Abstract
Genotype scores that predict relevant clinical outcomes may detect other disease features and help direct prevention efforts. We report data that validate a previously-established v1.0 smoking cessation quit success genotype score and describe striking differences in the score in individuals who display differing developmental trajectories of use of common addictive substances. In a cessation study, v1.0 genotype scores predicted ability to quit with p = 0.00056 and area under ROC curve 0.66. About 43 vs 13% quit in the upper vs lower genotype score terciles. Latent class growth analyses of a developmentally-assessed sample identified three latent classes based on substance use. Higher v1.0 scores were associated with a) higher probabilities of participant membership in a latent class that displayed low use of common addictive substances during adolescence (p = 0.0004) and b) lower probabilities of membership in a class that reported escalating use (p = 0.001). These results suggest that: a) we have identified genetic predictors of smoking cessation success, b) genetic influences on quit success overlap with those that influence the rate at which addictive substance use is taken up during adolescence and c) individuals at genetic risk for both escalating use of addictive substances and poor abilities to quit may provide especially urgent focus for prevention efforts.
Keywords: genetic scores, complex genetics, smoking, development, cannabis, predictive validity
INTRODUCTION
The complexity of genetic influences on many common disorders has motivated development and validation of complex genetic scores that are often composed of sums of risk genotypes that can be weighted in different fashions1–8. Fewer studies have identified which disease features are captured by such complex genetic scores, however.
Twin studies document substantial heritability for smokers’ abilities to successfully abstain from smoking; there are thus robust genetic influences likely for individual differences in abilities to quit 9,10. We have carried out genome wide association studies for success in quitting smoking in several independent samples of smokers who attempted to quit smoking in clinical trials or in the community 11–13. In 2007, we used data from three initial samples to develop a v1.0 quit success score. Risk alleles for each of 12,058 quit-success-associated SNPs 14 (Supplement Table III) make up this score. When we used this score prospectively in a clinical trial of smoking cessation success in which nicotine replacement (NRT) doses were randomly assigned to participants 15, interactions between this v1.0 score, level of dependence and NRT dose did successfully predict quit success.
It appears likely that there should be other behavioral implications of a bona fide genotype score that helps to predict quit success. Twin data documents shared genetic influences on smoking quantity/frequency and abilities to quit in the community 16. There is significant overlap between the genes identified in smoking cessation studies and those that are identified in molecular genetic studies of addiction vulnerability 14. However, there has been no direct evidence for other behavioral differences between individuals with higher and lower v1.0 scores.
We have wondered how genetic influences on quitting might overlap with those that might influence earlier manifestations of addiction-related behaviors. Individual differences in the development of involvement with addictive substances have been modeled, with substantial individual differences in likelihoods of falling into classes associated with different temporal trajectories of involvement with addictive substances 17–19. We hypothesized that individuals with more of the genomic variants that predict ability to quit (after dependence was established) might also display different trajectories whereby addictions were established. Failure of early attempts to terminate use might increase the rate of “uptake” of use of common abused substances, for example.
We thus now report application of this established v1.0 score to three samples, two positive and a negative control sample. In a new clinical trial in which NRT dose was matched to baseline smoking intensity, and in a negative control Alzheimer’s disease sample, we sought evidence for further validation of this score 14. In a community based sample of developmentally-assessed participants followed since first grade, we sought evidence that genetic determinants for ability to quit might influence the developmental trajectories of involvement with addictive substances. This third sample was of especial interest, since their addiction vulnerability genetic data identifies many of the same chromosomal regions found in larger research volunteer samples 20 (Supplement Table 2). Taken together, the results of these studies suggest that v1.0 scores can reproducibly and specifically aid in identifying ability to quit smoking and predict trajectories of earlier involvement with addictive substances.
METHODS
1. Subjects and assessments
a)Smoking cessation
Adult smokers seeking to quit provided written informed consent, reported smoking an average of ≥ 10 cigarettes/day that each yield ≥ 0.5 mg nicotine, displayed end-expired air CO ≥ 10 ppm, failed to display exclusionary features on history physical exam or laboratory evaluations, and were compensated up to $140 (Clinical trials.gov NCT00894166). Smokers with expired air CO levels > and < 30 ppm (the median CO for all subjects) were assigned to 42 mg/24 h and 21 mg/24 h nicotine patch doses, respectively (GlaxoSmithKline). Participants wore the patches daily, were provided with usual brand cigarettes to smoke during the 2-week pre-cessation period without instructions to quit during this period (Figure S1) and were followed for seven study sessions with brief counseling and assessments of end-expired air CO and smoking self reports. Individuals who reduced baseline end expired air CO levels by more than 50% during the precessation period were maintained on NRT. Those who did not were randomized to: a) maintenance on NRT, b) maintenance on NRT with the addition of bupropion, 150 mg bid or c) discontinuation of NRT and treatment with an ascending then stabilized dose regimen of varenecline, beginning from 0.5 mg/d and escalating to 1 mg bid for 12 weeks.
The primary endpoint was continuous abstinence for the 11 weeks following the quit date, based on daily diary self reports of continuous abstinence and end-expired CO levels ≤ 10 ppm (Vitalograph; Lenexa, KS). Individuals screened for participation in this study reported 4 week mean duration of their longest abstinence during their average 25 years’ smoking histories prior to study participation. DNAs were selected for genotyping from all abstinent subjects from self-reported European American racial/ethnic backgrounds (n = 50), and more than twice the number of comparison European American individuals reported failure to achieve and/or sustain abstinence through the 11 week followup period (n = 117) and were matched to successful quitters for gender, baseline FTND scores and arm of the study. Self-reported European American ancestry correlated >99% with genetic background determined by principal component analyses in a previously-reported samples of smokers recruited in identical fashion 14.
b) Alzheimer’s disease (negative controls)
Alzheimer’s disease was diagnosed clinically and pathologically in brains of 664 individuals who were > 65 and excluded by clinical and pathological examination of 442 other control individuals >65 as described 21.
c) Prevention study developmentally-assessed samples
Baltimore first graders in 1993 (cohort III; 6.2 +/−0.4 years old) were group-randomized to control or to one of two universal, school-based interventions 22,23 targeting behavior, school performance, drug use and antisocial behavior 24,25 (Fig S2). 799 students, 54% male, 85% African American and 13% European American, were recruited from 27 classrooms in 9 Baltimore elementary schools 22,23,24,25 Participants provided data at a median of nine of ten follow-up attempts that asked questions about drug use from 8th grade until age 24. There was about 20% loss of subjects to follow-up over this time period. During follow-up visits in 2007 – 2011, almost 80% of contacted participants provided consent for genotyping and blood and/or buccal material for DNA extraction. Analyses here focus on 555 cohort III individuals, of both European- and African-American heritages, for whom we have both genotype and detailed clinical information.
2. Genotyping and assignment of v1.0 quit success genotype scores
DNA from samples a and c was extracted from blood or buccal material 26–28, quantitated and genotyped using Affymetrix 6.0 microarrays according to manufacturer’s instructions by investigators blinded to clinical phenotype. Genotypes for each individual passed Affymetrix quality control metrics with contrast quality control threshold > 0.4 and provided calls for 100% (median) and 99.1% (average) of the 12,058 SNPs that comprise the v1.0 score. Affymetrix 500k genotypes from sample b also passed Affymetrix quality control standards, were generously provided by TGEN investigators, were subject to imputation using PLINK to generate genotypes for the 12,058 v1.0 score SNPs. The v1.0 score weights the susceptibility allele for each of 12,058 SNPs as described in table S3. 5616 pairs of these v1.0 SNPs display linkage disequilibrium with r2 > 0.2 (Table S4).
3. Analyses
a) Smoking cessation success and Alzheimer’s disease negative controls
1) The directional hypothesis that higher v1.0 scores would be associated with greater likelihood of quitting was assessed using one tailed t tests, while differences between Alzheimer’s and control subjects were assessed with two tailed tests. The significance of the difference in quit success between the subjects with the highest vs lowest tercile v1.0 scores was assessed with Fisher’s exact test 29.
2) ROC analysis: Receiver operating characteristic analyses of the v1.0 quit success score data and comparative data for CO reductions during the first week of precessation NRT used a web based tool 30.
3) Power for quit success comparisons: Power for v1.0 score application used current sample sizes and standard deviations, as well as the standard deviations derived from prior independent samples14, the program PS v2.1.31 31,32 and α = 0.05.
b) Prevention study samples with longitudinal followup
Beginning in eighth grade, subjects provided data about their past year use of the common addictive substances, tobacco, alcohol and cannabis. Use of these substances was highly correlated among individuals (p < 0.001 for all correlations at all timepoints; Pearson correlation coefficients 0.3 – 0.6). At each time point, a summed score ranged from 0 to 21 with past year use of each of these substances weighted as follows: 0= none, 1= once, 2 = twice, 3=3–4 times, 4 = 5–9 times, 5 = 10–19 times, 6 = 20–39 times, 7 = 40 or more times. Latent class growth analysis (LCGA) was performed for this data using M plus v6 33. 34. We selected a three class model based on results of goodness-of-fit, Bayesian information criterion (BIC), Vuong-Lo-Mendell-Rubin likelihood ratio test and bootstrap likelihood ratio test parameters for 2, 3, 4 and 5 class models (Table S1). We used gender and race/ethnicity covariates so that each subject’s probabilities of membership in each of the three classes was calculated with statistical correction for effects of these covariates (Fig S3).
Primary preplanned analyses of these data paralleled primary analyses of the quit success data. Class membership probabilities from subjects displaying v1.0 quit success scores in the top tercile were compared to those of subjects displaying v1.0 scores in the lowest tercile by t test.
RESULTS
Smokers: unsuccessful vs successful quitters
The 50 successful European-American trial participants who maintained continuous abstinence for the 11 week duration of the quit success study, matched to 117 European-American individuals who did not abstain for 11 weeks, provided 0.95 power to detect the differences in v1.0 scores and standard deviations that we actually observed (see also Supplementary analysis D).
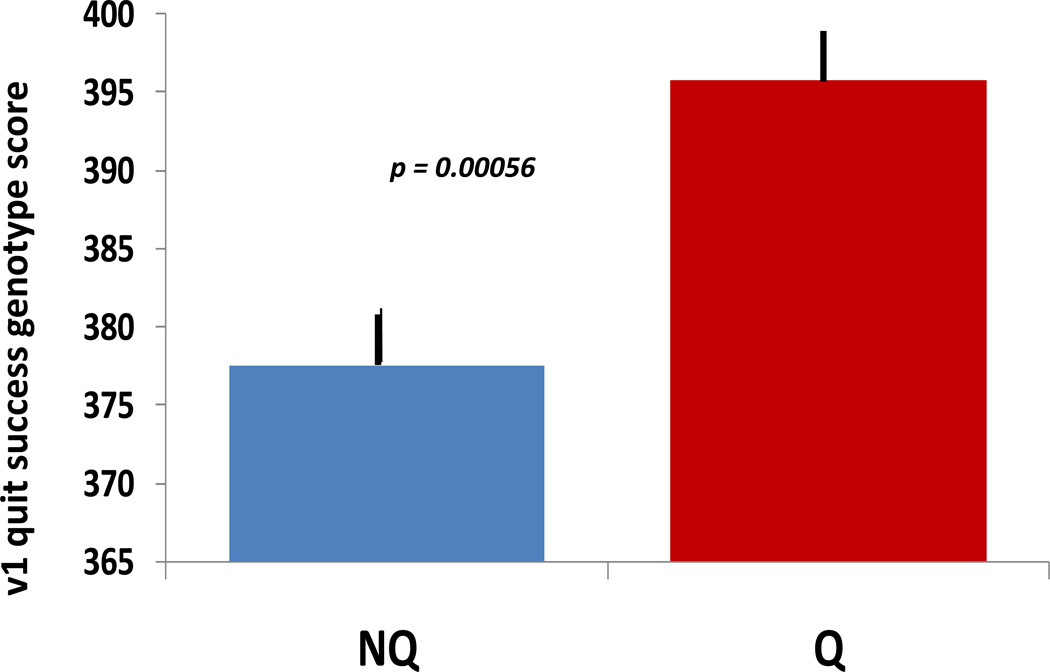
Mean v1.0 scores for these successful vs unsuccessful quitters displayed highly significant differences (p = 0.0005, one tailed t test) (Fig 1).The quit success scores were significantly higher in the successful quitters than in the matched comparison group of nonquitters in ways that were not readily explained by several other comparisons (Supplementary analysis E). When we ranked these trial participants based on their quit success scores, we found that about 43% of individuals in the upper tercile achieved continuous abstinence for at least 11 weeks, compared with 14% of individuals in the lower tercile (p = 0.0006; Fisher exact test). By contrast, there were no significant differences or trends in this direction in comparison of v1.0 scores in Alzeimer’s disease vs control individuals. Identical scores for cases and controls provided p = 0.91.
Figure 1.
v1.0 scores for nonquitters (NQ) and successful quitters (Q) in this clinical trial. Scores could range from 0 to 1000. Quitters reported continuous abstinence, confirmed by monitoring of CO in exhaled breath, for at least 11 weeks after the targeted quit date for this trial. *** P = 0.0005, t test. SEMs are 2.5 and 4.5, respectively.
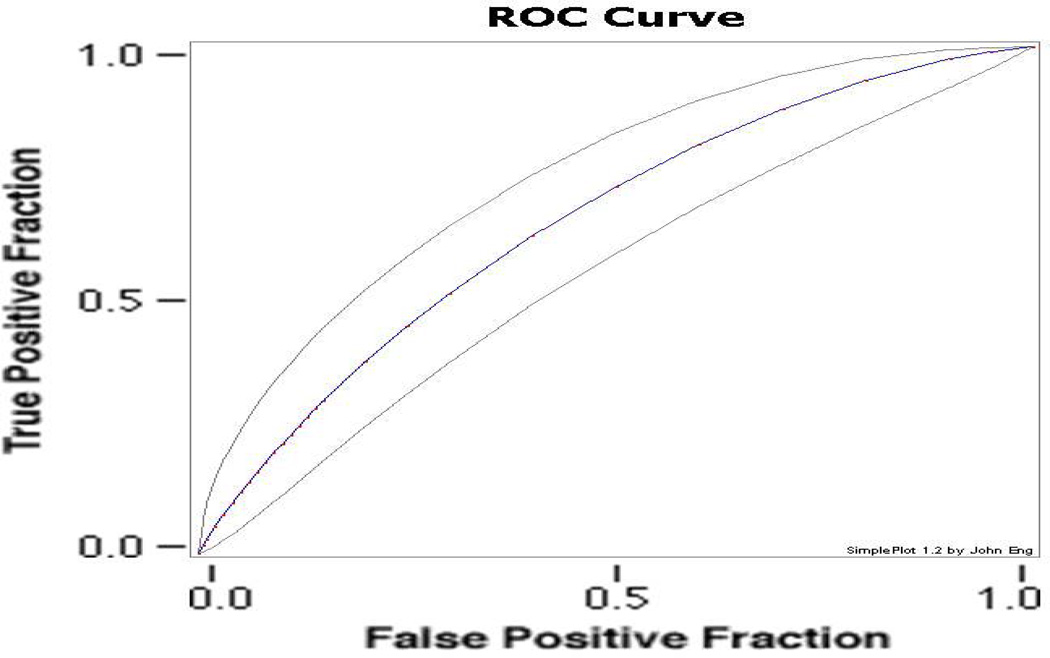
Receiver operating characteristic (ROC) curves evaluate the likely distributions of true and false positive results, so that a score that predicted quit success at chance levels would provide, on average, 0.5 area under the ROC curve. Analyses of the present data (Fig 2) provide an area under the ROC curve of 0.66.
Figure 2.
Receiver operating characteristic curve fitted to data for v1.0 scores ability to predict continuous abstinence (11 weeks) in the smoking cessation clinical trial described herein. Blue line indicates the area under the fitted curve. Grey lines indicate 95% confidence intervals for this estimate. Area under the curve: 0.657 (http://www.rad.jhmi.edu/jeng/javaradroc/JROCFITi.html).
Prevention study subjects: latent class growth analysis and development of a three-class model
Trajectory modeling approaches revealed sizable individual differences in the developmental profiles of frequency of use of the common addictive substances alcohol, tobacco and cannabis, among subgroups of the 555 individuals available for these analyses, as anticipated from prior analyses of other similarly-treated cohorts 22. The genome wide data for development of substance dependence for these and other prevention study subjects fit remarkably well with data from research volunteers that we had previously obtained (Table S2), supporting the validity of this sample. Latent class growth analyses (LCGA) of a three-class models (Table S1) provided favorable Vuong-Lo-Mendell-Rubin test results, allowing us to add covariates of gender and race, estimate trajectories, and assess probabilities of membership in each trajectory class for each participant (Fig 3) prior to testing association with v1.0 scores.
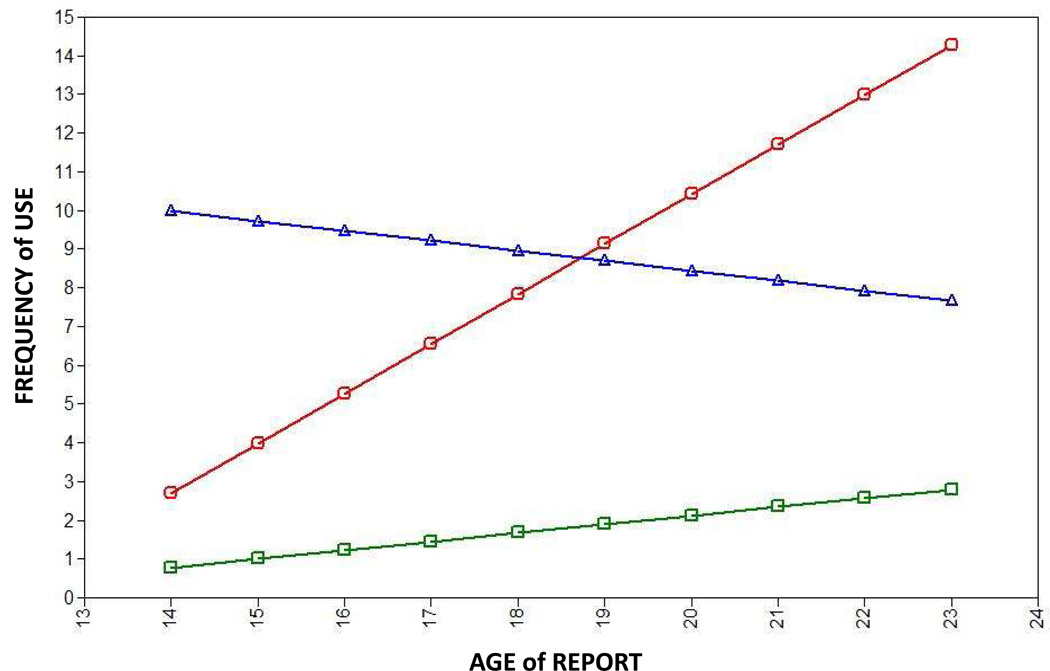
Figure 3.
Trajectories of involvement with common abused substances for classes of prevention study subjects as derived using latent class growth analysis implemented in Mplus. Members of class 1 (green; 80.8% of subjects) used few substances during the followup period. Members of class 2 (blue; 8.8%) stably used a number of substances during the followup period. Members of class 3 (red; 10.6%) escalated use of substances during the followup period. X axis: age. Y axis: aggregate score for past year frequency of use of tobacco, alcohol and cannabis derived from self report data from followup interviews.
Class 1 consists of individuals (about 81% of the total) who use common addictive substances at low levels, if at all, both in eighth grade and beyond. Class 2 contains individuals (about 9%) who already report substantial frequencies of use of common addictive substances by eighth grade, and maintain that use through adolescence and early adulthood. Class 3 consists of individuals (about 11%) who report only modest frequencies of addictive substance use in eighth grade, but who escalate their drug use through much of the period of observation. About 96% of individuals have high probabilities of membership in one of these three classes (Fig S3).
We identified individuals who displayed upper- or lower-tercile v1.0 scores and assessed differences in their probabilities of membership in each of the three classes. There were significant differences in the probabilities of membership in each of the classes (p = 0.00036, 0.046 and 0.00098, respectively) in subjects with upper vs lower tercile v1.0 scores. High v1.0 scores were associated with strikingly increased likelihood of membership in the class (1) that displays slow/low level uptake of addictive substances. Conversely, lower v1.0 scores are associated with strikingly increased likelihood of membership in the class (3) that displays increasing and escalating use of addictive substances during development (see also Supplementary analyses I).
DISCUSSION
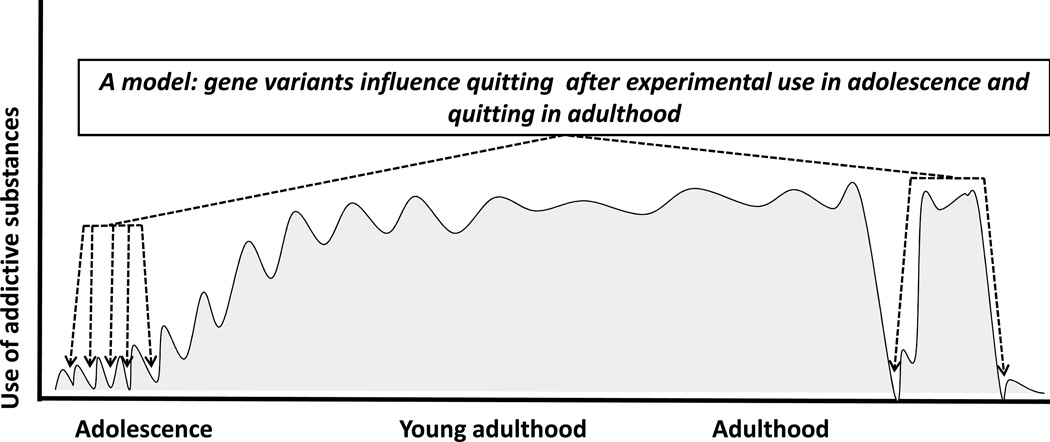
The current results provide additional and independent support for validity of a v1.0 smoking cessation genotype score in predicting ability to quit smoking in the setting of a randomized controlled clinical trial that provided pharmacological and modest behavioral support. Results from the longitudinally-assessed sample support shared molecular genetic determinants between quit success and the rate/degree to which use of common addictive substances is taken up during adolescence (Fig 4).
Figure 4.
Cartoon suggesting one mechanism by which quit success genetics might influence trajectories of uptake of substance use, dependence and quitting over time. If initial bouts of use were terminated by processes shared with those involved in quitting after an extended course of substance use and dependence, current results might be explained. (Please note that the current results are also compatible with other explanatory models.)
Classical genetic evidence suggests that about half of individual differences in abilities to quit smoking are heritable, and that half are mediated by environmental influences 35,36. We would thus anticipate that even a perfect genetic score would be able to predict quit success with less than perfect accuracy. The robust predictive ability of the v1.0 score described here is thus remarkable. The 3-fold difference in quit success in individuals in the upper vs lower terciles of v1.0 score provides an area under the ROC curve of the same magnitude as those provided by genotype scores for other complex disorders in which there are also strong genetic and environmental components of roughly similar magnitudes 1–8 The area under ROC curve provided by the v1.0 score is similar to that provided by one of the best clinical predictors of quit success, ability to reduce CO levels during precessation nicotine replacement therapy (Supplementary analysis C). Evidence for specificity of these observations comes from the failure to find any difference between scores in pathologically-verified Alzheimer’s disease vs control individuals. Genotyping is highly desirable in clinical trials, in which very costly false-negative results can emanate from trials in which stochastic mechanisms provide unfavorable distributions of quit success genotypes in placebo vs active treatment arms 37. As genotyping costs drop, such scores are also likely to provide valuable tools for more routine clinical use.
The latent class growth analyses used in this work have differences from, and potential advantages in comparison to, the latent growth mixture and other modeling approaches that have been applied to developmental datasets 34. Like growth mixture models, latent class growth analyses explore substantively meaningful groups under the assumption that there are unobserved subpopulations that display different patterns of development. Unlike growth mixture models, however, latent class growth analyses allow the latent class variable to capture all of the heterogeneity in the growth factors, based on key postulates that the variance of the intercept and slope factors within classes are zero and that there is no covariance between the growth factors.
Latent class growth modeling differentiates three classes with strikingly different patterns of substance involvement during adolescence and early adulthood. Two of the three classes provide evidence for striking association with v1.0 score genotypes in both primary preplanned and secondary analyses (Supplementary analysis I). These data thus provide some of the first direct molecular genetic evidence for overlaps between genetic influences on the extent of use of common addictive substance use during adolescence and the ability to quit use of at least one of these substances, tobacco, later in life.
Strengths of these results include the quality control and high genotype call rates for both samples; the parallel recruitment of smokers who were ultimately successful and unsuccessful in smoking cessation, the careful clinical and biochemical monitoring of the smoking cessation participants, the matching of successful quitters with twice the number of unsuccessful quitters in the same arm of the study and the contrasting striking negative data for Alzheimer’s disease vs control samples. The relatively high rates of follow-up and consent for genetic studies of the prevention sample participants that display strong overlaps between the addiction genetics documented in larger research volunteer samples, the clearcut differences between the trajectories established by the 3 class latent class growth analyses, the favorable distributions of probabilities for membership in distinct classes from most prevention study participants; and the fit between clinical characteristics during development and at the end of addictive trajectories are also strengths of these datasets. While it is plausible that increasing use of addictive substances in adolescence might represent, in part, failure of early attempts to quit use (Fig 4), other mechanisms that might link genetic influences on acquisition and cessation including neuroticism, disinhibition and/or tolerance of stress.
There are also limitations to these analyses. Their power is 0.95 for quit success, but difficult to calculate for LCGA trajectories. Latent classes identified here are unlikely to be identical to those produced in other samples, possibly limiting the generalizability of these findings. Two of the classes from this analysis are represented by modest n’s (120 and 56). While these modest sample sizes provide caution for lower-powered searches for specific influences on developmental patterns of use tobacco or other single substances, longitudinal latent class analyses based on only two classes do reveal significantly higher v1.0 scores (p < 0.05) in members of the classes that provided lower and slower use of tobacco and of marijuana (RM, NI, GRU in preparation). Small sample sizes also yield low power for seeking genotype × treatment interactions (see Supplementary analysis E). There is no consensus about any single approach that characterizes individual differences in trajectories of involvement with addictive substances. While we report data from a single, preplanned approach to the prevention study cohort and a three class solution, only the Vuong-Lo-Mendell-Rubin test indicated superiority of three vs two class solution. Failure to identify the third class studied here would have made the effect of the v1.0 score smaller, since the association with membership in class 2 was less robust despite the larger number of members of class 2; 5). An additional limitation may come from our prior lack of success in improving v1.0 scores. In interim analyses based on the first part of the quit success sample, there was poorer performance of subsets of v1.0 SNPs selected based on: i) Bayesian network analyses of data from a previous smoking cessation trial and ii) a trimmed v1.0 score in which the SNPs with the lowest weights were removed (GRU, DW JER, unpublished results, 2010). We have thus retained the full v1.0 score for the current preplanned analysis of the complete dataset.
The current data add appreciably to the increasingly-robust set of studies that document molecular genetic contributions to the ability to quit smoking and the ability of the v1.0 score to predict quit success. They increase conviction that complex genetic scores, in the appropriate clinical contexts, can significantly reduce noise in clinical trial settings in highly-cost effective ways. As genotyping costs are reduced and as strategies that allow differing therapeutic approaches to groups of individuals with high vs low genetic propensity for quitting smoking are tailored, these data suggest that the results should be useful for more generalized use in clinical practice and general health promotion strategies as well. The influences of “quit success” variants on trajectories of involvement with common addictive substances during adolescence also support efforts to define the best type and/or intensity of prevention strategies to maximize their benefits might aid in preventing development of dependence in otherwise vulnerable individuals. Focus on preventing substance use in individuals with low v1.0 scores, who appear to be genetically predisposed to display both escalating patterns of substance use and difficulty in quitting once they are dependent, appears to provide an attractive strategy for effective targeting of prevention resources.
Supplementary Material
ACKNOWLEDGEMENTS
This study was supported by: 1) the National Institutes of Health (NIH)–Intramural Research Program, National Institute on Drug Abuse, Department of Health and Human Services (Dr. Uhl); 2) a grant to Duke University (PI, Dr. Rose) from Philip Morris, USA for work performed prior to January, 2012; 3) NIDA grants R01-DA009897 (WE) and 4R37DA011796-11 (NI) . The funders had no role in the planning or execution of the study, data analysis or publication of results. We are grateful to TGEN investigators for generous access to Alzheimer’s disease GWAS genotype data and D Sisto for its analysis, to E Westman for assistance with the smoking cessation clinical trial, for each of the prevention study investigators, especially S Kellam, for the sustained cooperation of the study participants and for help and thoughtful advice from C Johnson, J Schroder, P Zandi and K Masyn. The underlying smoking cessation clinical trial was registered with clinicaltrials.gov (NCT00894166).
Footnotes
CONFLICT OF INTEREST
Drs. Rose and Uhl are listed as inventors for a patent application filed by Duke University based on genomic markers that distinguish successful quitters from unsuccessful quitters in data from other clinical trials.
REFERENCES
- 1.Zheng J, et al. Predictive Performance of prostate cancer risk in Chinese men using 33 reported prostate cancer risk-associated SNPs. Prostate. 2011 doi: 10.1002/pros.21462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang Y, et al. Predictive role of multilocus genetic polymorphisms in cardiovascular disease and inflammation-related genes on chronic kidney disease in Type 2 diabetes--an 8-year prospective cohort analysis of 1163 patients. Nephrol Dial Transplant. 2011 doi: 10.1093/ndt/gfr343. [DOI] [PubMed] [Google Scholar]
- 3.Xu M, et al. Genome Wide Association Study to predict severe asthma exacerbations in children using random forests classifiers. BMC Med Genet. 2011;12:90. doi: 10.1186/1471-2350-12-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kang J, Kugathasan S, Georges M, Zhao H, Cho JH. Improved risk prediction for Crohn's disease with a multi-locus approach. Hum Mol Genet. 2011;20:2435–2442. doi: 10.1093/hmg/ddr116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mealiffe ME, et al. Assessment of clinical validity of a breast cancer risk model combining genetic and clinical information. J Natl Cancer Inst. 2010;102:1618–1627. doi: 10.1093/jnci/djq388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Renstrom F, et al. Genetic predisposition to long-term nondiabetic deteriorations in glucose homeostasis: Ten-year follow-up of the GLACIER study. Diabetes. 2011;60:345–354. doi: 10.2337/db10-0933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu Y, et al. Exploring genetic determinants of plasma total cholesterol levels and their predictive value in a longitudinal study. Atherosclerosis. 2010;213:200–205. doi: 10.1016/j.atherosclerosis.2010.08.053. [DOI] [PubMed] [Google Scholar]
- 8.Lluis-Ganella C, et al. Additive effect of multiple genetic variants on the risk of coronary artery disease. Rev Esp Cardiol. 2010;63:925–933. doi: 10.1016/s1885-5857(10)70186-9. [DOI] [PubMed] [Google Scholar]
- 9.Broms U, Silventoinen K, Madden PA, Heath AC, Kaprio J. Genetic architecture of smoking behavior: a study of Finnish adult twins. Twin Res Hum Genet. 2006;9:64–72. doi: 10.1375/183242706776403046. [DOI] [PubMed] [Google Scholar]
- 10.Lessov CN, et al. Defining nicotine dependence for genetic research: evidence from Australian twins. Psychol Med. 2004;34:865–879. doi: 10.1017/s0033291703001582. [DOI] [PubMed] [Google Scholar]
- 11.Uhl GR, et al. Molecular genetics of successful smoking cessation: convergent genome-wide association study results. Archives of general psychiatry. 2008;65:683–693. doi: 10.1001/archpsyc.65.6.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drgon T, et al. Genome-wide association for smoking cessation success: participants in a trial with adjunctive denicotinized cigarettes. Molecular medicine. 2009;15:268–274. doi: 10.2119/molmed.2009.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uhl GR, et al. Genome-wide association for smoking cessation success: participants in the Patch in Practice trial of nicotine replacement. Pharmacogenomics. 2010;11:357–367. doi: 10.2217/pgs.09.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uhl GR, et al. Genome-wide association for smoking cessation success in a trial of precessation nicotine replacement. Molecular medicine. 2010;16:513–526. doi: 10.2119/molmed.2010.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rose JE, Behm FM, Drgon T, Johnson C, Uhl GR. Personalized smoking cessation: interactions between nicotine dose, dependence and quit-success genotype score. Molecular medicine. 2010;16:247–253. doi: 10.2119/molmed.2009.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morley KI, et al. Exploring the inter-relationship of smoking age-at-onset, cigarette consumption and smoking persistence: genes or environment? Psychol Med. 2007;37:1357–1367. doi: 10.1017/S0033291707000748. [DOI] [PubMed] [Google Scholar]
- 17.Lynne-Landsman SD, Graber JA, Nichols TR, Botvin GJ. Trajectories of aggression, delinquency, and substance use across middle school among urban, minority adolescents. Aggressive behavior. 2011;37:161–176. doi: 10.1002/ab.20382. [DOI] [PubMed] [Google Scholar]
- 18.Lynne-Landsman SD, Bradshaw CP, Ialongo NS. Testing a developmental cascade model of adolescent substance use trajectories and young adult adjustment. Development and psychopathology. 2010;22:933–948. doi: 10.1017/S0954579410000556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marsiglia FF, Kulis S, Yabiku ST, Nieri TA, Coleman E. When to intervene: elementary school, middle school or both? Effects of keepin' it REAL on substance use trajectories of Mexican heritage youth. Prevention science : the official journal of the Society for Prevention Research. 2011;12:48–62. doi: 10.1007/s11121-010-0189-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Drgon T, et al. Genome wide association for addiction: replicated results and comparisons of two analytic approaches. PloS one. 2010;5:e8832. doi: 10.1371/journal.pone.0008832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coon KD, et al. A high-density whole-genome association study reveals that APOE is the major susceptibility gene for sporadic late-onset Alzheimer's disease. J Clin Psychiatry. 2007;68:613–618. doi: 10.4088/jcp.v68n0419. [DOI] [PubMed] [Google Scholar]
- 22.Kellam SG, et al. Effects of a universal classroom behavior management program in first and second grades on young adult behavioral, psychiatric, and social outcomes. Drug Alcohol Depend. 2008;95(Suppl 1):S5–S28. doi: 10.1016/j.drugalcdep.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y, et al. Depressed mood and the effect of two universal first grade preventive interventions on survival to the first tobacco cigarette smoked among urban youth. Drug Alcohol Depend. 2009;100:194–203. doi: 10.1016/j.drugalcdep.2008.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ialongo NS, Poduska JM, Werthamer-Larsson L. The distal impact of two first grade preventive interventions on conduct problems and disorder and mental health service need and utilization in early adolescence. Journal of Emotional and Behavioral Disorders. 2001;9:146–160. [Google Scholar]
- 25.Kellam SG, et al. Developmental epidemiologically based preventive trials: baseline modeling of early target behaviors and depressive symptoms. Am J Community Psychol. 1991;19:563–584. doi: 10.1007/BF00937992. [DOI] [PubMed] [Google Scholar]
- 26.Uhl GR, Liu QR, Walther D, Hess J, Naiman D. Polysubstance abuse-vulnerability genes: genome scans for association, using 1,004 subjects and 1,494 single-nucleotide polymorphisms. American journal of human genetics. 2001;69:1290–1300. doi: 10.1086/324467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith SS, et al. Genetic vulnerability to drug abuse. The D2 dopamine receptor Taq I B1 restriction fragment length polymorphism appears more frequently in polysubstance abusers. Archives of general psychiatry. 1992;49:723–727. doi: 10.1001/archpsyc.1992.01820090051009. [DOI] [PubMed] [Google Scholar]
- 28.Persico AM, Bird G, Gabbay FH, Uhl GR. D2 dopamine receptor gene TaqI A1 and B1 restriction fragment length polymorphisms: enhanced frequencies in psychostimulant-preferring polysubstance abusers. Biological psychiatry. 1996;40:776–784. doi: 10.1016/0006-3223(95)00483-1. [DOI] [PubMed] [Google Scholar]
- 29. http://www.langsrud.com/fisher.htm#INTRO.
- 30. http://www.rad.jhmi.edu/jeng/ravaradroc/JROCFITi.html.
- 31.Dupont WD, Plummer WD., Jr Power and sample size calculations. A review and computer program. Control Clin Trials. 1990;11:116–128. doi: 10.1016/0197-2456(90)90005-m. [DOI] [PubMed] [Google Scholar]
- 32.Dupont WD, Plummer WD., Jr Power and sample size calculations for studies involving linear regression. Control Clin Trials. 1998;19:589–601. doi: 10.1016/s0197-2456(98)00037-3. [DOI] [PubMed] [Google Scholar]
- 33. www.statmodel.com/
- 34.Muthen B, Muthen LK. Integrating person-centered and variable-centered analyses: growth mixture modeling with latent trajectory classes. Alcoholism, clinical and experimental research. 2000;24:882–891. [PubMed] [Google Scholar]
- 35.Morley KI, et al. Exploring the inter-relationship of smoking age-at-onset, cigarette consumption and smoking persistence: genes or environment? Psychological medicine. 2007;37:1357–1367. doi: 10.1017/S0033291707000748. [DOI] [PubMed] [Google Scholar]
- 36.Broms U, Silventoinen K, Madden PA, Heath AC, Kaprio J. Genetic architecture of smoking behavior: a study of Finnish adult twins. Twin research and human genetics : the official journal of the International Society for Twin Studies. 2006;9:64–72. doi: 10.1375/183242706776403046. [DOI] [PubMed] [Google Scholar]
- 37.Uhl GR, Drgon T, Johnson C, Rose JE. Nicotine abstinence genotyping: assessing the impact on smoking cessation clinical trials. The pharmacogenomics journal. 2009;9:111–115. doi: 10.1038/tpj.2008.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.