Abstract
Strong genetic contributions to individual differences in vulnerability to addictions are well supported by classical genetic studies. Linkage and association genome scans for addiction vulnerability have provided converging evidence for several chromosomal regions which are likely to harbor allelic variants that contribute to such vulnerability. We and others have delineated a candidate addiction-associated chromosome 4p12 “rSA3” region based on convergent data from association genome scanning studies in polysubstance abusers (Uhl and others 2001), linkage based studies in alcoholism (Long and others 1998; Reich and others 1998) and association-based studies for alcoholism and association-based studies for individual differences in electroencephalographic (EEG) spectral power phenotypes (Edenberg and others 2004; Porjesz and others 2002). The rSA3 region contains interesting candidate genes that encode the alpha 2, alpha 4, beta 1 and gamma 1 receptor subunits for the principal brain inhibitory neurotransmitter, GABA (Covault and others 2004; Edenberg and others 2004; Lappalainen and others 2005). We now report assessment of single nucleotide polymorphism (SNP) genotypes in this region in three samples of substance abusers and controls. These results delineate the haplotypes and patterns of linkage disequilibrium in this region, focus attention of the GABRA2 gene and identify modest associations between GABRA2 genotypes and addiction phenotypes. These results are consistent with modest roles for GABRA2 variants in addiction vulnerabilities.
Keywords: GABA receptors, addiction, linkage disequilibrium
Introduction
Substantial genetic contributions to human vulnerability to substance abuse are well documented (Johnson and others 1996; Kaprio and others 1982; Kendler and others 1999; Kendler and others 2000; Kendler and Prescott 1998; Maes and others 1999; Tsuang and others 1999). While identification of the specific genomic variants that contribute to this overall genetic vulnerability is ongoing, current results from association- and linkage-based genome scans have delineated chromosomal regions that are likely to harbor some of these functional variants, (Long and others 1998; Reich and others 1998; Uhl and others 2001; Liu and others, 2005). Taken together these results support polygenic genetic architectures for addiction. There are thus no consistently-large effects from variants at any locus in either European- or African-American samples.
Fine mapping studies of the regions that have been identified via convergent results from several genome scanning efforts are thus 1) necessary to identify the specific gene variants that contribute to addiction vulnerability and 2) likely to be difficult since contributions of each locus may well prove to be modest.
Chromosome 4p12 contains an interesting region for addiction vulnerability. We have delineated the reproducible substance abuse vulnerability region 3 “rSA3” on 4p12 based on convergent data from association genome scanning results in two samples of polysubstance abuse (Uhl and others 2001; Uhl and others 2002), linkage (and association) based transmission disequilibrium studies of alcoholics (Long and others 1998; Reich and others 1998), linkage (and association) based transmission disequilibrium studies for individual differences in alcoholism-related electroencephalographic (EEG) spectral power phenotypes (Edenberg and others 2004; Porjesz and others 2002) and association-based studies of alcoholism. The rSA3 region contains interesting candidate genes that encode alpha 2, alpha 4, beta 1 and gamma 1 receptor subunit for the ligand gated channels that recognize the principal brain inhibitory neurotransmitter, γ-aminobutyric acid (GABA). Edenberg and colleagues (Edenberg and others 2004) have recently described family-based association of alcoholism and EEG beta power phenotypes with SNPs and haplotypes spanning the alpha2 subunit (GABRA2) that lies within this GABA receptor gene cluster.
Genes that encode subunits of the inotropic GABAA receptors are good candidates for addiction vulnerability for several reasons (Edwards and Kuffler 1959; Kuffler and Edwards 1958). GABA binding to GABAA receptors increases chloride conductance, hyperpolarizes neurons and reduces their firing rates. GABA receptors are 1) directly influenced by abused substances that include ethanol, benzodiazepines and barbiturates and also 2) indirectly influenced by actions of virtually all abused substances on important GABAergic brain circuits (Davies 2003; Olsen and others 1977; Ticku and Olsen 1977). Inhibitory projections onto reward-associated meso-corticolimbic dopaminergic neurons in the ventral tegmental area use GABA (Mathon and others 2003), for example, so that changes in this GABAergic system can alter functions of reward-related dopaminergic systems. Pharmacologic modulation of GABA functions can alter the effects of abused substances. Ethanol intake can be increased by GABAA agonists and decreased by GABAA antagonists, for example (Boyle and others 1993; Nowak and others 1998; Tomkins and Fletcher 1996).
For each of these reasons, improved understanding of possible roles for variants in the chromosome 4 GABAA receptor gene cluster in human addiction vulnerability is of substantial interest. We now report fine mapping studies that focus on the GABRA2 and adjacent genes, attempt to improve definition of the haplotypes and patterns of linkage disequilibrium at this locus, and address association with addiction in three distinct samples of substance abusers and controls. We compare these results with those reported by other groups as this work was in preparation.
Materials and Methods
Subjects
Subjects included three groups: 1) 239 control individuals and 415 polysubstance abusers of self-reported European-American ancestry who were research volunteers at the NIDA IRP in Baltimore Maryland and who were characterized using Diagnostic Interview Schedule dependence criteria from DSMIII-R and DMSIV and quantity-frequency criteria as described (Uhl and others 2001; Liu et al 2005), 2) 99 control individuals and 252 polysubstance abusers of self-reported African-American ancestry who were NIDA research volunteers characterized as described above and 3) 112 unrelated control and 65 unrelated alcohol dependent individuals of reported European-American ancestry selected from COGA, Wave I pedigrees from whom DNA was generously made available through COGA investigators and NIAAA (Schuckit and others 1996)
Genotyping
Genotyping was performed by primer extension and MALDI-TOF based allele detection (Sequenom, San Diego, CA) using PCR and extension primer sequences listed in Table VI. Primary PCRs were performed in 5μl reaction volumes with 2.5 ng genomic DNA, 200 μM dNTP mix, 1.5 mM 10X PCR buffer and 2.5mM MgCl2, 1μM each of the oligonucleotide primers, and 0.1 U/reaction Taq polymerase (Applied Biosystems, Foster City, CA). Samples were held at 95°C for 15 minutes, treated with 45 cycles that consisted of 95°C for 20 seconds, 56°C for 30 seconds and 72°C for 1 minute, and then held at 72°C for 3 minutes (Sequenom, San Diego, CA). After PCR amplification, unincorporated dNTPs were removed by 20 min 37°C incubation with shrimp alkaline phosphatase, enzymes were inactivated by 5 min incubation at 85°C, and primer extension was performed according to manufacturers’ instructions (Sequenom, San Diego, CA). Extension primers (Table VI) were added together with 2.25 mM of the appropriate combination of deoxy dNTP and di-deoxy ddNTP and thermosequenase (32 U/μl). Reactions were incubated in thermal cyclers with initial denaturation for1 min at 96°C followed by 50 cycles of 96°C for 10 s, 43°C for 15 s and 60°C for 1 min. Reactions were then heated to 96°C for 30s, cooled on ice, purified and spotted in matrix on Sequenom DNA chips. Samples were subjected to MALDI-TOF mass spectrographic analyses with automatic allele detection and manual allele confirmation. The performance of each SNP assay was validated using DNA from 24 CEPH individuals prior to use with “unknown” samples.
Table VI. rs1391168-rs279871 haplotype association with polysubstance abuse.
p-value of GA haplotype in African American marked with an asterisk was Bonferroni corrected for multiple comparisons.
| HAPLOTYPER
| |||||||
|---|---|---|---|---|---|---|---|
| haplotype | AA control (n=182) | AA abuser (n=434) | p=0.052 | haplotype | Caucasian control (n=350) | Caucasian abuser (n=650) | p=0.59 |
| AA | 0.58 | 0.51 | p=0.08 | AA | 0.18 | 0.19 | |
| AG | 0.21 | 0.20 | AG | 0.35 | 0.30 | ||
| GA | 0.20 | 0.29 | p=0.051* | GA | 0.42 | 0.44 | |
| GG | 0.06 | 0.07 | |||||
| EH
| |||||||
|---|---|---|---|---|---|---|---|
| haplotype | AA control (n=182) | AA abuser (n=434) | p=0.11 | haplotype | Caucasian control (n=350) | Caucasian abuser (n=650) | p=0.12 |
| AA | 0.59 | 0.51 | p=0.07 | AA | 0.19 | 0.24 | |
| AG | 0.20 | 0.19 | AG | 0.33 | 0.26 | ||
| GA | 0.19 | 0.28 | p=0.048* | GA | 0.40 | 0.40 | |
| GG | 0.02 | 0.01 | GG | 0.07 | 0.10 | ||
Linkage disequilibrium mapping, haplotype generation and statistical analysis
Linkage disequilibrium between SNPs was calculated by the program Ldmax (EH) embedded in the Genetic Analysis of Linkage Disequilibrium program (GOLD) (Abecasis and Cookson 2000). Haplotypes were reconstructed from individual genotypes. Most likely haplotype assignment was performed by a partition-ligation algorithm (HAPLOTYPER) (Niu and others 2002) and haplotype frequencies estimated using the EH algorithm (Trewilliger and Ott 1994; Xie and Ott 1993) obtained from http://linkage.rockefeller.edu/ott/eh.htm. SNP and haplotype associations were analyzed using χ2 statistics. Power analysis was performed using the program PS v2.1.3.1 (Dupont and Plummer 1997).
Results
The 140 kb on chromosome 4p12 that contains the GABRA2 gene contains over 300 annotated SNPs. Coding SNPs (cSNPs) include only the relatively low-frequency non-synonymous rs519972 and the more frequent synonymous rs270858 (http://www.ncbi.nlm.nih.gov/SNP/). This paucity of cSNPs is consistent with the idea that common allelic variants at this locus are more likely to provide functional effects by altering gene expression and/or regulation through influences on transcription, mRNA stability, mRNA processing and/or translation.
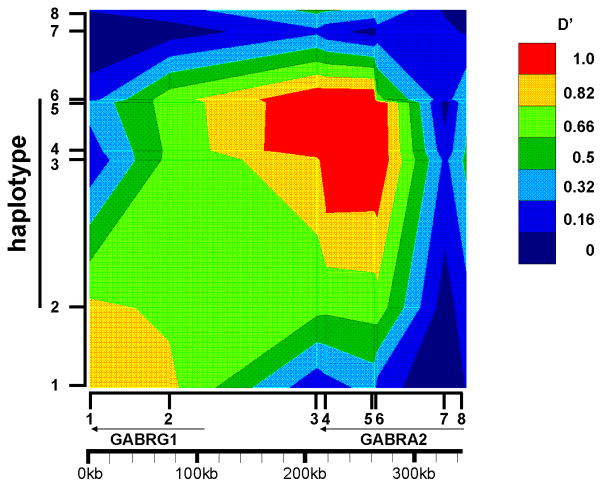
We constructed a linkage disequilibrium map (fig. 2) of the region that includes GABRA2 and GABRG1. A block of restricted haplotype diversity and high linkage disequilibrium spans the 3′end of GABRA2 and the 5′ end of GABRG1, with haplotypes defined by alleles of SNPs rs1391168, rs573400, rs505474, rs279871 and rs279867 (Fig. 2). Five haplotypes with frequencies above 5% are found; thirty-two would be expected in the absence of linkage disequilibrium [Table III]. Linkage disequilibrium patterns do appear to differ between European- and African-Americans. Linkage disequilibrium between the rs1391168 and rs279871 SNPs was higher in NIDA African American [D′=0.76 (p=5×10−5)] than in NIDA European-American [D′=0.52 (p=10−5)] or COGA samples [D′=0.666; (p=10−5)] (Table V).
Figure 2. GOLD plot of linkage disequilibrium in the region of the GABA receptor cluster constructed from COGA population.
A region of high linkage disequilibrium spans the 3′ end of GABRA2. The color-code for strength of linkage disequilibrium (D′) is displayed in the figure. Numbers on the X and Y
Table IIIa.
Association of haplotypes based on rs1391168, rs573400, rs505474, rs279871 and rs279867 with alcoholism in COGA samples (HAPLOTYPER)
| haplotypes | Alcoholic n=122 | control n=201 | p=0.27 |
|---|---|---|---|
| GTGAT | 0.33 | 0.36 | |
| GCAGG | 0.12 | 0.11 | |
| ATGAT | 0.25 | 0.18 | |
| ACAAG | 0.03 | 0.08 | |
| ACAGG | 0.26 | 0.27 |
Table V.
Linkage disequilibrium between rs279871 and rs139116 in Caucasian and African American population.
| African american | D′=0.76 p=0.00005 |
Caucasian | D′=0.527 p<0.00001 |
||
|---|---|---|---|---|---|
|
| |||||
| Haplotype | n | Frequency | Haplotype | N | frequency |
| AA | 326 | 0.53 | AA | 189 | 0.19 |
| AG | 125 | 0.20 | AG | 319 | 0.32 |
| GA | 162 | 0.26 | GA | 429 | 0.43 |
| GG | 3 | 0.00 | GG | 63 | 0.06 |
(n is the number of alleles in analysis)
In unrelated European-American alcohol-dependent and control individuals sampled from COGA Wave I pedigrees, these haplotypes failed to display significant association with alcohol dependence (p=0.27 [haplotyper] and p=0.76 [EH] for the overall association). Frequencies of alleles and genotypes of twelve SNPs, including those that define the haplotypes, were also assessed (Fig. 1, Table I). No SNP displayed allele frequency differences between alcohol dependent and control individuals that reached significance. rs1391168, located in the adjacent GABRG1 gene, displayed nominally-significant genotypic association (p=0.04) (Table II). Allelic association for this SNP did not reach significance (p=0.75), but this SNP’s alleles do display strong departure from Hardy-Weinberg equilibrium in COGA control individuals (data not shown).
Figure 1. Genomic view of the GABA receptor cluster on chromosome 4.
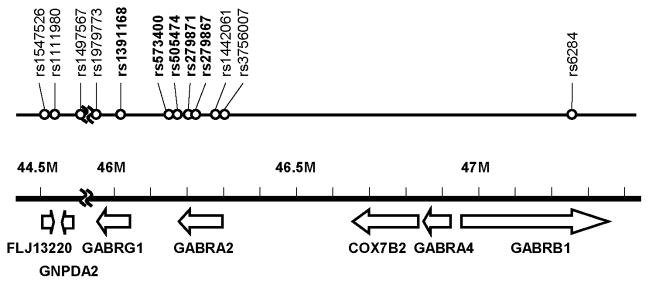
The SNPs used to construct haplotypes in this study are in boldface.
Table I.
SNPs assessed
| SNP | Genomic location | gene | variant | comment |
|---|---|---|---|---|
| rs1547526 | 44615272 | FLJ13220 | C/G | intron |
| rs1111980 | 44877021 | A/C | ||
| rs1497567 | 45956280 | A/G | ||
| rs1979773 | 45965363 | GABRG1 | A/C | intron |
| rs1391168 | 46030701 | GABRG1 | A/G | intron |
| rs573400 | 46167608 | GABRA2 | A/G | untranslated |
| rs505474 | 46176129 | GABRA2 | G/A | intron |
| rs279871 | 46221275 | GABRA2 | A/G | intron |
| rs279867 | 46223845 | GABRA2 | T/G | intron |
| rs1442061 | 46286762 | GABRA2 | C/G | intron |
| rs3756007 | 46306606 | GABRA2 | T/C | untranslated |
| rs6284 | 47237761 | GABRB1 | C/A | synonymous |
Table II. Genotypes: COGA individuals.
Nominal p-values from χ2 statistics are listed. “n” indicates number of individuals genotyped.
| SNP | genotype | alcoholic | control | p-value | allele | alcoholic | control | p-value | chromosomal position | Gene |
|---|---|---|---|---|---|---|---|---|---|---|
| rs1547526 n=175 |
C | 0.34 | 0.36 | 0.83 | C | 0.60 | 0.63 | 0.61 | 44615995 | FLJ13220 |
| CG | 0.52 | 0.53 | G | 0.40 | 0.37 | |||||
| G | 0.14 | 0.11 | ||||||||
|
| ||||||||||
| rs1111980 n=168 |
G | 0.59 | 0.68 | 0.39 | G | 0.77 | 0.81 | 0.43 | 44877021 | – |
| GT | 0.36 | 0.26 | T | 0.23 | 0.19 | |||||
| T | 0.05 | 0.06 | ||||||||
|
| ||||||||||
| rs1497567 n=170 |
C | 0.29 | 0.36 | 0.49 | C | 0.54 | 0.57 | 0.52 | 45956280 | – |
| CT | 0.51 | 0.42 | T | 0.46 | 0.43 | |||||
| T | 0.21 | 0.21 | ||||||||
|
| ||||||||||
| rs1979773 n=51 |
A | 0.09 | 0.38 | 0.14 | A | 0.50 | 0.68 | 0.13 | 45965363 | GABRG1 |
| CA | 0.82 | 0.60 | C | 0.50 | 0.33 | |||||
| C | 0.09 | 0.03 | ||||||||
|
| ||||||||||
| rs1391168 n=177 |
A | 0.26 | 0.34 | 0.04 | A | 0.53 | 0.51 | 0.75 | 46030701 | GABRG1 |
| AG | 0.54 | 0.35 | G | 0.47 | 0.49 | |||||
| G | 0.20 | 0.31 | ||||||||
|
| ||||||||||
| rs573400 n=169 |
C | 0.14 | 0.21 | 0.48 | C | 0.39 | 0.45 | 0.98 | 46167608 | GABRA2 |
| CT | 0.51 | 0.47 | T | 0.61 | 0.55 | |||||
| T | 0.35 | 0.32 | ||||||||
|
| ||||||||||
| rs505474 n=174 |
A | 0.15 | 0.23 | 0.44 | A | 0.42 | 0.45 | 0.48 | 46176129 | GABRA2 |
| AG | 0.52 | 0.45 | G | 0.58 | 0.55 | |||||
| G | 0.32 | 0.32 | ||||||||
|
| ||||||||||
| rs279871 n=117 |
A | 0.37 | 0.39 | 0.51 | A | 0.64 | 0.68 | 0.57 | 46221275 | GABRA2 |
| AG | 0.53 | 0.57 | G | 0.36 | 0.32 | |||||
| G | 0.09 | 0.04 | ||||||||
|
| ||||||||||
| rs279867 n=176 |
G | 0.16 | 0.21 | 0.71 | G | 0.44 | 0.45 | 0.79 | 46223845 | GABRA2 |
| TG | 0.50 | 0.46 | T | 0.56 | 0.55 | |||||
| T | 0.34 | 0.34 | ||||||||
|
| ||||||||||
| rs1442061 n=174 |
C | 0.09 | 0.07 | 0.80 | C | 0.27 | 0.26 | 0.89 | 46286762 | GABRA2 |
| CG | 0.34 | 0.37 | G | 0.73 | 0.74 | |||||
| G | 0.56 | 0.55 | ||||||||
|
| ||||||||||
| rs3756007 n=162 |
C | 0.03 | 0.05 | 0.79 | C | 0.09 | 0.09 | 0.99 | 46306606 | GABRA2 |
| CT | 0.10 | 0.08 | T | 0.91 | 0.91 | |||||
| T | 0.86 | 0.88 | ||||||||
|
| ||||||||||
| rs6284 n=175 |
A | 0.05 | 0.04 | 0.50 | A | 0.20 | 0.16 | 0.32 | 47237761 | GABRB1 |
| CA | 0.30 | 0.22 | C | 0.80 | 0.84 | |||||
| C | 0.65 | 0.73 | ||||||||
We tested the haplotypes defined by SNPs rs1391168 and rs279871 in African-American polysubstance abusers and controls (Table IV). The three common haplotypes identified in African-American samples displayed trends toward association with polysubstance abuse with borderline statistical significance (p=0.052 [haplotyper] and 0.11 [EH]). The haplotype defined by rs1391168 = G and rs279871 =A displayed nominally-significant differences between abuser and control groups (nominal p=0.017 [haplotyper] and p=0.012 [EH]; after Bonferroni correction for multiple comparisons p = 0.051 [haplotyper] and p=0.048 [EH]). The AA haplotype displayed a trend that did not reach nominal significance (p=0.08 [haplotyper] and 0.07 [EH]) (Table VI). rs1391168 displayed trends toward genotypic and allelic associations in this sample (p =0.079 and p= 0.063, respectively). However, rs279871 revealed no trends toward either genotypic or allelic association (p=0.55 and p=0.73).
Table IV.
SNP association with polysubstance abuse in NIDA African- and European–American samples
| rs1391168 | |||||||
|---|---|---|---|---|---|---|---|
| genotype | AA control (n=97) | AA abuser (n=231) | p=0.079 | genotype | Caucasian control (n=194) | Caucasian abuser (n=365) | p=0.317 |
| A | 0.62 | 0.48 | A | 0.32 | 0.26 | ||
| AG | 0.32 | 0.45 | AG | 0.44 | 0.49 | ||
| G | 0.06 | 0.07 | G | 0.24 | 0.25 | ||
|
| |||||||
| Allele | AA control (n=194) | AA abuser (n=462) | p=0.063 | allele | Caucasian control (n=388) | Caucasian abuser (n=730) | p=0.271 |
|
| |||||||
| A | 0.78 | 0.71 | A | 0.54 | 0.50 | ||
| G | 0.22 | 0.29 | G | 0.46 | 0.50 | ||
| rs279871 | |||||||
|---|---|---|---|---|---|---|---|
| genotype | AA control (n=92) | AA abuser (n=223) | p=0.73 | genotype | Caucasian control (n=205) | Caucasian abuser (n=373) | p=0.5×10−13 |
| A | 0.57 | 0.61 | A | 0.37 | 0.29 | ||
| AG | 0.42 | 0.38 | AG | 0.48 | 0.71 | ||
| G | 0.01 | 0.01 | G | 0.16 | 0.01 | ||
|
| |||||||
| allele freq | AA control (n=184) | AA abuser (n=446) | p=0.55 | allele | Caucasian control (n=410) | Caucasian abuser (n=746) | p=0.24 |
|
| |||||||
| A | 0.78 | 0.80 | A | 0.60 | 0.64 | ||
| G | 0.22 | 0.20 | G | 0.40 | 0.36 | ||
When we tested these markers in European-American NIDA polysubstance abusers and controls, we found that these samples displayed the same four common haplotypes found in COGA European-American samples. There were no significant associations between any haplotype and substance abuse in these samples (Table VI). There were neither genotypic nor allelic associations for rs1391168 in European-American polysubstance abuser/control comparisons (p=0.31 and p=0.27) There was a robust association of rs279871 genotypes with addiction in the NIDA European-American samples (p < 10−13), although no particular allele displayed nominally significant association. There was also a strong departure from Hardy-Weinberg equilibrium in the European-American polysubstance abuser sample, although not in the control sample, possibly providing evidence for association (Luo and others 2005; Nielsen and Zaykin 1999).
Discussion
We discuss the current results in the context of the a priori chances that variations in inotrophic GABAA receptors might influence vulnerability to addictions, the patterns of linkage disequilibrium and Hardy-Weinberg disequilibrium that are present for markers in this chromosome 4 GABAA receptor gene cluster, the failure of current results to support any large and consistent role for variants at this locus in human addiction vulnerability, and the evidence from the present data that modest influences on addiction vulnerability appear likely to come from variants in this genomic region.
A priori, this region was an attractive candidate region to harbor addiction vulnerability alleles. Positional cloning data from a number of linkage and association studies repeatedly identify the chromosomal region that contains this GABAA receptor gene cluster. The GABA receptor subunits encoded by this region are expressed in brain regions that include many that are implicated in drug reward and/or mnemonic processes likely to lie at the core of the genetic influences on human addiction vulnerability (Uhl 2004; Uhl 2004). Studies in mice that express point mutations that alter drug affinities for different GABAA receptor subunits implicate GABRA2 receptors in modulating anxiety phenotypes (Low and others 2000), possibly providing one mechanism for effects on addiction vulnerability.
As this work was unfolding, additional data also increased interest in this locus. Markers at this locus were strongly associated with EEG power in the β1 segment of the spectrum. Association between alcohol dependence and with similar SNPs were described in work from others (Covault and others 2004; Edenberg and others 2004; Lappalainen and others 2005). As we elucidated the patterns of linkage disequilibrium across this locus, they were confirmed by HapMap data obtained in different populations. These results each demonstrate a relatively strong block of restricted haplotype diversity that encompasses both the 3′end of the GABRA2 gene and the 5′GABRG1 gene.
The current results provide evidence for effects of variants at this locus, though no strong association is reproducibly present in the multiple samples studied here. These data contrast with results for markers in the chromosome 4 alcohol dehydrogenase/acetaldehyde dehydrogenase gene cluster region, which are more consistently linked to and associated with differential vulnerability to addictions in numerous European- and African-American samples that include each of the samples studied here. Our findings in unrelated individuals sampled from COGA, wave I populations of alcohol dependent and control individuals provide modest support for the haplotype associations with alcohol dependence reported by Edenberg et al as the current work was being written (Covault and others 2004; Edenberg and others 2004; Lappalainen and others 2005) (Table III). There was a robust (p < 10−13) association of rs279871 genotypes with addiction vulnerability in a large European-American sample of polysubstance abusers and controls. The G/A haplotype defined using this rs279871 SNP and the nearby rs1391168 SNP is present at significantly-different frequencies in African-American polysubstance abusers and controls. The rs1391168 SNP did display significant differences in allelic frequencies between COGA Wave I alcohol dependent individuals and controls. Each of these observations needs to be tempered by the failure to identify a consistent pattern of significant association across these distinct samples. Conceivably, effects of selection acting on variants at this locus might have helped to produce some of the difficulties in identifying consistent allelic, genotypic and haplotypic association patterns across these three samples. Alternatively, these variable results might come from the modest effect sizes whose magnitudes provide none of the samples with sufficient power to display significant associations in allelic, genotypic and haplotype analyses.
Vulnerability to substance abuse in humans is likely to result from complex genetic influences, multifactorial environmental influences, and both gene x gene and gene x environment interactions. Most of the results from genome scanning studies available to date, taken together, support polygenic contributions from allelic variants at most of the gene loci that these studies nominate. Our current data, along with other work, appears to support modest contributions of variations at the GABRA2 locus on human addiction vulnerability in individuals sampled from two distinct ethnic/racial groups and from studies using two distinct methods for ascertainment of substance dependent individuals. As we identify locus-specific phenotypes relevant to GABRA2-locus variants and as we identify more and more of the other, potentially-interacting genetic and environmental features that provide noise for the current analyses, the specific roles played by specific GABRA2-locus variation in addiction vulnerability are likely to become clearer.
Table IIIb.
Association of haplotypes based on rs1391168 and rs279871 with alcoholism in COGA samples (EH)
| haplotypes | Alcoholic N=122 | Control N=201 | P=0.76 |
|---|---|---|---|
| AA | 0.16 | 0.13 | |
| AG | 0.37 | 0.38 | |
| GA | 0.26 | 0.31 | |
| GG | 0.20 | 0.17 |
Table VII.
List of genotyping oligonucleotides.
| SNP rs# | Forward PCR primer | Reverse PCR primer | Extension primer |
|---|---|---|---|
| rs573400 | GCAGACAGAAAGCACTCCAT | GGTTGTACAGGATCCCCATT | AGAATAGGAAATTAATCAGGTCA |
| rs279867 | TAACTGGGATGCTATGAATGTG | TAACGCTATACTTGCATCAAGT | GATATCTGTGTGCATTGATTCAT |
| rs3756007 | CTGCCAGGAACGTCCCCC | CCTTTCCAGCTGCTATGCC | CTGTTTTGCGCACACGTAATAA |
| rs1391168 | GATCACTATTTGCTCTTCTATCCAA | CTCAGTCTTGGGCAGTTCTTTATA | ATTCCACACCTCCTTGCCC |
| rs1442061 | CAGCACAGTACTTCCTGGTCAA | CCAATGACAGTAGCTCAATGAAATT | CATACACTGGATAGATCTCTG |
| rs505474 | GATTCTCTGCCAGAGTTCAGAGT | GTGAGAATCACGCAGTGAATACT | GTTCTTGTTTATCCCTAAAGAC |
| rs6284 | CTATCAGAATCACAACCACAGCT | CCTGTCCAAGTAACTCACAACTTT | CAGAACTGCACCCTGGAGAT |
| rs1547526 | CAGCGGCATCAGTACAACCT | GAAGACAGTAAAATTAGATCTG | CAACCATACCAAATTATTATC |
| rs1111980 | GCGGGAGTTGCTAGTCTTAT | GATACTTAAATCTTTTTGTTG | ATATAATGTCCTTCTCTGACT |
| rs1979773 | GCATGAGAAATTATTCATCTAATTGT | AGGGATGATTACATCCAGTTGTG | GTTTAATTTTGATCTTTATCTTGGA |
| rs279871 | ACTCATGCTATGCTAAGGAG | GAAGGGATCAGAGGTAGAACA | TCCTGACATGTATGTGATATATT |
| rs1497567 | AGCTGTATTTGATTTCTTGAGAA | ATATACCATCAAGCAAGCACTT | TAAATGTGTGATCAGAAAGAATGG |
Acknowledgments
We are grateful to COGA investigators and NIAAA for providing access to COGA DNAs, and to NIDA for financial support.
References
- Abecasis GR, Cookson WO. GOLD--graphical overview of linkage disequilibrium. Bioinformatics. 2000;16(2):182–3. doi: 10.1093/bioinformatics/16.2.182. [DOI] [PubMed] [Google Scholar]
- Boyle AE, Segal R, Smith BR, Amit Z. Bidirectional effects of GABAergic agonists and antagonists on maintenance of voluntary ethanol intake in rats. Pharmacol Biochem Behav. 1993;46(1):179–82. doi: 10.1016/0091-3057(93)90338-t. [DOI] [PubMed] [Google Scholar]
- Covault J, Gelernter J, Hesselbrock V, Nellissery M, Kranzler HR. Allelic and haplotypic association of GABRA2 with alcohol dependence. Am J Med Genet B Neuropsychiatr Genet. 2004;129(1):104–9. doi: 10.1002/ajmg.b.30091. [DOI] [PubMed] [Google Scholar]
- Davies M. The role of GABAA receptors in mediating the effects of alcohol in the central nervous system. J Psychiatry Neurosci. 2003;28(4):263–74. [PMC free article] [PubMed] [Google Scholar]
- Dupont W, Plummer W. PS power and sample size program available for free on the Internet. Controlled Clin Trials. 1997;18:274. [Google Scholar]
- Edenberg HJ, Dick DM, Xuei X, Tian H, Almasy L, Bauer LO, Crowe RR, Goate A, Hesselbrock V, Jones K, et al. Variations in GABRA2, Encoding the alpha 2 Subunit of the GABAA Receptor, Are Associated with Alcohol Dependence and with Brain Oscillations. Am J Hum Genet. 2004;74(4):705–14. doi: 10.1086/383283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards C, Kuffler SW. The blocking effect of gamma-aminobutyric acid (GABA) and the action of related compounds on single nerve cells. J Neurochem. 1959;4(1):19–30. doi: 10.1111/j.1471-4159.1959.tb13170.x. [DOI] [PubMed] [Google Scholar]
- Johnson EO, van den Bree MB, Uhl GR, Pickens RW. Indicators of genetic and environmental influences in drug abusing individuals. Drug Alcohol Depend. 1996;41(1):17–23. doi: 10.1016/0376-8716(96)01223-9. [DOI] [PubMed] [Google Scholar]
- Kaprio J, Hammar N, Koskenvuo M, Floderus-Myrhed B, Langinvainio H, Sarna S. Cigarette smoking and alcohol use in Finland and Sweden: a cross-national twin study. Int J Epidemiol. 1982;11(4):378–86. doi: 10.1093/ije/11.4.378. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Karkowski LM, Corey LA, Prescott CA, Neale MC. Genetic and environmental risk factors in the aetiology of illicit drug initiation and subsequent misuse in women. Br J Psychiatry. 1999;175:351–6. doi: 10.1192/bjp.175.4.351. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Karkowski LM, Neale MC, Prescott CA. Illicit psychoactive substance use, heavy use, abuse, and dependence in a US population-based sample of male twins. Arch Gen Psychiatry. 2000;57(3):261–9. doi: 10.1001/archpsyc.57.3.261. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA. Cannabis use, abuse, and dependence in a population-based sample of female twins. Am J Psychiatry. 1998;155(8):1016–22. doi: 10.1176/ajp.155.8.1016. [DOI] [PubMed] [Google Scholar]
- Kuffler SW, Edwards C. Mechanism of gamma aminobutyric acid (GABA) action and its relation to synaptic inhibition. J Neurophysiol. 1958;21(6):589–610. doi: 10.1152/jn.1958.21.6.589. [DOI] [PubMed] [Google Scholar]
- Lappalainen J, Krupitsky E, Remizov M, Pchelina S, Taraskina A, Zvartau E, Somberg LK, Covault J, Kranzler HR, Krystal JH, et al. Association Between Alcoholism and gamma-Amino Butyric Acid alpha2 Receptor Subtype in a Russian Population. Alcohol Clin Exp Res. 2005;29(4):493–498. doi: 10.1097/01.alc.0000158938.97464.90. [DOI] [PubMed] [Google Scholar]
- Long JC, Knowler WC, Hanson RL, Robin RW, Urbanek M, Moore E, Bennett PH, Goldman D. Evidence for genetic linkage to alcohol dependence on chromosomes 4 and 11 from an autosome-wide scan in an American Indian population. Am J Med Genet. 1998;81(3):216–21. doi: 10.1002/(sici)1096-8628(19980508)81:3<216::aid-ajmg2>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Low K, Crestani F, Keist R, Benke D, Brunig I, Benson JA, Fritschy JM, Rulicke T, Bluethmann H, Mohler H, et al. Molecular and neuronal substrate for the selective attenuation of anxiety. Science. 2000;290(5489):131–4. doi: 10.1126/science.290.5489.131. [DOI] [PubMed] [Google Scholar]
- Luo X, Kranzler HR, Zuo L, Lappalainen J, Yang BZ, Gelernter J. ADH4 Gene Variation is Associated with Alcohol Dependence and Drug Dependence in European Americans: Results from HWD Tests and Case-Control Association Studies. Neuropsychopharmacology. 2005 doi: 10.1038/sj.npp.1300925. [DOI] [PubMed] [Google Scholar]
- Maes HH, Woodard CE, Murrelle L, Meyer JM, Silberg JL, Hewitt JK, Rutter M, Simonoff E, Pickles A, Carbonneau R, et al. Tobacco, alcohol and drug use in eight- to sixteen-year-old twins: the Virginia Twin Study of Adolescent Behavioral Development. J Stud Alcohol. 1999;60(3):293–305. doi: 10.15288/jsa.1999.60.293. [DOI] [PubMed] [Google Scholar]
- Mathon DS, Kamal A, Smidt MP, Ramakers GM. Modulation of cellular activity and synaptic transmission in the ventral tegmental area. Eur J Pharmacol. 2003;480(1–3):97–115. doi: 10.1016/j.ejphar.2003.08.097. [DOI] [PubMed] [Google Scholar]
- Nielsen D, Zaykin D. Novel tests for marker-disease association using the Collaborative Study on the Genetics of Alcoholism data. Genet Epidemiol. 1999;17(Suppl 1):S265–70. doi: 10.1002/gepi.1370170745. [DOI] [PubMed] [Google Scholar]
- Niu T, Qin ZS, Xu X, Liu JS. Bayesian haplotype inference for multiple linked single-nucleotide polymorphisms. Am J Hum Genet. 2002;70(1):157–69. doi: 10.1086/338446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak KL, McBride WJ, Lumeng L, Li TK, Murphy JM. Blocking GABA(A) receptors in the anterior ventral tegmental area attenuates ethanol intake of the alcohol-preferring P rat. Psychopharmacology (Berl) 1998;139(1–2):108–16. doi: 10.1007/s002130050695. [DOI] [PubMed] [Google Scholar]
- Olsen RW, Meiners B, Kehoe P, Ticku MK. Properties of gamma-aminobutyric acid receptor/ionophore proteins from crayfish muscle [proceedings] Biochem Soc Trans. 1977;5(4):863–6. doi: 10.1042/bst0050863. [DOI] [PubMed] [Google Scholar]
- Porjesz B, Almasy L, Edenberg HJ, Wang K, Chorlian DB, Foroud T, Goate A, Rice JP, O’Connor SJ, Rohrbaugh J, et al. Linkage disequilibrium between the beta frequency of the human EEG and a GABAA receptor gene locus. Proc Natl Acad Sci U S A. 2002;99(6):3729–33. doi: 10.1073/pnas.052716399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich T, Edenberg HJ, Goate A, Williams JT, Rice JP, Van Eerdewegh P, Foroud T, Hesselbrock V, Schuckit MA, Bucholz K, et al. Genome-wide search for genes affecting the risk for alcohol dependence. Am J Med Genet. 1998;81(3):207–15. [PubMed] [Google Scholar]
- Schuckit MA, Tsuang JW, Anthenelli RM, Tipp JE, Nurnberger JI., Jr Alcohol challenges in young men from alcoholic pedigrees and control families: a report from the COGA project. J Stud Alcohol. 1996;57(4):368–77. doi: 10.15288/jsa.1996.57.368. [DOI] [PubMed] [Google Scholar]
- Ticku MK, Olsen RW. gamma-Aminobutyric acid-stimulated chloride permeability in crayfish muscle. Biochim Biophys Acta. 1977;464(3):519–29. doi: 10.1016/0005-2736(77)90027-x. [DOI] [PubMed] [Google Scholar]
- Tomkins DM, Fletcher PJ. Evidence that GABA(A) but not GABA(B) receptor activation in the dorsal raphe nucleus modulates ethanol intake in Wistar rats. Behav Pharmacol. 1996;7(1):85–93. [PubMed] [Google Scholar]
- Trewilliger J, Ott J. Handbook of Human Genetic Linkage. Baltimore: Johns Hopkins University Press; 1994. [Google Scholar]
- Tsuang MT, Lyons MJ, Harley RM, Xian H, Eisen S, Goldberg J, True WR, Faraone SV. Genetic and environmental influences on transitions in drug use. Behav Genet. 1999;29(6):473–9. doi: 10.1023/a:1021635223370. [DOI] [PubMed] [Google Scholar]
- Uhl GR. Molecular genetic underpinnings of human substance abuse vulnerability: likely contributions to understanding addiction as a mnemonic process. Neuropharmacology. 2004;47(Suppl 1):140–7. doi: 10.1016/j.neuropharm.2004.07.029. [DOI] [PubMed] [Google Scholar]
- Uhl GR. Molecular genetics of substance abuse vulnerability: remarkable recent convergence of genome scan results. Ann N Y Acad Sci. 2004;1025:1–13. doi: 10.1196/annals.1316.001. [DOI] [PubMed] [Google Scholar]
- Uhl GR, Liu QR, Walther D, Hess J, Naiman D. Polysubstance abuse-vulnerability genes: genome scans for association, using 1,004 subjects and 1,494 single-nucleotide polymorphisms. Am J Hum Genet. 2001;69(6):1290–300. doi: 10.1086/324467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Ott J. Testing linkage disequilibrium between a disease gene and marker loci. Am J Hum Genet. 1993;53:1107. [Google Scholar]