteomics and gene ontology analysis. Int J Exp Pathol. 2003;84:267–279. doi: 10.1111/j.0959-9673.2003.00362.x. doi:10.1111/j.0959-9673.2003.00362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aitken E, Khaund A, Hamid SA, Millan D, Campbell S. The normal human myometrium has a vascular spatial gradient absent in small fibroids. Hum Reprod. 2006;21:2669–2678. doi: 10.1093/humrep/del220. doi:10.1093/humrep/del220. [DOI] [PubMed] [Google Scholar]
- Alam NA, Rowan AJ, Wortham NC, Pollard PJ, Mitchell M, Tyrer JP, Barclay E, Calonje E, Manek S, Adams SJ, et al. Genetic and functional analyses of FH mutations in multiple cutaneous and uterine leiomyomatosis, hereditary leiomyomatosis and renal cancer, and fumarate hydratase deficiency. Hum Mol Genet. 2003;12:1241–1252. doi: 10.1093/hmg/ddg148. doi:10.1093/hmg/ddg148. [DOI] [PubMed] [Google Scholar]
- Alatas G, Aksoy E, Akarsu C, Yakin K, Bahceci M. The effect of uterine volume on uterine artery Doppler velocimetry in the myomatous state. Gynecol Obstet Invest. 1997;43:55–59. doi: 10.1159/000291820. doi:10.1159/000291820. [DOI] [PubMed] [Google Scholar]
- Anania CA, Stewart EA, Quade BJ, Hill JA, Nowak RA. Expression of the fibroblast growth factor receptor in women with leiomyomas and abnormal uterine bleeding. Mol Hum Reprod. 1997;3:685–691. doi: 10.1093/molehr/3.8.685. doi:10.1093/molehr/3.8.685. [DOI] [PubMed] [Google Scholar]
- Arici A, Sozen I. Transforming growth factor-beta3 is expressed at high levels in leiomyoma where it stimulates fibronectin expression and cell proliferation. Fertil Steril. 2000;73:1006–1011. doi: 10.1016/s0015-0282(00)00418-0. doi:10.1016/S0015-0282(00)00418-0. [DOI] [PubMed] [Google Scholar]
- Arslan AA, Gold LI, Mittal K, Suen TC, Belitskaya-Levy I, Tang MS, Toniolo P. Gene expression studies provide clues to the pathogenesis of uterine leiomyoma: new evidence and a systematic review. Hum Reprod. 2005;20:852–863. doi: 10.1093/humrep/deh698. doi:10.1093/humrep/deh698. [DOI] [PubMed] [Google Scholar]
- Asahara T, Takahashi T, Masuda H, Kalka C, Chen D, Iwaguro H, Inai Y, Silver M, Isner JM. VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. EMBO J. 1999;18:3964–3972. doi: 10.1093/emboj/18.14.3964. doi:10.1093/emboj/18.14.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astrup T, Beller FK, Glas P, Rasmussen J. Fibronolytic activity of the human uterine tube. Obstet Gynecol. 1965;25:853–857. [PubMed] [Google Scholar]
- Attisano L, Wrana JL. Signal transduction by the TGF-beta superfamily. Science. 2002;296:1646–1647. doi: 10.1126/science.1071809. doi:10.1126/science.1071809. [DOI] [PubMed] [Google Scholar]
- Baird DD, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100–107. doi: 10.1067/mob.2003.99. doi:10.1067/mob.2003.99. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay A, Lopez-Casillas F, Malik SN, Montiel JL, Mendoza V, Yang J, Sun LZ. Antitumor activity of a recombinant soluble betaglycan in human breast cancer xenograft. Cancer Res. 2002;62:4690–4695. [PubMed] [Google Scholar]
- Bandyopadhyay A, Wang L, Lopez-Casillas F, Mendoza V, Yeh IT, Sun L. Systemic administration of a soluble betaglycan suppresses tumor growth, angiogenesis, and matrix metalloproteinase-9 expression in a human xenograft model of prostate cancer. Prostate. 2005;63:81–90. doi: 10.1002/pros.20166. doi:10.1002/pros.20166. [DOI] [PubMed] [Google Scholar]
- Baranova O, Miranda LF, Pichiule P, Dragatsis I, Johnson RS, Chavez JC. Neuron-specific inactivation of the hypoxia inducible factor 1 alpha increases brain injury in a mouse model of transient focal cerebral ischemia. J Neurosci. 2007;27:6320–6332. doi: 10.1523/JNEUROSCI.0449-07.2007. doi:10.1523/JNEUROSCI.0449-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbarisi A, Petillo O, Di Lieto A, Melone MA, Margarucci S, Cannas M, Peluso G. 17-beta estradiol elicits an autocrine leiomyoma cell proliferation: evidence for a stimulation of protein kinase-dependent pathway. J Cell Physiol. 2001;186:414–424. doi: 10.1002/1097-4652(2000)9999:999<000::AID-JCP1040>3.0.CO;2-E. doi:10.1002/1097-4652(2000)9999:999<000::AID-JCP1040>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Bardella C, Olivero M, Lorenzato A, Geuna M, Adam J, O'Flaherty L, Rustin P, Tomlinson I, Pollard PJ, Di Renzo MF. Cells lacking the fumarase tumor suppressor are protected from apoptosis through a hypoxia-inducible factor-independent, AMPK-dependent mechanism. Mol Cell Biol. 2012;32:3081–3094. doi: 10.1128/MCB.06160-11. doi:10.1128/MCB.06160-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin LE, Golijanin D, Itin A, Pode D, Keshet E. Selective ablation of immature blood vessels in established human tumors follows vascular endothelial growth factor withdrawal. J Clin Invest. 1999;103:159–165. doi: 10.1172/JCI5028. doi:10.1172/JCI5028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm KD, Daimon M, Gorodeski IG, Sheean LA, Utian WH, Ilan J. Expression of the insulin-like and platelet-derived growth factor genes in human uterine tissues. Mol Reprod Dev. 1990;27:93–101. doi: 10.1002/mrd.1080270203. doi:10.1002/mrd.1080270203. [DOI] [PubMed] [Google Scholar]
- Bowden W, Skorupski J, Kovanci E, Rajkovic A. Detection of novel copy number variants in uterine leiomyomas using high-resolution SNP arrays. Mol Hum Reprod. 2009;15:563–568. doi: 10.1093/molehr/gap050. doi:10.1093/molehr/gap050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon DD, Bethea CL, Strawn EY, Novy MJ, Burry KA, Harrington MS, Erickson TE, Warner C, Keenan EJ, Clinton GM. Progesterone receptor messenger ribonucleic acid and protein are overexpressed in human uterine leiomyomas. Am J Obstet Gynecol. 1993;169:78–85. doi: 10.1016/0002-9378(93)90135-6. doi:10.1016/0002-9378(93)90135-6. [DOI] [PubMed] [Google Scholar]
- Brown LF, Detmar M, Tognazzi K, Abu-Jawdeh G, Iruela-Arispe ML. Uterine smooth muscle cells express functional receptors (flt-1 and KDR) for vascular permeability factor/vascular endothelial growth factor. Lab Invest. 1997;76:245–255. [PubMed] [Google Scholar]
- Brugarolas J, Lei K, Hurley RL, Manning BD, Reiling JH, Hafen E, Witters LA, Ellisen LW, Kaelin WG., Jr Regulation of mTOR function in response to hypoxia by REDD1 and the TSC1/TSC2 tumor suppressor complex. Genes Dev. 2004;18:2893–2904. doi: 10.1101/gad.1256804. doi:10.1101/gad.1256804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruick RK, McKnight SL. A conserved family of prolyl-4-hydroxylases that modify HIF. Science. 2001;294:1337–1340. doi: 10.1126/science.1066373. doi:10.1126/science.1066373. [DOI] [PubMed] [Google Scholar]
- Bullock JJ, Mehta SL, Lin Y, Lolla P, Li PA. Hyperglycemia-enhanced ischemic brain damage in mutant manganese SOD mice is associated with suppression of HIF-1alpha. Neurosci Lett. 2009;456:89–92. doi: 10.1016/j.neulet.2009.03.076. doi:10.1016/j.neulet.2009.03.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbank F. Childbirth and myoma treatment by uterine artery occlusion: do they share a common biology? J Am Assoc Gynecol Laparosc. 2004;11:138–152. doi: 10.1016/s1074-3804(05)60189-2. doi:10.1016/S1074-3804(05)60189-2. [DOI] [PubMed] [Google Scholar]
- Burn PR, McCall JM, Chinn RJ, Vashisht A, Smith JR, Healy JC. Uterine fibroleiomyoma: MR imaging appearances before and after embolization of uterine arteries. Radiology. 2000;214:729–734. doi: 10.1148/radiology.214.3.r00fe07729. [DOI] [PubMed] [Google Scholar]
- Cardozo ER, Clark AD, Banks NK, Henne MB, Stegmann BJ, Segars JH. The estimated annual cost of uterine leiomyomata in the United States. Am J Obstet Gynecol. 2012;206:211e211–219. doi: 10.1016/j.ajog.2011.12.002. doi:10.1016/j.ajog.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–660. doi: 10.1038/nm0603-653. doi:10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Dor Y, Herbert JM, Fukumura D, Brusselmans K, Dewerchin M, Neeman M, Bono F, Abramovitch R, Maxwell P, et al. Role of HIF-1alpha in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature. 1998;394:485–490. doi: 10.1038/28867. doi:10.1038/28867. [DOI] [PubMed] [Google Scholar]
- Carpenter G, Cohen S. Epidermal growth factor. J Biol Chem. 1990;265:7709–7712. [PubMed] [Google Scholar]
- Casey R, Rogers PA, Vollenhoven BJ. An immunohistochemical analysis of fibroid vasculature. Hum Reprod. 2000;15:1469–1475. doi: 10.1093/humrep/15.7.1469. doi:10.1093/humrep/15.7.1469. [DOI] [PubMed] [Google Scholar]
- Catherino WH, Leppert PC, Stenmark MH, Payson M, Potlog-Nahari C, Nieman LK, Segars JH. Reduced dermatopontin expression is a molecular link between uterine leiomyomas and keloids. Genes Chromosomes Cancer. 2004;40:204–217. doi: 10.1002/gcc.20035. doi:10.1002/gcc.20035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha PC, Takahashi A, Hosono N, Low SK, Kamatani N, Kubo M, Nakamura Y. A genome-wide association study identifies three loci associated with susceptibility to uterine fibroids. Nat Genet. 2011;43:447–450. doi: 10.1038/ng.805. doi:10.1038/ng.805. [DOI] [PubMed] [Google Scholar]
- Charnock-Jones DS, Sharkey AM, Rajput-Williams J, Burch D, Schofield JP, Fountain SA, Boocock CA, Smith SK. Identification and localization of alternately spliced mRNAs for vascular endothelial growth factor in human uterus and estrogen regulation in endometrial carcinoma cell lines. Biol Reprod. 1993;48:1120–1128. doi: 10.1095/biolreprod48.5.1120. doi:10.1095/biolreprod48.5.1120. [DOI] [PubMed] [Google Scholar]
- Chegini N, Zhao Y, Williams RS, Flanders KC. Human uterine tissue throughout the menstrual cycle expresses transforming growth factor-beta 1 (TGF beta 1), TGF beta 2, TGF beta 3, and TGF beta type II receptor messenger ribonucleic acid and protein and contains [125I]TGF beta 1-binding sites. Endocrinology. 1994;135:439–449. doi: 10.1210/endo.135.1.8013382. doi:10.1210/en.135.1.439. [DOI] [PubMed] [Google Scholar]
- Chegini N, Luo X, Ding L, Ripley D. The expression of Smads and transforming growth factor beta receptors in leiomyoma and myometrium and the effect of gonadotropin releasing hormone analogue therapy. Mol Cell Endocrinol. 2003a;209:9–16. doi: 10.1016/j.mce.2003.08.007. doi:10.1016/j.mce.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Chegini N, Verala J, Luo X, Xu J, Williams RS. Gene expression profile of leiomyoma and myometrium and the effect of gonadotropin releasing hormone analogue therapy. J Soc Gynecol Investig. 2003b;10:161–171. doi: 10.1016/s1071-5576(03)00004-2. doi:10.1016/S1071-5576(03)00004-2. [DOI] [PubMed] [Google Scholar]
- Chen W, Ohara N, Wang J, Xu Q, Liu J, Morikawa A, Sasaki H, Yoshida S, Demanno DA, Chwalisz K, et al. A novel selective progesterone receptor modulator asoprisnil (J867) inhibits proliferation and induces apoptosis in cultured human uterine leiomyoma cells in the absence of comparable effects on myometrial cells. J Clin Endocrinol Metab. 2006;91:1296–1304. doi: 10.1210/jc.2005-2379. doi:10.1210/jc.2005-2379. [DOI] [PubMed] [Google Scholar]
- Cheng Z, Xie Y, Dai H, Hu L, Zhu Y, Gong J. Unequal tissue expression of proteins from the PA/PAI system, myoma necrosis, and uterus survival after uterine artery occlusion. Int J Gynaecol Obstet. 2008;102:55–59. doi: 10.1016/j.ijgo.2008.02.006. doi:10.1016/j.ijgo.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Chin BY, Mohsenin A, Li SX, Choi AM, Choi ME. Stimulation of pro-alpha(1)(I) collagen by TGF-beta(1) in mesangial cells: role of the p38 MAPK pathway. Am J Physiol Renal Physiol. 2001;280:F495–F504. doi: 10.1152/ajprenal.2001.280.3.F495. [DOI] [PubMed] [Google Scholar]
- Chobotova K, Muchmore ME, Carver J, Yoo HJ, Manek S, Gullick WJ, Barlow DH, Mardon HJ. The mitogenic potential of heparin-binding epidermal growth factor in the human endometrium is mediated by the epidermal growth factor receptor and is modulated by tumor necrosis factor-alpha. J Clin Endocrinol Metab. 2002;87:5769–5777. doi: 10.1210/jc.2002-020069. doi:10.1210/jc.2002-020069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chwalisz K, Larsen L, Mattia-Goldberg C, Edmonds A, Elger W, Winkel CA. A randomized, controlled trial of asoprisnil, a novel selective progesterone receptor modulator, in women with uterine leiomyomata. Fertil Steril. 2007;87:1399–1412. doi: 10.1016/j.fertnstert.2006.11.094. doi:10.1016/j.fertnstert.2006.11.094. [DOI] [PubMed] [Google Scholar]
- Ciarmela P, Islam MS, Reis FM, Gray PC, Bloise E, Petraglia F, Vale W, Castellucci M. Growth factors and myometrium: biological effects in uterine fibroid and possible clinical implications. Hum Reprod Update. 2011;17:772–790. doi: 10.1093/humupd/dmr031. doi:10.1093/humupd/dmr031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colgan TJ, Pron G, Mocarski EJ, Bennett JD, Asch MR, Common A. Pathologic features of uteri and leiomyomas following uterine artery embolization for leiomyomas. Am J Surg Pathol. 2003;27:167–177. doi: 10.1097/00000478-200302000-00004. doi:10.1097/00000478-200302000-00004. [DOI] [PubMed] [Google Scholar]
- Cook JD, Walker CL. Treatment strategies for uterine leiomyoma: the role of hormonal modulation. Semin Reprod Med. 2004;22:105–111. doi: 10.1055/s-2004-828616. doi:10.1055/s-2004-828616. [DOI] [PubMed] [Google Scholar]
- Costa B, Dettori D, Lorenzato A, Bardella C, Coltella N, Martino C, Cammarata C, Carmeliet P, Olivero M, Di Renzo MF. Fumarase tumor suppressor gene and MET oncogene cooperate in upholding transformation and tumorigenesis. FASEB J. 2010;24:2680–2688. doi: 10.1096/fj.09-146928. doi:10.1096/fj.09-146928. [DOI] [PubMed] [Google Scholar]
- Crabtree JS, Jelinsky SA, Harris HA, Choe SE, Cotreau MM, Kimberland ML, Wilson E, Saraf KA, Liu W, McCampbell AS, et al. Comparison of human and rat uterine leiomyomata: identification of a dysregulated mammalian target of rapamycin pathway. Cancer Res. 2009;69:6171–6178. doi: 10.1158/0008-5472.CAN-08-4471. doi:10.1158/0008-5472.CAN-08-4471. [DOI] [PubMed] [Google Scholar]
- Cramer SF, Mann L, Calianese E, Daley J, Williamson K. Association of seedling myomas with myometrial hyperplasia. Hum Pathol. 2009;40:218–225. doi: 10.1016/j.humpath.2008.07.004. doi:10.1016/j.humpath.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Cui L, Ren Y, Yin H, Wang Y, Li D, Liu M, Zhu Y, Lin W, Tang XD, Gui Y, et al. Increased expression of tuberin in human uterine leiomyoma. Fertil Steril. 2011;95:1805–1808. doi: 10.1016/j.fertnstert.2010.11.028. doi:10.1016/j.fertnstert.2010.11.028. [DOI] [PubMed] [Google Scholar]
- De Falco M, Staibano S, D'Armiento FP, Mascolo M, Salvatore G, Busiello A, Carbone IF, Pollio F, Di Lieto A. Preoperative treatment of uterine leiomyomas: clinical findings and expression of transforming growth factor-beta3 and connective tissue growth factor. J Soc Gynecol Investig. 2006;13:297–303. doi: 10.1016/j.jsgi.2006.02.008. doi:10.1016/j.jsgi.2006.02.008. [DOI] [PubMed] [Google Scholar]
- De Leo V, Morgante G, La Marca A, Musacchio MC, Sorace M, Cavicchioli C, Petraglia F. A benefit-risk assessment of medical treatment for uterine leiomyomas. Drug Saf. 2002;25:759–779. doi: 10.2165/00002018-200225110-00002. doi:10.2165/00002018-200225110-00002. [DOI] [PubMed] [Google Scholar]
- Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. doi:10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- deSouza NM, Williams AD. Uterine arterial embolization for leiomyomas: perfusion and volume changes at MR imaging and relation to clinical outcome. Radiology. 2002;222:367–374. doi: 10.1148/radiol.2222010584. doi:10.1148/radiol.2222010584. [DOI] [PubMed] [Google Scholar]
- Dhingra S, Rodriguez ME, Shen Q, Duan X, Stanton ML, Chen L, Zhang R, Brown RE. Constitutive activation with overexpression of the mTORC2-phospholipase D1 pathway in uterine leiomyosarcoma and STUMP: morphoproteomic analysis with therapeutic implications. Int J Clin Exp Pathol. 2011;4:134–146. [PMC free article] [PubMed] [Google Scholar]
- Di Lieto A, De Falco M, Staibano S, Iannotti F, Scaramellino M, Salvatore G, Mansueto G, Granata P, Pontillo M, Pollio F, et al. Effects of gonadotropin-releasing hormone agonists on uterine volume and vasculature and on the immunohistochemical expression of basic fibroblast growth factor (bFGF) in uterine leiomyomas. Int J Gynecol Pathol. 2003a;22:353–358. doi: 10.1097/01.PGP.0000070849.25718.73. doi:10.1097/01.PGP.0000070849.25718.73. [DOI] [PubMed] [Google Scholar]
- Di Lieto A, Iannotti F, De Falco M, Staibano S, Pollio F, Ciociola F, De Rosa G. Immunohistochemical detection of insulin-like growth factor type I receptor and uterine volume changes in gonadotropin-releasing hormone analog-treated uterine leiomyomas. Am J Obstet Gynecol. 2003b;188:702–706. doi: 10.1067/mob.2003.180. doi:10.1067/mob.2003.180. [DOI] [PubMed] [Google Scholar]
- Di Lieto A, De Falco M, Mansueto G, De Rosa G, Pollio F, Staibano S. Preoperative administration of GnRH-a plus tibolone to premenopausal women with uterine fibroids: evaluation of the clinical response, the immunohistochemical expression of PDGF, bFGF and VEGF and the vascular pattern. Steroids. 2005a;70:95–102. doi: 10.1016/j.steroids.2004.10.008. doi:10.1016/j.steroids.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Di Lieto A, De Falco M, Pollio F, Mansueto G, Salvatore G, Somma P, Ciociola F, De Rosa G, Staibano S. Clinical response, vascular change, and angiogenesis in gonadotropin-releasing hormone analogue-treated women with uterine myomas. J Soc Gynecol Investig. 2005b;12:123–128. doi: 10.1016/j.jsgi.2004.10.008. doi:10.1016/j.jsgi.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Dixon D, He H, Haseman JK. Immunohistochemical localization of growth factors and their receptors in uterine leiomyomas and matched myometrium. Environ Health Perspect. 2000;108(Suppl 5):795–802. doi: 10.1289/ehp.00108s5795. [DOI] [PubMed] [Google Scholar]
- Dou Q, Zhao Y, Tarnuzzer RW, Rong H, Williams RS, Schultz GS, Chegini N. Suppression of transforming growth factor-beta (TGF beta) and TGF beta receptor messenger ribonucleic acid and protein expression in leiomyomata in women receiving gonadotropin-releasing hormone agonist therapy. J Clin Endocrinol Metab. 1996;81:3222–3230. doi: 10.1210/jcem.81.9.8784073. doi:10.1210/jc.81.9.3222. [DOI] [PubMed] [Google Scholar]
- Dye J, Lawrence L, Linge C, Leach L, Firth J, Clark P. Distinct patterns of microvascular endothelial cell morphology are determined by extracellular matrix composition. Endothelium. 2004;11:151–167. doi: 10.1080/10623320490512093. doi:10.1080/10623320490512093. [DOI] [PubMed] [Google Scholar]
- Eckle T, Kohler D, Lehmann R, El Kasmi K, Eltzschig HK. Hypoxia-inducible factor-1 is central to cardioprotection: a new paradigm for ischemic preconditioning. Circulation. 2008;118:166–175. doi: 10.1161/CIRCULATIONAHA.107.758516. doi:10.1161/CIRCULATIONAHA.107.758516. [DOI] [PubMed] [Google Scholar]
- Eggert SL, Huyck KL, Somasundaram P, Kavalla R, Stewart EA, Lu AT, Painter JN, Montgomery GW, Medland SE, Nyholt DR, et al. Genome-wide linkage and association analyses implicate FASN in predisposition to Uterine Leiomyomata. Am J Hum Genet. 2012;91:621–628. doi: 10.1016/j.ajhg.2012.08.009. doi:10.1016/j.ajhg.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisinger SH, Meldrum S, Fiscella K, le Roux HD, Guzick DS. Low-dose mifepristone for uterine leiomyomata. Obstet Gynecol. 2003;101:243–250. doi: 10.1016/s0029-7844(02)02511-5. doi:10.1016/S0029-7844(02)02511-5. [DOI] [PubMed] [Google Scholar]
- Eisinger SH, Fiscella J, Bonfiglio T, Meldrum S, Fiscella K. Open-label study of ultra-low-dose mifepristone for the treatment of uterine leiomyomata. Eur J Obstet Gynecol Reprod Biol. 2009;146:215–218. doi: 10.1016/j.ejogrb.2009.06.004. doi:10.1016/j.ejogrb.2009.06.004. [DOI] [PubMed] [Google Scholar]
- Eldar-Geva T, Meagher S, Healy DL, MacLachlan V, Breheny S, Wood C. Effect of intramural, subserosal, and submucosal uterine fibroids on the outcome of assisted reproductive technology treatment. Fertil Steril. 1998;70:687–691. doi: 10.1016/s0015-0282(98)00265-9. doi:10.1016/S0015-0282(98)00265-9. [DOI] [PubMed] [Google Scholar]
- Elenius K, Paul S, Allison G, Sun J, Klagsbrun M. Activation of HER4 by heparin-binding EGF-like growth factor stimulates chemotaxis but not proliferation. EMBO J. 1997;16:1268–1278. doi: 10.1093/emboj/16.6.1268. doi:10.1093/emboj/16.6.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O'Rourke J, Mole DR, Mukherji M, Metzen E, Wilson MI, Dhanda A, et al. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107:43–54. doi: 10.1016/s0092-8674(01)00507-4. doi:10.1016/S0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- Fahmy RG, Dass CR, Sun LQ, Chesterman CN, Khachigian LM. Transcription factor Egr-1 supports FGF-dependent angiogenesis during neovascularization and tumor growth. Nat Med. 2003;9:1026–1032. doi: 10.1038/nm905. doi:10.1038/nm905. [DOI] [PubMed] [Google Scholar]
- Farhi J, Ashkenazi J, Feldberg D, Dicker D, Orvieto R, Ben Rafael Z. Effect of uterine leiomyomata on the results of in-vitro fertilization treatment. Hum Reprod. 1995;10:2576–2578. doi: 10.1093/oxfordjournals.humrep.a135748. doi:10.1093/humrep/10.1.85. [DOI] [PubMed] [Google Scholar]
- Farquhar CM, Steiner CA. Hysterectomy rates in the United States 1990–1997. Obstet Gynecol. 2002;99:229–234. doi: 10.1016/s0029-7844(01)01723-9. doi:10.1016/S0029-7844(01)01723-9. [DOI] [PubMed] [Google Scholar]
- Farquhar CM, Harvey SA, Yu Y, Sadler L, Stewart AW. A prospective study of 3 years of outcomes after hysterectomy with and without oophorectomy. Am J Obstet Gynecol. 2006;194:711–717. doi: 10.1016/j.ajog.2005.08.066. doi:10.1016/j.ajog.2005.08.066. [DOI] [PubMed] [Google Scholar]
- Farrer-Brown G, Beilby JO, Tarbit MH. The vascular patterns in myomatous uteri. J Obstet Gynaecol Br Commonw. 1970;77:967–975. doi: 10.1111/j.1471-0528.1970.tb03439.x. doi:10.1111/j.1471-0528.1970.tb03439.x. [DOI] [PubMed] [Google Scholar]
- Faulkner RL. The blood vessels of the myomatous uterus. Am J Obstet Gynecol. 1944;47:185–197. [Google Scholar]
- Fayed YM, Tsibris JC, Langenberg PW, Robertson AL., Jr Human uterine leiomyoma cells: binding and growth responses to epidermal growth factor, platelet-derived growth factor, and insulin. Lab Invest. 1989;60:30–37. [PubMed] [Google Scholar]
- Fennessy FM, Tempany CM, McDannold NJ, So MJ, Hesley G, Gostout B, Kim HS, Holland GA, Sarti DA, Hynynen K, et al. Uterine leiomyomas: MR imaging-guided focused ultrasound surgery—results of different treatment protocols. Radiology. 2007;243:885–893. doi: 10.1148/radiol.2433060267. doi:10.1148/radiol.2433060267. [DOI] [PubMed] [Google Scholar]
- Fernig DG, Gallagher JT. Fibroblast growth factors and their receptors: an information network controlling tissue growth, morphogenesis and repair. Prog Growth Factor Res. 1994;5:353–377. doi: 10.1016/0955-2235(94)00007-8. doi:10.1016/0955-2235(94)00007-8. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997;18:4–25. doi: 10.1210/edrv.18.1.0287. doi:10.1210/er.18.1.4. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Hillan KJ, Gerber HP, Novotny W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov. 2004;3:391–400. doi: 10.1038/nrd1381. doi:10.1038/nrd1381. [DOI] [PubMed] [Google Scholar]
- Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. doi:10.1056/NEJM197108122850711. [DOI] [PubMed] [Google Scholar]
- Folkman J. Angiogenesis. Annu Rev Med. 2006;57:1–18. doi: 10.1146/annurev.med.57.121304.131306. doi:10.1146/annurev.med.57.121304.131306. [DOI] [PubMed] [Google Scholar]
- Forssman L. Blood flow in myomatous uteri as measured by intra-arterial 133Xenon. Acta Obstet Gynecol Scand. 1976a;55:21–24. doi: 10.3109/00016347609156778. doi:10.3109/00016347609156778. [DOI] [PubMed] [Google Scholar]
- Forssman L. Distribution of blood flow in myomatous uteri as measured by locally injected 133Xenon. Acta Obstet Gynecol Scand. 1976b;55:101–104. doi: 10.3109/00016347609156794. doi:10.3109/00016347609156794. [DOI] [PubMed] [Google Scholar]
- Fraser HM, Duncan WC. SRB reproduction, fertility and development award lecture 2008. Regulation and manipulation of angiogenesis in the ovary and endometrium. Reprod Fertil Dev. 2009;21:377–392. doi: 10.1071/rd08272. doi:10.1071/RD08272. [DOI] [PubMed] [Google Scholar]
- Fraser HM, Lunn SF. Angiogenesis and its control in the female reproductive system. Br Med Bull. 2000;56:787–797. doi: 10.1258/0007142001903364. doi:10.1258/0007142001903364. [DOI] [PubMed] [Google Scholar]
- Fujii S, Konishi I, Horiuchi A, Orii A, Nikaido T. Mesenchymal cell differentiation: speculation about the histogenesis of uterine leiomyomas. In: Brosens IA, Donnez J, Lunenfeld B, editors. Pathogenesis and Medical Management of Uterine Fibroids. New York: The Parthenon Publishing Group; 1999. pp. 3–15. [Google Scholar]
- Fujimoto J. Novel strategy of anti-angiogenic therapy for uterine cervical carcinomas. Anticancer Res. 2009;29:2665–2669. [PubMed] [Google Scholar]
- Garg K, Tickoo SK, Soslow RA, Reuter VE. Morphologic features of uterine leiomyomas associated with hereditary leiomyomatosis and renal cell carcinoma syndrome: a case report. Am J Surg Pathol. 2011;35:1235–1237. doi: 10.1097/PAS.0b013e318223ca01. doi:10.1097/PAS.0b013e318223ca01. [DOI] [PubMed] [Google Scholar]
- Gargett CE, Rogers PA. Human endometrial angiogenesis. Reproduction. 2001;121:181–186. doi: 10.1530/rep.0.1210181. doi:10.1530/rep.0.1210181. [DOI] [PubMed] [Google Scholar]
- Gentry CC, Okolo SO, Fong LF, Crow JC, Maclean AB, Perrett CW. Quantification of vascular endothelial growth factor-A in leiomyomas and adjacent myometrium. Clin Sci (Lond) 2001;101:691–695. doi:10.1042/CS20010096. [PubMed] [Google Scholar]
- Gross KL, Morton CC. Genetics and the development of fibroids. Clin Obstet Gynecol. 2001;44:335–349. doi: 10.1097/00003081-200106000-00020. doi:10.1097/00003081-200106000-00020. [DOI] [PubMed] [Google Scholar]
- Grubb RL, 3rd, Franks ME, Toro J, Middelton L, Choyke L, Fowler S, Torres-Cabala C, Glenn GM, Choyke P, Merino MJ, et al. Hereditary leiomyomatosis and renal cell cancer: a syndrome associated with an aggressive form of inherited renal cancer. J Urol. 2007;177:2074–2079. doi: 10.1016/j.juro.2007.01.155. discussion 2079–2080 doi:10.1016/j.juro.2007.01.155. [DOI] [PubMed] [Google Scholar]
- Gururaj AE, Belakavadi M, Venkatesh DA, Marme D, Salimath BP. Molecular mechanisms of anti-angiogenic effect of curcumin. Biochem Biophys Res Commun. 2002;297:934–942. doi: 10.1016/s0006-291x(02)02306-9. doi:10.1016/S0006-291X(02)02306-9. [DOI] [PubMed] [Google Scholar]
- Hague S, Zhang L, Oehler MK, Manek S, MacKenzie IZ, Bicknell R, Rees MC. Expression of the hypoxically regulated angiogenic factor adrenomedullin correlates with uterine leiomyoma vascular density. Clin Cancer Res. 2000;6:2808–2814. [PubMed] [Google Scholar]
- Hague S, MacKenzie IZ, Bicknell R, Rees MC. In-vivo angiogenesis and progestogens. Hum Reprod. 2002;17:786–793. doi: 10.1093/humrep/17.3.786. doi:10.1093/humrep/17.3.786. [DOI] [PubMed] [Google Scholar]
- Harris AL. Hypoxia—a key regulatory factor in tumour growth. Nat Rev Cancer. 2002;2:38–47. doi: 10.1038/nrc704. doi:10.1038/nrc704. [DOI] [PubMed] [Google Scholar]
- Harrison-Woolrych ML, Charnock-Jones DS, Smith SK. Quantification of messenger ribonucleic acid for epidermal growth factor in human myometrium and leiomyomata using reverse transcriptase polymerase chain reaction. J Clin Endocrinol Metab. 1994;78:1179–1184. doi: 10.1210/jcem.78.5.8175976. doi:10.1210/jc.78.5.1179. [DOI] [PubMed] [Google Scholar]
- Harrison-Woolrych ML, Sharkey AM, Charnock-Jones DS, Smith SK. Localization and quantification of vascular endothelial growth factor messenger ribonucleic acid in human myometrium and leiomyomata. J Clin Endocrinol Metab. 1995;80:1853–1858. doi: 10.1210/jcem.80.6.7775632. doi:10.1210/jc.80.6.1853. [DOI] [PubMed] [Google Scholar]
- Hassan MH, Eyzaguirre E, Arafa HM, Hamada FM, Salama SA, Al-Hendy A. Memy I: a novel murine model for uterine leiomyoma using adenovirus-enhanced human fibroid explants in severe combined immune deficiency mice. Am J Obstet Gynecol. 2008;199:156 e151–158. doi: 10.1016/j.ajog.2008.02.010. doi:10.1016/j.ajog.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan M, Zhang D, Salama S, Hamada F, Arafa H, Fouad H, Walker C, Al-Hendy A. Towards fibroid gene therapy: adenovirus-mediated delivery of herpes simplex virus 1 thymidine kinase gene/ganciclovir shrinks uterine leiomyoma in the Eker rat model. Gynecol Obstet Invest. 2009;68:19–32. doi: 10.1159/000209675. doi:10.1159/000209675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan MH, Salama SA, Zhang D, Arafa HM, Hamada FM, Fouad H, Walker CC, Al-Hendy A. Gene therapy targeting leiomyoma: adenovirus-mediated delivery of dominant-negative estrogen receptor gene shrinks uterine tumors in Eker rat model. Fertil Steril. 2010;93:239–250. doi: 10.1016/j.fertnstert.2008.09.086. doi:10.1016/j.fertnstert.2008.09.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatcher H, Planalp R, Cho J, Torti FM, Torti SV. Curcumin: from ancient medicine to current clinical trials. Cell Mol Life Sci. 2008;65:1631–1652. doi: 10.1007/s00018-008-7452-4. doi:10.1007/s00018-008-7452-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldin CH. Platelet-derived growth factor–an introduction. Cytokine Growth Factor Rev. 2004;15:195–196. doi: 10.1016/j.cytogfr.2004.03.001. doi:10.1016/j.cytogfr.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Heldin CH, Westermark B. Mechanism of action and in vivo role of platelet-derived growth factor. Physiol Rev. 1999;79:1283–1316. doi: 10.1152/physrev.1999.79.4.1283. [DOI] [PubMed] [Google Scholar]
- Heldin CH, Eriksson U, Ostman A. New members of the platelet-derived growth factor family of mitogens. Arch Biochem Biophys. 2002;398:284–290. doi: 10.1006/abbi.2001.2707. doi:10.1006/abbi.2001.2707. [DOI] [PubMed] [Google Scholar]
- Hellstrom M, Kalen M, Lindahl P, Abramsson A, Betsholtz C. Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development. 1999;126:3047–3055. doi: 10.1242/dev.126.14.3047. [DOI] [PubMed] [Google Scholar]
- Hernando E, Charytonowicz E, Dudas ME, Menendez S, Matushansky I, Mills J, Socci ND, Behrendt N, Ma L, Maki RG, et al. The AKT-mTOR pathway plays a critical role in the development of leiomyosarcomas. Nat Med. 2007;13:748–753. doi: 10.1038/nm1560. doi:10.1038/nm1560. [DOI] [PubMed] [Google Scholar]
- Higashiyama S, Abraham JA, Miller J, Fiddes JC, Klagsbrun M. A heparin-binding growth factor secreted by macrophage-like cells that is related to EGF. Science. 1991;251:936–939. doi: 10.1126/science.1840698. doi:10.1126/science.1840698. [DOI] [PubMed] [Google Scholar]
- Higashiyama S, Abraham JA, Klagsbrun M. Heparin-binding EGF-like growth factor stimulation of smooth muscle cell migration: dependence on interactions with cell surface heparan sulfate. J Cell Biol. 1993;122:933–940. doi: 10.1083/jcb.122.4.933. doi:10.1083/jcb.122.4.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge JC, Cuenco KT, Huyck KL, Somasundaram P, Panhuysen CI, Stewart EA, Morton CC. Uterine leiomyomata and decreased height: a common HMGA2 predisposition allele. Hum Genet. 2009;125:257–263. doi: 10.1007/s00439-008-0621-6. doi:10.1007/s00439-008-0621-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekstra AV, Sefton EC, Berry E, Lu Z, Hardt J, Marsh E, Yin P, Clardy J, Chakravarti D, Bulun S, et al. Progestins activate the AKT pathway in leiomyoma cells and promote survival. J Clin Endocrinol Metab. 2009;94:1768–1774. doi: 10.1210/jc.2008-2093. doi:10.1210/jc.2008-2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman PJ, Milliken DB, Gregg LC, Davis RR, Gregg JP. Molecular characterization of uterine fibroids and its implication for underlying mechanisms of pathogenesis. Fertil Steril. 2004;82:639–649. doi: 10.1016/j.fertnstert.2004.01.047. doi:10.1016/j.fertnstert.2004.01.047. [DOI] [PubMed] [Google Scholar]
- Hong T, Shimada Y, Uchida S, Itami A, Li Z, Ding Y, Kaganoi J, Komoto I, Sakurai T, Imamura M. Expression of angiogenic factors and apoptotic factors in leiomyosarcoma and leiomyoma. Int J Mol Med. 2001;8:141–148. doi: 10.3892/ijmm.8.2.141. [DOI] [PubMed] [Google Scholar]
- Horree N, van Diest PJ, van der Groep P, Sie-Go DM, Heintz AP. Hypoxia and angiogenesis in endometrioid endometrial carcinogenesis. Cell Oncol. 2007;29:219–227. doi: 10.1155/2007/434731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang SC, Yu CH, Huang RT, Hsu KF, Tsai YC, Chou CY. Intratumoral blood flow in uterine myoma correlated with a lower tumor size and volume, but not correlated with cell proliferation or angiogenesis. Obstet Gynecol. 1996;87:1019–1024. doi: 10.1016/0029-7844(96)00073-7. doi:10.1016/0029-7844(96)00073-7. [DOI] [PubMed] [Google Scholar]
- Hudson CC, Liu M, Chiang GG, Otterness DM, Loomis DC, Kaper F, Giaccia AJ, Abraham RT. Regulation of hypoxia-inducible factor 1alpha expression and function by the mammalian target of rapamycin. Mol Cell Biol. 2002;22:7004–7014. doi: 10.1128/MCB.22.20.7004-7014.2002. doi:10.1128/MCB.22.20.7004-7014.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwu YM, Li SH, Lee RK, Tsai YH, Yeh TS, Lin SY. Increased expression of platelet-derived growth factor C messenger ribonucleic acid in uterine leiomyomata. Fertil Steril. 2008;89:468–471. doi: 10.1016/j.fertnstert.2007.02.031. doi:10.1016/j.fertnstert.2007.02.031. [DOI] [PubMed] [Google Scholar]
- Hyder SM, Stancel GM. Regulation of VEGF in the reproductive tract by sex-steroid hormones. Histol Histopathol. 2000;15:325–334. doi: 10.14670/HH-15.325. [DOI] [PubMed] [Google Scholar]
- Hyder SM, Huang JC, Nawaz Z, Boettger-Tong H, Makela S, Chiappetta C, Stancel GM. Regulation of vascular endothelial growth factor expression by estrogens and progestins. Environ Health Perspect. 2000;108(Suppl 5):785–790. doi: 10.1289/ehp.00108s5785. [DOI] [PubMed] [Google Scholar]
- Hyman KM, Seghezzi G, Pintucci G, Stellari G, Kim JH, Grossi EA, Galloway AC, Mignatti P. Transforming growth factor-beta1 induces apoptosis in vascular endothelial cells by activation of mitogen-activated protein kinase. Surgery. 2002;132:173–179. doi: 10.1067/msy.2002.125304. doi:10.1067/msy.2002.125304. [DOI] [PubMed] [Google Scholar]
- Ignotz RA, Endo T, Massague J. Regulation of fibronectin and type I collagen mRNA levels by transforming growth factor-beta. J Biol Chem. 1987;262:6443–6446. [PubMed] [Google Scholar]
- Ingber D, Fujita T, Kishimoto S, Sudo K, Kanamaru T, Brem H, Folkman J. Synthetic analogues of fumagillin that inhibit angiogenesis and suppress tumour growth. Nature. 1990;348:555–557. doi: 10.1038/348555a0. doi:10.1038/348555a0. [DOI] [PubMed] [Google Scholar]
- Isaacs JS, Jung YJ, Mole DR, Lee S, Torres-Cabala C, Chung YL, Merino M, Trepel J, Zbar B, Toro J, et al. HIF overexpression correlates with biallelic loss of fumarate hydratase in renal cancer: novel role of fumarate in regulation of HIF stability. Cancer Cell. 2005;8:143–153. doi: 10.1016/j.ccr.2005.06.017. doi:10.1016/j.ccr.2005.06.017. [DOI] [PubMed] [Google Scholar]
- Ishikawa H, Shozu M, Okada M, Inukai M, Zhang B, Kato K, Kasai T, Inoue M. Early growth response gene-1 plays a pivotal role in down-regulation of a cohort of genes in uterine leiomyoma. J Mol Endocrinol. 2007;39:333–341. doi: 10.1677/JME-06-0069. doi:10.1677/JME-06-0069. [DOI] [PubMed] [Google Scholar]
- Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, Kriegsheim A, Hebestreit HF, Mukherji M, Schofield CJ, et al. Targeting of HIF-alpha to the Von Hippel–Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. doi:10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- Jackson C. Matrix metalloproteinases and angiogenesis. Curr Opin Nephrol Hypertens. 2002;11:295–299. doi: 10.1097/00041552-200205000-00005. doi:10.1097/00041552-200205000-00005. [DOI] [PubMed] [Google Scholar]
- Jaffe RB. Importance of angiogenesis in reproductive physiology. Semin Perinatol. 2000;24:79–81. doi: 10.1016/s0146-0005(00)80062-2. doi:10.1016/S0146-0005(00)80062-2. [DOI] [PubMed] [Google Scholar]
- Jaffer S, Shynlova O, Lye S. Mammalian target of rapamycin is activated in association with myometrial proliferation during pregnancy. Endocrinology. 2009;150:4672–4680. doi: 10.1210/en.2009-0419. doi:10.1210/en.2009-0419. [DOI] [PubMed] [Google Scholar]
- Jain RK. Molecular regulation of vessel maturation. Nat Med. 2003;9:685–693. doi: 10.1038/nm0603-685. doi:10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- Jha RC, Ascher SM, Imaoka I, Spies JB. Symptomatic fibroleiomyomata: MR imaging of the uterus before and after uterine arterial embolization. Radiology. 2000;217:228–235. doi: 10.1148/radiology.217.1.r00se49228. [DOI] [PubMed] [Google Scholar]
- Joseph DS, Malik M, Nurudeen S, Catherino WH. Myometrial cells undergo fibrotic transformation under the influence of transforming growth factor beta-3. Fertil Steril. 2010;93:1500–1508. doi: 10.1016/j.fertnstert.2009.01.081. doi:10.1016/j.fertnstert.2009.01.081. [DOI] [PubMed] [Google Scholar]
- Kamphaus GD, Colorado PC, Panka DJ, Hopfer H, Ramchandran R, Torre A, Maeshima Y, Mier JW, Sukhatme VP, Kalluri R. Canstatin, a novel matrix-derived inhibitor of angiogenesis and tumor growth. J Biol Chem. 2000;275:1209–1215. doi: 10.1074/jbc.275.2.1209. doi:10.1074/jbc.275.2.1209. [DOI] [PubMed] [Google Scholar]
- Karashima T, Sweeney P, Slaton JW, Kim SJ, Kedar D, Izawa JI, Fan Z, Pettaway C, Hicklin DJ, Shuin T, et al. Inhibition of angiogenesis by the antiepidermal growth factor receptor antibody ImClone C225 in androgen-independent prostate cancer growing orthotopically in nude mice. Clin Cancer Res. 2002;8:1253–1264. [PubMed] [Google Scholar]
- Katsumori T, Kasahara T, Kin Y, Nozaki T. Infarction of uterine fibroids after embolization: relationship between postprocedural enhanced MRI findings and long-term clinical outcomes. Cardiovasc Intervent Radiol. 2008;31:66–72. doi: 10.1007/s00270-007-9187-2. doi:10.1007/s00270-007-9187-2. [DOI] [PubMed] [Google Scholar]
- Khan KN, Kitajima M, Hiraki K, Fujishita A, Sekine I, Ishimaru T, Masuzaki H. Changes in tissue inflammation, angiogenesis and apoptosis in endometriosis, adenomyosis and uterine myoma after GnRH agonist therapy. Hum Reprod. 2010;25:642–653. doi: 10.1093/humrep/dep437. doi:10.1093/humrep/dep437. [DOI] [PubMed] [Google Scholar]
- Kim KJ, Li B, Winer J, Armanini M, Gillett N, Phillips HS, Ferrara N. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature. 1993;362:841–844. doi: 10.1038/362841a0. doi:10.1038/362841a0. [DOI] [PubMed] [Google Scholar]
- Klagsbrun M, Dluz S. Smooth muscle cell and endothelial cell growth factors. Trends Cardiovasc Med. 1993;3:213–217. doi: 10.1016/1050-1738(93)90042-5. doi:10.1016/1050-1738(93)90042-5. [DOI] [PubMed] [Google Scholar]
- Klauber N, Rohan RM, Flynn E, D'Amato RJ. Critical components of the female reproductive pathway are suppressed by the angiogenesis inhibitor AGM-1470. Nat Med. 1997;3:443–446. doi: 10.1038/nm0497-443. doi:10.1038/nm0497-443. [DOI] [PubMed] [Google Scholar]
- Klemke M, Meyer A, Nezhad MH, Bartnitzke S, Drieschner N, Frantzen C, Schmidt EH, Belge G, Bullerdiek J. Overexpression of HMGA2 in uterine leiomyomas points to its general role for the pathogenesis of the disease. Genes Chromosomes Cancer. 2009;48:171–178. doi: 10.1002/gcc.20627. doi:10.1002/gcc.20627. [DOI] [PubMed] [Google Scholar]
- Kovacs KA, Lengyel F, Vertes Z, Kornyei JL, Gocze PM, Sumegi B, Szabo I, Vertes M. Phosphorylation of PTEN (phosphatase and tensin homologue deleted on chromosome ten) protein is enhanced in human fibromyomatous uteri. J Steroid Biochem Mol Biol. 2007;103:196–199. doi: 10.1016/j.jsbmb.2006.08.006. doi:10.1016/j.jsbmb.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Kroencke TJ, Scheurig C, Poellinger A, Gronewold M, Hamm B. Uterine artery embolization for leiomyomas: percentage of infarction predicts clinical outcome. Radiology. 2010;255:834–841. doi: 10.1148/radiol.10090977. doi:10.1148/radiol.10090977. [DOI] [PubMed] [Google Scholar]
- Kurjak A, Kupesic-Urek S, Miric D. The assessment of benign uterine tumor vascularization by transvaginal color Doppler. Ultrasound Med Biol. 1992;18:645–649. doi: 10.1016/0301-5629(92)90079-p. doi:10.1016/0301-5629(92)90079-P. [DOI] [PubMed] [Google Scholar]
- Laiho M, DeCaprio JA, Ludlow JW, Livingston DM, Massague J. Growth inhibition by TGF-beta linked to suppression of retinoblastoma protein phosphorylation. Cell. 1990;62:175–185. doi: 10.1016/0092-8674(90)90251-9. doi:10.1016/0092-8674(90)90251-9. [DOI] [PubMed] [Google Scholar]
- Lametschwandtner A, Lametschwandtner U, Weiger T. Scanning electron microscopy of vascular corrosion casts—technique and applications: updated review. Scanning Microsc. 1990;4:889–940. discussion 941. [PubMed] [Google Scholar]
- Lando D, Peet DJ, Whelan DA, Gorman JJ, Whitelaw ML. Asparagine hydroxylation of the HIF transactivation domain a hypoxic switch. Science. 2002;295:858–861. doi: 10.1126/science.1068592. doi:10.1126/science.1068592. [DOI] [PubMed] [Google Scholar]
- Laping NJ, Everitt JI, Frazier KS, Burgert M, Portis MJ, Cadacio C, Gold LI, Walker CL. Tumor-specific efficacy of transforming growth factor-beta RI inhibition in Eker rats. Clin Cancer Res. 2007;13:3087–3099. doi: 10.1158/1078-0432.CCR-06-1811. doi:10.1158/1078-0432.CCR-06-1811. [DOI] [PubMed] [Google Scholar]
- Laschke MW, Menger MD. Anti-angiogenic treatment strategies for the therapy of endometriosis. Hum Reprod Update. 2012;18:682–702. doi: 10.1093/humupd/dms026. doi:10.1093/humupd/dms026. [DOI] [PubMed] [Google Scholar]
- Laschke MW, Elitzsch A, Scheuer C, Vollmar B, Menger MD. Selective cyclo-oxygenase-2 inhibition induces regression of autologous endometrial grafts by down-regulation of vascular endothelial growth factor-mediated angiogenesis and stimulation of caspase-3-dependent apoptosis. Fertil Steril. 2007;87:163–171. doi: 10.1016/j.fertnstert.2006.05.068. doi:10.1016/j.fertnstert.2006.05.068. [DOI] [PubMed] [Google Scholar]
- Launonen V, Vierimaa O, Kiuru M, Isola J, Roth S, Pukkala E, Sistonen P, Herva R, Aaltonen LA. Inherited susceptibility to uterine leiomyomas and renal cell cancer. Proc Natl Acad Sci USA. 2001;98:3387–3392. doi: 10.1073/pnas.051633798. doi:10.1073/pnas.051633798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BS, Nowak RA. Human leiomyoma smooth muscle cells show increased expression of transforming growth factor-beta 3 (TGF beta 3) and altered responses to the antiproliferative effects of TGF beta. J Clin Endocrinol Metab. 2001;86:913–920. doi: 10.1210/jcem.86.2.7237. doi:10.1210/jc.86.2.913. [DOI] [PubMed] [Google Scholar]
- Lehtonen HJ, Kiuru M, Ylisaukko-Oja SK, Salovaara R, Herva R, Koivisto PA, Vierimaa O, Aittomaki K, Pukkala E, Launonen V, et al. Increased risk of cancer in patients with fumarate hydratase germline mutation. J Med Genet. 2006;43:523–526. doi: 10.1136/jmg.2005.036400. doi:10.1136/jmg.2005.036400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei J, Jiang A, Pei D. Identification and characterization of a new splicing variant of vascular endothelial growth factor: VEGF183. Biochim Biophys Acta. 1998;1443:400–406. doi: 10.1016/s0167-4781(98)00240-1. doi:10.1016/S0167-4781(98)00240-1. [DOI] [PubMed] [Google Scholar]
- Leppert PC, Catherino WH, Segars JH. A new hypothesis about the origin of uterine fibroids based on gene expression profiling with microarrays. Am J Obstet Gynecol. 2006;195:415–420. doi: 10.1016/j.ajog.2005.12.059. doi:10.1016/j.ajog.2005.12.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letterio JJ, Roberts AB. Regulation of immune responses by TGF-beta. Annu Rev Immunol. 1998;16:137–161. doi: 10.1146/annurev.immunol.16.1.137. doi:10.1146/annurev.immunol.16.1.137. [DOI] [PubMed] [Google Scholar]
- Levens ED, Potlog-Nahari C, Armstrong AY, Wesley R, Premkumar A, Blithe DL, Blocker W, Nieman LK. CDB-2914 for uterine leiomyomata treatment: a randomized controlled trial. Obstet Gynecol. 2008;111:1129–1136. doi: 10.1097/AOG.0b013e3181705d0e. doi:10.1097/AOG.0b013e3181705d0e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy G, Hill MJ, Beall S, Zarek SM, Segars JH, Catherino WH. Leiomyoma: genetics, assisted reproduction, pregnancy and therapeutic advances. J Assist Reprod Genet. 2012;29:703–712. doi: 10.1007/s10815-012-9784-0. doi:10.1007/s10815-012-9784-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewicka A, Osuch B, Cendrowski K, Zegarska J, Stelmachow J. Expression of vascular endothelial growth factor mRNA in human leiomyomas. Gynecol Endocrinol. 2010;26:451–455. doi: 10.3109/09513591003632159. doi:10.3109/09513591003632159. [DOI] [PubMed] [Google Scholar]
- Li C, Guo B, Bernabeu C, Kumar S. Angiogenesis in breast cancer: the role of transforming growth factor beta and CD105. Microsc Res Tech. 2001;52:437–449. doi: 10.1002/1097-0029(20010215)52:4<437::AID-JEMT1029>3.0.CO;2-G. doi:10.1002/1097-0029(20010215)52:4<437::AID-JEMT1029>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Liang M, Wang H, Zhang Y, Lu S, Wang Z. Expression and functional analysis of platelet-derived growth factor in uterine leiomyomata. Cancer Biol Ther. 2006;5:28–33. doi: 10.4161/cbt.5.1.2234. doi:10.4161/cbt.5.1.2234. [DOI] [PubMed] [Google Scholar]
- Lindner V, Reidy MA. Proliferation of smooth muscle cells after vascular injury is inhibited by an antibody against basic fibroblast growth factor. Proc Natl Acad Sci USA. 1991;88:3739–3743. doi: 10.1073/pnas.88.9.3739. doi:10.1073/pnas.88.9.3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Matulonis UA. Anti-angiogenic agents in ovarian cancer: dawn of a new era? Curr Oncol Rep. 2011;13:450–458. doi: 10.1007/s11912-011-0194-8. doi:10.1007/s11912-011-0194-8. [DOI] [PubMed] [Google Scholar]
- Liu L, Cash TP, Jones RG, Keith B, Thompson CB, Simon MC. Hypoxia-induced energy stress regulates mRNA translation and cell growth. Mol Cell. 2006;21:521–531. doi: 10.1016/j.molcel.2006.01.010. doi:10.1016/j.molcel.2006.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucerna M, Mechtcheriakova D, Kadl A, Schabbauer G, Schafer R, Gruber F, Koshelnick Y, Muller HD, Issbrucker K, Clauss M, et al. NAB2, a corepressor of EGR-1, inhibits vascular endothelial growth factor-mediated gene induction and angiogenic responses of endothelial cells. J Biol Chem. 2003;278:11433–11440. doi: 10.1074/jbc.M204937200. doi:10.1074/jbc.M204937200. [DOI] [PubMed] [Google Scholar]
- Luttun A, Dewerchin M, Collen D, Carmeliet P. The role of proteinases in angiogenesis, heart development, restenosis, atherosclerosis, myocardial ischemia, and stroke: insights from genetic studies. Curr Atheroscler Rep. 2000;2:407–416. doi: 10.1007/s11883-000-0079-z. doi:10.1007/s11883-000-0079-z. [DOI] [PubMed] [Google Scholar]
- Mahon PC, Hirota K, Semenza GL. FIH-1: a novel protein that interacts with HIF-1alpha and VHL to mediate repression of HIF-1 transcriptional activity. Genes Dev. 2001;15:2675–2686. doi: 10.1101/gad.924501. doi:10.1101/gad.924501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N, et al. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997;277:55–60. doi: 10.1126/science.277.5322.55. doi:10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- Makinen N, Heinonen HR, Moore S, Tomlinson IP, van der Spuy ZM, Aaltonen LA. MED12 exon 2 mutations are common in uterine leiomyomas from South African patients. Oncotarget. 2011a;2:966–969. doi: 10.18632/oncotarget.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makinen N, Mehine M, Tolvanen J, Kaasinen E, Li Y, Lehtonen HJ, Gentile M, Yan J, Enge M, Taipale M, et al. MED12, the mediator complex subunit 12 gene, is mutated at high frequency in uterine leiomyomas. Science. 2011b;334:252–255. doi: 10.1126/science.1208930. doi:10.1126/science.1208930. [DOI] [PubMed] [Google Scholar]
- Malik M, Catherino WH. Development and validation of a three-dimensional in vitro model for uterine leiomyoma and patient-matched myometrium. Fertil Steril. 2012;97:1287–1293. doi: 10.1016/j.fertnstert.2012.02.037. doi:10.1016/j.fertnstert.2012.02.037. [DOI] [PubMed] [Google Scholar]
- Malik M, Webb J, Catherino WH. Retinoic acid treatment of human leiomyoma cells transformed the cell phenotype to one strongly resembling myometrial cells. Clin Endocrinol (Oxf) 2008;69:462–470. doi: 10.1111/j.1365-2265.2008.03207.x. doi:10.1111/j.1365-2265.2008.03207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik M, Mendoza M, Payson M, Catherino WH. Curcumin, a nutritional supplement with antineoplastic activity, enhances leiomyoma cell apoptosis and decreases fibronectin expression. Fertil Steril. 2009;91:2177–2184. doi: 10.1016/j.fertnstert.2008.03.045. doi:10.1016/j.fertnstert.2008.03.045. [DOI] [PubMed] [Google Scholar]
- Mangrulkar RS, Ono M, Ishikawa M, Takashima S, Klagsbrun M, Nowak RA. Isolation and characterization of heparin-binding growth factors in human leiomyomas and normal myometrium. Biol Reprod. 1995;53:636–646. doi: 10.1095/biolreprod53.3.636. doi:10.1095/biolreprod53.3.636. [DOI] [PubMed] [Google Scholar]
- Marikovsky M, Breuing K, Liu PY, Eriksson E, Higashiyama S, Farber P, Abraham J, Klagsbrun M. Appearance of heparin-binding EGF-like growth factor in wound fluid as a response to injury. Proc Natl Acad Sci USA. 1993;90:3889–3893. doi: 10.1073/pnas.90.9.3889. doi:10.1073/pnas.90.9.3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh EE, Bulun SE. Steroid hormones and leiomyomas. Obstet Gynecol Clin North Am. 2006;33:59–67. doi: 10.1016/j.ogc.2005.12.001. doi:10.1016/j.ogc.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Maruo T, Ohara N, Wang J, Matsuo H. Sex steroidal regulation of uterine leiomyoma growth and apoptosis. Hum Reprod Update. 2004;10:207–220. doi: 10.1093/humupd/dmh019. doi:10.1093/humupd/dmh019. [DOI] [PubMed] [Google Scholar]
- Massague J. The transforming growth factor-beta family. Annu Rev Cell Biol. 1990;6:597–641. doi: 10.1146/annurev.cb.06.110190.003121. doi:10.1146/annurev.cb.06.110190.003121. [DOI] [PubMed] [Google Scholar]
- Masson N, Willam C, Maxwell PH, Pugh CW, Ratcliffe PJ. Independent function of two destruction domains in hypoxia-inducible factor-alpha chains activated by prolyl hydroxylation. Embo J. 2001;20:5197–5206. doi: 10.1093/emboj/20.18.5197. doi:10.1093/emboj/20.18.5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda H, Kalka C, Takahashi T, Yoshida M, Wada M, Kobori M, Itoh R, Iwaguro H, Eguchi M, Iwami Y, et al. Estrogen-mediated endothelial progenitor cell biology and kinetics for physiological postnatal vasculogenesis. Circ Res. 2007;101:598–606. doi: 10.1161/CIRCRESAHA.106.144006. doi:10.1161/CIRCRESAHA.106.144006. [DOI] [PubMed] [Google Scholar]
- Matsuo H, Kurachi O, Shimomura Y, Samoto T, Maruo T. Molecular bases for the actions of ovarian sex steroids in the regulation of proliferation and apoptosis of human uterine leiomyoma. Oncology. 1999;57(Suppl 2):49–58. doi: 10.1159/000055275. doi:10.1159/000055275. [DOI] [PubMed] [Google Scholar]
- Matta WH, Stabile I, Shaw RW, Campbell S. Doppler assessment of uterine blood flow changes in patients with fibroids receiving the gonadotropin-releasing hormone agonist Buserelin. Fertil Steril. 1988;49:1083–1085. doi: 10.1016/s0015-0282(16)59966-x. [DOI] [PubMed] [Google Scholar]
- Mayer A, Hockel M, Wree A, Leo C, Horn LC, Vaupel P. Lack of hypoxic response in uterine leiomyomas despite severe tissue hypoxia. Cancer Res. 2008;68:4719–4726. doi: 10.1158/0008-5472.CAN-07-6339. doi:10.1158/0008-5472.CAN-07-6339. [DOI] [PubMed] [Google Scholar]
- Mayer A, Hoeckel M, von Wallbrunn A, Horn LC, Wree A, Vaupel P. HIF-mediated hypoxic response is missing in severely hypoxic uterine leiomyomas. Adv Exp Med Biol. 2010;662:399–405. doi: 10.1007/978-1-4419-1241-1_58. [DOI] [PubMed] [Google Scholar]
- Mazzocca A, Fransvea E, Lavezzari G, Antonaci S, Giannelli G. Inhibition of transforming growth factor beta receptor I kinase blocks hepatocellular carcinoma growth through neo-angiogenesis regulation. Hepatology. 2009;50:1140–1151. doi: 10.1002/hep.23118. doi:10.1002/hep.23118. [DOI] [PubMed] [Google Scholar]
- McGuire MM, Yatsenko A, Hoffner L, Jones M, Surti U, Rajkovic A. Whole exome sequencing in a random sample of North American women with leiomyomas identifies MED12 mutations in majority of uterine leiomyomas. PLoS One. 2012;7:e33251. doi: 10.1371/journal.pone.0033251. doi:10.1371/journal.pone.0033251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLatchie LM, Fraser NJ, Main MJ, Wise A, Brown J, Thompson N, Solari R, Lee MG, Foord SM. RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature. 1998;393:333–339. doi: 10.1038/30666. doi:10.1038/30666. [DOI] [PubMed] [Google Scholar]
- Mehta VB, Besner GE. HB-EGF promotes angiogenesis in endothelial cells via PI3-kinase and MAPK signaling pathways. Growth Factors. 2007;25:253–263. doi: 10.1080/08977190701773070. doi:10.1080/08977190701773070. [DOI] [PubMed] [Google Scholar]
- Miller MJ, Martinez A, Unsworth EJ, Thiele CJ, Moody TW, Elsasser T, Cuttitta F. Adrenomedullin expression in human tumor cell lines. Its potential role as an autocrine growth factor. J Biol Chem. 1996;271:23345–23351. doi: 10.1074/jbc.271.38.23345. doi:10.1074/jbc.271.38.23345. [DOI] [PubMed] [Google Scholar]
- Naganuma Y, Choijamts B, Shirota K, Nakajima K, Ogata S, Miyamoto S, Kawarabayashi T, Emoto M. Metronomic doxifluridine chemotherapy combined with the anti-angiogenic agent TNP-470 inhibits the growth of human uterine carcinosarcoma xenografts. Cancer Sci. 2011;102:1545–1552. doi: 10.1111/j.1349-7006.2011.01998.x. doi:10.1111/j.1349-7006.2011.01998.x. [DOI] [PubMed] [Google Scholar]
- Nap AW, Griffioen AW, Dunselman GA, Bouma-Ter Steege JC, Thijssen VL, Evers JL, Groothuis PG. Antiangiogenesis therapy for endometriosis. J Clin Endocrinol Metab. 2004;89:1089–1095. doi: 10.1210/jc.2003-031406. doi:10.1210/jc.2003-031406. [DOI] [PubMed] [Google Scholar]
- Nikitenko LL, MacKenzie IZ, Rees MC, Bicknell R. Adrenomedullin is an autocrine regulator of endothelial growth in human endometrium. Mol Hum Reprod. 2000;6:811–819. doi: 10.1093/molehr/6.9.811. doi:10.1093/molehr/6.9.811. [DOI] [PubMed] [Google Scholar]
- Nikitenko LL, Cross T, Campo L, Turley H, Leek R, Manek S, Bicknell R, Rees MC. Expression of terminally glycosylated calcitonin receptor-like receptor in uterine leiomyoma: endothelial phenotype and association with microvascular density. Clin Cancer Res. 2006;12:5648–5658. doi: 10.1158/1078-0432.CCR-06-0852. doi:10.1158/1078-0432.CCR-06-0852. [DOI] [PubMed] [Google Scholar]
- Nisolle M, Gillerot S, Casanas-Roux F, Squifflet J, Berliere M, Donnez J. Immunohistochemical study of the proliferation index, oestrogen receptors and progesterone receptors A and B in leiomyomata and normal myometrium during the menstrual cycle and under gonadotrophin-releasing hormone agonist therapy. Hum Reprod. 1999;14:2844–2850. doi: 10.1093/humrep/14.11.2844. doi:10.1093/humrep/14.11.2844. [DOI] [PubMed] [Google Scholar]
- Norian JM, Malik M, Parker CY, Joseph D, Leppert PC, Segars JH, Catherino WH. Transforming growth factor beta3 regulates the versican variants in the extracellular matrix-rich uterine leiomyomas. Reprod Sci. 2009;16:1153–1164. doi: 10.1177/1933719109343310. doi:10.1177/1933719109343310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak RA. Novel therapeutic strategies for leiomyomas: targeting growth factors and their receptors. Environ Health Perspect. 2000;108(Suppl 5):849–853. doi: 10.1289/ehp.00108s5849. [DOI] [PubMed] [Google Scholar]
- O'Reilly MS, Holmgren L, Shing Y, Chen C, Rosenthal RA, Moses M, Lane WS, Cao Y, Sage EH, Folkman J. Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell. 1994;79:315–328. doi: 10.1016/0092-8674(94)90200-3. doi:10.1016/0092-8674(94)90200-3. [DOI] [PubMed] [Google Scholar]
- O'Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR, Folkman J. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277–285. doi: 10.1016/s0092-8674(00)81848-6. doi:10.1016/S0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- Oehler MK, Norbury C, Hague S, Rees MC, Bicknell R. Adrenomedullin inhibits hypoxic cell death by upregulation of Bcl-2 in endometrial cancer cells: a possible promotion mechanism for tumour growth. Oncogene. 2001;20:2937–2945. doi: 10.1038/sj.onc.1204422. doi:10.1038/sj.onc.1204422. [DOI] [PubMed] [Google Scholar]
- Oehler MK, Hague S, Rees MC, Bicknell R. Adrenomedullin promotes formation of xenografted endometrial tumors by stimulation of autocrine growth and angiogenesis. Oncogene. 2002;21:2815–2821. doi: 10.1038/sj.onc.1205374. doi:10.1038/sj.onc.1205374. [DOI] [PubMed] [Google Scholar]
- Ohara N, Morikawa A, Chen W, Wang J, DeManno DA, Chwalisz K, Maruo T. Comparative effects of SPRM asoprisnil (J867) on proliferation, apoptosis, and the expression of growth factors in cultured uterine leiomyoma cells and normal myometrial cells. Reprod Sci. 2007;14:20–27. doi: 10.1177/1933719107311464. doi:10.1177/1933719107311464. [DOI] [PubMed] [Google Scholar]
- Okolo S. Incidence, aetiology and epidemiology of uterine fibroids. Best Pract Res Clin Obstet Gynaecol. 2008;22:571–588. doi: 10.1016/j.bpobgyn.2008.04.002. doi:10.1016/j.bpobgyn.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Olufowobi O, Sharif K, Papaionnou S, Neelakantan D, Mohammed H, Afnan M. Are the anticipated benefits of myomectomy achieved in women of reproductive age? A 5-year review of the results at a UK tertiary hospital. J Obstet Gynaecol. 2004;24:434–440. doi: 10.1080/01443610410001685600. doi:10.1080/01443610410001685600. [DOI] [PubMed] [Google Scholar]
- Ongusaha PP, Kwak JC, Zwible AJ, Macip S, Higashiyama S, Taniguchi N, Fang L, Lee SW. HB-EGF is a potent inducer of tumor growth and angiogenesis. Cancer Res. 2004;64:5283–5290. doi: 10.1158/0008-5472.CAN-04-0925. doi:10.1158/0008-5472.CAN-04-0925. [DOI] [PubMed] [Google Scholar]
- Ono M, Maruyama T, Masuda H, Kajitani T, Nagashima T, Arase T, Ito M, Ohta K, Uchida H, Asada H, et al. Side population in human uterine myometrium displays phenotypic and functional characteristics of myometrial stem cells. Proc Natl Acad Sci USA. 2007;104:18700–18705. doi: 10.1073/pnas.0704472104. doi:10.1073/pnas.0704472104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono M, Qiang W, Serna VA, Yin P, Coon JS.t, Navarro A, Monsivais D, Kakinuma T, Dyson M, Druschitz S, et al. Role of stem cells in human uterine leiomyoma growth. PLoS One. 2012;7:e36935. doi: 10.1371/journal.pone.0036935. doi:10.1371/journal.pone.0036935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pambuccian CA, Oprea GM, Lakatua DJ. Reduced expression of early growth response-1 gene in leiomyoma as identified by mRNA differential display. Gynecol Oncol. 2002;84:431–436. doi: 10.1006/gyno.2001.6549. doi:10.1006/gyno.2001.6549. [DOI] [PubMed] [Google Scholar]
- Patyna S, Haznedar J, Morris D, Freshwater K, Peng G, Sukbuntherng J, Chmielewski G, Matsumoto D. Evaluation of the safety and pharmacokinetics of the multi-targeted receptor tyrosine kinase inhibitor sunitinib during embryo-fetal development in rats and rabbits. Birth Defects Res B Dev Reprod Toxicol. 2009;86:204–213. doi: 10.1002/bdrb.20194. doi:10.1002/bdrb.20194. [DOI] [PubMed] [Google Scholar]
- Pauli SA, Tang H, Wang J, Bohlen P, Posser R, Hartman T, Sauer MV, Kitajewski J, Zimmermann RC. The vascular endothelial growth factor (VEGF)/VEGF receptor 2 pathway is critical for blood vessel survival in corpora lutea of pregnancy in the rodent. Endocrinology. 2005;146:1301–1311. doi: 10.1210/en.2004-0765. doi:10.1210/en.2004-0765. [DOI] [PubMed] [Google Scholar]
- Peddada SD, Laughlin SK, Miner K, Guyon JP, Haneke K, Vahdat HL, Semelka RC, Kowalik A, Armao D, Davis B, et al. Growth of uterine leiomyomata among premenopausal black and white women. Proc Natl Acad Sci USA. 2008;105:19887–19892. doi: 10.1073/pnas.0808188105. doi:10.1073/pnas.0808188105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekonen F, Nyman T, Rutanen EM. Differential expression of keratinocyte growth factor and its receptor in the human uterus. Mol Cell Endocrinol. 1993;95:43–49. doi: 10.1016/0303-7207(93)90027-h. doi:10.1016/0303-7207(93)90027-H. [DOI] [PubMed] [Google Scholar]
- Pepper MS. Extracellular proteolysis and angiogenesis. Thromb Haemost. 2001;86:346–355. [PubMed] [Google Scholar]
- Pollard P, Wortham N, Barclay E, Alam A, Elia G, Manek S, Poulsom R, Tomlinson I. Evidence of increased microvessel density and activation of the hypoxia pathway in tumours from the hereditary leiomyomatosis and renal cell cancer syndrome. J Pathol. 2005a;205:41–49. doi: 10.1002/path.1686. doi:10.1002/path.1686. [DOI] [PubMed] [Google Scholar]
- Pollard PJ, Briere JJ, Alam NA, Barwell J, Barclay E, Wortham NC, Hunt T, Mitchell M, Olpin S, Moat SJ, et al. Accumulation of Krebs cycle intermediates and over-expression of HIF1alpha in tumours which result from germline FH and SDH mutations. Hum Mol Genet. 2005b;14:2231–2239. doi: 10.1093/hmg/ddi227. doi:10.1093/hmg/ddi227. [DOI] [PubMed] [Google Scholar]
- Poltorak Z, Cohen T, Sivan R, Kandelis Y, Spira G, Vlodavsky I, Keshet E, Neufeld G. VEGF145, a secreted vascular endothelial growth factor isoform that binds to extracellular matrix. J Biol Chem. 1997;272:7151–7158. doi: 10.1074/jbc.272.11.7151. doi:10.1074/jbc.272.11.7151. [DOI] [PubMed] [Google Scholar]
- Poncelet C, Madelenat P, Feldmann G, Walker F, Darai E. Expression of von Willebrand's factor, CD34, CD31, and vascular endothelial growth factor in uterine leiomyomas. Fertil Steril. 2002;78:581–586. doi: 10.1016/s0015-0282(02)03301-0. doi:10.1016/S0015-0282(02)03301-0. [DOI] [PubMed] [Google Scholar]
- Pouyssegur J, Dayan F, Mazure NM. Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature. 2006;441:437–443. doi: 10.1038/nature04871. doi:10.1038/nature04871. [DOI] [PubMed] [Google Scholar]
- Presta M. Sex hormones modulate the synthesis of basic fibroblast growth factor in human endometrial adenocarcinoma cells: implications for the neovascularization of normal and neoplastic endometrium. J Cell Physiol. 1988;137:593–597. doi: 10.1002/jcp.1041370329. doi:10.1002/jcp.1041370329. [DOI] [PubMed] [Google Scholar]
- Quade BJ, Wang TY, Sornberger K, Dal Cin P, Mutter GL, Morton CC. Molecular pathogenesis of uterine smooth muscle tumors from transcriptional profiling. Genes Chromosomes Cancer. 2004;40:97–108. doi: 10.1002/gcc.20018. doi:10.1002/gcc.20018. [DOI] [PubMed] [Google Scholar]
- Ramsey M. Anatomy of the human uterus. In: Chard T, Grudzinskas JG, editors. The Uterus. Cambridge, UK: Cambridge University Press; 1994. pp. 18–29. [Google Scholar]
- Rasila KK, Burger RA, Smith H, Lee FC, Verschraegen C. Angiogenesis in gynecological oncology-mechanism of tumor progression and therapeutic targets. Int J Gynecol Cancer. 2005;15:710–726. doi: 10.1111/j.1525-1438.2005.00132.x. doi:10.1111/j.1525-1438.2005.00132.x. [DOI] [PubMed] [Google Scholar]
- Ravina JH, Herbreteau D, Ciraru-Vigneron N, Bouret JM, Houdart E, Aymard A, Merland JJ. Arterial embolisation to treat uterine myomata. Lancet. 1995;346:671–672. doi: 10.1016/s0140-6736(95)92282-2. doi:10.1016/S0140-6736(95)92282-2. [DOI] [PubMed] [Google Scholar]
- Rein MS, Barbieri RL, Friedman AJ. Progesterone: a critical role in the pathogenesis of uterine myomas. Am J Obstet Gynecol. 1995;172:14–18. doi: 10.1016/0002-9378(95)90077-2. doi:10.1016/0002-9378(95)90077-2. [DOI] [PubMed] [Google Scholar]
- Ren W, Korchin B, Lahat G, Wei C, Bolshakov S, Nguyen T, Merritt W, Dicker A, Lazar A, Sood A, et al. Combined vascular endothelial growth factor receptor/epidermal growth factor receptor blockade with chemotherapy for treatment of local, uterine, and metastatic soft tissue sarcoma. Clin Cancer Res. 2008;14:5466–5475. doi: 10.1158/1078-0432.CCR-08-0562. doi:10.1158/1078-0432.CCR-08-0562. [DOI] [PubMed] [Google Scholar]
- Reynolds LP, Redmer DA. Angiogenesis in the placenta. Biol Reprod. 2001;64:1033–1040. doi: 10.1095/biolreprod64.4.1033. doi:10.1095/biolreprod64.4.1033. [DOI] [PubMed] [Google Scholar]
- Reynolds LP, Grazul-Bilska AT, Redmer DA. Angiogenesis in the female reproductive organs: pathological implications. Int J Exp Pathol. 2002;83:151–163. doi: 10.1046/j.1365-2613.2002.00277.x. doi:10.1046/j.1365-2613.2002.00277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribatti D. Endogenous inhibitors of angiogenesis: a historical review. Leuk Res. 2009;33:638–644. doi: 10.1016/j.leukres.2008.11.019. doi:10.1016/j.leukres.2008.11.019. [DOI] [PubMed] [Google Scholar]
- Ribatti D, Conconi MT, Nico B, Baiguera S, Corsi P, Parnigotto PP, Nussdorfer GG. Angiogenic response induced by a cellular brain scaffolds grafted onto the chick embryo chorioallantoic membrane. Brain Res. 2003;989:9–15. doi: 10.1016/s0006-8993(03)03225-6. doi:10.1016/S0006-8993(03)03225-6. [DOI] [PubMed] [Google Scholar]
- Rider V, Carlone DL, Foster RT. Oestrogen and progesterone control basic fibroblast growth factor mRNA in the rat uterus. J Endocrinol. 1997;154:75–84. doi: 10.1677/joe.0.1540075. doi:10.1677/joe.0.1540075. [DOI] [PubMed] [Google Scholar]
- Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–674. doi: 10.1038/386671a0. doi:10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- Ross R, Raines EW, Bowen-Pope DF. The biology of platelet-derived growth factor. Cell. 1986;46:155–169. doi: 10.1016/0092-8674(86)90733-6. doi:10.1016/0092-8674(86)90733-6. [DOI] [PubMed] [Google Scholar]
- Rossi MJ, Chegini N, Masterson BJ. Presence of epidermal growth factor, platelet-derived growth factor, and their receptors in human myometrial tissue and smooth muscle cells: their action in smooth muscle cells in vitro. Endocrinology. 1992;130:1716–1727. doi: 10.1210/endo.130.3.1311246. doi:10.1210/en.130.3.1716. [DOI] [PubMed] [Google Scholar]
- Rotello RJ, Lieberman RC, Purchio AF, Gerschenson LE. Coordinated regulation of apoptosis and cell proliferation by transforming growth factor beta 1 in cultured uterine epithelial cells. Proc Natl Acad Sci USA. 1991;88:3412–3415. doi: 10.1073/pnas.88.8.3412. doi:10.1073/pnas.88.8.3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson JA. The blood supply of uterine myomata. Surg Gynecol Obstet. 1912;XIV:15–234. [Google Scholar]
- Sanci M, Dikis C, Inan S, Turkoz E, Dicle N, Ispahi C. Immunolocalization of VEGF, VEGF receptors, EGF-R and Ki-67 in leiomyoma, cellular leiomyoma and leiomyosarcoma. Acta Histochem. 2011;113:317–325. doi: 10.1016/j.acthis.2010.01.001. doi:10.1016/j.acthis.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Sanz-Ortega J, Vocke C, Stratton P, Linehan WM, Merino MJ. Morphologic and Molecular Characteristics of Uterine Leiomyomas in Hereditary Leiomyomatosis and Renal Cancer (HLRCC) Syndrome. Am J Surg Pathol. 2013;37:74–80. doi: 10.1097/PAS.0b013e31825ec16f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar K, Cai Z, Gupta R, Parajuli N, Fox-Talbot K, Darshan MS, Gonzalez FJ, Semenza GL. Hypoxia-inducible factor 1 transcriptional activity in endothelial cells is required for acute phase cardioprotection induced by ischemic preconditioning. Proc Natl Acad Sci USA. 2012;109:10504–10509. doi: 10.1073/pnas.1208314109. doi:10.1073/pnas.1208314109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterwhite DJ, Moses HL. Mechanisms of transforming growth factor-beta 1-induced cell cycle arrest. Invasion Metastasis. 1994;14:309–318. [PubMed] [Google Scholar]
- Scheurig-Muenkler C, Wagner M, Franiel T, Hamm B, Kroencke TJ. Effect of uterine artery embolization on uterine and leiomyoma perfusion: evidence of transient myometrial ischemia on magnetic resonance imaging. J Vasc Interv Radiol. 2010;21:1347–1353. doi: 10.1016/j.jvir.2010.05.008. doi:10.1016/j.jvir.2010.05.008. [DOI] [PubMed] [Google Scholar]
- Selak MA, Armour SM, MacKenzie ED, Boulahbel H, Watson DG, Mansfield KD, Pan Y, Simon MC, Thompson CB, Gottlieb E. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-alpha prolyl hydroxylase. Cancer Cell. 2005;7:77–85. doi: 10.1016/j.ccr.2004.11.022. doi:10.1016/j.ccr.2004.11.022. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. doi:10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene. 2010;29:625–634. doi: 10.1038/onc.2009.441. doi:10.1038/onc.2009.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp HT. Assessment of new technology in the treatment of idiopathic menorrhagia and uterine leiomyomata. Obstet Gynecol. 2006;108:990–1003. doi: 10.1097/01.AOG.0000232618.26261.75. doi:10.1097/01.AOG.0000232618.26261.75. [DOI] [PubMed] [Google Scholar]
- Shichiri M, Kato H, Doi M, Marumo F, Hirata Y. Induction of max by adrenomedullin and calcitonin gene-related peptide antagonizes endothelial apoptosis. Mol Endocrinol. 1999;13:1353–1363. doi: 10.1210/mend.13.8.0324. doi:10.1210/me.13.8.1353. [DOI] [PubMed] [Google Scholar]
- Shimomura Y, Matsuo H, Samoto T, Maruo T. Up-regulation by progesterone of proliferating cell nuclear antigen and epidermal growth factor expression in human uterine leiomyoma. J Clin Endocrinol Metab. 1998;83:2192–2198. doi: 10.1210/jcem.83.6.4879. doi:10.1210/jc.83.6.2192. [DOI] [PubMed] [Google Scholar]
- Shinozaki K, Suominen E, Carrick F, Sauter B, Kahari VM, Lieber A, Woo SL, Savontaus M. Efficient infection of tumor endothelial cells by a capsid-modified adenovirus. Gene Ther. 2006;13:52–59. doi: 10.1038/sj.gt.3302598. doi:10.1038/sj.gt.3302598. [DOI] [PubMed] [Google Scholar]
- Shozu M, Murakami K, Segawa T, Kasai T, Ishikawa H, Shinohara K, Okada M, Inoue M. Decreased expression of early growth response-1 and its role in uterine leiomyoma growth. Cancer Res. 2004;64:4677–4684. doi: 10.1158/0008-5472.CAN-03-0560. doi:10.1158/0008-5472.CAN-03-0560. [DOI] [PubMed] [Google Scholar]
- Shushan A, Rojansky N, Laufer N, Klein BY, Shlomai Z, Levitzki R, Hartzstark Z, Ben-Bassat H. The AG1478 tyrosine kinase inhibitor is an effective suppressor of leiomyoma cell growth. Hum Reprod. 2004;19:1957–1967. doi: 10.1093/humrep/deh355. doi:10.1093/humrep/deh355. [DOI] [PubMed] [Google Scholar]
- Shushan A, Ben-Bassat H, Mishani E, Laufer N, Klein BY, Rojansky N. Inhibition of leiomyoma cell proliferation in vitro by genistein and the protein tyrosine kinase inhibitor TKS050. Fertil Steril. 2007;87:127–135. doi: 10.1016/j.fertnstert.2006.05.056. doi:10.1016/j.fertnstert.2006.05.056. [DOI] [PubMed] [Google Scholar]
- Shynlova O, Oldenhof A, Dorogin A, Xu Q, Mu J, Nashman N, Lye SJ. Myometrial apoptosis: activation of the caspase cascade in the pregnant rat myometrium at midgestation. Biol Reprod. 2006;74:839–849. doi: 10.1095/biolreprod.105.048124. doi:10.1095/biolreprod.105.048124. [DOI] [PubMed] [Google Scholar]
- Shynlova O, Dorogin A, Lye SJ. Stretch-induced uterine myocyte differentiation during rat pregnancy: involvement of caspase activation. Biol Reprod. 2010;82:1248–1255. doi: 10.1095/biolreprod.109.081158. doi:10.1095/biolreprod.109.081158. [DOI] [PubMed] [Google Scholar]
- Smith SK. Angiogenesis, vascular endothelial growth factor and the endometrium. Hum Reprod Update. 1998;4:509–519. doi: 10.1093/humupd/4.5.509. doi:10.1093/humupd/4.5.509. [DOI] [PubMed] [Google Scholar]
- Sosic A, Skupski DW, Streltzoff J, Yun H, Chervenak FA. Vascularity of uterine myomas: assessment by color and pulsed Doppler ultrasound. Int J Gynaecol Obstet. 1996;54:245–250. doi: 10.1016/0020-7292(96)02722-1. doi:10.1016/0020-7292(96)02722-1. [DOI] [PubMed] [Google Scholar]
- Sozen I, Arici A. Interactions of cytokines, growth factors, and the extracellular matrix in the cellular biology of uterine leiomyomata. Fertil Steril. 2002;78:1–12. doi: 10.1016/s0015-0282(02)03154-0. doi:10.1016/S0015-0282(02)03154-0. [DOI] [PubMed] [Google Scholar]
- Sperandio S, Fortin J, Sasik R, Robitaille L, Corbeil J, de Belle I. The transcription factor Egr1 regulates the HIF-1alpha gene during hypoxia. Mol Carcinog. 2009;48:38–44. doi: 10.1002/mc.20454. doi:10.1002/mc.20454. [DOI] [PubMed] [Google Scholar]
- Stewart L, Glenn GM, Stratton P, Goldstein AM, Merino MJ, Tucker MA, Linehan WM, Toro JR. Association of germline mutations in the fumarate hydratase gene and uterine fibroids in women with hereditary leiomyomatosis and renal cell cancer. Arch Dermatol. 2008;144:1584–1592. doi: 10.1001/archdermatol.2008.517. doi:10.1001/archdermatol.2008.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suo G, Jiang Y, Cowan B, Wang JY. Platelet-derived growth factor C is upregulated in human uterine fibroids and regulates uterine smooth muscle cell growth. Biol Reprod. 2009;81:749–758. doi: 10.1095/biolreprod.109.076869. doi:10.1095/biolreprod.109.076869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surrey ES, Lietz AK, Schoolcraft WB. Impact of intramural leiomyomata in patients with a normal endometrial cavity on in vitro fertilization-embryo transfer cycle outcome. Fertil Steril. 2001;75:405–410. doi: 10.1016/s0015-0282(00)01714-3. doi:10.1016/S0015-0282(00)01714-3. [DOI] [PubMed] [Google Scholar]
- Takeda T, Osuga K, Morishige K, Tasaka K, Nakamura H, Murata Y. Changes of plasma vascular endothelial growth factor level after uterine artery embolisation for leiomyomata. BJOG. 2005;112:1437–1439. doi: 10.1111/j.1471-0528.2005.00690.x. doi:10.1111/j.1471-0528.2005.00690.x. [DOI] [PubMed] [Google Scholar]
- Tamaya T, Fujimoto J, Okada H. Comparison of cellular levels of steroid receptors in uterine leiomyoma and myometrium. Acta Obstet Gynecol Scand. 1985;64:307–309. doi: 10.3109/00016348509155136. doi:10.3109/00016348509155136. [DOI] [PubMed] [Google Scholar]
- Tang XM, Dou Q, Zhao Y, McLean F, Davis J, Chegini N. The expression of transforming growth factor-beta s and TGF-beta receptor mRNA and protein and the effect of TGF-beta s on human myometrial smooth muscle cells in vitro. Mol Hum Reprod. 1997;3:233–240. doi: 10.1093/molehr/3.3.233. doi:10.1093/molehr/3.3.233. [DOI] [PubMed] [Google Scholar]
- Taniguchi Y, Morita I, Kubota T, Murota S, Aso T. Human uterine myometrial smooth muscle cell proliferation and vascular endothelial growth-factor production in response to platelet-derived growth factor. J Endocrinol. 2001;169:79–86. doi: 10.1677/joe.0.1690079. doi:10.1677/joe.0.1690079. [DOI] [PubMed] [Google Scholar]
- Tavassoli FA, Devilee P. Tumors of the Breast and Female Genital Organs- Pathology and Genetics. Lyon, France: WHO Classification of Tumours, IARC; 2003. pp. 64–67. 231–232; 265–266. [Google Scholar]
- Tischer E, Mitchell R, Hartman T, Silva M, Gospodarowicz D, Fiddes JC, Abraham JA. The human gene for vascular endothelial growth factor: multiple protein forms are encoded through alternative exon splicing. J Biol Chem. 1991;266:11947–11954. [PubMed] [Google Scholar]
- Tomlinson IP, Alam NA, Rowan AJ, Barclay E, Jaeger EE, Kelsell D, Leigh I, Gorman P, Lamlum H, Rahman S, et al. Germline mutations in FH predispose to dominantly inherited uterine fibroids, skin leiomyomata and papillary renal cell cancer. Nat Genet. 2002;30:406–410. doi: 10.1038/ng849. doi:10.1038/ng849. [DOI] [PubMed] [Google Scholar]
- Toro JR, Nickerson ML, Wei MH, Warren MB, Glenn GM, Turner ML, Stewart L, Duray P, Tourre O, Sharma N, et al. Mutations in the fumarate hydratase gene cause hereditary leiomyomatosis and renal cell cancer in families in North America. Am J Hum Genet. 2003;73:95–106. doi: 10.1086/376435. doi:10.1086/376435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torry RJ, Rongish BJ. Angiogenesis in the uterus: potential regulation and relation to tumor angiogenesis. Am J Reprod Immunol. 1992;27:171–179. doi: 10.1111/j.1600-0897.1992.tb00746.x. doi:10.1111/j.1600-0897.1992.tb00746.x. [DOI] [PubMed] [Google Scholar]
- Toschi A, Lee E, Gadir N, Ohh M, Foster DA. Differential dependence of hypoxia-inducible factors 1 alpha and 2 alpha on mTORC1 and mTORC2. J Biol Chem. 2008;283:34495–34499. doi: 10.1074/jbc.C800170200. doi:10.1074/jbc.C800170200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treloar SA, Do KA, Martin NG. Genetic influences on the age at menopause. Lancet. 1998;352:1084–1085. doi: 10.1016/S0140-6736(05)79753-1. doi:10.1016/S0140-6736(05)79753-1. [DOI] [PubMed] [Google Scholar]
- Triozzi PL, Borden EC. VB-111 for cancer. Expert Opin Biol Ther. 2011;11:1669–1676. doi: 10.1517/14712598.2011.618122. doi:10.1517/14712598.2011.618122. [DOI] [PubMed] [Google Scholar]
- Tsibris JC, Segars J, Coppola D, Mane S, Wilbanks GD, O'Brien WF, Spellacy WN. Insights from gene arrays on the development and growth regulation of uterine leiomyomata. Fertil Steril. 2002;78:114–121. doi: 10.1016/s0015-0282(02)03191-6. doi:10.1016/S0015-0282(02)03191-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiligiannis SE, Zaitseva M, Coombs PR, Shekleton P, Olshansky M, Hickey M, Vollenhoven B, Rogers PA. Fibroid-associated heavy menstrual bleeding: correlation between clinical features, Doppler ultrasound assessment of vasculature, and tissue gene expression profiles. Reprod Sci. 2013;20:361–370. doi: 10.1177/1933719112459233. doi:10.1177/1933719112459233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uliana V, Marcocci E, Mucciolo M, Meloni I, Izzi C, Manno C, Bruttini M, Mari F, Scolari F, Renieri A, et al. Alport syndrome and leiomyomatosis: the first deletion extending beyond COL4A6 intron 2. Pediatr Nephrol. 2011;26:717–724. doi: 10.1007/s00467-010-1693-9. doi:10.1007/s00467-010-1693-9. [DOI] [PubMed] [Google Scholar]
- van Cruijsen H, Giaccone G, Hoekman K. Epidermal growth factor receptor and angiogenesis: opportunities for combined anticancer strategies. Int J Cancer. 2005;117:883–888. doi: 10.1002/ijc.21479. doi:10.1002/ijc.21479. [DOI] [PubMed] [Google Scholar]
- Varghese BV, Koohestani F, McWilliams M, Colvin A, Gunewardena S, Kinsey WH, Nowak RA, Nothnick WB, Chennathukuzhi VM. Loss of the repressor REST in uterine fibroids promotes aberrant G protein-coupled receptor 10 expression and activates mammalian target of rapamycin pathway. Proc Natl Acad Sci USA. 2013;110:2187–2192. doi: 10.1073/pnas.1215759110. doi:10.1073/pnas.1215759110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vikhlyaeva EM, Khodzhaeva ZS, Fantschenko ND. Familial predisposition to uterine leiomyomas. Int J Gynaecol Obstet. 1995;51:127–131. doi: 10.1016/0020-7292(95)02533-i. doi:10.1016/0020-7292(95)02533-I. [DOI] [PubMed] [Google Scholar]
- Vincent KA, Feron O, Kelly RA. Harnessing the response to tissue hypoxia: HIF-1 alpha and therapeutic angiogenesis. Trends Cardiovasc Med. 2002;12:362–367. doi: 10.1016/s1050-1738(02)00186-x. doi:10.1016/S1050-1738(02)00186-X. [DOI] [PubMed] [Google Scholar]
- Viville B, Charnock-Jones DS, Sharkey AM, Wetzka B, Smith SK. Distribution of the A and B forms of the progesterone receptor messenger ribonucleic acid and protein in uterine leiomyomata and adjacent myometrium. Hum Reprod. 1997;12:815–822. doi: 10.1093/humrep/12.4.815. doi:10.1093/humrep/12.4.815. [DOI] [PubMed] [Google Scholar]
- Vollenhoven BJ, Herington AC, Healy DL. Epidermal growth factor and transforming growth factor-beta in uterine fibroids and myometrium. Gynecol Obstet Invest. 1995;40:120–124. doi: 10.1159/000292319. doi:10.1159/000292319. [DOI] [PubMed] [Google Scholar]
- Vott S, Bonilla SM, Goodwin SC, Chen G, Wong GC, Lai A, Yegul T, DeLeon M. CT findings after uterine artery embolization. J Comput Assist Tomogr. 2000;24:846–848. doi: 10.1097/00004728-200011000-00004. doi:10.1097/00004728-200011000-00004. [DOI] [PubMed] [Google Scholar]
- Walker WJ, Pelage JP. Uterine artery embolisation for symptomatic fibroids: clinical results in 400 women with imaging follow up. BJOG. 2002;109:1262–1272. doi: 10.1046/j.1471-0528.2002.01449.x. doi:10.1046/j.1471-0528.2002.01449.x. [DOI] [PubMed] [Google Scholar]
- Walker CL, Stewart EA. Uterine fibroids: the elephant in the room. Science. 2005;308:1589–1592. doi: 10.1126/science.1112063. doi:10.1126/science.1112063. [DOI] [PubMed] [Google Scholar]
- Walocha JA, Litwin JA, Miodonski AJ. Vascular system of intramural leiomyomata revealed by corrosion casting and scanning electron microscopy. Hum Reprod. 2003;18:1088–1093. doi: 10.1093/humrep/deg213. doi:10.1093/humrep/deg213. [DOI] [PubMed] [Google Scholar]
- Wang XN, Das SK, Damm D, Klagsbrun M, Abraham JA, Dey SK. Differential regulation of heparin-binding epidermal growth factor-like growth factor in the adult ovariectomized mouse uterus by progesterone and estrogen. Endocrinology. 1994;135:1264–1271. doi: 10.1210/endo.135.3.8070372. doi:10.1210/en.135.3.1264. [DOI] [PubMed] [Google Scholar]
- Wang H, Mahadevappa M, Yamamoto K, Wen Y, Chen B, Warrington JA, Polan ML. Distinctive proliferative phase differences in gene expression in human myometrium and leiomyomata. Fertil Steril. 2003;80:266–276. doi: 10.1016/s0015-0282(03)00730-1. doi:10.1016/S0015-0282(03)00730-1. [DOI] [PubMed] [Google Scholar]
- Wang J, Ohara N, Takekida S, Xu Q, Maruo T. Comparative effects of heparin-binding epidermal growth factor-like growth factor on the growth of cultured human uterine leiomyoma cells and myometrial cells. Hum Reprod. 2005;20:1456–1465. doi: 10.1093/humrep/deh842. doi:10.1093/humrep/deh842. [DOI] [PubMed] [Google Scholar]
- Wang J, Ohara N, Wang Z, Chen W, Morikawa A, Sasaki H, DeManno DA, Chwalisz K, Maruo T. A novel selective progesterone receptor modulator asoprisnil (J867) down-regulates the expression of EGF, IGF-I, TGFbeta3 and their receptors in cultured uterine leiomyoma cells. Hum Reprod. 2006;21:1869–1877. doi: 10.1093/humrep/del035. doi:10.1093/humrep/del035. [DOI] [PubMed] [Google Scholar]
- Wei JJ, Zhang XM, Chiriboga L, Yee H, Perle MA, Mittal K. Spatial differences in biologic activity of large uterine leiomyomata. Fertil Steril. 2006;85:179–187. doi: 10.1016/j.fertnstert.2005.07.1294. doi:10.1016/j.fertnstert.2005.07.1294. [DOI] [PubMed] [Google Scholar]
- Weichert W, Denkert C, Gauruder-Burmester A, Kurzeja R, Hamm B, Dietel M, Kroencke TJ. Uterine arterial embolization with tris-acryl gelatin microspheres: a histopathologic evaluation. Am J Surg Pathol. 2005;29:955–961. doi: 10.1097/01.pas.0000159776.77912.ce. doi:10.1097/01.pas.0000159776.77912.ce. [DOI] [PubMed] [Google Scholar]
- Weston G, Trajstman AC, Gargett CE, Manuelpillai U, Vollenhoven BJ, Rogers PA. Fibroids display an anti-angiogenic gene expression profile when compared with adjacent myometrium. Mol Hum Reprod. 2003;9:541–549. doi: 10.1093/molehr/gag066. doi:10.1093/molehr/gag066. [DOI] [PubMed] [Google Scholar]
- Wilkens J, Chwalisz K, Han C, Walker J, Cameron IT, Ingamells S, Lawrence AC, Lumsden MA, Hapangama D, Williams AR, et al. Effects of the selective progesterone receptor modulator asoprisnil on uterine artery blood flow, ovarian activity, clinical symptoms in patients with uterine leiomyomata scheduled for hysterectomy. J Clin Endocrinol Metab. 2008;93:4664–4671. doi: 10.1210/jc.2008-1104. doi:10.1210/jc.2008-1104. [DOI] [PubMed] [Google Scholar]
- Wilson EA, Yang F, Rees ED. Estradiol and progesterone binding in uterine leiomyomata and in normal uterine tissues. Obstet Gynecol. 1980;55:20–24. [PubMed] [Google Scholar]
- Wolanska M, Bankowski E. Fibroblast growth factors (FGF) in human myometrium and uterine leiomyomas in various stages of tumour growth. Biochimie. 2006;88:141–146. doi: 10.1016/j.biochi.2005.07.014. doi:10.1016/j.biochi.2005.07.014. [DOI] [PubMed] [Google Scholar]
- Work LM, Ritchie N, Nicklin SA, Reynolds PN, Baker AH. Dual targeting of gene delivery by genetic modification of adenovirus serotype 5 fibers and cell-selective transcriptional control. Gene Ther. 2004;11:1296–1300. doi: 10.1038/sj.gt.3302292. doi:10.1038/sj.gt.3302292. [DOI] [PubMed] [Google Scholar]
- Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. doi:10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Xu Q, Takekida S, Ohara N, Chen W, Sitruk-Ware R, Johansson ED, Maruo T. Progesterone receptor modulator CDB-2914 down-regulates proliferative cell nuclear antigen and Bcl-2 protein expression and up-regulates caspase-3 and poly(adenosine 5′-diphosphate-ribose) polymerase expression in cultured human uterine leiomyoma cells. J Clin Endocrinol Metab. 2005;90:953–961. doi: 10.1210/jc.2004-1569. doi:10.1210/jc.2004-1569. [DOI] [PubMed] [Google Scholar]
- Xu Q, Ohara N, Chen W, Liu J, Sasaki H, Morikawa A, Sitruk-Ware R, Johansson ED, Maruo T. Progesterone receptor modulator CDB-2914 down-regulates vascular endothelial growth factor, adrenomedullin and their receptors and modulates progesterone receptor content in cultured human uterine leiomyoma cells. Hum Reprod. 2006;21:2408–2416. doi: 10.1093/humrep/del159. doi:10.1093/humrep/del159. [DOI] [PubMed] [Google Scholar]
- Yamagata Y, Maekawa R, Asada H, Taketani T, Tamura I, Tamura H, Ogane J, Hattori N, Shiota K, Sugino N. Aberrant DNA methylation status in human uterine leiomyoma. Mol Hum Reprod. 2009;15:259–267. doi: 10.1093/molehr/gap010. doi:10.1093/molehr/gap010. [DOI] [PubMed] [Google Scholar]
- Yan SF, Lu J, Zou YS, Soh-Won J, Cohen DM, Buttrick PM, Cooper DR, Steinberg SF, Mackman N, Pinsky DJ, et al. Hypoxia-associated induction of early growth response-1 gene expression. J Biol Chem. 1999;274:15030–15040. doi: 10.1074/jbc.274.21.15030. doi:10.1074/jbc.274.21.15030. [DOI] [PubMed] [Google Scholar]
- Yang EY, Moses HL. Transforming growth factor beta 1-induced changes in cell migration, proliferation, and angiogenesis in the chicken chorioallantoic membrane. J Cell Biol. 1990;111:731–741. doi: 10.1083/jcb.111.2.731. doi:10.1083/jcb.111.2.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh J, Rein M, Nowak R. Presence of messenger ribonucleic acid for epidermal growth factor (EGF) and EGF receptor demonstrable in monolayer cell cultures of myometria and leiomyomata. Fertil Steril. 1991;56:997–1000. doi: 10.1016/s0015-0282(16)54681-0. [DOI] [PubMed] [Google Scholar]
- Yu L, Saile K, Swartz CD, He H, Zheng X, Kissling GE, Di X, Lucas S, Robboy SJ, Dixon D. Differential expression of receptor tyrosine kinases (RTKs) and IGF-I pathway activation in human uterine leiomyomas. Mol Med. 2008;14:264–275. doi: 10.2119/2007-00101.Yu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Jia Z, Liu S, Zhang G, Xiao Z, Yang D, Gan L, Wang Z, Li Y. Efficient cage-opening cascade process for the preparation of water-encapsulated [60]fullerene derivatives. Org Lett. 2009;11:2772–2774. doi: 10.1021/ol9009305. doi:10.1021/ol9009305. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Cao H, Hu YY, Wang H, Zhang CJ. Inhibitory effect of curcumin on angiogenesis in ectopic endometrium of rats with experimental endometriosis. Int J Mol Med. 2011;27:87–94. doi: 10.3892/ijmm.2010.552. doi:10.3892/ijmm.2011.613. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Hague S, Manek S, Zhang L, Bicknell R, Rees MC. PCR display identifies tamoxifen induction of the novel angiogenic factor adrenomedullin by a non-estrogenic mechanism in the human endometrium. Oncogene. 1998;16:409–415. doi: 10.1038/sj.onc.1201768. doi:10.1038/sj.onc.1201768. [DOI] [PubMed] [Google Scholar]