Abstract
Objective:
The purpose of this study was to seek radiation dose responses separately for primary hepatocellular carcinoma (HCC) and metastatic (MET) colorectal liver tumours to establish tumour control probabilities (TCPs) for radiotherapy (RT) of liver tumours.
Methods:
The records of 36 HCC and 26 MET colorectal liver tumour patients were reviewed. The median dose per fraction and total dose were 4 Gy (2–10 Gy) and 52 Gy (29–83 Gy) for the HCC group and 3.6 Gy (2.0–13.0 Gy) and 55 Gy (30–80 Gy) for the MET group, respectively. Median tumour diameter was 6.6 cm (3.0–18.0 cm) and 5.0 cm (1.0–13.0 cm) for the HCC and MET groups, respectively. A logistic TCP model was fitted to the response data for each group using the maximum likelihood method.
Results:
50% and 90% probabilities of 6-month local control were estimated to be achievable by 2 Gy per fraction equivalent doses (α/β=10 Gy) of 53 Gy and 84 Gy for the HCC group and 70 Gy and 95 Gy for the MET group, respectively. Actuarial 1-year local control for the HCC and MET groups was 65% (45–85%) and 32% (6–58%), respectively, whereas median time to failure was 543 days (374–711 days) and 183 days (72–294 days), respectively.
Conclusion:
Dose–response relationships were found and modelled for the HCC and MET patient groups, with a higher dose required to control MET tumours. RT offers better local control for HCC than for MET colorectal liver tumours at our institution.
Advances in knowledge:
An improved understanding of radiation dose–response relationships for primary and MET colorectal liver tumours will help inform future dose prescriptions.
Hepatocellular carcinoma (HCC) is the fourth most common cancer worldwide [1]. The liver is also the most common site of metastasis from colorectal carcinoma, with the incidence of liver metastases exceeding 25% [2,3]. Although transplantation and surgical resection offer significant survival benefits among these two groups [4–7], impaired liver function, tumour size and the number of lesions can limit a patient’s eligibility for these treatments. Consequently, there has been a push to develop alternative locoregional therapies for treatment of primary and metastatic liver cancer.
Stereotactic body radiotherapy (SBRT) has shown promise as a new method to safely and non-invasively treat liver tumours [8–11]. SBRT involves precise image-guided delivery of a high dose of radiation to the tumour in a small number of fractions and usually employs motion suppression or gating techniques. A positive association between outcome and dose has previously been reported [11–13]. However, unlike other tumour sites [14–18], there is currently a paucity of explicit liver tumour dose–response modelling within the literature [11]. An improved understanding of radiation dose response is necessary to help better inform future dose prescriptions.
At our institution, patients with liver lesions are prescribed the highest possible radiation dose while maintaining ≤5% normal tissue complication probability (NTCP) with respect to an end point of radiation-induced liver disease (RILD) [19]. As a result, patients are treated with different doses depending on their tumour size relative to the size of remaining healthy liver. This provides for a valuable opportunity to investigate the dose response and to determine the radiation dose required to control liver tumours.
Here, our primary goal is to retrospectively determine separate dose–response relationships for patients with HCC and metastatic (MET) colorectal liver tumours using tumour control probability (TCP) modelling. Our secondary goal is to evaluate local control rates between these two groups at our institution.
PATIENTS AND METHODS
Patient data
The records of patients treated with radiotherapy (RT) at our institution for HCC or MET colorectal liver tumours from 2004 to 2011 were reviewed. Patients who had previous regional or systemic therapy were included in the analysis as long as their treatment concluded prior to the start of RT. Patients who underwent additional concurrent therapy were excluded from the study. Patients who received alternative therapy, post RT, were included; however, their follow-up data were censored on initiation of the additional therapy. No limit was placed on the size or the number of target lesions. A total of 36 patients treated for HCC and 26 patients treated for MET colorectal liver tumours were analysed. Follow-up data typically included CT-based measurements of tumour size and measurements of α-fetoprotein (AFP) and carcinoembryonic antigen (CEA) biomarkers. The times of all follow-up observations were reported with respect to the treatment end date and were typically at 1 month, 3 months, 6 months, 1 year and 2 years after treatment. Patients' data were collected, quality assured and analysed in a database approved by our Institutional Review Board (Health Sciences REB#: 16487E.
Radiotherapy
The clinical target volume (CTV) was defined based on four-dimensional CT scans. Motion management involved patient immobilisation (Vac-Lok™; CIVCO Medical Systems, Orange City, IA) and respiratory gating (Varian® Real-Time Position Management™; Varian Medical Systems, Inc., Palo Alto, CA) to minimise internal motion. Depending on patient motion and residual motion measurements, an additional 2–10-mm, 3–25-mm and 3–7-mm planning target volume (PTV) margin was added to the CTV in the anteriror–posterior, superior–inferior and lateral directions, respectively. Patients were treated with three-dimensional conformal RT, intensity-modulated radiotherapy or tomotherapy.
Diverse dose fractionation regimens were employed in the two patient groups, including both traditional and hypofractionation schedules. The dose prescribed to each patient was maximised while maintaining ≤5% Lyman–Kutcher NTCP for the remaining healthy liver with respect to an end point of Grade 3 or higher RILD, as defined by the Radiation Therapy Oncology Group [19]. The NTCP model employed parameter values reported by Dawson et al [19], who had separate parameter sets for calculating the probability of liver toxicity between HCC and MET patient groups. Doses were prescribed to the PTV, such that 95% of the PTV received at least 95% of the prescribed dose. The maximum PTV dose was limited to no more than 107% of the prescribed dose. Consequently, the PTV mean dose was similar to the prescribed dose. The doses received by the small bowel, lung, heart, stomach, kidneys and spinal cord were also constrained to prevent toxicity.
Tumour control probability
A TCP model was used to investigate the relationship between the radiation dose and the tumour control. 6-month local control was chosen as the end point for the TCP model as detailed 6-month local control data were available for the largest number of patients and were considered clinically important a priori. Patients who had failed locally or died owing to liver disease progression prior to the 6-month time point were considered to be uncontrolled at 6 months. Since the patients included in this study were treated with diverse dose-fractionation regimens, prescription doses were converted to 2 Gy per fraction equivalent doses prior to TCP modelling to ensure biological comparability. Equivalent doses were computed using the standard linear quadratic model (LQM) approach. Currently, there is no strong consensus on liver tumour α/β ratios with published values ranging from 3.1 Gy to 15 Gy [20]. Consequently, a typical tumour value of α/β =10 Gy was used for this conversion. A sensitivity analysis was also performed to assess the impact of using other α/β values on dose–response parameters. A uniform dose distribution within the target was assumed owing to the dose uniformity constraints described in the section “Radiotherapy”.
To facilitate comparisons with Chang et al [11], we elected to use the same logistic TCP model [21] to fit the 6-month tumour control data:
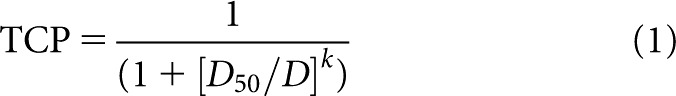 |
where D50 is the dose that would result in a 50% probability of local control, D is the prescription dose and k controls the slope of the TCP curve. Similar to other response studies [22,23], the model was fitted to the data using the maximum likelihood method, which can provide both the fitted parameters and the estimates of their standard deviations [24].
In our context, the maximum likelihood method determines the values of D50 and k, which maximise the probability of predicting response within our patient groups using the TCP from Equation (1). We employed a binary relationship where patients either respond or do not respond to treatment, which we denote for the ith patient by Ri=1 or Ri=0, respectively. Here, response corresponds to observing local control approximately 6 months (±1 month) post RT. The probability that patient i responds or does not respond given a prescribed dose Di is given by
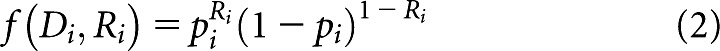 |
where pi is equal to the TCP defined by Equation (1), evaluated at D=Di. A constrained minimisation procedure from the MATLAB® optimisation toolkit (fmincon) (The Mathworks, Inc., Natick, MA) was used to search for the values of D50 and k, which minimise the negative of the log-likelihood function
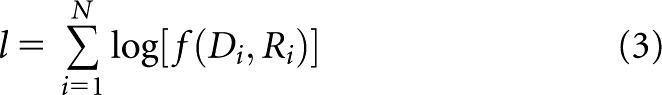 |
where N is the number of patients in the patient group of interest and f(Di, Ri) is defined by Equation (2).
Local control definition
Tumour response was evaluated in the largest treated lesion in each patient. Local control was defined using radiographic or tumour biomarker information depending on data availability. Using radiographic information, local control corresponded to observing at least stable disease criteria or better using the Response Evaluation Criteria in Solid Tumours v. 1.1 [25]. Using biomarker information, local control corresponded to observing a ≤20% increase from baseline measurements of AFP for HCC patients and CEA for MET patients.
AFP has recently been shown to be a reliable biomarker of radiological response after locoregional therapy for HCC [26,27]. CEA has also previously been used as a biomarker for response and metastasis after surgical resection and chemotherapy for colorectal carcinoma [28,29]. Biomarker levels were required to exceed the normal values either prior to or after RT in order for them to be included in the definition of local control. The normal values used for AFP and CEA levels were 6 ng ml−1 and 5 ng ml−1, respectively [30,31]. If a treated lesion met the criteria for radiographic local control but not biomarker local control (or vice versa), then the lesion was still considered to be locally controlled. If a patient did not have follow-up CT data, then local control was assessed using biomarker data and vice versa. Data were censored starting at the time of the last follow-up if a patient was lost to follow-up prior to the loss of local control.
Local control analysis
Local control follow-up data were visualised using what we have termed “dose–control history plots”. These plots contain horizontal timelines for each patient, which indicate follow-up history and outcomes. The timelines are displayed in order of increasing prescription dose. Standard Kaplan–Meier analyses and log-rank tests were also performed using SPSS® Statistics v. 19 (IBM Corporation, Armonk, NY) to quantitatively assess local control as a function of time.
Potential factors affecting local control, such as the radiation dose and pre-treatment tumour volume, were explored using plots of patient pre-treatment tumour volume vs the prescribed equivalent dose, with colour coding indicating time to loss of local control or time to censor (last follow-up before loss of patient contact). Two-tailed Spearman rank tests were used to test for the possible correlations. Censored data (patients lost to follow-up before loss of local control) were included in correlative analyses, provided the data were censored at least 6 months post RT. We expect that RT had an effect if the tumour was controlled for at least 6 months, and, therefore, that time to last follow-up for censored data may be correlated with the radiation dose or pre-treatment tumour volume.
RESULTS
Patient and treatment characteristics for the HCC and MET groups are summarised in Table 1. The median follow-up time was 197 days (27–1095 days) and 178 days (51–1101 days) for the HCC and MET groups, respectively.
Table 1.
Patient characteristics and treatment data
| Characteristics | HCC (n=36) | MET (n=26) | p-value |
| Age (years) | 74 (22–87) | 68 (42–90) | 0.0634 |
| Gender | |||
| Male (%) | 29 (81) | 16 (62) | |
| Female (%) | 7 (19) | 10 (38) | |
| Child–Pugh score | |||
| Class A (%) | 27 (75) | 21 (81) | |
| Class B (%) | 8 (22) | 5 (19) | |
| Class C (%) | 1 (3) | 0 | |
| Previous treatment | |||
| Surgical resection (liver, %) | 0 | 8 (31) | |
| Chemotherapy (%) | 5 (14) | 23 (88) | |
| TACE (%) | 9 (25) | 0 | |
| RFA (%) | 1 (3) | 1 (4) | |
| Active extrahepatic disease (%) | 11 (31) | 12(46) | |
| Tumour diameter (cm) | 6.6 (3.0–18.0) | 5.0 (1.0–13.0) | 0.0329 |
| CTV (ml) | 186 (8–995) | 57 (5–1804) | 0.0156 |
| Radiotherapy technique | |||
| 3D-CRT (%) | 25 (70) | 21 (81) | |
| IMRT (%) | 8 (22) | 4 (15) | |
| Tomotherapy (%) | 3 (8) | 1 (4) | |
| Gating (%) | 21 (58) | 15 (58) | |
| Number of fractions | 15 (6–20) | 15 (6–21) | 0.3657 |
| Dose per fraction (Gy) | 4.0 (2.0–10.0) | 3.6 (2.0–13.0) | 0.2363 |
| Total dose (Gy) | 52 (29–83) | 55 (30–80) | 0.7752 |
| Equivalent dose (Gy) | 63 (33–107) | 61 (33–154) | 0.8753 |
3D-CRT, three-dimensional radiotherapy; CTV, clinical target volume; HCC, hepatocellular carcinoma; IMRT, intensity-modulated radiotherapy; MET, metastatic colorectal liver tumours; RFA, radiofrequency ablation; TACE, transarterial chemoembolisation.
All summary statistics are medians with ranges displayed in brackets. p-values were calculated using the Wilcoxon rank sum test.
Sufficient follow-up data were available to assess 6-month local control for 27/36 (75%) HCC and 19/26 (73%) MET cases. 5/27 HCC and 1/19 MET patients died prior to true observation of loss of local control. However, in all six cases, patient charts indicated that there was no extrahepatic progression and that liver disease progression was the cause of death. Therefore, these tumours were considered to be uncontrolled at 6 months. 16/27 (59%) and 7/19 (32%) patients were locally controlled at approximately 6 months post RT in the HCC and MET groups, respectively.
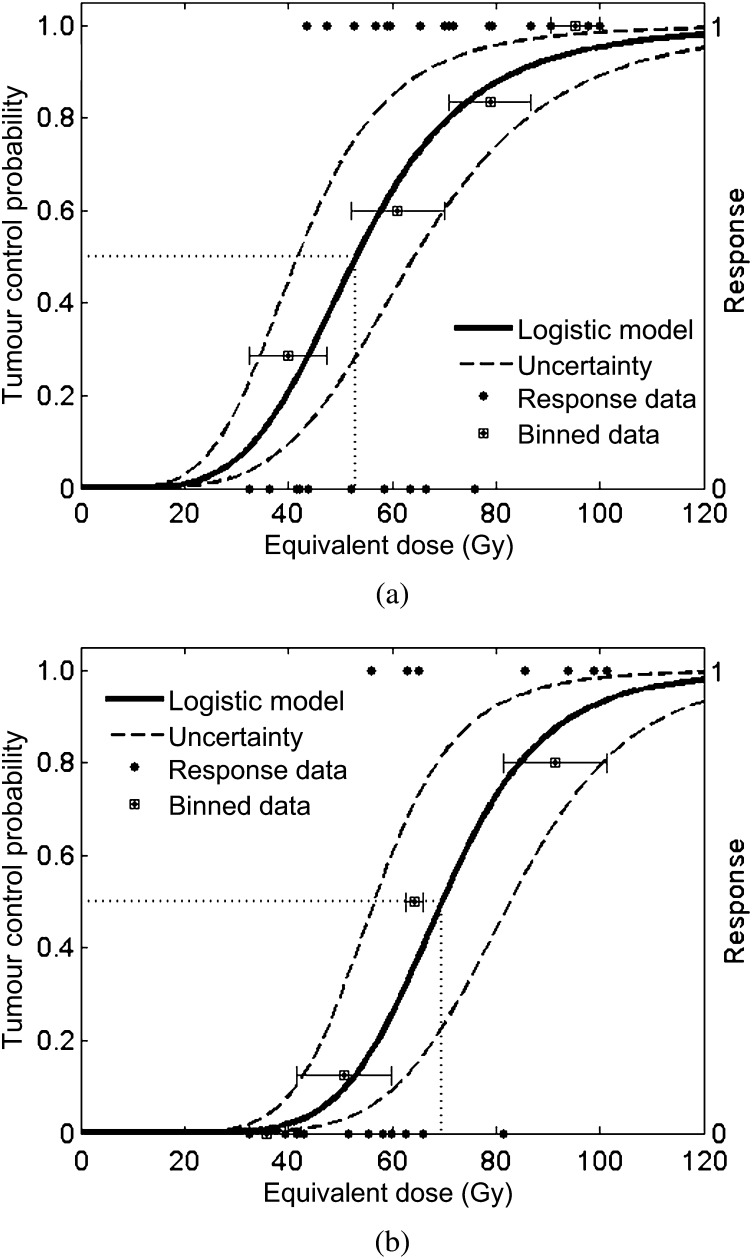
The logistic TCP model was fitted independently to the response data for each subgroup using the end point of 6 months of local control (Figure 1). One outlier within the HCC group was omitted prior to model fitting (107 Gy, uncontrolled at 6 months) owing to its severe contradiction with the trends indicated by the other data. For reference purposes, the observed patient response data used by the maximum likelihood fitting method are also indicated in Figure 1 as a function of equivalent dose. These data were then binned and averaged to generate estimates of the observed TCP at various dose levels and to assist in evaluating the quality of the model fits. Horizontal whiskers indicate the range of doses included in each bin.
Figure 1.
Tumour control probability curves and patient response data for the (a) hepatocellular carcinoma and (b) metastatic colorectal liver tumours patient groups approximately 6 months post treatment.
D50 was determined to be 53 Gy (σ=5.6 Gy) and 70 Gy (σ=6.6 Gy), and the slope parameter k was estimated to be 4.8 (σ=2) and 7.1 (σ=3.3) for the HCC and MET groups, respectively. 2 Gy per fraction equivalent doses of 84 Gy and 95 Gy are predicted to result in 90% 6-month local control rates for patients with HCC and MET colorectal liver tumour, respectively. Uncertainty in the dose–response curves was illustrated in Figure 1 by plotting the TCP model using the fitted D50 parameters plus or minus two standard deviations while keeping the fitted k parameter constant. The standard deviations of the parameter estimates serve as surrogates for assessing the quality of the model fits, with higher values indicating greater parameter uncertainty.
General treatment-related toxicities (e.g. nausea, abdominal pain, fatigue) were scored using the Common Terminology Criteria for Adverse Events v. 3.0 (National Cancer Institute, Bethesda, MD) for the subset of patients included in TCP analysis. For the HCC group after 6 months, there were 5 asymptomatic patients, 9 patients with Grade 1, 10 patients with Grade 2 and 2 patients with Grade 3 complications. For the MET group after 6 months, there were five asymptomatic patients, three patients with Grade 1, eight patients with Grade 2 and three patients with Grade 3 complications. Liver-specific toxicities were scored using the same RILD end point as the NTCP model used during planning [19]. No instances of Grade 3 or higher Radiation Therapy Oncology Group RILD were observed among any of the HCC or MET patients.
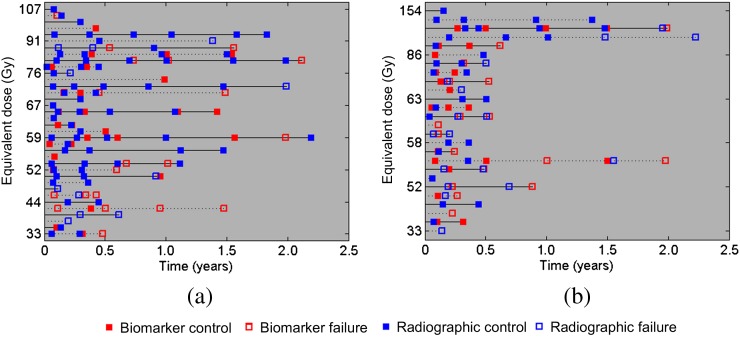
Figure 2 summarises the local control data acquired for the two patient groups using dose–control history plots. The plots indicate all the radiographic and biomarker local control data that were available for each HCC and MET patient. Between the full HCC (n=36) and MET (n=26) cohorts, local control was determined by radiographic data for 21/36 (58%) HCC and 13/26 (50%) MET patients at the time of last follow-up, with local control determined by the AFP and CEA data for the remaining patients. The median follow-up time was 197 days (range, 27–1095 days) and 178 days (range, 51–1101 days) for the HCC and MET groups, respectively. At the time of last follow-up, 21/36 (58%) HCC and 14/26 (54%) MET patients had lost local control.
Figure 2.
Dose control histories for the (a) hepatocellular carcinoma and (b) metastatic colorectal liver tumours patient groups. Horizontal lines correspond to individual patient histories and are displayed in the increasing order of the radiation treatment dose.
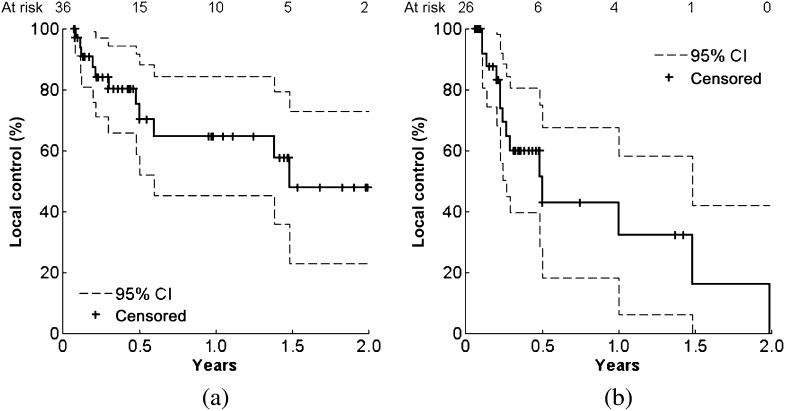
Kaplan–Meier analysis was performed on the local control data for the full HCC (n=36) and MET (n=26) cohorts (Figure 3). The HCC and MET curves were found to be significantly different (log-rank p=0.03). For the HCC group, actuarial 1- and 2-year local control rates were 65% (45–85%) and 48% (23–73%), respectively. For the MET group, actuarial 1- and 2-year local control rates were 32% (6–58%) and 0% (0–42%), respectively. The median time to failure (loss of local control) between HCC and MET groups was estimated to be 543 days [95% confidence interval (CI) 374–711] and 183 days (95% CI 72–294), respectively.
Figure 3.
Local control Kaplan–Meier curves for the (a) hepatocellular carcinoma and (b) metastatic colorectal liver tumours patient groups (log-rank p=0.03). CI, confidence interval.
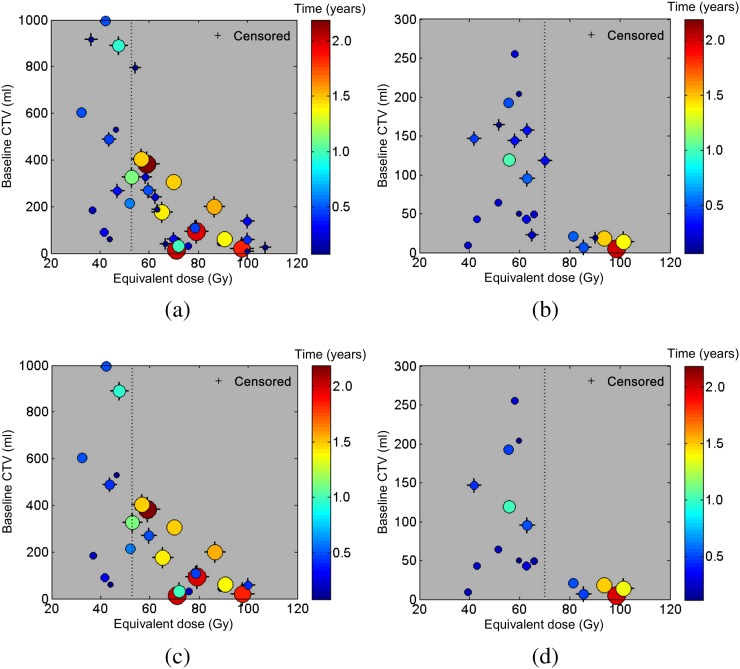
To visualise how the data are distributed about the D50 parameters, plots of pre-treatment tumour volume vs equivalent prescribed dose with time to failure/censor colour coding were generated (Figure 4). Data point radii were also related to time to failure/censor for improved visualisation.
Figure 4.
Pre-treatment clinical target volume (CTV) vs the prescribed equivalent dose with time to loss of local control/censor colour coding. (a) and (b) Plots for the hepatocellular carcinoma and metastatic colorectal liver tumours patient groups, respectively. (c) and (d) contain the same information except we have omitted the data censored prior to 6 months. Outliers (35 Gy, 1804 ml, 0.3 years), (33 Gy, 1122 ml, 0.14 years) and (154 Gy, 7 ml, 0.14 years) have been omitted from (b) to better visualise trends. D50 is indicated by the dashed vertical line.
We performed two-tailed Spearman's rank correlation tests on the subset of data included in Figure 4c (n=27/36) and 4d (n=19/26). Dose was significantly correlated with tumour volume for both the HCC (ρ=−0.73, p<0.001) and the MET groups (ρ=−0.62, p=0.005). However, baseline tumour volume was not significantly correlated with time to failure/censor for either of the HCC (ρ=−0.047, p=0.82) or of the MET groups (ρ=−0.44, p=0.06). Dose and time to failure/censor were significantly correlated for the MET group (ρ=0.50, p=0.03) but not for the HCC group (ρ=0.22, p=0.26).
DISCUSSION
In this study, we have demonstrated 6-month dose–response relationships for patients treated with RT for HCC or MET colorectal liver tumour groups. The heterogeneous doses prescribed to the patients within the HCC and MET groups provided a valuable opportunity to evaluate dose response in the liver. Although dose–response relationships have previously been established for HCC [13] and MET [11] patients, currently, there is a lack of literature on dose response and explicit TCP modelling for the liver. These data are critical if personalised radiobiologically guided dose-escalated RT is to be applied to patients. This study employed the maximum likelihood method to fit a TCP model to HCC and MET tumour response data, adding to this body of literature. To our knowledge, this is the first study to explicitly model TCP for HCC patients and the second to do so for MET patients [11].
Park et al [13] reported a 77% partial response rate, 4–6 weeks post RT, among a subset of patients with HCC (n=83) who were prescribed a radiation dose >50 Gy. In the present study, we found that a 50% response rate at 6 months post RT could be achievable with a higher dose of 53 Gy (σ=5.6 Gy). This suggests that dose escalation may improve the durability (duration of local control) of tumour response that is consistent with the findings of Park et al [13].
Chang et al [11] reported a D50 value of 68 Gy (standard error=6 Gy) with an end point of 12 months of local control among patients (n=65) treated with SBRT for MET colorectal liver tumours in a multicentre pooled analysis. Here, we found a slightly higher D50 value of 70 Gy (σ=6.6 Gy) for an end point of 6 months of local control among a similar group of patients, although this difference is within the uncertainties of the two D50 estimates. If we had similar patient demographics, we would expect that higher doses should translate to improved local tumour response durability. Therefore, a 12-month response D50 value as in Chang et al [11] would be expected to be higher than a 6-month response value. However, a difference between the patient population in Chang et al [11] and the MET group is the median tumour volume that is smaller in Chang et al [11] than in the present study [30 ml, range=(0.5, 3008) vs 57 ml, range=(5, 1804)]. A steeper dose–response relationship was found in our study with a slope parameter of 7.1 (σ=3.3) compared with 4.2 (standard error=1.6) as reported by Chang et al.
Prior to TCP modelling, the LQM was used to convert doses to 2 Gy per fraction equivalents. The LQM has been shown to overestimate cell kill for dose fractionations beyond 8–10 Gy per fraction [32]. Although some patients within this study received higher ablative doses, 96% of the HCC patients and 95% of the MET patients included in the TCP analyses received ≤8 Gy per fraction, supporting our use of the LQM. None of the included patients received doses exceeding 10 Gy per fraction.
Currently, there is no strong consensus on α/β ratios for HCC, and, to our knowledge, there are no published data on ratios for MET colorectal liver tumours. Consequently, a single α/β ratio of 10 Gy was used to determine the 2 Gy per fraction equivalent doses for both the HCC and the MET groups prior to dose–response modelling. Therefore, we investigated the sensitivity of our D50 and k dose–response parameter values to the α/β ratio. Wigg et al [20] have compiled a short list of reported HCC α/β ratios. We used these alternative values to recompute the 2 Gy per fraction equivalent doses and then recalculate the dose–response parameter values (Table 2). The parameter variability for values of α/β ≥7.2 Gy was much smaller than the uncertainty (standard deviation) in the parameter estimation itself. Therefore, our reported values should be robust to current α/β uncertainty provided that liver tumours are early responding tissues.
Table 2.
Influence of using different α/β ratios on estimated dose–response parameters
| α/β (Gy) | HCC | MET | ||
| D50 (Gy) | k | D50 (Gy) | k | |
| 3.1 | 63 (6.7) | 4.8 (2.0) | 80 (8.4) | 6.1 (2.7) |
| 7.2 | 55 (5.8) | 4.9 (2.0) | 72 (7.2) | 6.8 (3.1) |
| 10 | 53 (5.6) | 4.8 (2.0) | 70 (6.6) | 7.1 (3.3) |
| 15 | 51 (5.6) | 4.6 (2.0) | 67 (6.0) | 7.4 (3.5) |
D50, dose that would result in a 50% probability of local control; HCC, hepatocellular carcinoma; MET, metastatic colorectal liver tumours.
The prescription doses were converted to 2 Gy per fraction equivalent doses using each α/β ratio followed by tumour control probability model fitting. Parameter results for α/β=10 Gy were included for comparison. Standard deviations are shown in brackets.
In this study, our primary aim was to examine whether dose–response relationships exist for HCC and MET patients. Since limited research has been done on liver tumour dose response, we employed the commonly used logistic TCP model to fit the observed data. This provided a simple way to demonstrate dose response in the liver and facilitated comparison with the only other existing literature. In the future, more refined TCP models will be investigated, which account for non-uniform tumour doses and tumour volumes. Furthermore, the predictive utility of the models will then be investigated in independent data sets.
Toxicity among the patients included in the TCP analyses was comparable to previous liver irradiation studies [10,11,33–35]. The large tumour sizes in the HCC and MET patient groups may have contributed to marginally higher toxicities. However, HCC patients who experienced Grade 3 complications post RT had pre-existing Grade 2 complications before treatment. Similarly, for the MET group, one patient with post-RT Grade 3 complications had pre-RT Grade 3 complications and the remaining two had pre-RT Grade 1 complications. In addition, no instances of Grade 3 or higher Radiation Therapy Oncology Group RILD were observed among either of the two groups.
We found that the dose and time to loss of local control/censor were correlated for the MET patients but not for the HCC patients. This could be because of the increased percentage of patients in the HCC group who were lost during follow-up prior to observation of loss of local control (18/27, 67% for HCC vs 5/19, 26% for MET). HCC patients tend to lose local control later than MET patients, and therefore patients are more frequently lost to follow-up prior to observation of loss of local control. Although data censored at later times may have a connection to treatment parameters, inclusion of these data in the analysis may weaken correlations in the HCC group since time to last follow-up (or censor) can be related to factors aside from the dose.
Tumour volume was not found to be correlated with time to loss of local control/censor for both the HCC and MET groups, which is in agreement with the studies by Andolino et al [10] and Chang et al [11], respectively. Therefore, although the HCC tumours were significantly larger than the MET tumours (Table 1), tumour size could not be used to explain the significant differences between HCC and MET dose–response parameters or local control rates. Prescription dose was also not significantly different between the two groups (Table 1).
However, a key difference between the two demographics was the heavily pre-treated nature of the MET group. 88% of MET patients had received previous chemotherapy compared with only 14% of HCC patients. This is consistent with the pattern of referral at our institution, whereby MET patients are usually treated with RT after failing multiple chemotherapy regimens. Consequently, the MET patients tend to be further along in their disease than the HCC patients. This may explain why the median time to loss of local control for the MET group (183 days) was much lower than for the HCC group (543 days) as well as why higher doses were required to control MET colorectal liver tumours.
The local control rates determined for the HCC and MET patients in this investigation may seem low when compared with the current literature. For example, Andolino et al [10] found that HCC patients (n=37) who were ineligible for transplant had a 2-year local control rate of 87% compared with the 48% (23–73%) rate observed in this study. Similarly for the MET group, Chang et al [11] reported 1- and 2-year local control rates of 67% and 55%, respectively, for patients with MET colorectal liver tumours (n=65) compared with the 32% (6–58%) and 0% (0–42%) local control rates reported here. Currently, there is a lack of consensus on whether liver tumour size correlates with radiotherapy outcome [10,11,34,35]. However, a notable difference between the present and aforementioned studies is tumour size, which will be summarised in the subsequent paragraph. The lower local control rates observed in this study is the reason for our choice of a 6-month local control TCP end point (instead of 1 year), particularly among the MET patient group whose median time to loss of local control was approximately 6 months. This ensures that patient response within the two groups can be maximally stratified as a function of dose.
For Andolino et al [10], the median tumour diameter was 3.5 cm [range=(1, 6.5)] compared with 6.6 cm [range=(3, 18)] for our HCC group. In contrast, Tse et al [33] investigated a group of HCC and intrahepatic cholangiocarcinoma patients (n=49) with more comparable tumour sizes and reported a 65% 1-year local control rate that is similar to the 1-year local control rate reported here. The median tumour volume in their investigation was 173 ml [range=(9, 1913)] compared with 186 ml [range=(8, 995)] for our HCC group. For Chang et al [11], the median tumour volume was 30 ml [range=(0.5, 3008)] compared with 57 ml [range=(5, 1804)] for the MET group in this study.
In this study, we used a combination of biomarker and radiographic response data to help define local control. However, for MET patients with untreated metastatic tumour burden, multiple tumours could be contributing to increased CEA levels that hide decreases caused by treatment of the target lesion. We attempted to mitigate this effect by allowing for a <20% increase in biomarker levels within the local control definition and by supplementing biomarker data with local radiographic information.
CONCLUSIONS
In conclusion, we have found radiation dose–response relationships for patients with HCC and MET groups. D50 was determined to be 53 Gy (σ=5.6 Gy) and 70 Gy (σ=6.6 Gy) and the slope parameter k was estimated to be 4.8 (σ=2) and 7.1 (σ=3.3) for the HCC and MET groups, respectively. 2 Gy per fraction equivalent doses of 84 Gy and 95 Gy are predicted to result in 90% 6-month local control rates for patients in the HCC and MET groups, respectively. RT for HCC and MET results in significantly different local control rates at our institution, which may warrant an investigation into the effect of earlier RT referral for patients with MET. Improved understanding of the dose–response relationships for patients with primary or metastatic liver cancer will help to inform future dose prescriptions.
Funding
This research is funded by the Canadian Cancer Society (grant no. 700386). The study sponsors had no involvement in the study design, collection, analysis and interpretation of data and in the writing of the manuscript or in the decision to submit this manuscript for publication. The authors have no financial interest in the submitted work.
REFERENCES
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010;127:2893–917 10.1002/ijc.25516 [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin 2009;59:225–49 [DOI] [PubMed] [Google Scholar]
- 3.Mainfredi S, Lepage C, Hatem C, Coatmeur O, Faivre J, Bouvier AM. Epidemiology and management of liver metastases from colorectal cancer. Ann Surg 2006;244:254–9 10.1097/01.sla.0000217629.94941.cf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996;334:693–9 10.1056/NEJM199603143341104 [DOI] [PubMed] [Google Scholar]
- 5.Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology 1999;30:1434–40 10.1002/hep.510300629 [DOI] [PubMed] [Google Scholar]
- 6.Scheele J, Stangl R, Altendorf-Hoffmann A. Hepatic metastases from colorectal carcinoma: impact of surgical resection on the natural history. Br J Surg 1990;77:1241–6 [DOI] [PubMed] [Google Scholar]
- 7.Fong Y, Blumgart LH, Cohen AM. Surgical treatment of colorectal metastases to the liver. CA Cancer J Clin 1995;45:50–62 [DOI] [PubMed] [Google Scholar]
- 8.Wulf J, Guckenberger M, Haedinger U, Oppitz U, Mueller G, Baier K, et al. Stereotactic radiotherapy of primary liver cancer and hepatic metastases. Acta Oncol 2006;45:838–47 10.1080/02841860600904821 [DOI] [PubMed] [Google Scholar]
- 9.Katz AW, Carey-Sampson M, Muhs AG, Milano MT, Schell MC, Okunieff P. Hypofractionated stereotactic body radiation therapy (SBRT) for limited hepatic metastases. Int J Radiat Oncol Biol Phys 2007;67:793–8 10.1016/j.ijrobp.2006.10.025 [DOI] [PubMed] [Google Scholar]
- 10.Andolino DL, Johnson CS, Maluccio M, Kwo P, Tector AJ, Zook J, et al. Stereotactic body radiotherapy for primary hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 2011;81:e447–53 10.1016/j.ijrobp.2011.04.011 [DOI] [PubMed] [Google Scholar]
- 11.Chang DT, Swaminath A, Kozak M, Weintraub J, Koong AC, Kim J, et al. Stereotactic body radiotherapy for colorectal liver metastases: a pooled analysis. Cancer 2011;117:4060–9 10.1002/cncr.25997 [DOI] [PubMed] [Google Scholar]
- 12.Dawson LA, McGinn CJ, Normolle D, Ten Haken RK, Walker S, Ensminger W, et al. Escalated focal liver radiation and concurrent hepatic artery fluorodeoxyuridine for unresectable intrahepatic malignancies. J Clin Oncol 2000;18:2210–18 [DOI] [PubMed] [Google Scholar]
- 13.Park HC, Seong J, Han KH, Chon CY, Moon YM, Suh CO. Dose-response relationship in local radiotherapy for hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 2002;54:150–5 [DOI] [PubMed] [Google Scholar]
- 14.Partridge M, Ramos M, Sardaro A, Brada M. Dose escalation for non-small cell lung cancer: analysis and modeling of published literature. Radiother Oncol 2011;99:6–11 10.1016/j.radonc.2011.02.014 [DOI] [PubMed] [Google Scholar]
- 15.Dimopoulos JCA, Pötter R, Lang S, Fidarova E, Georg P, Dörr W, et al. Dose-effect relationship for local control of cervical cancer by magnetic resonance image-guided brachytherapy. Radiother Oncol 2009;93:311–15 10.1016/j.radonc.2009.07.001 [DOI] [PubMed] [Google Scholar]
- 16.Appelt AL, Pløen J, Vogelius IR, Bentzen SM, Jakobsen A. Radiation dose-response model for locally advanced rectal cancer after preoperative chemoradiation therapy. Int J Radiat Oncol Biol Phys 2013;85:74–80 10.1016/j.ijrobp.2012.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rockne R, Alvord EC, Jr, Rockhill JK, Swanson KR. A mathematical model for brain tumor response to radiation therapy. J Math Biol 2009;58:561–78 10.1007/s00285-008-0219-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ohri N, Dicker AP, Trabulsi EJ, Showalter TN. Can early implementation of salvage radiotherapy for prostate cancer improve therapeutic ratio? A systemic review and regression meta-analysis with radiobiological modeling. Eur J Cancer 2012;48:837–44 10.1016/j.ejca.2011.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dawson LA, Normolle D, Balter JM, McGinn CJ, Lawrence TS, Ten Haken RK. Analysis of radiation-induced liver disease using the Lyman NTCP model. Int J Radiat Oncol Biol Phys 2002;53:810–21 [DOI] [PubMed] [Google Scholar]
- 20.Wigg AJ, Palumbo K, Wigg DR. Radiotherapy for hepatocellular carcinoma: systematic review of radiobiology and modeling projections indicate reconsideration of its use. J Gastroenterol Hepatol 2010;25:663–71 10.1111/j.1440-1746.2009.06126.x [DOI] [PubMed] [Google Scholar]
- 21.Goiten M, Schultheiss TE. Strategies for treating possible tumor extension: some theoretical considerations. Int J Radiat Oncol Biol Phys 1985;11:1519–28 [DOI] [PubMed] [Google Scholar]
- 22.Levegrün S, Jackson A, Zelefsky MJ, Skwarchuk MW, Venkatraman ES, Schlegel W, et al. Fitting tumor control probability models to biopsy outcome after three-dimensional conformal radiation therapy of prostate cancer: pitfalls in deducing radiobiologic parameters for tumors from clinical data. Int J Radiat Oncol Biol Phys 2001;51:1064–80 [DOI] [PubMed] [Google Scholar]
- 23.Jackson A, Ten Haken RK, Robertson JM, Kessler ML, Kutcher GJ, Lawrence TS. Analysis of clinical complication data for radiation hepatitis using a parallel architecture model. Int J Radiat Oncol Biol Phys 1995;31:883–91 10.1016/0360-3016(94)00471-4 [DOI] [PubMed] [Google Scholar]
- 24.Aldrich JRA. Fisher and the making of maximum likelihood 1912-1922. Stat Sci 1997;12:162–76 [Google Scholar]
- 25.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumors: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 26.Riaz A, Ryu RK, Kulik LM, Mulcahy MF, Lewandowski RJ, Minocha J, et al. Alpha-fetoprotein response after locoregional therapy for hepatocellular carcinoma: oncologic marker of radiologic response, progression, survival. J Clin Oncol 2009;27:5734–42 10.1200/JCO.2009.23.1282 [DOI] [PubMed] [Google Scholar]
- 27.Kim BK, Ahn SH, Seong JS, Park JY, Kim Y, do, Kim JK, et al. Early α-fetoprotein response as a predictor of clinical outcome after localized concurrent chemoradiotherapy for advanced hepatocellular carcinoma. Liver Int 2011;31:369–76 10.1111/j.1478-3231.2010.02368.x [DOI] [PubMed] [Google Scholar]
- 28.Chau I, Allen MJ, Cunningham D, Norman AR, Brown G, Ford HE, et al. The value of routine serum carcino-embryonic antigen measurement and computed tomography in the surveillance of patients after adjuvant chemotherapy for colorectal cancer. J Clin Oncol 2004;22:1420–9 [DOI] [PubMed] [Google Scholar]
- 29.Tan E, Gouvas N, Nicholls RJ, Ziprin P, Xynos E, Tekkis PP. Diagnostic precision of carcinoembryonic antigen in the detection of recurrence of colorectal cancer. Surg Oncol 2009;18:15–24 10.1016/j.suronc.2008.05.008 [DOI] [PubMed] [Google Scholar]
- 30.Taketa K. Alpha-fetoprotein: reevaluation in hepatology. Hepatology 1990;12:1420–32 [DOI] [PubMed] [Google Scholar]
- 31.Goldstein MJ, Mitchell EP. Carcinoembryonic antigen in the staging and follow-up of patients with colorectal cancer. Cancer Invest 2005;23:338–51 [DOI] [PubMed] [Google Scholar]
- 32.Park C, Papiez L, Zhang S, Story M, Timmerman RD. Universal survival curve and single fraction equivalent dose: useful tools in understanding potency of ablative radiotherapy. Int J Radiat Oncol Biol Phys 2008;70:847–52 10.1016/j.ijrobp.2007.10.059 [DOI] [PubMed] [Google Scholar]
- 33.Tse RV, Hawkins M, Lockwood G, Kim JJ, Cummings B, Knox J, et al. Phase I study of individualized stereotactic body radiotherapy for hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J Clin Oncol 2008;26:657–64 10.1200/JCO.2007.14.3529 [DOI] [PubMed] [Google Scholar]
- 34.Lee MT, Kim JJ, Dinniwell R, Brierley J, Lockwood G, Wong R, et al. Phase I study of individualized stereotactic body radiotherapy of liver metastases. J Clin Oncol 2009;27:1585–91 10.1200/JCO.2008.20.0600 [DOI] [PubMed] [Google Scholar]
- 35.Rusthoven KE, Kavanagh BD, Cardenes H, Stieber VW, Burri SH, Feigenberg SJ, et al. Multi-institutional phase I/II trial of stereotactic body radiation therapy for liver metastases. J Clin Oncol 2009;27:1572–8 10.1200/JCO.2008.19.6329 [DOI] [PubMed] [Google Scholar]