Abstract
The nucleotide sequence of the mRNA encoding the F protein of respiratory syncytial (RS) virus (strain A2) was determined from cDNA clones that contain the complete mRNA sequence. The mRNA is 1899 nucleotides long exclusive of polyadenylylate. The single major open reading frame encodes a protein of 574 amino acids, with a calculated molecular weight of 63,453. Major structural features predicted from the amino acid sequence include an NH2-terminal signal sequence (residues 1-22), hydrophobic transmembrane anchor sequence (residues 525-550), five potential acceptor sites for asparagine-linked carbohydrate, and a potential site (residues 131-136) for the proteolytic cleavage that generates the disulfide-linked F1 and F2 subunits, which, by analogy to other paramyxoviruses, constitute the biologically active form of the F protein. The sequence also contains an internal hydrophobic domain (residues 137-154) that, as a consequence of the activating proteolytic cleavage described above, would become the NH2 terminus of the larger, F1 subunit. The amino acid sequence of the hydrophobic terminus of the F1 subunit is known to be highly conserved among several paramyxoviruses but is markedly dissimilar for RS virus. The F2 subunit is relatively hydrophilic and contains four of the five potential carbohydrate acceptor sites. The subunit order is NH2-F2-F1-COOH. The nucleotide sequences at the 5' and 3' mRNA termini are conserved among the eight RS viral mRNAs sequenced to date. The conserved sequences are: 5' G-G-G-G-C-A-A-A-U ... A-G-U-AU-A-(N)0-2-AU-U-poly(A). These are candidates to be signals for viral transcription. The nucleotide and amino acid sequences described further define the relationship between RS virus and other paramyxoviruses.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austen B. M. Predicted secondary structures of amino-terminal extension sequences of secreted proteins. FEBS Lett. 1979 Jul 15;103(2):308–313. doi: 10.1016/0014-5793(79)81351-4. [DOI] [PubMed] [Google Scholar]
- Blobel G. Intracellular protein topogenesis. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1496–1500. doi: 10.1073/pnas.77.3.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen H. A., Lyles D. S. Structure of Sendai viral proteins in plasma membranes of virus-infected cells. J Virol. 1981 Mar;37(3):1079–1082. doi: 10.1128/jvi.37.3.1079-1082.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choppin P. W., Scheid A. The role of viral glycoproteins in adsorption, penetration, and pathogenicity of viruses. Rev Infect Dis. 1980 Jan-Feb;2(1):40–61. doi: 10.1093/clinids/2.1.40. [DOI] [PubMed] [Google Scholar]
- Collins P. L., Huang Y. T., Wertz G. W. Identification of a tenth mRNA of respiratory syncytial virus and assignment of polypeptides to the 10 viral genes. J Virol. 1984 Feb;49(2):572–578. doi: 10.1128/jvi.49.2.572-578.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins P. L., Wertz G. W. cDNA cloning and transcriptional mapping of nine polyadenylylated RNAs encoded by the genome of human respiratory syncytial virus. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3208–3212. doi: 10.1073/pnas.80.11.3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elango N., Venkatesan S. Amino acid sequence of human respiratory syncytial virus nucleocapsid protein. Nucleic Acids Res. 1983 Sep 10;11(17):5941–5951. doi: 10.1093/nar/11.17.5941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernie B. F., Gerin J. L. Immunochemical identification of viral and nonviral proteins of the respiratory syncytial virus virion. Infect Immun. 1982 Jul;37(1):243–249. doi: 10.1128/iai.37.1.243-249.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gething M. J., White J. M., Waterfield M. D. Purification of the fusion protein of Sendai virus: analysis of the NH2-terminal sequence generated during precursor activation. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2737–2740. doi: 10.1073/pnas.75.6.2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgi C., Blumberg B. M., Kolakofsky D. Sendai virus contains overlapping genes expressed from a single mRNA. Cell. 1983 Dec;35(3 Pt 2):829–836. doi: 10.1016/0092-8674(83)90115-0. [DOI] [PubMed] [Google Scholar]
- Gupta K. C., Kingsbury D. W. Complete sequences of the intergenic and mRNA start signals in the Sendai virus genome: homologies with the genome of vesicular stomatitis virus. Nucleic Acids Res. 1984 May 11;12(9):3829–3841. doi: 10.1093/nar/12.9.3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta K. C., Kingsbury D. W. Conserved polyadenylation signals in two negative-strand RNA virus families. Virology. 1982 Jul 30;120(2):518–523. doi: 10.1016/0042-6822(82)90055-1. [DOI] [PubMed] [Google Scholar]
- Homma M., Ouchi M. Trypsin action on the growth of Sendai virus in tissue culture cells. 3. Structural difference of Sendai viruses grown in eggs and tissue culture cells. J Virol. 1973 Dec;12(6):1457–1465. doi: 10.1128/jvi.12.6.1457-1465.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu M., Scheid A., Choppin P. W. Activation of the Sendai virus fusion protein (f) involves a conformational change with exposure of a new hydrophobic region. J Biol Chem. 1981 Apr 10;256(7):3557–3563. [PubMed] [Google Scholar]
- Huang Y. T., Wertz G. W. Respiratory syncytial virus mRNA coding assignments. J Virol. 1983 May;46(2):667–672. doi: 10.1128/jvi.46.2.667-672.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y. T., Wertz G. W. The genome of respiratory syncytial virus is a negative-stranded RNA that codes for at least seven mRNA species. J Virol. 1982 Jul;43(1):150–157. doi: 10.1128/jvi.43.1.150-157.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
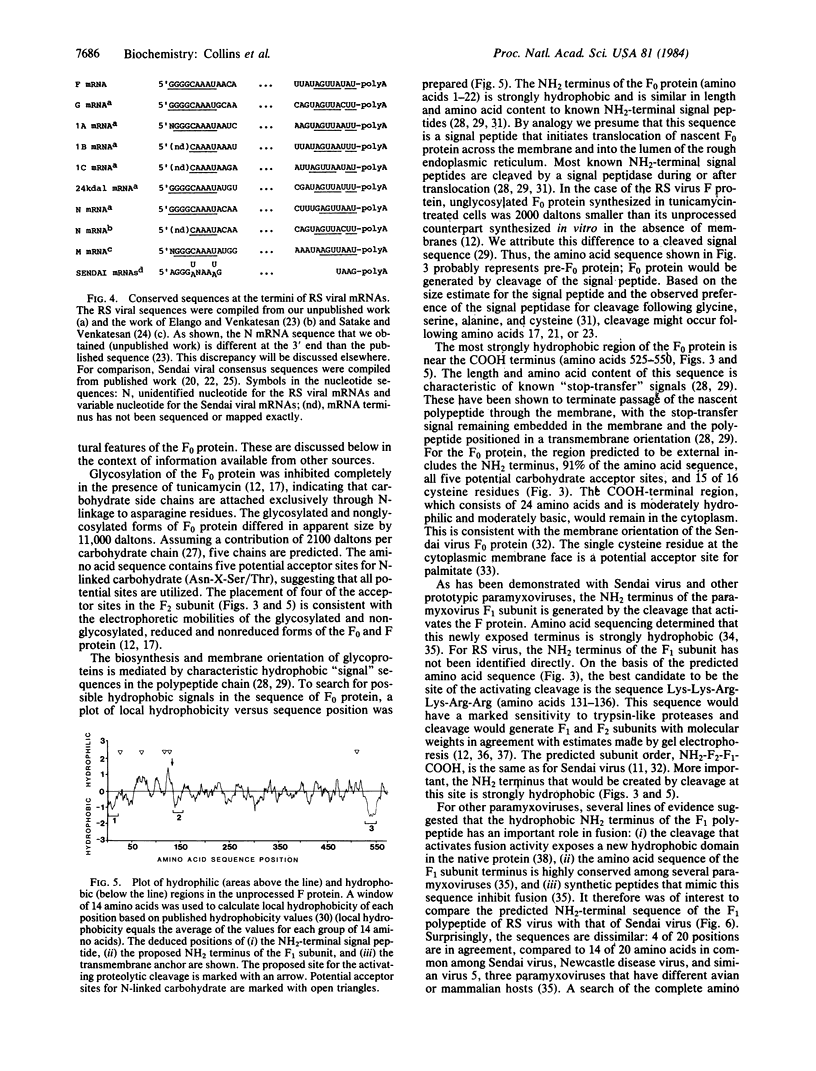
- Hunter E., Hill E., Hardwick M., Bhown A., Schwartz D. E., Tizard R. Complete sequence of the Rous sarcoma virus env gene: identification of structural and functional regions of its product. J Virol. 1983 Jun;46(3):920–936. doi: 10.1128/jvi.46.3.920-936.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert D. M., Pons M. W. Respiratory syncytial virus glycoproteins. Virology. 1983 Oct 15;130(1):204–214. doi: 10.1016/0042-6822(83)90128-9. [DOI] [PubMed] [Google Scholar]
- Land H., Grez M., Hauser H., Lindenmaier W., Schütz G. 5'-Terminal sequences of eucaryotic mRNA can be cloned with high efficiency. Nucleic Acids Res. 1981 May 25;9(10):2251–2266. doi: 10.1093/nar/9.10.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt M. A simplified representation of protein conformations for rapid simulation of protein folding. J Mol Biol. 1976 Jun 14;104(1):59–107. doi: 10.1016/0022-2836(76)90004-8. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Merz D. C., Scheid A., Choppin P. W. Importance of antibodies to the fusion glycoprotein of paramyxoviruses in the prevention of spread of infection. J Exp Med. 1980 Feb 1;151(2):275–288. doi: 10.1084/jem.151.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai Y., Klenk H. D., Rott R. Proteolytic cleavage of the viral glycoproteins and its significance for the virulence of Newcastle disease virus. Virology. 1976 Jul 15;72(2):494–508. doi: 10.1016/0042-6822(76)90178-1. [DOI] [PubMed] [Google Scholar]
- Nakada S., Creager R. S., Krystal M., Aaronson R. P., Palese P. Influenza C virus hemagglutinin: comparison with influenza A and B virus hemagglutinins. J Virol. 1984 Apr;50(1):118–124. doi: 10.1128/jvi.50.1.118-124.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson C. D., Scheid A., Choppin P. W. Specific inhibition of paramyxovirus and myxovirus replication by oligopeptides with amino acid sequences similar to those at the N-termini of the F1 or HA2 viral polypeptides. Virology. 1980 Aug;105(1):205–222. doi: 10.1016/0042-6822(80)90168-3. [DOI] [PubMed] [Google Scholar]
- Rose J. K., Adams G. A., Gallione C. J. The presence of cysteine in the cytoplasmic domain of the vesicular stomatitis virus glycoprotein is required for palmitate addition. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2050–2054. doi: 10.1073/pnas.81.7.2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. K. Complete intergenic and flanking gene sequences from the genome of vesicular stomatitis virus. Cell. 1980 Feb;19(2):415–421. doi: 10.1016/0092-8674(80)90515-2. [DOI] [PubMed] [Google Scholar]
- Sabatini D. D., Kreibich G., Morimoto T., Adesnik M. Mechanisms for the incorporation of proteins in membranes and organelles. J Cell Biol. 1982 Jan;92(1):1–22. doi: 10.1083/jcb.92.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satake M., Venkatesan S. Nucleotide sequence of the gene encoding respiratory syncytial virus matrix protein. J Virol. 1984 Apr;50(1):92–99. doi: 10.1128/jvi.50.1.92-99.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheid A., Choppin P. W. Identification of biological activities of paramyxovirus glycoproteins. Activation of cell fusion, hemolysis, and infectivity of proteolytic cleavage of an inactive precursor protein of Sendai virus. Virology. 1974 Feb;57(2):475–490. doi: 10.1016/0042-6822(74)90187-1. [DOI] [PubMed] [Google Scholar]
- Scheid A., Choppin P. W. Protease activation mutants of sendai virus. Activation of biological properties by specific proteases. Virology. 1976 Jan;69(1):265–277. doi: 10.1016/0042-6822(76)90213-0. [DOI] [PubMed] [Google Scholar]
- Scheid A., Choppin P. W. Two disulfide-linked polypeptide chains constitute the active F protein of paramyxoviruses. Virology. 1977 Jul 1;80(1):54–66. doi: 10.1016/0042-6822(77)90380-4. [DOI] [PubMed] [Google Scholar]
- Shaw M. W., Choppin P. W., Lamb R. A. A previously unrecognized influenza B virus glycoprotein from a bicistronic mRNA that also encodes the viral neuraminidase. Proc Natl Acad Sci U S A. 1983 Aug;80(16):4879–4883. doi: 10.1073/pnas.80.16.4879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh E. E., Hruska J. Monoclonal antibodies to respiratory syncytial virus proteins: identification of the fusion protein. J Virol. 1983 Jul;47(1):171–177. doi: 10.1128/jvi.47.1.171-177.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh E. E., Schlesinger J. J., Brandriss M. W. Purification and characterization of GP90, one of the envelope glycoproteins of respiratory syncytial virus. J Gen Virol. 1984 Apr;65(Pt 4):761–767. doi: 10.1099/0022-1317-65-4-761. [DOI] [PubMed] [Google Scholar]
- Wilson I. A., Skehel J. J., Wiley D. C. Structure of the haemagglutinin membrane glycoprotein of influenza virus at 3 A resolution. Nature. 1981 Jan 29;289(5796):366–373. doi: 10.1038/289366a0. [DOI] [PubMed] [Google Scholar]