Abstract
Most reports in the literature have shown that the effects of opioid analgesics are primarily mediated by μ-opioid receptor (MOR), whereas other potential targets of opioid analgesics have not been thoroughly characterized. In this study, we found that extracellular application of morphine, fentanyl or oxycodone, which are all considered to be MOR agonists, at relatively high concentrations, but not endogenous μ-opioid peptides, produced a concentration-dependent suppression of sodium currents in cultured thalamic neurons. These effects of opioids were not affected by either a MOR antagonist naloxone or a deletion of MOR gene. Among these opioids, fentanyl strongly suppressed sodium currents to the same degree as lidocaine, and both morphine and oxycodone slightly but significantly reduced sodium currents when they were present extracellularly. In contrast, the intracellular application of morphine, but not oxycodone, fentanyl or lidocaine, reduced sodium currents. These results suggest that morphine, fentanyl and oxycodone each produce the MOR-independent suppression of sodium currents by distinct mechanisms in thalamic neurons.
Keywords: Morphine, fentanyl, oxycodone, lidocaine, voltage-gated sodium channels
μ-Opioid receptor (MOR) is the principle physiological target for most clinically important opioid analgesics, including morphine, fentanyl and oxycodone [9, 12]. It is well known that opioid receptors transduce signals through pertussis toxin-sensitive Gi/Go proteins to inhibit adenylyl cyclase, increase membrane K+ conductance and reduce Ca2+ current, which leads to cell hyperpolarization and exerts an inhibitory effect [3, 4, 14]. Several physiological studies have also demonstrated that opioid receptors can activate phospholipase C/protein kinase C-linked pathways [15] in a diverse range of opioid-modulated events, such as pain regulation [11, 13] and the response to neuronal excitability [11]. Although there are interesting pharmacological differences in the analgesic potency and the frequency and intensity of adverse events among opioid analgesics classified as MOR agonists, the potential targets of opioid analgesics other than MOR have not been thoroughly characterized.
There is broad agreement on the general outlines of the afferent transmission pathways from primary afferent nociceptors through the dorsal horn to the thalamus. Although the thalamus constitutes the main gateway through which the cerebral cortex receives external sensory signals, there have been relatively few studies on the mechanism of the analgesic effect of opioid analgesics on the thalamus.
Voltage-gated sodium channels play an important role in excitable cells such as nerve and muscle cells. Sodium channels generate rapid and transient inward currents that permit neuronal firing and axonal conduction. Sodium channels are also the target of several classes of drugs, including anesthetics, analgesics, antiepileptics, antidepressants and antiarrhythmics. Therefore, in this study we investigated whether clinically used opioid analgesics could affect voltage-gated sodium channels in rat thalamic neurons, and examined the mechanism by which they affect this channel.
The present study was conducted in accordance with the Guiding Principles for the Care and Use of Laboratory Animals (Hoshi University), as adopted by the Committee on Animal Research of Hoshi University, which is accredited by the Ministry of Education, Culture, Sports, Science, and Technology of Japan. Thalamic neuron/glia co-cultures were grown as follows. The thalamic region was obtained from Sprague-Dawley rat (Tokyo Laboratory Animals Science, Tokyo, Japan) embryos on embryonic day 17 or MOR−/− mice [16] at postnatal 1 day, minced, and treated with papain (9 U/ml, Worthington Biochemical, Lakewood, NJ, USA). After being treated with enzyme at 37 °C for 15 min, cells were seeded on poly-l-lysine-coated coverslips at a density of 2 × 106 cells/cm3. The cells were maintained for 10-14 days in Dulbecco’s modified Eagle’s medium (Invitrogen, Grand Island, NY, USA) supplemented with 10 % precolostrum newborn calf serum (Invitrogen), 10 U/ml penicillin and 10 μg/ml streptomycin. Standard whole-cell voltage-clamp recordings were made from cultured thalamic neurons at room temperature using an Axopatch 200B amplifier (Axon Instruments, Foster City, CA, USA). To selectively record sodium currents, the pipette solution contained: 115 CsCl, 25 NaCl, 2 MgCl2, 1 CaCl2, 11 EGTA and 10 HEPES, pH 7.4 with CsOH. The external bath solution contained the following (in mM): 100 NaCl, 40 tetraethylammonium-Cl, 0.03 CaCl2, 10 HEPES, 10 MgCl2, 10 d-glucose, pH 7.4 with TEA-OH. The patch microelectrodes were made from borosilicate capillary glass and had resistances of 5-10 MΩ for the whole-cell recording. Under voltage-clamp recording conditions, the series resistance was 4-7 MΩ. Leak currents were subtracted by a P/4 pulse protocol except for the experiments on use-dependent block, and the series resistance was compensated by 70 to 80 % in all experiments. The signals filtered at 2 kHz were directly digitized and stored on a personal computer. These sampled measurements were analyzed using the pCLAMP8 program (Axon Instruments). In repeated tests, voltage steps of 20 ms duration were applied every 20 s from a resting potential of −70 mV to a test potential of −20 mV. Availability protocols consisted of a series of prepulses between −100 mV and −20 mV in 10-mV increments lasting 1 sec, from a holding potential of −70 mV, followed by a 20-ms depolarization to −20 mV. The normalized curves were fitted using a Boltzmann distribution equation: I/Imax = 1/(1+ exp((Vm - V1/2)/k)), where Imax is the peak sodium current elicited after the most hyperpolarized prepulse, Vm is the preconditioning pulse potential, V1/2 is the potential at which inactivation is half-maximal, and k is the slope factor. Unless otherwise noted, statistical analyses were performed using Student’s t-test. The opioid analgesics used in the present study were morphine hydrochloride (Daiichi–Sankyo Co., Tokyo, Japan), fentanyl citrate (a kind gift from Hisamitsu Pharmaceutical Co. Inc., Tokyo, Japan) and oxycodone hydrochloride (a kind gift from Shionogi Pharmaceutical Co. Inc., Osaka, Japan). Lidocaine, naloxone, β-endorphin, endomorphin-1, endomorphin-2 and QX-314 were obtained from Sigma-Aldrich (St Louis, Mo, USA).
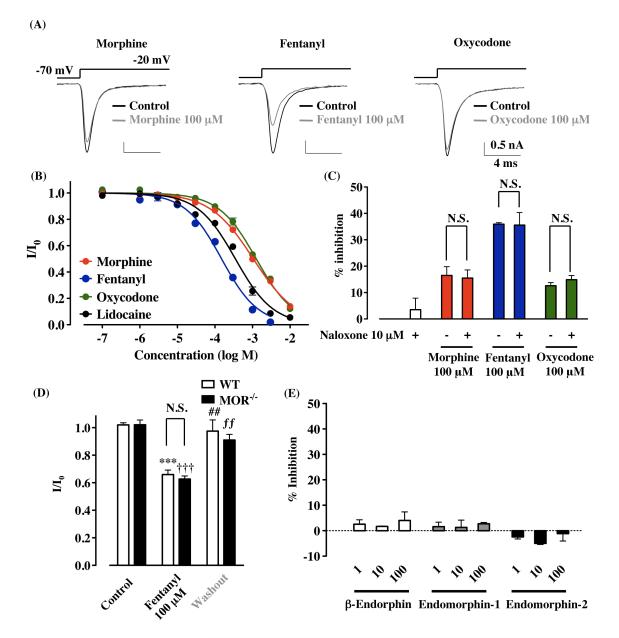
To determine if morphine, fentanyl and oxycodone could affect voltage-dependent sodium currents, whole-cell sodium currents were recorded in rat cultured thalamic neurons. Each opioid analgesic and the sodium channel blocker lidocaine reduced sodium currents in a concentration-dependent manner in rat cultured thalamic neurons (Fig. 1B). The IC50 values for morphine, fentanyl, oxycodone and lidocaine were 1053 (920-1206 μM), 153.2 (132.7-170.2 μM), 1260 (1135-1398 μM) and 350.2 μM (303.0-404.6 μM), respectively. The suppression of sodium currents was observed immediately after bath application of 100 μM morphine, fentanyl and oxycodone, and these inhibitions were reversible on washing (Supplementary Figure 1). Under these recording conditions, tetrodotoxin blocked all channel currents (data not shown). In acute thalamic slices, sodium currents were suppressed by 100 μM morphine (Supplementary Figure 2). To determine whether MOR contributes to the reduction of sodium currents by opioid analgesics, we first tested the effects of the opioid receptor antagonist naloxone on the suppression of sodium currents by opioid analgesics in cultured thalamic neurons. Bath application of 10 μM naloxone failed to block the inhibitory effects of 100 μM morphine (16.5 ± 3.3 %; morphine + naloxone, 15.5 ± 3.1 %, n = 7), fentanyl (35.9 ± 0.6 %; fentanyl + naloxone, 35.6 ± 4.8 %, n = 3) and oxycodone (12.6 ± 1.2 %; oxycodone + naloxone, 14.9 ± 1.6 %, n = 4) (Fig. 1C). Furthermore, to confirm the MOR-independent fashion of the fentanyl-induced suppression of sodium currents, we used thalamic neurons obtained from MOR−/− mice. After the bath application of 100 μM fentanyl, sodium currents were clearly suppressed in thalamic neurons with no detectable MORs (I/I0 = 0.659 ± 0.033, p<0.001, n = 5, Fig. 1D). We next tested whether endogenous μ-opioid peptides could affect the sodium currents in rat cultured thalamic neurons. Application of β-endorphin, endomorphin-1 or endomorphin-2 (1, 10 or 100 μM) did not affect the amplitude of sodium current (100 μM; decrease by 4.0 ± 1.5 %, 2.7 ± 0.5 %, −1.1 ± 2.9 %, respectively, Fig. 1E).
Figure 1.
Suppression of sodium currents by opioid analgesics in rat thalamic neuron through non-opioidergic mechanisms. Voltage steps were applied from a holding potential of −70 mV to a test potential of −20 mV. (A) Representative current traces obtained in the presence or absence of 100 μM morphine, fentanyl or oxycodone. (B) Concentration-dependent inhibition of the sodium currents by morphine, fentanyl, oxycodone and lidocaine in rat cultured thalamic neurons. Each symbol represents the mean value of normalized peak currents in the presence of drug (I/Io, mean ± S.E.M.), derived from 3-8 independent experiments for each concentration tested. (C) No effects of the opioid receptor antagonist naloxone (10 μM) on suppression of sodium currents by the bath application of 100 μM morphine, fentanyl or oxycodone. (D) No involvement of MORs in fentanyl-induced suppression of sodium currents in thalamic neurons using MOR−/− mice. Each column represents the normalized peak current amplitudes (I/I0, mean ± S.E.M., n = 5). ***p<0.001 control (WT) vs. fentanyl (WT), ##p<0.01 fentanyl (WT) vs. washout (WT), †††p<0.001 control (MOR−/−) vs. fentanyl (MOR−/−), ƒƒp<0.01 fentanyl (MOR−/−) vs. washout (MOR−/−). (E) Effects of endogenous μ-opioid peptides on voltage-gated sodium channels in rat cultured thalamic neurons. No significant changes in sodium currents were observed with the bath application of 1-100 μM β-endorphin (n = 5-7), endomorphin-1 (n = 3) or endomorphin-2 (n = 3-8). Each column represents the mean value of the % inhibition of the peak amplitude of the sodium currents in the presence of drug (mean ± S.E.M.).
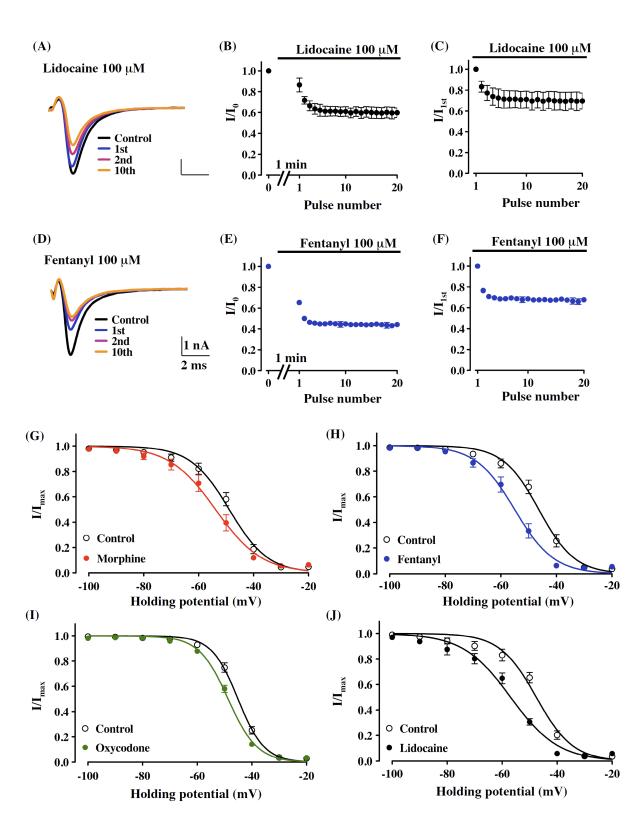
A common property of local anesthetics used clinically is that they preferentially affect the channel at a specific stage in its cycle of rest, activation and inactivation, often by delaying recovery from the inactivated state, thereby producing a cumulative reduction of sodium currents called ‘use-dependent’ block [5]. In the present study, the suppression of sodium currents by 100 μM fentanyl or lidocaine was use-dependent, as demonstrated by a progressive decrease in current during repetitive stimulation from −100 mV to −20 mV at 10 Hz (Fig. 2A, D). After the bath application of 100 μM fentanyl or lidocaine, the ratio of current amplitude was decreased at the first pulse (34.7 ± 2.1 %, n = 5, or 13.6 ± 6.6 %, n = 4, respectively), and at the 10th pulse (55.2 ± 1.9 % or 31.0 ± 4.6 %, respectively), compared with before drugs (Fig. 2B, E). A further analysis of the pulse-by-pulse decrease revealed that the suppression of sodium current by fentanyl or lidocaine was use-dependent (first pulse versus 10th pulse; 31.5 ± 1.6 % or 29.0 ± 7.9 %, respectively, Fig. 2C, F).
Figure 2.
A common feature of the effects of opioid analgesics and local anesthetics on sodium current suppression. (A-F) Use-dependent block of sodium currents by lidocaine and fentanyl. Use-dependent block was examined at 10 Hz with 30 20-ms test pulses to −20 mV from a holding potential of −100 mV. Repeated test potentials were given 1 min after the bath application of 100 μM lidocaine or fentanyl. (A, D) Representative traces show currents elicited by the pre-drug (Control) and first (1 st), second (2 nd) or 10 th pulses after the bath application of lidocaine or fentanyl. (B, C, E, F) Time-course changes in use-dependent block. Peak current amplitudes were normalized to the control peak current amplitude (I/I0, mean ± S.E.M., B, E) or to the initial peak current amplitude in the presence of drug (I/I1st, mean ± S.E.M., C, F) and plotted against the pulse number. (G-J) Voltage-dependence of inactivation for the sodium current in rat cultured thalamic neurons. The voltage-dependence of channel inactivation in the absence (control) and presence of 100 μM morphine (G), fentanyl (H), oxycodone (I) or lidocaine (J) was estimated by measuring the peak amplitude of the sodium current during a test potential (−20 mV) from a variable holding potential. The current at each membrane potential was divided by the electrochemical driving force for sodium ions and normalized to the maximum sodium current (Imax).
Local anesthetics can produce hyperpolarizing shifts in steady-state inactivation by binding to a receptor site on the ion channel [1, 7]. In the present study, the application of 100 μM morphine (−4.8 ± 1.1 mV, n = 7), fentanyl (−8.6 ± 0.9 mV, n = 7), oxycodone (−3.6 ± 0.4 mV, n = 4) and lidocaine (−9.3 ± 0.6 mV, n = 3) caused hyperpolarizing shifts in V1/2 of steady-state inactivation (Fig. 2G-J).
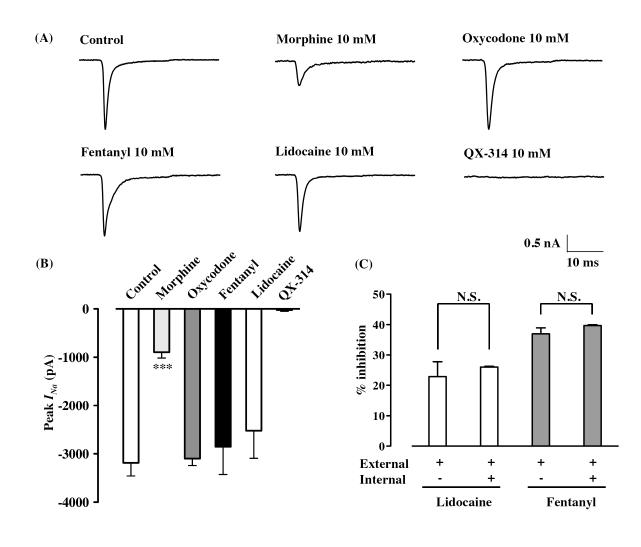
Most local anesthetics used clinically are relatively hydrophobic molecules that gain access to their blocking site on the sodium channel by diffusing into or through the cell membrane [7]. To determine the apparent sidedness of drug action, we measured the sodium currents elicited by a depolarizing step pulse (from −70 mV to −20 mV, 20 ms) in cells filled with each opioid analgesic or lidocaine. QX-314, a positively charged derivative of lidocaine, has no effect on neuronal sodium channels when applied extracellularly, but blocks sodium channels when applied intracellularly [2, 6, 17]. Consistent with this finding, QX-314 (10 mM) totally suppressed sodium current in our experiments (Fig. 3A, B). The addition of oxycodone, fentanyl or lidocaine at 10 mM to the recording pipette had no effect on sodium currents compared with a control pipette solution (peak amplitude = −3099.4 ± 140.4 pA with oxycodone, n = 4, −2850.5 ± 572.8 pA with fentanyl, n = 5, −2522.4 ± 569.6 pA with lidocaine, n = 4, Fig. 3A, B), whereas the sodium current was almost totally abolished by bath application of these drugs at the same concentration (Fig. 1B). On the other hand, the addition of 10 mM morphine to the recording pipette significantly reduced sodium currents (peak amplitude = −897.7 ± 119.0 pA, p<0.001, n = 6, Fig. 3A, B). We also asked whether the intracellular application of fentanyl or lidocaine could affect the suppression of sodium currents caused by the extracellular application of fentanyl or lidocaine. In the presence of intracellular fentanyl or lidocaine at 10 mM, extracellular application of 100 μM fentanyl or lidocaine caused current suppression (39.7 ± 0.2 % or 26.0 ± 0.2 %, respectively, Fig. 3C). Furthermore, this was observed at approximately the same level as in the absence of intracellular fentanyl or lidocaine (37.0 ± 2.0 % or 22.9 ± 1.8 %, respectively, Fig. 3C).
Figure 3.
The effect of intracellular application of opioid analgesics in whole-cell configurations. (A) Representative traces show the currents in a control pipette solution and in the presence of intracellular morphine (10 mM), oxycodone (10 mM), fentanyl (10 mM), lidocaine (10 mM) and the lidocaine derivative QX-314 (10 mM). Currents were elicited by 20-ms depolarizing steps from a holding potential of −70 mV to a test potential of −20 mV. Pulses were applied until steady-state sodium currents were achieved. (B) Bar graphs summarize steady-state sodium currents with the internal application of morphine, oxycodone, fentanyl, lidocaine and QX-314. Each column represents the mean value of the peak currents with S.E.M. ***p<0.001 vs. control. (C) Test of sidedness of fentanyl block using whole-cell recordings. Suppression of sodium currents by the extracellular application of 100 μM lidocaine or fentanyl was measured in the absence or presence of intracellular lidocaine (10 mM) or fentanyl (10 mM). Each column represents the mean value of the % inhibition of the peak amplitude of the sodium currents in the presence of drugs (mean ± S.E.M.).
It has been well established that opioid analgesics inhibit cAMP formation, close voltage-sensitive Ca2+ channels and open K+ channels though MOR, which leads to cell hyperpolarization and exerts an inhibitory effect [3, 4, 14]. In this study, we used whole-cell voltage-clamp recording and found that, as with lidocaine, extracellular application of morphine, fentanyl or oxycodone produced a concentration-dependent suppression of sodium currents, which was not influenced by the opioid receptor antagonist naloxone. In agreement with these findings, the effect of fentanyl on sodium current suppression was clearly shown in thalamic neurons obtained from MOR−/− mice. Moreover, endogenous μ-opioid peptides did not affect the amplitude of sodium currents. These findings clearly indicate that morphine, fentanyl and oxycodone suppress thalamic sodium currents through non-opioidergic mechanisms.
We also found that, among these opioids, the inhibitory potency of the extracellular application of fentanyl was much stronger than those of the other two opioids, to the same degree as lidocaine. Although further study is required, the similarity of the chemical structures of fentanyl and lidocaine may help to explain the similarity of their potencies, as well as the difference in the sodium channel-blocking effects of fentanyl, morphine or oxycodone. There is a broad consensus that most local anesthetics used clinically gain access to their blocking site on the sodium channel by diffusing into or through the cell membrane [7]. In addition, the site at which local anesthetics act, at least in their charged form, is accessible only from the inner surface of the membrane [10]. Lidocaine, morphine, oxycodone and fentanyl have pKa values of 7.8, 7.9, 8.5 and 8.4, respectively, which means that more than 80% of the drug is ionized at physiological pH. Even though large amounts of lidocaine, fentanyl and oxycodone are ionized at experimental pH, the present data clearly show that the internal application of fentanyl, oxycodone or lidocaine failed to suppress sodium currents, whereas external application of the same drugs suppressed sodium currents with different potencies. Furthermore, in the presence of intracellular fentanyl or lidocaine, extracellular application of fentanyl or lidocaine, respectively, caused current suppression to approximately the same level as that in the absence of intracellular fentanyl or lidocaine. This phenomenon induced by lidocaine is consistent with a recent report that external, but not internal, application of the sodium channel blocker flecainide promotes use-dependent block of heart sodium channels [8]. Thus, the present data strongly suggest that either fentanyl- or lidocaine-induced sodium current suppression may occur when drugs are present extracellularly. In contrast, morphine caused a significant suppression of sodium currents when applied either internally or externally, but with different potencies. These findings suggest that, like lidocaine, either fentanyl or oxycodone may have a blocking effect on sodium currents by acting through the extracellular pathway, whereas morphine may act through both the extracellular and intracellular pathway.
Use-dependent block, where the inhibitory effect on channels by a drug cumulatively increases with repetitive stimulation at high frequency, which mostly indicates that the drug can block the channel current when the channel is activated (open), is the hallmark for local anesthetics. In the present study, depending on the pulse number, both lidocaine and fentanyl progressively decreased the peak amplitude of sodium currents at a holding potential of −100 mV, indicating that fentanyl effectively blocks the sodium channel current when the channel gate is open. We also found that fentanyl, but not lidocaine, caused the tonic suppression of sodium currents with no depolarizing stimulation at a holding potential of −100 mV, and that almost all thalamic sodium channels were maintained with a resting state. These findings suggest that the inhibition of sodium channels by fentanyl may result from its multiple effects on sodium channels in both the resting and activated (channel open) states.
It has been reported that a local anesthetic binds more tightly to and stabilizes the inactivated state of the sodium channel [1, 5, 7]. Consistent with these observations with local anesthetics, we demonstrated that lidocaine showed hyperpolarizing shifts in the steady-state inactivation of voltage-gated sodium channels. Under the present conditions, we found that the extracellular application of these three opioids gave the same performance as with exposure to lidocaine. The present data suggest that, like lidocaine, morphine, fentanyl and oxycodone suppress sodium currents by facilitating inactivation of a thalamic sodium channel at a normal resting membrane potential when these three opioids are applied extracellularly.
In conclusion, we have shown that opioid analgesics in thalamic neurons may promote the inhibition of neuronal activity by blocking voltage-gated sodium channels without MOR activation. Furthermore, our data suggest that three MOR agonists exhibit different blocking potencies toward sodium channels with distinct mechanisms. In future studies, it might be worthwhile to ascertain whether these opioid analgesics directly access their binding domains on voltage-gated sodium channels and how to facilitate their blocking effects.
Supplementary Material
References
- [1].Bean BP, Cohen CJ, Tsien RW. Lidocaine block of cardiac sodium channels. J. Gen. Physiol. 1983;81:613–642. doi: 10.1085/jgp.81.5.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Cahalan MD, Almers W. Interactions between quaternary lidocaine, the sodium channel gates, and tetrodotoxin. Biophys. J. 1979;27:39–55. doi: 10.1016/S0006-3495(79)85201-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Childers SR. Opioid receptor-coupled second messenger systems. Life Sci. 1991;48:1991–2003. doi: 10.1016/0024-3205(91)90154-4. [DOI] [PubMed] [Google Scholar]
- [4].Christie MJ, North RA. Agonists at mu-opioid, M2-muscarinic and GABAB-receptors increase the same potassium conductance in rat lateral parabrachial neurones. Br. J. Pharmacol. 1988;95:896–902. doi: 10.1111/j.1476-5381.1988.tb11719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Courtney KR. Mechanism of frequency-dependent inhibition of sodium currents in frog myelinated nerve by the lidocaine derivative GEA. J. Pharmacol. Exp. Ther. 1975;195:225–236. [PubMed] [Google Scholar]
- [6].Frazier DT, Narahashi T, Yamada M. The site of action and active form of local anesthetics. II. Experiments with quaternary compounds. J. Pharmacol. Exp. Ther. 1970;171:45–51. [PubMed] [Google Scholar]
- [7].Hille B. Local anesthetics: hydrophilic and hydrophobic pathways for the drug-receptor reaction. J. Gen. Physiol. 1977;69:497–515. doi: 10.1085/jgp.69.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Liu H, Atkins J, Kass RS. Common molecular determinants of flecainide and lidocaine block of heart Na+ channels: evidence from experiments with neutral and quaternary flecainide analogues. J. Gen. Physiol. 2003;121:199–214. doi: 10.1085/jgp.20028723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Mizoguchi H, Wu HE, Narita M, Sora I, Hall SF, Uhl GR, Loh HH, Nagase H, Tseng LF. Lack of mu-opioid receptor-mediated G-protein activation in the spinal cord of mice lacking Exon 1 or Exons 2 and 3 of the MOR-1 gene. J. Pharmacol. Sci. 2003;93:423–429. doi: 10.1254/jphs.93.423. [DOI] [PubMed] [Google Scholar]
- [10].Narahashi T, Frazier DT. Site of action and active form of local anesthetics. Neurosci. Res. 1971;4:65–99. doi: 10.1016/b978-0-12-512504-8.50009-3. [DOI] [PubMed] [Google Scholar]
- [11].Narita M, Hashimoto K, Amano T, Niikura K, Nakamura A, Suzuki T. Post-synaptic action of morphine on glutamatergic neuronal transmission related to the descending antinociceptive pathway in the rat thalamus. J. Neurochem. 2008;104:469–478. doi: 10.1111/j.1471-4159.2007.05059.x. [DOI] [PubMed] [Google Scholar]
- [12].Narita M, Nakamura A, Ozaki M, Imai S, Miyoshi K, Suzuki M, Suzuki T. Comparative Pharmacological Profiles of Morphine and Oxycodone under a Neuropathic Pain-Like State in Mice: Evidence for Less Sensitivity to Morphine. Neuropsychopharmacology. 2008;33:1097–1112. doi: 10.1038/sj.npp.1301471. [DOI] [PubMed] [Google Scholar]
- [13].Narita M, Ohsawa M, Mizoguchi H, Aoki T, Suzuki T, Tseng LF. Role of the phosphatidylinositol-specific phospholipase C pathway in delta-opioid receptor-mediated antinociception in the mouse spinal cord. Neuroscience. 2000;99:327–331. doi: 10.1016/s0306-4522(00)00202-5. [DOI] [PubMed] [Google Scholar]
- [14].Rhim H, Miller RJ. Opioid receptors modulate diverse types of calcium channels in the nucleus tractus solitarius of the rat. J. Neurosci. 1994;14:7608–7615. doi: 10.1523/JNEUROSCI.14-12-07608.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Smart D, Smith G, Lambert DG. Mu-opioids activate phospholipase C in SH-SY5Y human neuroblastoma cells via calcium-channel opening. Biochem. J. 1995;305:577–581. doi: 10.1042/bj3050577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Sora I, Takahashi N, Funada M, Ujike H, Revay RS, Donovan DM, Miner LL, Uhl GR. Opiate receptor knockout mice define mu receptor roles in endogenous nociceptive responses and morphine-induced analgesia. Proc. Natl. Acad. Sci. USA. 1997;94:1544–1549. doi: 10.1073/pnas.94.4.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Strichartz GR. The inhibition of sodium currents in myelinated nerve by quaternary derivatives of lidocaine. J. Gen. Physiol. 1973;62:37–57. doi: 10.1085/jgp.62.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.