We used marginal structural models to evaluate the rate of hepatic decompensation by initiation of combination antiretroviral therapy (ART) in a population of HIV/hepatitis C virus–coinfected male veterans, and found a 28%–41% reduction among ART initiators.
Keywords: HIV, hepatitis C, coinfection, hepatic decompensation, marginal structural model
Abstract
Background. Human immunodeficiency virus (HIV) coinfection accelerates the rate of liver disease outcomes in individuals chronically infected with hepatitis C virus (HCV). It remains unclear to what degree combination antiretroviral therapy (ART) protects against HCV-associated liver failure.
Methods. We evaluated 10 090 HIV/HCV-coinfected males from the Veterans Aging Cohort Study Virtual Cohort, who had not initiated ART at entry, for incident hepatic decompensation between 1996 and 2010. We defined ART initiation as the first pharmacy fill date of a qualifying ART regimen of ≥3 drugs from ≥2 classes. Hepatic decompensation was defined as the first occurrence of 1 hospital discharge diagnosis or 2 outpatient diagnoses for ascites, spontaneous bacterial peritonitis, or esophageal variceal hemorrhage. To account for potential confounding by indication, marginal structural models were used to estimate hazard ratios (HRs) of hepatic decompensation, comparing initiation of ART to noninitiation.
Results. We observed 645 hepatic decompensation events in 46 444 person-years of follow-up (incidence rate, 1.4/100 person-years). Coinfected patients who initiated ART had a significantly reduced rate of hepatic decompensation relative to noninitiators (HR = 0.72; 95% confidence interval [CI], .54–.94). When we removed individuals with HIV RNA ≤400 copies/mL at baseline from the analysis (assuming that they may have received undocumented ART at entry), the hazard ratio became more pronounced (HR = 0.59; 95% CI, .43–.82).
Conclusions. Initiation of ART significantly reduced the rate of hepatic decompensation by 28%–41% on average. These results suggest that ART should be administered to HIV/HCV-coinfected patients to lower the risk of end-stage liver disease.
Liver complications, particularly those due to chronic hepatitis C virus (HCV) infection, have emerged as leading non-AIDS-related causes of morbidity and mortality among patients infected with human immunodeficiency virus (HIV) [1, 2]. HIV coinfection accelerates progression to clinical HCV-related liver outcomes, although the mechanisms have yet to be fully characterized [3]. Given the high prevalence of HIV/HCV coinfection in the United States (250 000–300 000 individuals) and the rapid HCV-associated fibrosis progression rate among coinfected patients, therapies to reduce the incidence of advanced liver disease are needed [4, 5].
Although the benefits of combination antiretroviral therapy (ART) on the course of HIV disease have been well established, the effects of ART on the course of chronic HCV–related liver outcomes remain less clear. ART could help reduce the risk of HCV-associated hepatic decompensation by controlling HIV-mediated immune dysfunction and dysregulation through CD4 T-lymphocyte recovery. This mechanism is consistent with prior studies suggesting that CD4 count gain and suppression of HIV RNA are associated with reduced rates of advanced hepatic fibrosis, cirrhosis, hepatic decompensation, and/or death due to end-stage liver disease [6–11], although not with others [12–14]. In contrast, immune reconstitution due to ART could exacerbate host-mediated hepatic inflammation and chronic antiretroviral-associated hepatotoxicity and subsequently increase the risk of hepatic decompensation [11, 15–17]. Results from previous studies assessing the direct impact of ART on liver-related outcomes have been inconclusive [6, 18–23]. Most have relied on cross-sectional analyses of liver biopsies, which can be susceptible to bias. Generally these studies have evaluated the stage or rate of fibrosis as the outcome of interest, and not clinical liver disease progression events.
Given that HIV coinfection hastens liver disease progression in individuals with chronic HCV, elucidation of the impact of treating HIV on hepatic decompensation events in this population is warranted. However, studies using longitudinal data to evaluate the effects of clinical interventions on health outcomes are in general susceptible to biases. This is primarily because patient factors that predict the likelihood of treatment are also correlates of poor outcomes, and may in turn be affected by treatment (confounding by indication). Whereas standard regression methods will be inherently biased in this context, marginal structural models can be used to account for these biases and are preferred when confounding by indication may be present [24]. Thus, the primary goal of this study was to estimate the effect of ART initiation on the rate of hepatic decompensation in a large cohort of HIV/HCV-coinfected veterans, using marginal structural model methods.
METHODS
Study Population
We evaluated HIV/HCV-coinfected patients in the Veterans Aging Cohort Study Virtual Cohort (VACS-VC), an electronic medical records database of HIV-infected adults enrolled in the US Department of Veterans Affairs (VA) healthcare system from 1996 to the present [25]. This database contains demographic information, laboratory results, pharmacy data, clinical diagnoses (recorded using International Classification of Diseases, Ninth Revision [ICD-9] diagnostic codes), and dates of death. The pharmacy database includes information on medication fill dates, dosages, numbers dispensed, and dosing frequency. Dates of death were available from the VA vital status file, which records information from the Social Security Administration death master file, the Beneficiary Identification and Records Locator subsystem, and the VA medical Statistical Analysis Systems inpatient data sets.
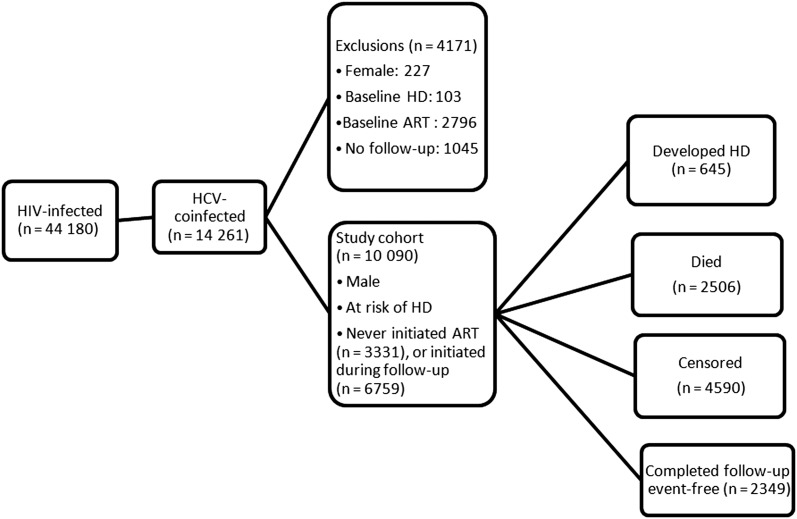
Patients were included in the analysis if they (1) had laboratory evidence of HIV infection (HIV antibody and RNA positive); (2) had a recorded diagnosis of HCV infection [26], a positive HCV antibody [27], and/or quantifiable HCV RNA; and (3) had not initiated ART at or prior to entry in the VA healthcare system. Patients were excluded if they (1) were female (given the small sample size and possible sex-based differences in HCV-associated liver disease progression rates); (2) had a baseline diagnosis of hepatic decompensation; (3) were on ART at VACS-VC entry; or (4) lacked follow-up visits (Figure 1). The VACS-VC has been granted a waiver of informed consent and has been approved by the institutional review boards (IRBs) of the VA Connecticut healthcare system and Yale University; this analysis was additionally approved by the IRB at the Harvard School of Public Health.
Figure 1.
Study population flow diagram. Abbreviations: ART, antiretroviral therapy; HCV, hepatitis C virus; HD, hepatic decompensation; HIV, human immunodeficiency virus.
Data Collection
Individuals were considered to have initiated ART on the filled prescription date of their first treatment regimen consisting of ≥3 antiretroviral medications from at least 2 different drug classes, or 3 nucleoside analogues. Until the first documented date these criteria were fulfilled, individuals were considered to be noninitiators. To account for the possibility that patients may have received undocumented antiretrovirals prior to entry in the VACS-VC, we repeated the primary analyses after removing from consideration patients with a baseline HIV RNA of ≤400 copies/mL yet no record of ART initiation. In a recent validation study within the VACS, this algorithm correctly identified 86% as truly ART-naive based on medical records review [28]. For all analyses, we assumed that patients stayed on ART once having initiated, for ease in interpretation and to parallel the intent-to-treat approach of a hypothetical clinical trial.
Covariates included age at enrollment (years); calendar year at enrollment; race/ethnicity (white non-Hispanic, black non-Hispanic, Hispanic, other/unknown); hepatitis B virus (HBV) coinfection; history of alcohol abuse; history of drug abuse; CD4 cell count (cells/µL); HIV RNA level (copies/mL); diabetes; FIB-4 score; pegylated interferon therapy for HCV; and AIDS-defining conditions. HBV coinfection status was determined by a positive test for HBV surface antigen at any time under observation. Alcohol and/or drug abuse were considered to be present if an individual had documented alcohol- and/or drug-related ICD-9 diagnosis codes [29]. FIB-4, a noninvasive index used to identify advanced hepatic fibrosis, was calculated and categorized as described by Sterling et al [30]. Individuals were considered to have initiated HCV therapy at the filled prescription date for their first regimen of pegylated interferon lasting at least 28 days. AIDS-defining conditions were identified using ICD-9 diagnoses and established criteria [31]. HCV genotype (1, other) and HCV RNA (IU/mL) were additionally evaluated in preliminary analyses but removed from primary models due to a large proportion of missing data (61% and 60%, respectively) and a lack of observed associations of these factors with both ART initiation and hepatic decompensation.
Outcome
The outcome under study was incident hepatic decompensation, defined as a primary hospital discharge diagnosis or ≥2 outpatient diagnoses for ascites, spontaneous bacterial peritonitis, or esophageal variceal hemorrhage. Determination of hepatic decompensation using this diagnostic algorithm was found to be highly valid in a previous study, with 91% of events confirmed via medical records [32]. The decompensation date was determined by the hospital admission date (if identified by discharge diagnosis) or initial outpatient diagnosis date (if identified by outpatient diagnoses). We did not include hepatic encephalopathy or nonobstructive jaundice, which might also indicate decompensation, as these diagnoses often identified unrelated conditions [32].
Statistical Analysis
The baseline date for analysis was considered to be the first visit on record with available values for both CD4 count and HIV RNA. Study patients were followed and considered to be at risk from the baseline date until the first occurrence of hepatic decompensation, death, censoring, or 30 September 2010. Individuals were conservatively considered to be lost to follow-up if there was any gap in observations of at least 1 year; these individuals were censored at their last prior visit. Values for CD4 count and HIV RNA were carried forward until a new measure was available but not for more than 1 year. Values for FIB-4 were carried forward indefinitely until an updated value was available. To reflect the reality that the clinical decision to initiate ART is based on previously available (and not simultaneous) values of covariates (ie, CD4 count precedes and informs treatment status), we lagged covariate values to ensure the proper sequence for analysis.
To appropriately account for time-dependent confounding by indication, marginal structural (weighted Cox) models were constructed as previously described with minor modifications [33–35]. First, logistic regression models were constructed to calculate the likelihood of initiating ART at each time point, given an individual's covariate history. Models for probabilities of death and censoring were similarly constructed to account for potential biases due to competing risks and/or differential loss to follow-up [33, 35]. These predicted probabilities were then combined to create stabilized weights for unbiased estimation of the marginal structural model. All aforementioned baseline covariates were considered for inclusion in these weight estimation models, plus time-dependent variables for CD4, HIV RNA, diabetes, FIB-4 score, HCV therapy, and AIDS-defining diagnoses. Collectively these covariates were initially selected based on relevance in the context of HIV/HCV coinfection, as well as their availability in the source data. Individual covariates were then removed sequentially from a given model if they (1) were not statistically significant predictors of the given outcome, and (2) removal did not materially change the coefficient for treatment in the marginal structural model. Second, marginal structural models were specified using weighted Cox regression and used to estimate the effect of ART initiation on the rate of hepatic decompensation. Applying weights in this way can correct for time-dependent confounding and selection biases (due to selective attrition by censoring/death) under specific assumptions, whereas standard unweighted regression models remain inherently biased in this context [24, 35]. Results are reported as hazard ratios (HRs) with 95% confidence intervals (CIs), which were calculated using robust variances. We also constructed standard (unweighted) unadjusted and adjusted Cox models for comparison, and explored several supplemental analyses in which alterations in various modeling parameters were evaluated. An expanded description of our analytical approach can be found in the Supplementary Appendix. All analyses were conducted using SAS software, version 9.2 (SAS Institute Inc, Cary, North Carolina). A statistical significance level of .05 was assumed throughout, and any reported P values are 2-sided.
RESULTS
Among 44 180 HIV-infected individuals in the VACS-VC, 14 261 (32%) had evidence of HCV coinfection. After exclusions (Figure 1), the study cohort consisted of 10 090 HIV/HCV-coinfected male veterans who contributed 46 444 person-years of follow-up (median, 3.1 years; maximum, 14.6 years). HCV coinfection was confirmed in 6006 patients (60%) by detectable HCV RNA; 625 (6%) were identified as HCV-coinfected by ICD-9 diagnosis alone. Baseline characteristics of these patients are summarized in Table 1. The median age at baseline was 47 years (range, 20–89 years), and 61% were black non-Hispanic. Of 9843 tested for HBV surface antigen (98%), 820 (8%) screened positive. Four percent received pegylated interferon–based therapy during follow-up. Of those with genotype information available (39%), most (87%) were infected with HCV genotype 1. Approximately one-quarter had a history of alcohol dependence/abuse, and 32% had a history of drug abuse. More than half of the population had an AIDS-defining diagnosis by the end of follow-up. At baseline, 36% had a low CD4 count (<200 cells/µL), and 82% had a detectable HIV RNA load (>400 copies/mL). In unadjusted models, we observed a statistically significant association with hepatic decompensation for age, year at entry, race, diabetes, HBV, HCV therapy, FIB-4 score, alcohol abuse, AIDS-defining conditions, CD4 count, and HIV RNA (Table 1). Of note, African American coinfected patients appeared to progress to hepatic decompensation more slowly relative to other reported ethnicities, consistent with previous reports [36].
Table 1.
Baseline Distributions of Selected Patient Characteristics and Time-Dependent Unadjusted Associations With Hepatic Decompensation
| Characteristic | No. (%)a | Unadjusted HR (95% CI) | P for Trend |
|---|---|---|---|
| Total patients | 10 090 (100) | ||
| Age, y | |||
| 20–39 | 1459 (14.5) | Referent | .007 |
| 40–49 | 5364 (53.2) | 1.55 (1.19–2.00) | |
| 50–59 | 2718 (26.9) | 1.72 (1.30–2.27) | |
| 60–89 | 549 (5.4) | 1.35 (.86–2.10) | |
| Year at baseline | |||
| 1996–2000 | 6780 (67.2) | Referent | .048 |
| 2001–2009 | 3310 (32.8) | 0.82 (.68–1.00) | |
| Race | |||
| White non-Hispanic | 2703 (26.8) | Referent | <.001b |
| Black non-Hispanic | 6135 (60.8) | 0.69 (.58–.83) | |
| Hispanic | 945 (9.4) | 1.40 (1.10–1.76) | |
| Other | 307 (3.0) | 0.86 (.49–1.50) | |
| Diabetes | |||
| No | 9789 (97.0) | Referent | <.001 |
| Yes | 301 (3.0) | 1.88 (1.54–2.29) | |
| HBV surface antigen | |||
| No | 9023 (91.7) | Referent | <.001 |
| Yes | 820 (8.3) | 1.57 (1.24–1.98) | |
| HCV therapy | |||
| No | 10 081 (99.9) | Referent | .002 |
| Yes | 9 (0.1) | 1.80 (1.23–2.62) | |
| HCV genotype 1 | |||
| No | 507 (13.0) | Referent | .95 |
| Yes | 3393 (87.0) | 0.99 (.68–1.44) | |
| FIB-4 score | |||
| <1.45 (mild fibrosis) | 3072 (39.0) | Referent | <.001 |
| 1.45–3.25 (moderate) | 3216 (40.9) | 1.82 (1.28–2.59) | |
| >3.25 (advanced) | 1580 (20.1) | 25.80 (19.18–34.70) | |
| Alcohol abuse condition | |||
| No | 7385 (74.7) | Referent | <.001 |
| Yes | 2495 (25.3) | 1.41 (1.19–1.67) | |
| Drug abuse condition | |||
| No | 6672 (67.5) | Referent | .076 |
| Yes | 3208 (32.5) | 0.85 (.72–1.02) | |
| AIDS-defining condition | |||
| No | 8534 (84.6) | Referent | <.001 |
| Yes | 1556 (15.4) | 1.73 (1.47–2.04) | |
| CD4 count, cells/µL | |||
| <50 | 1353 (13.4) | 2.86 (2.10–3.91) | <.001 |
| 50–199 | 2331 (23.1) | 3.52 (2.79–4.43) | |
| 200–349 | 2360 (23.4) | 1.82 (1.42–2.33) | |
| 350–499 | 1792 (17.8) | 1.43 (1.10–1.87) | |
| ≥500 | 2254 (22.3) | Referent | |
| HIV RNA, copies/mL | <.001 | ||
| ≤400 | 1876 (18.6) | Referent | |
| 401–10 000 | 2622 (26.0) | 0.92 (.75–1.14) | |
| 10 001–100 000 | 3395 (33.6) | 1.25 (1.01–1.54) | |
| >100 000 | 2197 (21.8) | 1.81 (1.39–2.35) | |
Missing observations (No. [%]): HBV (247 [2.4%]); HCV genotype (6190 [61.3%]); baseline FIB-4 (2222 [22.0%]); alcohol (210 [2.1%]); drug abuse (210 [2.1%]).
Abbreviations: CI, confidence interval; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; HR, hazard ratio.
a Percentages are among the nonmissing.
b The P value for race is testing the type 3 null hypothesis that none of the categorical β estimates are significantly different from each other.
During observed follow-up, 6935 individuals initiated ART (69%; median time to initiation, 3 months). In the multivariable model, later calendar year, white non-Hispanic race/ethnicity, no history of drug abuse, lower CD4 count, higher HIV RNA load, and a history of AIDS-defining conditions were positively associated with initiating ART (Table 2). These variables were used to compute, at each visit, the predicted probability of receiving the treatment an individual actually received, given covariate and treatment history. In turn, these predicted probabilities were used to compute stabilized inverse probability weights, in conjunction with additional models for attrition due to death (n = 2506) and censoring (n = 4590), respectively. Predictors of death in multivariable regression included older age; race/ethnicity designated as unknown or other than white, black or Hispanic; lower CD4 count; higher HIV RNA load; history of diabetes; higher FIB-4 score; AIDS-defining diagnoses; and noninitiation of ART (estimates not shown). Predictors of censoring in multivariable regression included younger age; later calendar year; race/ethnicity classified as black non-Hispanic or as unknown or other than white, black, or Hispanic; history of drug abuse; higher CD4 count; higher HIV RNA; no history of diabetes; no prior pegylated interferon therapy; no history of AIDS-defining conditions; and noninitiation of ART (estimates not shown).
Table 2.
Predictors of Antiretroviral Therapy Initiation
| Variable | Adjusteda HR (95% CI) | P for Trend |
|---|---|---|
| Baseline variables | ||
| Year at baseline >2000 | 1.22 (1.16–1.29) | <.001 |
| Race | ||
| White non-Hispanic | Referent | .002b |
| Black non-Hispanic | 0.90 (.85–.95) | |
| Hispanic | 0.89 (.82–.97) | |
| Other | 0.95 (.81–1.11) | |
| Drug abuse condition | 0.85 (.81–.90) | <.001 |
| Time-dependent variables | ||
| Current CD4, cells/µL | ||
| <50 | 3.37 (2.81–4.02) | <.001 |
| 50–199 | 3.38 (2.93–3.89) | |
| 200–349 | 2.54 (2.25–2.86) | |
| 350–499 | 1.56 (1.40–1.74) | |
| ≥500 | Referent | |
| Current HIV RNA, copies/mL | ||
| ≤400 | Referent | <.001 |
| 401–10 000 | 0.91 (.83–1.00) | |
| 10 001–100 000 | 1.05 (.95–1.16) | |
| >100 000 | 1.20 (1.07–1.35) | |
| AIDS-defining condition | 1.40 (1.31–1.50) | <.001 |
Patients missing values at initiation: current CD4 (33 [0.5%]); current HIV RNA (13 [0.2%]).
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus; HR, hazard ratio.
a The adjusted model contains all the variables listed in the table, as well as baseline measures of the time-dependent variables for purposes of creating stabilized inverse probability weights.
b Type 3 P value assessing race categories as a whole, testing whether any of the β estimates are significantly different from each other.
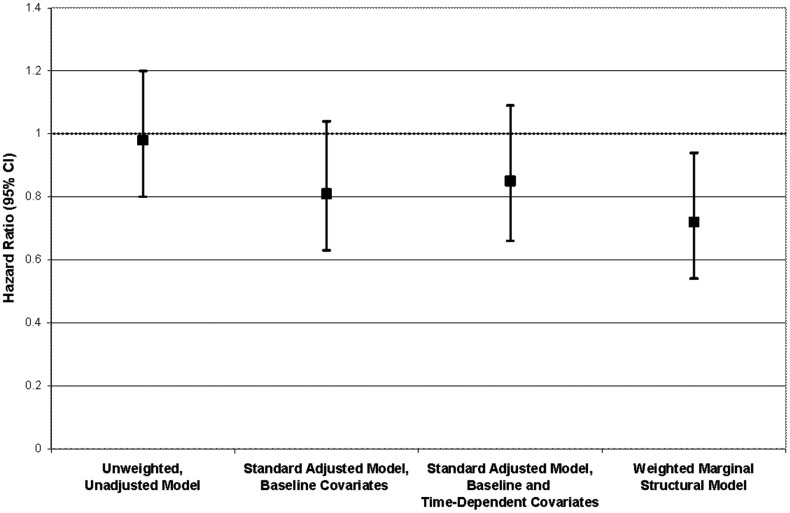
Hepatic decompensation events occurred in 645 individuals (6.4%; incidence rate, 1.4/100 person-years). A frequency distribution of qualifying diagnoses is given in Table 3. Most (82.3%) included a diagnosis of ascites. Of those who initiated ART, 457 events occurred in 35 553 person-years, whereas 188 events occurred in 10 891 person-years among those who had not yet initiated ART. In the primary marginal structural model, the hazard ratio of hepatic decompensation for ART initiation vs noninitiation was 0.72 (95% CI, .54–.94), representing a 28% average reduction in the rate of hepatic decompensation for treated vs untreated within levels of baseline covariates (Table 4). As illustrated in Figure 2, the estimate from the marginal structural model was more pronounced and conclusive when compared to those from unweighted models (unadjusted HR = 0.98; 95% CI, .80–1.20; baseline covariate adjusted HR = 0.81; 95% CI, .63–1.04; baseline and time-varying covariate adjusted HR = 0.85; 95% CI, .66–1.09). In evaluating whether the effect of ART initiation on hepatic decompensation changed with time, we found a suggestion of increased protection over time (Table 4).
Table 3.
Distribution of Qualifying Diagnoses for the 645 Hepatic Decompensation Events
| Diagnoses | No. | % |
|---|---|---|
| Ascites only | 445 | 69.0 |
| Variceal hemorrhage only | 65 | 10.1 |
| Ascites plus variceal hemorrhage | 49 | 7.6 |
| Spontaneous bacterial peritonitis only | 46 | 7.1 |
| Ascites plus spontaneous bacterial peritonitis | 37 | 5.7 |
| Spontaneous bacterial peritonitis plus variceal hemorrhage | 3 | 0.5 |
Table 4.
Hazard Ratios for Hepatic Decompensation by Antiretroviral Therapy Initiation Status and Time Since Initiation From Marginal Structural Modelsa
| Variable | Person-Years | Events | HR (95% CI) |
|---|---|---|---|
| Total | 46 444 | 645 | … |
| No initiation | 10 891 | 188 | Referent |
| ART initiation | 35 553 | 457 | 0.72 (.54–.94) |
| No initiation | 10 891 | 188 | Referent |
| <2 y since initiation | 10 727 | 154 | 0.75 (.56–1.01) |
| 2 to <4 y since initiation | 8560 | 109 | 0.69 (.46–1.03) |
| ≥4 y since initiation | 16 266 | 194 | 0.53 (.34–.83) |
Abbreviations: ART, antiretroviral therapy; CI, confidence interval; HR, hazard ratio.
a Stabilized inverse probability of treatment/death/censoring weights, based on covariate history at each time point, were applied to models. Estimates are adjusted for the following baseline covariates: year (<2001, ≥2001); race/ethnicity (white non-Hispanic, black non-Hispanic, Hispanic, other/unknown); history of drug abuse (yes, no); CD4 count (<50, 50–199, 200–349, 350–499, ≥500 cells/µL); HIV RNA load (≤400, 401–10 000, 10 001–100 000, >100 000 copies/mL); FIB-4 score (<1.45 [mild fibrosis], 1.45–3.25 [moderate fibrosis], >3.25 [advanced fibrosis]); history of AIDS-defining diagnoses (yes, no); age (<40, 40–49, 50–59, ≥60 years); history of diabetes (yes, no); and history of pegylated interferon therapy (yes, no).
Figure 2.
Comparison between marginal structural model and standard (unweighted) Cox model estimates of the hazard ratio for hepatic decompensation for antiretroviral therapy initiators vs noninitiators. Abbreviation: CI, confidence interval.
To account for possible misclassification of ART-naive individuals at baseline, we repeated the primary analyses after removing the 1876 individuals with no evidence of ART yet highly controlled HIV viremia (≤400 copies/mL) at entry. In this restricted study population, ART initiation was associated with a 41% decreased rate of hepatic decompensation relative to noninitiation (HR = 0.59; 95% CI, .43–.82; Table 5). In contrast to the original study population, there was no evidence that estimates of the hazard ratio changed with time from ART initiation (Table 5). Comparison of marginal structural model estimates to unweighted models in the restricted study population followed a similar pattern to those reported above (not shown). Information on additional sensitivity analyses related to modeling parameters is given in the Supplementary Appendix.
Table 5.
Hazard Ratios for Hepatic Decompensation by Antiretroviral Therapy Initiation Status and Time Since Initiation From Marginal Structural Modelsa, Among Patients With Baseline HIV RNA >400 copies/mL (N = 8214)
| Treatment Status | HR (95% CI) |
|---|---|
| No initiation | Referent |
| ART initiation | 0.59 (.43–.82) |
| No initiation | Referent |
| <2 y since initiation | 0.60 (.42–.84) |
| 2 to <4 y since initiation | 0.60 (.37–.96) |
| ≥4 y since initiation | 0.53 (.31–.91) |
Abbreviations: ART, antiretroviral therapy; CI, confidence interval; HR, hazard ratio.
a Stabilized inverse probability of treatment/death/censoring weights, based on covariate history at each time point, were applied to models. Estimates are adjusted for the following baseline covariates: year (<2001, ≥2001); race/ethnicity (white non-Hispanic, black non-Hispanic, Hispanic, other/unknown); history of drug abuse (yes, no); CD4 count (<50, 50–199, 200–349, 350–499, ≥500 cells/µL); HIV RNA load (≤400, 401–10 000, 10 001–100 000, >100 000 copies/mL); FIB-4 score (<1.45 [mild fibrosis], 1.45–3.25 [moderate fibrosis], >3.25 [advanced fibrosis]); history of AIDS-defining diagnoses (yes, no); age (<40, 40–49, 50–59, ≥60 years); history of diabetes (yes, no); and history of pegylated interferon therapy (yes, no).
DISCUSSION
Initiation of ART significantly reduced the rate of hepatic decompensation events by 28%–41% on average in this cohort of HIV/HCV-coinfected veterans. In applying marginal structural model methods, we sought to evaluate the impact of ART on clinical liver disease progression without the inherent biases of standard regression modeling of longitudinal data, by appropriately addressing confounding by indication and selective attrition related to death and dropout. The hazard ratios estimated from marginal structural models were more strongly protective than those from unweighted models, suggesting residual bias in results from standard models. Our results favor treatment of HIV in patients coinfected with HCV, and provide direct evidence to support recent clinical guidelines recommending consideration of ART initiation in HIV/HCV-coinfected individuals, regardless of CD4 cell count [37, 38].
Prior studies of the association between ART and liver outcomes among those coinfected with HIV and HCV have collectively been indirect and inconclusive. Protective [6, 18, 21, 22], harmful [20, 21], and null [22, 23] associations of ART with outcomes related to progressive liver fibrosis have been reported. In addition to conflicting results, these studies were reliant on liver biopsies and thus may have been susceptible to selection and observation biases. Additional studies have explored the effect of ART on liver-related mortality and similarly reported protective [8, 11], harmful [39], and null [40] associations. Our study is novel in that it represents the first to apply causal inference methods to estimate the effect of ART on the rate of hepatic decompensation events.
This study has limitations to be considered when interpreting the results. First, information on HCV viral load and genotype was not uniformly recorded. However, where information was available, HCV genotype was not associated with hepatic decompensation (Table 1), and it is unlikely that either variable would be used by clinicians as a primary indication for initiating ART; thus, they are not likely to be strong confounders. It is possible that without information on HCV RNA, some patients may have been misclassified as HCV-coinfected, which could have diluted the association to some degree. Second, we did not have detailed information on possibly relevant lifestyle and/or behavioral factors; for alcohol and drug intake specifically, we used alcohol/drug-related diagnoses as proxy measures. Although these validated measures had low sensitivity (18%), they demonstrated a high specificity (94%) in the VA population [29]. Third, our data represent medical records for male veterans attending clinics at unstructured intervals; it is uncertain how generalizable our results would be to other settings or populations with different frequencies of clinic visits. Fourth, our outcomes were derived from documented ICD-9 diagnoses and not directly confirmed, although the coding algorithm we used to define outcomes demonstrated a high positive predictive value (91%) [32]. Misclassification of outcomes in this context is likely to be random; thus, residual bias should be toward the null value, leaving the possibility of an underestimate of the causal effect. Furthermore, a certain degree of treatment status misclassification was likely introduced by our intent-to-treat assumption and strict definition of ART, as adherence to treatment status may have been incomplete. Additionally, some individuals may have received undocumented antiretrovirals outside of the VA healthcare system and thus contributed unexposed person-time prior to their first ART regimen in the VA system. However, by repeating the primary analyses using a validated algorithm with a positive predictive value for identifying ART-naive patients of 86%, we have attempted to account for such treatment misclassification. Indeed, removal of individuals who were likely ART experienced at entry resulted in a more pronounced protection against hepatic decompensation.
In conclusion we have demonstrated an average 28%–41% reduction in the rate of hepatic decompensation for ART initiators relative to noninitiators in our study population of HIV/HCV-coinfected male veterans. Our study utilized inverse probability weighting methods to account for confounding by indication, and paralleled the intent-to-treat analysis of a hypothetical randomized trial. Taken together with the body of available literature, our results suggest a significant benefit of ART for coinfected patients, and serve to inform therapeutic strategies as caregivers face the continuing challenges of chronic hepatitis in HIV-infected individuals.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Disclaimer. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs.
Financial support. This work was supported by the National Institutes of Health (grant numbers T32 AI007358 and T32 AI007535 to J. P. A.). The Veterans Aging Cohort Study has been funded by the National Institute on Alcohol Abuse and Alcoholism (grant numbers U01 AA13566, U24 AA020794, U01 AA020790); the National Heart, Lung and Blood Institute (grant numbers R01 HL095136, R01 HL090342, RCI HL100347); the National Institute of Allergy and Infectious Diseases (grant number U01 A1069918); the Veterans Health Administration Office of Research and Development (grant number VA REA 08266); and an interagency agreement between the National Institute on Aging, the National Institute of Mental Health, and the Veterans Health Administration.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Antiretroviral Therapy Cohort Collaboration Causes of death in HIV-1-infected patients treated with antiretroviral therapy, 1996–2006: collaborative analysis of 13 HIV cohort studies. Clin Infect Dis. 2010;50:1387–96. doi: 10.1086/652283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith C, Sabin CA, Lundgren JD, et al. Factors associated with specific causes of death amongst HIV-positive individuals in the D:A:D Study. AIDS. 2010;24:1537–48. doi: 10.1097/QAD.0b013e32833a0918. [DOI] [PubMed] [Google Scholar]
- 3.Graham CS, Baden LR, Yu E, et al. Influence of human immunodeficiency virus infection on the course of hepatitis C virus infection: a meta-analysis. Clin Infect Dis. 2001;33:562–9. doi: 10.1086/321909. [DOI] [PubMed] [Google Scholar]
- 4.Alter MJ, Kruszon-Moran D, Nainan OV, et al. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N Engl J Med. 1999;341:556–62. doi: 10.1056/NEJM199908193410802. [DOI] [PubMed] [Google Scholar]
- 5.Sherman KE, Rouster SD, Chung RT, Rajicic N. Hepatitis C virus prevalence among patients infected with human immunodeficiency virus: a cross-sectional analysis of the US adult AIDS Clinical Trials Group. Clin Infect Dis. 2002;34:831–7. doi: 10.1086/339042. [DOI] [PubMed] [Google Scholar]
- 6.Benhamou Y, Di Martino V, Bochet M, et al. Factors affecting liver fibrosis in human immunodeficiency virus-and hepatitis C virus-coinfected patients: impact of protease inhibitor therapy. Hepatology. 2001;34:283–7. doi: 10.1053/jhep.2001.26517. [DOI] [PubMed] [Google Scholar]
- 7.Di Martino V, Rufat P, Boyer N, et al. The influence of human immunodeficiency virus coinfection on chronic hepatitis C in injection drug users: a long-term retrospective cohort study. Hepatology. 2001;34:1193–9. doi: 10.1053/jhep.2001.29201. [DOI] [PubMed] [Google Scholar]
- 8.Merchante N, Giron-Gonzalez JA, Gonzalez-Serrano M, et al. Survival and prognostic factors of HIV-infected patients with HCV-related end-stage liver disease. AIDS. 2006;20:49–57. doi: 10.1097/01.aids.0000198087.47454.e1. [DOI] [PubMed] [Google Scholar]
- 9.Mohsen AH, Easterbrook PJ, Taylor C, et al. Impact of human immunodeficiency virus (HIV) infection on the progression of liver fibrosis in hepatitis C virus infected patients. Gut. 2003;52:1035–40. doi: 10.1136/gut.52.7.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pineda JA, Garcia-Garcia JA, Aguilar-Guisado M, et al. Clinical progression of hepatitis C virus-related chronic liver disease in human immunodeficiency virus-infected patients undergoing highly active antiretroviral therapy. Hepatology. 2007;46:622–30. doi: 10.1002/hep.21757. [DOI] [PubMed] [Google Scholar]
- 11.Qurishi N, Kreuzberg C, Luchters G, et al. Effect of antiretroviral therapy on liver-related mortality in patients with HIV and hepatitis C virus coinfection. Lancet. 2003;362:1708–13. doi: 10.1016/S0140-6736(03)14844-1. [DOI] [PubMed] [Google Scholar]
- 12.Bonnard P, Lescure FX, Amiel C, et al. Documented rapid course of hepatic fibrosis between two biopsies in patients coinfected by HIV and HCV despite high CD4 cell count. J Viral Hepat. 2007;14:806–11. doi: 10.1111/j.1365-2893.2007.00874.x. [DOI] [PubMed] [Google Scholar]
- 13.Collazos J, Carton JA, Asensi V. Immunological status does not influence hepatitis C virus or liver fibrosis in HIV-hepatitis C virus-coinfected patients. AIDS Res Hum Retroviruses. 2011;27:383–9. doi: 10.1089/aid.2010.0168. [DOI] [PubMed] [Google Scholar]
- 14.Mendes-Correa MC, Widman A, Brussi ML, Guastini CF, Gianini RJ. Incidence and predictors of severe liver fibrosis in HIV-infected patients with chronic hepatitis C in Brazil. AIDS Patient Care STDS. 2008;22:701–7. doi: 10.1089/apc.2007.0216. [DOI] [PubMed] [Google Scholar]
- 15.Sulkowski MS, Thomas DL, Chaisson RE, Moore RD. Hepatotoxicity associated with antiretroviral therapy in adults infected with human immunodeficiency virus and the role of hepatitis C or B virus infection. JAMA. 2000;283:74–80. doi: 10.1001/jama.283.1.74. [DOI] [PubMed] [Google Scholar]
- 16.Sulkowski MS, Thomas DL, Mehta SH, Chaisson RE, Moore RD. Hepatotoxicity associated with nevirapine or efavirenz-containing antiretroviral therapy: role of hepatitis C and B infections. Hepatology. 2002;35:182–9. doi: 10.1053/jhep.2002.30319. [DOI] [PubMed] [Google Scholar]
- 17.Shelburne SA, 3rd, Hamill RJ, Rodriguez-Barradas MC, et al. Immune reconstitution inflammatory syndrome: emergence of a unique syndrome during highly active antiretroviral therapy. Medicine. 2002;81:213–27. doi: 10.1097/00005792-200205000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Tural C, Fuster D, Tor J, et al. Time on antiretroviral therapy is a protective factor for liver fibrosis in HIV and hepatitis C virus (HCV) co-infected patients. J Viral Hepat. 2003;10:118–25. doi: 10.1046/j.1365-2893.2003.00413.x. [DOI] [PubMed] [Google Scholar]
- 19.Berenguer J, Bellon JM, Miralles P, et al. Association between exposure to nevirapine and reduced liver fibrosis progression in patients with HIV and hepatitis C virus coinfection. Clin Infect Dis. 2008;46:137–43. doi: 10.1086/524080. [DOI] [PubMed] [Google Scholar]
- 20.Fuster D, Planas R, Muga R, et al. Advanced liver fibrosis in HIV/HCV-coinfected patients on antiretroviral therapy. AIDS Res Hum Retroviruses. 2004;20:1293–7. doi: 10.1089/aid.2004.20.1293. [DOI] [PubMed] [Google Scholar]
- 21.Macias J, Castellano V, Merchante N, et al. Effect of antiretroviral drugs on liver fibrosis in HIV-infected patients with chronic hepatitis C: harmful impact of nevirapine. AIDS. 2004;18:767–74. doi: 10.1097/00002030-200403260-00007. [DOI] [PubMed] [Google Scholar]
- 22.Macias J, Mira JA, Lopez-Cortes LF, et al. Antiretroviral therapy based on protease inhibitors as a protective factor against liver fibrosis progression in patients with chronic hepatitis C. Antivir Ther. 2006;11:839–46. [PubMed] [Google Scholar]
- 23.Mehta SH, Thomas DL, Torbenson M, et al. The effect of antiretroviral therapy on liver disease among adults with HIV and hepatitis C coinfection. Hepatology. 2005;41:123–31. doi: 10.1002/hep.20541. [DOI] [PubMed] [Google Scholar]
- 24.Robins JM, Hernan MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11:550–60. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 25.Fultz SL, Skanderson M, Mole LA, et al. Development and verification of a “virtual” cohort using the National VA Health Information System. Med Care. 2006;44(8 suppl 2):S25–30. doi: 10.1097/01.mlr.0000223670.00890.74. [DOI] [PubMed] [Google Scholar]
- 26.Kramer JR, Davila JA, Miller ED, Richardson P, Giordano TP, El-Serag HB. The validity of viral hepatitis and chronic liver disease diagnoses in Veterans Affairs administrative databases. Aliment Pharmacol Ther. 2008;27:274–82. doi: 10.1111/j.1365-2036.2007.03572.x. [DOI] [PubMed] [Google Scholar]
- 27.Thomas DL, Astemborski J, Rai RM, et al. The natural history of hepatitis C virus infection: host, viral, and environmental factors. JAMA. 2000;284:450–6. doi: 10.1001/jama.284.4.450. [DOI] [PubMed] [Google Scholar]
- 28.Gandhi NR, Tate JP, Rodriguez-Barradas MC, et al. Validation of an algorithm to identify antiretroviral-naïve status at time of entry into a large, observational cohort of HIV-infected patients. Pharmacoepidemiol Drug Saf. 2013;22:1019–25. doi: 10.1002/pds.3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McGinnis KA, Justice AC, Kraemer KL, Saitz R, Bryant KJ, Fiellin DA. Comparing alcohol screening measures among HIV infected and uninfected men. Alcohol Clin Exp Res. 2013;37:435–42. doi: 10.1111/j.1530-0277.2012.01937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317–25. doi: 10.1002/hep.21178. [DOI] [PubMed] [Google Scholar]
- 31.Centers for Disease Control and Prevention 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep. 1992;41(RR-17):1–19. [PubMed] [Google Scholar]
- 32.Lo Re V, 3rd, Lim JK, Goetz MB, et al. Validity of diagnostic codes and liver-related laboratory abnormalities to identify hepatic decompensation events in the Veterans Aging Cohort Study. Pharmacoepidemiol Drug Saf. 2011;20:689–99. doi: 10.1002/pds.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hernan MA, Brumback B, Robins JM. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology. 2000;11:561–70. doi: 10.1097/00001648-200009000-00012. [DOI] [PubMed] [Google Scholar]
- 34.Cole SR, Hernan MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol. 2008;168:656–64. doi: 10.1093/aje/kwn164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weuve J, Tchetgen Tchetgen EJ, Glymour MM, et al. Accounting for bias due to selective attrition: the example of smoking and cognitive decline. Epidemiology. 2012;23:119–28. doi: 10.1097/EDE.0b013e318230e861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sarkar M, Bacchetti P, French AL, et al. Lower liver-related death in African-American women with human immunodeficiency virus/hepatitis C virus coinfection, compared to Caucasian and Hispanic women. Hepatology. 2012;56:1699–705. doi: 10.1002/hep.25859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brook G, Main J, Nelson M, et al. British HIV Association guidelines for the management of coinfection with HIV-1 and hepatitis B or C virus 2010. HIV Med. 2010;11:1–30. doi: 10.1111/j.1468-1293.2009.00781.x. [DOI] [PubMed] [Google Scholar]
- 38.Thompson MA, Aberg JA, Hoy JF, et al. Antiretroviral treatment of adult HIV infection: 2012 recommendations of the International Antiviral Society-USA panel. JAMA. 2012;308:387–402. doi: 10.1001/jama.2012.7961. [DOI] [PubMed] [Google Scholar]
- 39.Weber R, Sabin CA, Friis-Moller N, et al. Liver-related deaths in persons infected with the human immunodeficiency virus: the D:A:D Study. Arch Intern Med. 2006;166:1632–41. doi: 10.1001/archinte.166.15.1632. [DOI] [PubMed] [Google Scholar]
- 40.Mocroft A, Soriano V, Rockstroh J, et al. Is there evidence for an increase in the death rate from liver-related disease in patients with HIV? AIDS. 2005;19:2117–25. doi: 10.1097/01.aids.0000194799.43799.ea. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.