Abstract
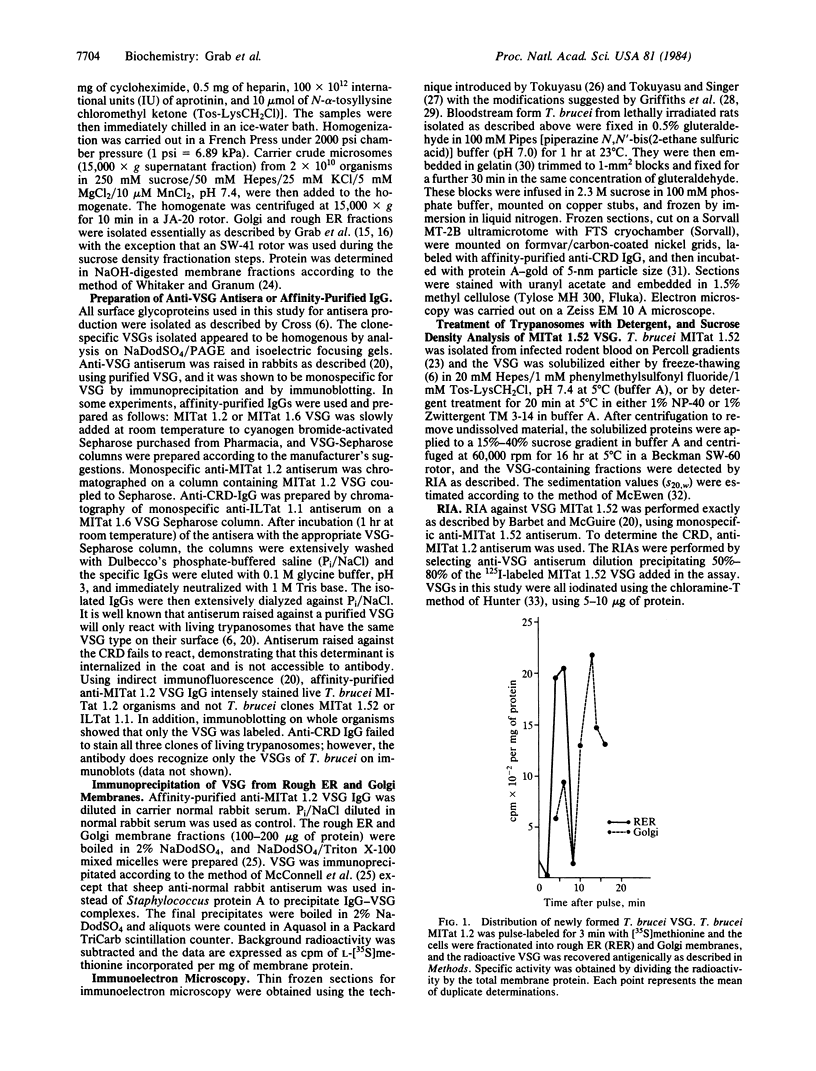
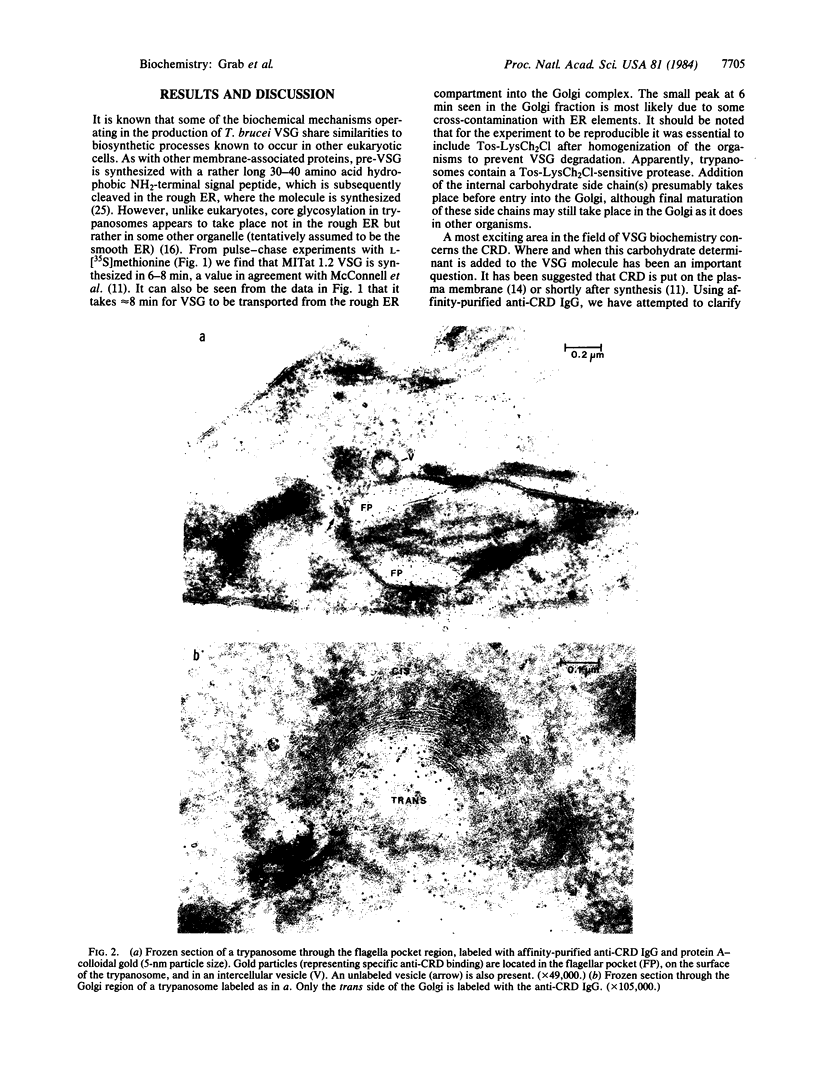
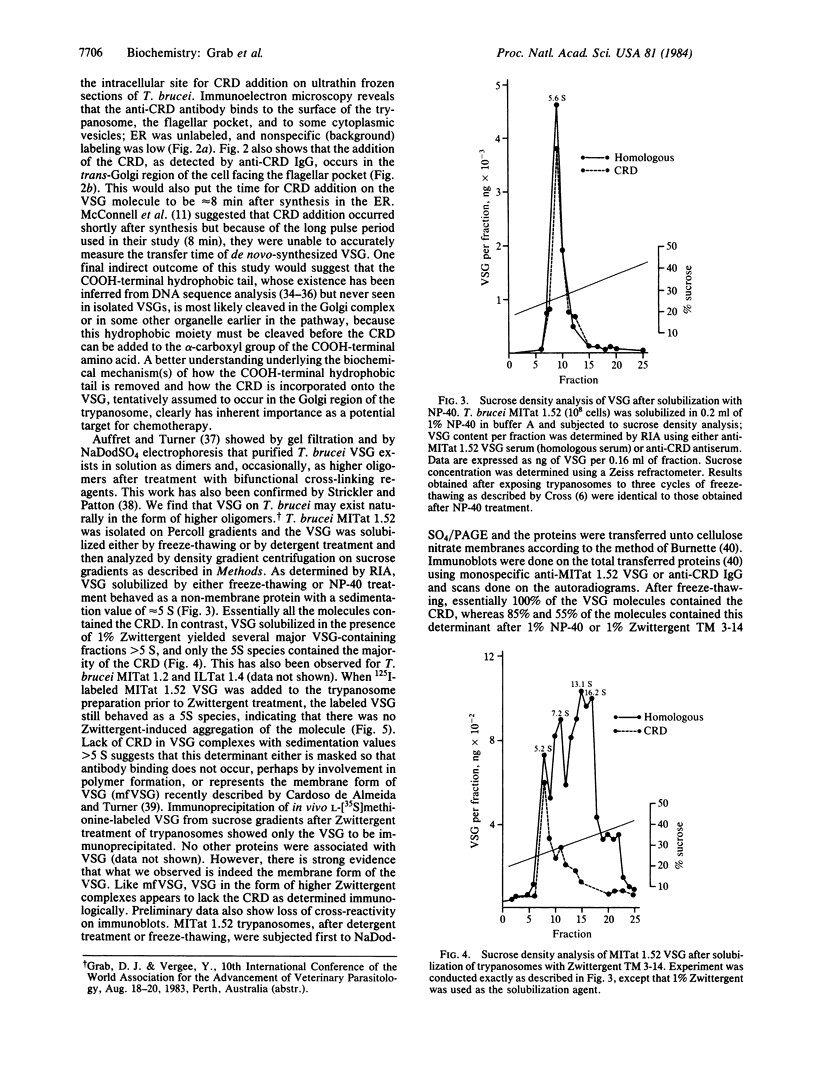
Pulse-chase experiments using L-[35S]methionine suggest that Trypanosoma brucei MITat 1.2 variable surface glycoprotein (VSG) synthesized in the rough endoplasmic reticulum, a process that takes 6-8 min, is shuttled to the Golgi complex 8 min later. Labeling of ultrathin frozen sections with affinity-purified anti-cross-reacting determinant (CRD) IgG followed by protein A-colloidal gold shows that the CRD is localized in the trans-Golgi region. cis-Golgi is not labeled. VSG, when solubilized by treatment with the detergent Nonidet P-40, behaves on sucrose density gradients as a non-membrane protein with a sedimentation value of 5 S. In contrast, VSG solubilized in the presence of Zwittergent TM 3-14 yielded several VSG-containing fractions greater than 5 S, and only the 5S fraction contained the CRD. Lack of the CRD in VSG complexes with sedimentation values greater than 5 S suggests that this determinant is either masked from antibody, perhaps by involvement in polymer formation, or represents the membrane form of VSG recently described by Cardoso de Almeida and Turner [Cardoso de Almeida, M. L. & Turner, M. J. (1983) Nature (London) 302, 349-352].
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auffret C. A., Turner M. J. Variant specific antigens of Trypanosoma brucei exist in solution as glycoprotein dimers. Biochem J. 1981 Feb 1;193(2):647–650. doi: 10.1042/bj1930647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbet A. F., McGuire T. C. Crossreacting determinants in variant-specific surface antigens of African trypanosomes. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1989–1993. doi: 10.1073/pnas.75.4.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boothroyd J. C., Cross G. A., Hoeijmakers J. H., Borst P. A variant surface glycoprotein of Trypanosoma brucei synthesized with a C-terminal hydrophobic 'tail' absent from purified glycoprotein. Nature. 1980 Dec 11;288(5791):624–626. doi: 10.1038/288624a0. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Cardoso de Almeida M. L., Turner M. J. The membrane form of variant surface glycoproteins of Trypanosoma brucei. Nature. 1983 Mar 24;302(5906):349–352. doi: 10.1038/302349a0. [DOI] [PubMed] [Google Scholar]
- Cross G. A. Crossreacting determinants in the C-terminal region of trypanosome variant surface antigens. Nature. 1979 Jan 25;277(5694):310–312. doi: 10.1038/277310a0. [DOI] [PubMed] [Google Scholar]
- Cross G. A. Identification, purification and properties of clone-specific glycoprotein antigens constituting the surface coat of Trypanosoma brucei. Parasitology. 1975 Dec;71(3):393–417. doi: 10.1017/s003118200004717x. [DOI] [PubMed] [Google Scholar]
- Cross G. A. Immunochemical aspects of antigenic variation on trypanosomes. The third Fleming Lecture. J Gen Microbiol. 1979 Jul;113(1):1–11. doi: 10.1099/00221287-113-1-1. [DOI] [PubMed] [Google Scholar]
- Duvillier G., Nouvelot A., Richet C., Baltz T., Degand P. Presence of glycerol and fatty acids in the C-terminal end of a variant surface glycoprotein from Trypanosoma equiperdum. Biochem Biophys Res Commun. 1983 Jul 18;114(1):119–125. doi: 10.1016/0006-291x(83)91602-9. [DOI] [PubMed] [Google Scholar]
- Geuze H. J., Slot J. W. Disproportional immunostaining patterns of two secretory proteins in guinea pig and rat exocrine pancreatic cells. An immunoferritin and fluorescence study. Eur J Cell Biol. 1980 Apr;21(1):93–100. [PubMed] [Google Scholar]
- Grab D. J., Bwayo J. J. Isopycnic isolation of African trypanosomes on Percoll gradients formed in situ. Acta Trop. 1982 Dec;39(4):363–366. [PubMed] [Google Scholar]
- Grab D. J., Ito S., Kara U. A., Rovis L. Glycosyltransferase activities in Golgi complex and endoplasmic reticulum fractions isolated from African trypanosomes. J Cell Biol. 1984 Aug;99(2):569–577. doi: 10.1083/jcb.99.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray A. R. Some principles of the immunology of trypanosomiasis. Bull World Health Organ. 1967;37(2):177–193. [PMC free article] [PubMed] [Google Scholar]
- Griffiths G., Brands R., Burke B., Louvard D., Warren G. Viral membrane proteins acquire galactose in trans Golgi cisternae during intracellular transport. J Cell Biol. 1982 Dec;95(3):781–792. doi: 10.1083/jcb.95.3.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths G., Simons K., Warren G., Tokuyasu K. T. Immunoelectron microscopy using thin, frozen sections: application to studies of the intracellular transport of Semliki Forest virus spike glycoproteins. Methods Enzymol. 1983;96:466–485. doi: 10.1016/s0076-6879(83)96041-x. [DOI] [PubMed] [Google Scholar]
- Holder A. A. Carbohydrate is linked through ethanolamine to the C-terminal amino acid of Trypanosoma brucei variant surface glycoprotein. Biochem J. 1983 Jan 1;209(1):261–262. doi: 10.1042/bj2090261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holder A. A., Cross G. A. Glycopeptides from variant surface glycoproteins of Trypanosoma Brucei. C-terminal location of antigenically cross-reacting carbohydrate moieties. Mol Biochem Parasitol. 1981 Feb;2(3-4):135–150. doi: 10.1016/0166-6851(81)90095-5. [DOI] [PubMed] [Google Scholar]
- Johnson J. G., Cross G. A. Carbohydrate composition of variant-specific surface antigen glycoproteins from Trypanosoma brucei. J Protozool. 1977 Nov;24(4):587–591. doi: 10.1111/j.1550-7408.1977.tb01020.x. [DOI] [PubMed] [Google Scholar]
- Matthyssens G., Michiels F., Hamers R., Pays E., Steinert M. Two variant surface glycoproteins of Trypanosoma brucei have a conserved C-terminus. Nature. 1981 Sep 17;293(5829):230–233. doi: 10.1038/293230a0. [DOI] [PubMed] [Google Scholar]
- McConnell J., Cordingley J. S., Turner M. J. The biosynthesis of Trypanosoma brucei variant surface glycoproteins--in vitro processing of signal peptide and glycosylation using heterologous rough endoplasmic reticulum vesicles. Mol Biochem Parasitol. 1982 Sep;6(3):161–174. doi: 10.1016/0166-6851(82)90075-5. [DOI] [PubMed] [Google Scholar]
- McConnell J., Gurnett A. M., Cordingley J. S., Walker J. E., Turner M. J. Biosynthesis of Trypanosoma brucei variant surface glycoprotein. I. Synthesis, size, and processing of an N-terminal signal peptide. Mol Biochem Parasitol. 1981 Dec;4(3-4):225–242. doi: 10.1016/0166-6851(81)90021-9. [DOI] [PubMed] [Google Scholar]
- McConnell J., Turner M., Rovis L. Biosynthesis of Trypanosoma brucei variant surface glycoproteins - analysis of carbohydrate heterogeneity and timing of post-translational modifications. Mol Biochem Parasitol. 1983 Jun;8(2):119–135. doi: 10.1016/0166-6851(83)90004-x. [DOI] [PubMed] [Google Scholar]
- McEwen C. R. Tables for estimating sedimentation through linear concentration gradients of sucrose solution. Anal Biochem. 1967 Jul;20(1):114–149. doi: 10.1016/0003-2697(67)90271-0. [DOI] [PubMed] [Google Scholar]
- Rip J. W., Chaudhary N., Carroll K. K. The submicrosomal distribution of dolichyl phosphate and dolichyl phosphate phosphatase in rat liver. J Biol Chem. 1983 Dec 25;258(24):14926–14930. [PubMed] [Google Scholar]
- Rovis L., Dube D. K. Studies on the biosynthesis of the variant surface glycoproteins of Trypanosoma brucei: sequence of glycosylation. Mol Biochem Parasitol. 1981 Nov;4(1-2):77–93. doi: 10.1016/0166-6851(81)90031-1. [DOI] [PubMed] [Google Scholar]
- Rovis L., Dube S. Identification and characterisation of two N-acetylglucosaminyltransferases associated with Trypanosoma Brucei microsomes. Mol Biochem Parasitol. 1982 Mar;5(3):173–187. doi: 10.1016/0166-6851(82)90019-6. [DOI] [PubMed] [Google Scholar]
- Slot J. W., Geuze H. J. Sizing of protein A-colloidal gold probes for immunoelectron microscopy. J Cell Biol. 1981 Aug;90(2):533–536. doi: 10.1083/jcb.90.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickler J. E., Patton C. L. Trypanosoma brucei brucei: inhibition of glycosylation of the major variable surface coat glycoprotein by tunicamycin. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1529–1533. doi: 10.1073/pnas.77.3.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickler J. E., Patton C. L. Trypanosoma brucei: nearest neighbor analysis on the major variable surface coat glycoprotein--crosslinking patterns with intact cells. Exp Parasitol. 1982 Feb;53(1):117–132. doi: 10.1016/0014-4894(82)90098-4. [DOI] [PubMed] [Google Scholar]
- Tokuyasu K. T. A technique for ultracryotomy of cell suspensions and tissues. J Cell Biol. 1973 May;57(2):551–565. doi: 10.1083/jcb.57.2.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuyasu K. T., Singer S. J. Improved procedures for immunoferritin labeling of ultrathin frozen sections. J Cell Biol. 1976 Dec;71(3):894–906. doi: 10.1083/jcb.71.3.894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickerman K., Luckins A. G. Localization of variable antigens in the surface coat of Trypanosoma brucei using ferritin conjugated antibody. Nature. 1969 Dec 13;224(5224):1125–1126. doi: 10.1038/2241125a0. [DOI] [PubMed] [Google Scholar]
- Whitaker J. R., Granum P. E. An absolute method for protein determination based on difference in absorbance at 235 and 280 nm. Anal Biochem. 1980 Nov 15;109(1):156–159. doi: 10.1016/0003-2697(80)90024-x. [DOI] [PubMed] [Google Scholar]