We present the results of a nationwide survey regarding HIV preexposure prophylaxis (PrEP) opinions and practices of infectious diseases physicians. Providers supported PrEP, but very few had provided it. Despite CDC guidance, real-world PrEP practices were highly variable.
Keywords: PrEP, HIV, survey, providers
Abstract
Background. Preexposure prophylaxis (PrEP) with tenofovir disoproxil fumarate and emtricitabine (Truvada) has demonstrated efficacy in placebo-controlled clinical trials involving men who have sex with men, high-risk heterosexuals, serodiscordant couples, and intravenous drug users. To assist in the real-world provision of PrEP, the Centers for Disease Control and Prevention (CDC) has released guidance documents for PrEP use.
Methods. Adult infectious disease physicians were surveyed about their opinions and current practices of PrEP through the Emerging Infections Network (EIN). Geographic information systems analysis was used to map out provider responses across the United States.
Results. Of 1175 EIN members across the country, 573 (48.8%) responded to the survey. A majority of clinicians supported PrEP but only 9% had actually provided it. Despite CDC guidance, PrEP practices were variable and clinicians reported many barriers to its real-world provision.
Conclusions. The majority of adult infectious disease physicians across the United States and Canada support PrEP but have vast differences of opinion and practice, despite the existence of CDC guidance documents. The success of real-world PrEP will likely require multifaceted programs addressing barriers to its provision and will be assisted with the development of comprehensive guidelines for real-world PrEP.
Implementation of measures to prevent the spread of human immunodeficiency virus (HIV), such as perinatal antiretroviral prophylaxis [1], needle-syringe programs in intravenous drug users (IVDUs) [2], and antiretroviral treatment as prevention [3], are having a dramatic impact on the HIV epidemic [4–6]. The observation that HIV preexposure prophylaxis (PrEP) decreases the risk of HIV infection in clinical trials of high-risk men who have sex with men (MSM), HIV-serodiscordant couples, heterosexual persons in areas of high HIV incidence [7–10] and IVDUs [11] has generated enthusiasm [12]. However, 2 studies of PrEP in women were not promising, with the Female Preexposure Prophylaxis (FEM-PrEP) [13] study requiring an early discontinuation for futility and the Vaginal and Oral Interventions to Control the Epidemic (VOICE) study reporting no effect in the intention-to-treat analyses [14]. The divergent results of PrEP studies are largely attributed to differences in PrEP adherence between studies, but other potential factors (biologic differences or complex sociodemographic issues) have not been evaluated [7]. The divergent results also raise concerns about the feasibility and efficacy of real-world PrEP. To assist clinicians in the implementation of PrEP, the Centers for Disease Control and Prevention (CDC) has published guidance documents detailing how to determine eligibility, begin, follow up, and discontinue PrEP [15, 16] until comprehensive US Public Health Service guidelines are available. The main purpose of this survey was to assess provider opinions, readiness, and current practices of PrEP in the United States and Canada.
METHODS
Study Population
The Infectious Diseases Society of America's (IDSA) Emerging Infections Network (EIN) is a provider-based network of infectious disease physicians actively involved in clinical practice who belong to IDSA. In June 2013, this electronic survey was sent by staff at the EIN coordinating center to members with an adult infectious disease practice. We sent 2 reminders to nonresponders at 1-week intervals.
PrEP Survey
A 10-question survey was developed to evaluate the current practices and attitudes of PrEP among infectious disease experts who are members of the EIN. This survey included a screening question to elicit provider opinions about PrEP in general. For participants who had or would provide PrEP, our survey inquired about the participant's HIV practice, to whom they had provided or would provide PrEP, how they assess eligibility, how they measure adherence, when PrEP would be discontinued, and what perceived barriers exist.
Geographic Information System
ArcGIS (ESRI, Redlands, California) was utilized to provide geographic visualization of provider responses from the survey. All surveys were geo-tagged according to the location of the provider's practice. To ensure provider anonymity, only the first 3 digits of the participating provider's practice zip code was used. Responses to questions 1, 2, 3, and 10 were mapped. We did not include the number of respondents per area. Given that Canadian respondents were only coded by country, we elected not to include them in our GIS analysis.
Statistical Analyses
For comparisons between respondents and nonrespondents, only active query members who have previously participated in at least 1 EIN survey were included (n = 115; the EIN has a percentage of members who register but never participate in the query process and they were not included). We analyzed the data using SAS software, version 9.3 (SAS Institute). We calculated differences in responses between participants who prescribed PrEP and those who would prescribe PrEP but had not yet prescribed it. For questions in which participants could select only 1 response, we used Fisher exact test. For questions in which participants could select >1 response, we used χ2 tests with a second-order Rao-Scott adjustment. For multiresponse questions, individual responses were analyzed separately using Fisher exact test.
RESULTS
Survey Population
Of 1175 active physician members, 573 (48.8%) responded to this survey from 5 June 2013 to 7 July 2013. Respondents included broad representation, from the United States and Canada with 51% of members responding from New England, 53% from the Mid-Atlantic, 49% from East North Central, 46% from West North Central, 46% from the South Atlantic, 45% from East South Central, 63% from West South Central, 42% from Mountain, and 50% from Pacific, and 35% from Canada (no responses were received from members in Puerto Rico). A similar breadth existed when respondents were evaluated by type of employment with responses from 48% of the members employed by hospital/clinic, 48% private practice, 49% university/medical school, and 49% VA and military. Respondents were significantly more likely than nonrespondents to have had at least 15 years of infectious disease experience (P < .0001). This response rate and difference in demographics is typical of EIN surveys [17, 18].
Providers Are Willing to Provide PrEP, but Few Have
A majority of respondents support the provision of PrEP (74%), but a fair proportion remained unsure (14%), and 12% did not support PrEP. Despite this strong support for PrEP, only 9% had actually provided PrEP, 43% had not provided PrEP but would, 34% believed PrEP was not relevant to their practice, and 14% would not provide PrEP. When asked why physicians would not provide PrEP, 77% were worried about adherence and the risk for future resistance, 57% were concerned about cost and reimbursement issues, 53% did not want to use potentially toxic drugs in healthy persons, and 53% felt there was insufficient evidence for the efficacy of real-world PrEP (Table 1). Other reasons for not prescribing PrEP included risk compensation (“Efficacy is limited and creates a false sense of security”; “Concern about irresponsible sexual activity”; “If they won't use condoms they won't use pills”), a lack of resources and information (“Don't have the capacity to see the potential large number”; “Don't know enough about it to feel comfortable”; “Lack of effectiveness data in local context”), limited resource allocation (“There are better prophylactics”; “Transmission can be prevented without medications”; “Concern about the selling of [PrEP] from HIV positives to HIV negative”) and personal ideology (“Moral issues”; “Medicine should not attempt to reverse bad behaviors artificially”; “The balance is not right in terms of risks/benefits”).
Table 1.
Survey Results
| Questions | No. (%) |
|---|---|
| Do you support the provision of HIV PrEP to at-risk individualsa | |
| No | 57 (12) |
| Yes | 351 (74) |
| Unsure | 66 (14) |
| Have you (or would you) provide PrEP? (select all that apply) | |
| Have not provided PrEP but would | 248 (43) |
| Have provided PrEP | 51 (9) |
| PrEP is not relevant to my practice | 197 (34) |
| Have not or would not provide PrEP because (all that apply): | 77 (14) |
| Concern about compliance and future resistance | 59 (77) |
| Cost/payer issues | 44 (57) |
| Concerns about potentially toxic drugs in healthy persons | 41 (53) |
| Insufficient evidence for efficacy of real-world PrEP | 41 (53) |
| Other reasons | 14 (18) |
| Rank order of barriers to provision of PrEPb, sum of ranks | |
| Time consuming to counsel and assess adherence | 835 |
| Starting a potentially toxic drug in healthy persons | 928 |
| Future drug resistance | 944 |
| Too costly and patients can't afford it | 988 |
| Patient population is not at risk for HIV infection | 813 |
| Concerns about efficacy of real-world PrEP | 911 |
For several of the questions, participants were asked to select all the answers that would apply; thus, the sum of percentages do not always equal 100.
Abbreviations: HIV, human immunodeficiency virus; PrEP, preexposure prophylaxis.
a Ninety-nine participants left this question blank and skipped to question 2 and filled out “PrEP is not relevant to my practice.”
b Each respondent ranked barriers from least important barrier (1) to greatest barrier (6). The number reported reflects the sum of all the ranks, so the overall least important barrier has the lowest number and the overall greatest barrier has the highest number.
Providers Would Use PrEP for Patients With a Range of Risk Factors
Respondents who had provided PrEP or who would provide PrEP were then asked questions regarding the “real-world” use of PrEP. A majority of respondents stated they would provide PrEP if their patients had risk factors. Similarly, a majority of respondents would provide PrEP if their patient requested it. The main risk factor that would prompt PrEP was an HIV-infected partner who was not on highly active antiretroviral therapy (HAART), followed by reported unprotected sex, multiple sex partners, and patients with an HIV-infected partner on HAART. A minority of respondents would provide PrEP for intravenous drug users (Table 2). Other reasons physicians would prescribe PrEP included for “discordant couple who are attempting to get pregnant,” in a “abusive or coercive sexual relationship,” for “heterosexual commercial sex workers,” and for “transgender females with risk factors.”
Table 2.
Differences Between Participants Who Provided Preexposure Prophylaxis (PrEP) Compared to Participants Who Would Provide PrEP
| Characteristics | Provided PrEP in Past Year, No. (%) |
Total (n = 285a) | P Value | |
|---|---|---|---|---|
| No (n = 238) | Yes (n = 47) | |||
| Region | .89 | |||
| New England/Mid-Atlantic | 58 (24.4) | 10 (21.3) | 68 (23.9) | |
| Midwest | 53 (22.3) | 13 (27.7) | 66 (23.2) | |
| South | 69 (29.0) | 12 (25.5) | 81 (28.4) | |
| Pacific and Mountain | 56 (23.5) | 12 (25.5) | 68 (23.9) | |
| Canada | 2 (0.8) | 0 (0.0) | 2 (0.7) | |
| Type of employment | .12 | |||
| Hospital/clinic | 67 (28.2) | 16 (34.0) | 83 (29.1) | |
| Private/group practice | 77 (32.4) | 18 (38.3) | 95 (33.3) | |
| University | 73 (30.7) | 13 (27.7) | 86 (30.2) | |
| Federal/VA | 21 (8.8) | 0 (0.0) | 21 (7.4) | |
| Treated HIV patients in past year | .04 | |||
| None | 10 (4.2) | 0 (0.0) | 10 (3.5) | |
| 1–20 | 31 (13.0) | 4 (8.5) | 35 (12.3) | |
| 21–50 | 59 (24.8) | 9 (19.1) | 68 (23.9) | |
| >50 | 138 (58.0) | 34 (72.3) | 172 (60.4) | |
| Would provide PrEP | ||||
| To MSMb (n = 268) | .07 | |||
| Regardless of risk factors | 22 (9.8) | 10 (22.7) | 32 (11.9) | |
| With certain risk factors | 167 (74.6) | 29 (65.9) | 196 (73.1) | |
| By request | 134 (59.8) | 30 (68.2) | 164 (61.2) | |
| To heterosexualsb (n = 269) | .36 | |||
| Regardless of risk | 12 (5.4) | 5 (10.9) | 17 (6.3) | |
| With certain risk factors | 174 (78.0) | 35 (76.1) | 209 (77.7) | |
| By request | 123 (55.2) | 28 (60.9) | 151 (56.1) | |
| Considerations when prescribing PrEP | ||||
| To MSMb (n = 267) | .40 | |||
| Reporting unprotected sex | 175 (78.1) | 35 (81.4) | 210 (78.7) | |
| Reporting multiple sex partners | 168 (75.0) | 30 (69.8) | 198 (74.2) | |
| With HIV partner on HAART | 130 (58.0) | 33 (76.7) | 163 (61.0) | |
| With HIV partner not on HAART | 201 (89.7) | 37 (86.0) | 238 (89.1) | |
| With IVDU | 76 (33.9) | 15 (34.9) | 91 (34.1) | |
| Other reasons | 5 (2.2) | 1 (2.3) | 6 (2.2) | |
| To heterosexualsb (n = 262) | .08 | |||
| With unprotected sex | 131 (60.6) | 29 (63.0) | 160 (61.1) | |
| With multiple sex partners | 132 (61.1) | 23 (50.0) | 155 (59.2) | |
| With HIV partner on HAART | 128 (59.3) | 36 (78.3) | 164 (62.6) | |
| With HIV partner not on HAART | 198 (91.7) | 37 (80.4) | 235 (89.7) | |
| With IVDU | 70 (32.4) | 12 (26.1) | 82 (31.3) | |
| Other reasons | 9 (4.2) | 5 (10.9) | 14 (5.3) | |
| Frequency of performing NAT in conjunction with PrEPb (n = 276) | .053 | |||
| Before starting PrEP | 142 (62.0) | 25 (53.2) | 167 (60.5) | |
| Only when symptoms consistent with acute HIV are present | 36 (15.7) | 17 (36.2) | 53 (19.2) | |
| Monthly | 9 (3.9) | 3 (6.4) | 12 (4.3) | |
| Every 3 mo | 119 (52.0) | 20 (42.6) | 139 (50.4) | |
| Less frequently than 3 mo | 23 (10.0) | 4 (8.5) | 27 (9.8) | |
| Never | 30 (13.1) | 4 (8.5) | 34 (12.3) | |
| Would measure PrEP adherence (n = 274) | 206 (90.7) | 40 (87.0) | 246 (90.1) | .42 |
| Method of measuring PrEP adherenceb (n = 246) | .75 | |||
| Self-report | 186 (90.3) | 37 (92.5) | 223 (90.7) | |
| Pill counts | 33 (16.0) | 4 (10.0) | 37 (15.0) | |
| Pharmacy refills | 104 (50.5) | 18 (45.0) | 122 (49.6) | |
| Blood drug levels | 6 (2.9) | 2 (5.0) | 8 (3.3) | |
| Other means | 4 (1.9) | 1 (2.5) | 5 (2.0) | |
| Frequency of measuring PrEP adherence among those who did not respond “It depends”b (n = 218) | .72 | |||
| Monthly | 31 (17.1) | 8 (21.6) | 39 (17.9) | |
| Every 3 mo | 121 (66.9) | 25 (67.6) | 146 (67.0) | |
| Every 6 mo | 28 (15.5) | 4 (10.8) | 32 (14.7) | |
| Annually | 1 (0.6) | 0 (0.0) | 1 (0.5) | |
| Reasons for discontinuing PrEPb (n = 274) | .36 | |||
| If adherence <80% | 132 (58.1) | 20 (42.6) | 152 (55.5) | |
| If adherence <50% | 76 (33.5) | 17 (36.2) | 93 (33.9) | |
| If pregnancy occurs | 119 (52.4) | 21 (44.7) | 140 (51.1) | |
| If any toxicity develops | 153 (67.4) | 36 (76.6) | 189 (69.0) | |
| If severe toxicity develops | 130 (57.3) | 22 (46.8) | 152 (55.5) | |
| If risk behavior decreases | 130 (57.3) | 23 (48.9) | 153 (55.8) | |
| If partner is virologically suppressed | 80 (35.2) | 10 (21.3) | 90 (32.8) | |
Abbreviations: HAART, highly active antiretroviral therapy; HIV, human immunodeficiency virus; IVDU, intravenous drug user; MSM, men who have sex with men; NAT, nucleic acid testing; PrEP, preexposure prophylaxis; VA, Veterans Affairs.
a Total number of responses unless indicated otherwise in Characteristics column.
b Column percentages sum to >100% because responses were not mutually exclusive (ie, participants were able to choose >1 response for each question). P values correspond to second-order Rao-Scott adjusted χ2 test.
Providers Report Variations in PrEP Practices
When specifically asked about the use of HIV nucleic acid testing (NAT) in determining eligibility and in follow-up, physicians report they would use NAT before starting PrEP and every 3 months while on PrEP. Fifteen percent reported they would not use HIV NAT for PrEP and 19% stated they would use it only when patients had symptoms of acute HIV. In terms of measuring adherence, 81% of clinicians would rely on patient self-report, 45% would rely on pharmacy refills, 14% would use pill counts, and 3% mentioned drug levels in the blood. Eleven percent of providers would not measure adherence. Most physicians would measure PrEP adherence every 3 months, with 16% evaluating more frequently and 13% less frequently (Table 2). Other respondents commented that they would measure PrEP “More frequently early on, then less frequently if adherent”; “Depends on the patient's risk”; and “Depends on the stability of patient relationships.” PrEP discontinuation would occur most commonly with the development of any toxicity, then with severe toxicity, when adherence is <80%, when risk behavior decreases, and, least commonly, if the sexual partner of the at-risk person is virologically suppressed on HAART (Table 2).
Perceived Barriers to PrEP Are Many
Physicians who have or would give PrEP were then asked to score barriers to PrEP from 1 (least) to 6 (greatest), but no one barrier could be differentiated as carrying more weight than another (based on mean and median value). Summing the ranks of the barriers demonstrated that the cost of PrEP was the highest concern for providers, followed by future drug resistance, reluctance to start a toxic drug in healthy persons, efficacy of real-world PrEP, the view that provision is time consuming, and the belief that patients were not at risk. Based on other comments provided, physicians polled did not seem to believe they were going to be seeing patients seeking PrEP (“Ours is HIV clinic and not HIV negative care”; “PrEP needs to be in the primary care setting”), had concerns about the cost-effectiveness of PrEP (“It's an expensive condom”; “Limited money to go around—is this the best use of such monies”; “Bigger bang for the buck is getting all the HIV positive patients on ART [antiretroviral therapy] and keeping them adherent”), did not believe the potential benefits outweigh the costs (“Is virtually a model for development and spread of resistant viruses”; “Not a good idea”), and reiterated doubt for the uptake and difficulty of implementation of real-world PrEP (“I have offered PrEP to partners of HIV positive patients and they have all declined”; “The patients most at risk [appropriate for PrEP] don't come into care”; “The details need to be fleshed out in guidance a lot better”).
Differences in Practices of Persons Who Have Provided PrEP Compared to Those Who Would
To evaluate if experience impacted PrEP opinions and practices, we evaluated the differences between participants who had provided PrEP and participants who had not but would. Not surprisingly, clinicians who had provided PrEP in the last 12 months saw more persons with HIV (P = .04; Table 2). Participants who had provided PrEP seemed more likely to provide PrEP to MSM regardless of risk factors, and more likely to provide PrEP to MSM or heterosexuals with a partner on HAART (Table 3). Participants who had provided PrEP also appeared more likely to use NAT only in settings of acute HIV infection and trends existed suggesting they were more likely to continue to use PrEP with patients with adherence <80% and in persons with a virologically suppressed HIV-positive partner (Table 3). There were no differences in provider demographics between those who had provided PrEP and those that would.
Table 3.
Significant Differences in Individual Responses in Participants Who Provided Preexposure Prophylaxis (PrEP) Compared to Participants Who Would Provide PrEP
| Characteristics | Provided PrEP in Past y, No. (%) |
Total (n = 285a) | P Value | |
|---|---|---|---|---|
| No (n = 238) | Yes (n = 47) | |||
| Would provide PrEP | ||||
| To MSMb (n = 268) | ||||
| Regardless of risk factors | 22 (9.8) | 10 (22.7) | 32 (11.9) | .022 |
| Considerations when prescribing PrEP | ||||
| To MSMb (n = 267) | ||||
| With HIV partner on HAART | 130 (58.0) | 33 (76.7) | 163 (61.0) | .026 |
| To heterosexualsb (n = 262) | ||||
| With HIV partner on HAART | 128 (59.3) | 36 (78.3) | 164 (62.6) | .018 |
| With HIV partner not on HAART | 198 (91.7) | 37 (80.4) | 235 (89.7) | .032 |
| Frequency of performing NAT in conjunction with PrEPb (n = 276) | ||||
| Only when symptoms consistent with acute HIV are present | 36 (15.7) | 17 (36.2) | 53 (19.2) | .002 |
| Reasons for discontinuing PrEPb (n = 274) | ||||
| If adherence <80% | 132 (58.1) | 20 (42.6) | 152 (55.5) | .055 |
| If partner is virologically suppressed | 80 (35.2) | 10 (21.3) | 90 (32.8) | .087 |
Abbreviations: HAART, highly active antiretroviral therapy; HIV, human immunodeficiency virus; MSM, men who have sex with men; NAT, nucleic acid testing; PrEP, preexposure prophylaxis.
a Total number of responses unless indicated otherwise in Characteristics column.
b Column percentages sum to >100% because responses were not mutually exclusive (ie, participants were able to choose >1 response for each question). P values correspond to second-order Rao-Scott adjusted χ2 test.
Interestingly, physicians who had provided PrEP had mixed feelings about the practice, with comments such as “This will never impact the overall incidence of HIV in the US”; “Not all are convinced by the data that is presented”; and “If a person has an HIV positive partner who is not on HAART, would first counsel HAART.”
A Geographic Dimension to Survey Responses
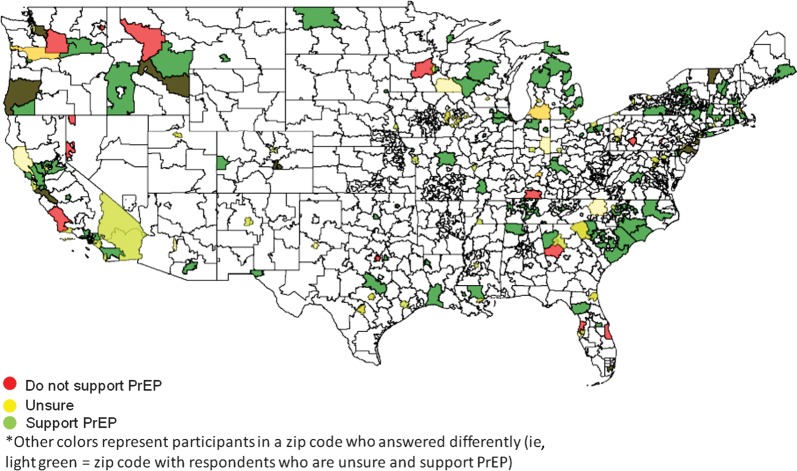
To illustrate the responses of physicians across the United States, ArcGIS software was utilized (Supplementary Figure 1). Respondents who support the idea of PrEP and those who do not did not appear to be clustered geographically. In fact, both populations were spread across the country and even within the same zip codes (Figure 1). Persons who had provided PrEP or would provide PrEP were also widespread and not exclusively located in areas of high HIV prevalence (Figure 2). Top-ranked barriers were also widespread, with most areas containing providers that prioritized different barriers (Supplementary Figure 2A–F). Statistical analyses of regional differences revealed that the only barrier that appeared to differ based on region was that provision of PrEP was time consuming, with providers in the Pacific and Mountain regions ranking this highly compared to providers in New England and the Mid-Atlantic (P = .0005; Supplementary Table 1).
Figure 1.
Geographic distribution of persons based on support of preexposure prophylaxis (PrEP). ArcGIS was utilized to map survey respondents based on the first 3 numbers of the provider's zip code. The number of providers responding was not included for simplicity. Red-colored zip codes contain at least 1 provider who does not support PrEP. Yellow-colored zip codes contain at least 1 provider who is unsure of PrEP. Green-colored zip codes represent at least 1 provider who supports PrEP. Zip codes that include providers with differences of opinion are a combination of colors (ie, orange zip codes have at least 1 provider who does not support PrEP and at least 1 provider who is unsure). Most providers surveyed support PrEP (green). Those who do not support PrEP or are unsure are spread across the country and do not appear to regionally cluster.
Figure 2.
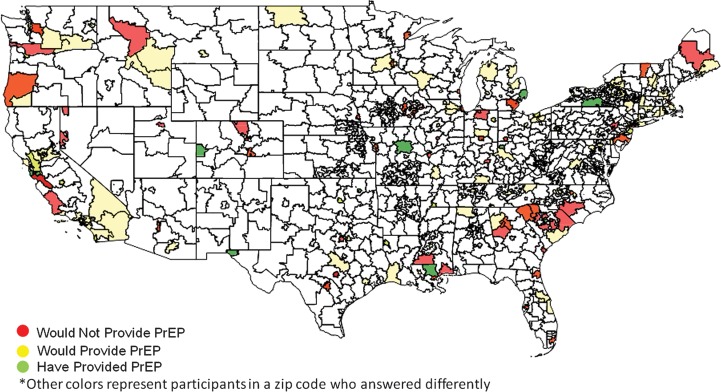
Geographic distribution of persons based on provision of preexposure prophylaxis (PrEP). Very few clinicians have actually provided PrEP (green); a larger percentage would provide PrEP (yellow) and would not provide PrEP (red). For simplicity, providers who commented that PrEP was not relevant to their practice were not included. Interestingly, there were several zip codes that contained providers with polar opinions, including persons who would not provide PrEP and those who would (orange) and providers who would not provide PrEP and those who had (dark red).
DISCUSSION
We provide the results of the largest survey to date of infectious disease physicians' opinions and practices of PrEP across the United States and Canada [19–21]. Following the release of CDC guidance documents and US Food and Drug Administration approval of Truvada for PrEP, strong support exists for PrEP, but very few clinicians (9%) had actually provided it. Additionally, a wide range of PrEP practices existed among those who have or would give PrEP, including differences in deciding who is eligible for PrEP, how persons on PrEP are followed up, and how PrEP is discontinued. Barriers to the provision of PrEP were many, with concerns about PrEP efficacy in the real world being the greatest concern. A previous study of in-depth interviews of healthcare providers (n = 22) performed in 2011 in San Francisco, Oakland, and Los Angeles revealed similar concerns with providers reporting little demand for PrEP, disagreement about appropriate PrEP patients, and insufficient capacity for PrEP despite beliefs in the potential for PrEP to impact the HIV epidemic [19].
Of interest was the finding that despite CDC guidance documents, great variability exists in the real-world practice of PrEP, suggesting either unawareness of, disagreement with, or ambiguity of CDC guidance (we did not ask specific questions about this). The providers surveyed reported that PrEP would be initiated for a wide variety of risk factors, although persons who had provided PrEP did appear to be less discriminating. Although guidance documents do provide the appropriate populations for PrEP (MSM, high-risk heterosexuals, serodiscordant couples, and high-risk IVDUs), they are vague about what constitutes “ongoing very high risk for acquiring HIV infection” [11, 15, 16]. Physicians stated they would use HIV NAT more frequently than what is currently recommended in the guidance documents. This opinion may have been bolstered by the findings that in published PrEP studies Preexposure Prophylaxis Initiative (iPrEX) and CDC 4940 Trial (TDF2), ART resistance only occurred in acutely infected participants who were seronegative at time of PrEP initiation [7, 8]. Providers were more consistent on how they would assess PrEP adherence, with a majority stating that patient self-report would be used. However, unlike with HIV-positive persons who demonstrate increases in HIV viral load with poor medication adherence, no objective point-of-care measure exists for determining adherence to PrEP. The experience of VOICE (participants reported 90%–91% adherence, but only approximately 30% had any evidence of tenofovir levels in the blood) and FEM-PrEP (95% of women reported taking pills as recommended, but only 25%–33% had detectable tenofovir in blood) suggests that self-report may not be a sufficient marker of adherence for PrEP [22, 23]. Last, providers varied on when PrEP would be discontinued, with half stopping for decreased adherence or decreases in risk behaviors and only a third stopping PrEP when an HIV-positive partner's viral load is completely suppressed. This likely represents a gap in current knowledge of the exact level of adherence that reaps the benefits of PrEP and whether PrEP provides additional protective benefit when used in serodiscordant couples when the HIV-positive partner is virally suppressed (the HIV-positive partners in Partners PrEP were not on suppressive ART) [9].
These results reveal that although PrEP acceptability is high, the uptake and practice are still low, and perception persists that multiple barriers exist to adequately provide PrEP. A modest level of skepticism about the efficacy of real-world PrEP also still exists. Many physicians expressed concern that PrEP may lead to risk compensation (ie, practice higher-risk behaviors) negating the PrEP benefit, and that PrEP would be resource-intensive and not cost-effective. These concerns may not be abated with increased provider education as has been recommend by previous studies [20, 21], but most likely requires the completion of ongoing open-label PrEP studies (eg, iPrEX OLE), future studies of real-world PrEP implementation, and an increase in the collective experiences of healthcare providers. GIS mapping of top-ranked barriers also demonstrated that individual providers in the same area had differently ranked barriers to PrEP provision. This suggests that successful real-world PrEP will require strategies to address multiple barriers.
Limitations of this study include the use of convenience sampling, which may introduce selection bias. The EIN is not a random sample of providers, and clinicians who participate in the EIN may not necessarily represent the opinions of clinicians who do not participate. Additionally, our response rate was 48.8%, and respondents were more likely to have had more years of infectious disease experience, but these providers may have a greater level of infectious disease knowledge. We attempted to minimize response fatigue by restricting the survey to 10 questions, but this does not completely eliminate bias. Finally, we did not perform an audit of actual practices, which may have been more accurate than reported practices.
Our results show that that the majority of adult infectious disease providers across the United States and Canada are supportive of PrEP but have vast differences of opinions and practices despite the publication of CDC guidance documents. CDC guidance is based on available clinical trial data and may be deliberately ambiguous because of the lack of information regarding real-world PrEP. The results of this survey and the additional comments provided by participants have highlighted the importance of future studies that specifically address the efficacy and risk compensation that occurs in open-label PrEP, the development of point-of-care objective adherence measures, description of long-term consequences of PrEP in HIV-negative persons, the design of successful and “resource-light” approaches to risk reduction and adherence counseling, and novel approaches to improving PrEP cost-effectiveness.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH) or the Centers for Disease Control and Prevention (CDC).
Financial support. This work was supported by the NIH (grant number KL2 RR031978, for years 1 and 2 of Clinical and Translation Science Award [CTSA] funding and grant number KL2TR00099 during year 3 and beyond of CTSA funding) and the CDC (cooperative agreement 5U50CK000187).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Fowler MG, Gable AR, Lampe MA, Etima M, Owor M. Perinatal HIV and its prevention: progress toward an HIV-free generation. Clin Perinatol. 2010;37:699–719. doi: 10.1016/j.clp.2010.09.002. vii. [DOI] [PubMed] [Google Scholar]
- 2.Kwon JA, Anderson J, Kerr CC, et al. Estimating the cost-effectiveness of needle-syringe programs in Australia. AIDS. 2012;26:2201–10. doi: 10.1097/QAD.0b013e3283578b5d. [DOI] [PubMed] [Google Scholar]
- 3.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lindegren ML, Byers RH, Jr, Thomas P, et al. Trends in perinatal transmission of HIV/AIDS in the United States. JAMA. 1999;282:531–8. doi: 10.1001/jama.282.6.531. [DOI] [PubMed] [Google Scholar]
- 5.Wodak A, Cooney A. Do needle syringe programs reduce HIV infection among injecting drug users: a comprehensive review of the international evidence. Subst Use Misuse. 2006;41:777–813. doi: 10.1080/10826080600669579. [DOI] [PubMed] [Google Scholar]
- 6.Granich RM, Gilks CF, Dye C, De Cock KM, Williams BG. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet. 2009;373:48–57. doi: 10.1016/S0140-6736(08)61697-9. [DOI] [PubMed] [Google Scholar]
- 7.Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587–99. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thigpen MC, Kebaabetswe PM, Paxton LA, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in. N Engl J Med. 2012;367:423–34. doi: 10.1056/NEJMoa1110711. [DOI] [PubMed] [Google Scholar]
- 9.Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367:399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baeten JK, Lingappa E, Coombs J, et al. Partners in Prevention HSV/HIV Transmission Study Team. Genital HIV-1 RNA predicts risk of heterosexual HIV-1 transmission. Sci Transl Med. 2011;3:77–ra29 doi: 10.1126/scitranslmed.3001888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Update to interim guidance for preexposure prophylaxis (PrEP) for the prevention of HIV infection: PrEP for injecting drug users. MMWR Morb Mortal Wkly Rep. 2013;62:463–5. [PMC free article] [PubMed] [Google Scholar]
- 12.Flash C, Krakower D, Mayer KH. The promise of antiretrovirals for HIV prevention. Curr Infect Dis Rep. 2012;14:185–93. doi: 10.1007/s11908-012-0242-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Early end for FEM-PrEP HIV prevention trial. AIDS Patient Care STDS. 2011;25:383. doi: 10.1089/apc.2011.9874. [DOI] [PubMed] [Google Scholar]
- 14.van der Straten A, Van Damme L, Haberer JE, Bangsberg DR. Unraveling the divergent results of pre-exposure prophylaxis trials for HIV prevention. AIDS. 2012;26:F13–9. doi: 10.1097/QAD.0b013e3283522272. [DOI] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention Interim guidance: preexposure prophylaxis for the prevention of HIV infection in men who have sex with men. MMWR Morb Mortal Wkly Rep. 2011;60:65–8. [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention Interim guidance for clinicians considering the use of preexposure prophylaxis. MMWR Morb Mortal Wkly Rep. 2012;61:586–9. [PubMed] [Google Scholar]
- 17.Marschall J, Lane MA, Beekmann SE, Polgreen PM, Babcock HM. Current management of prosthetic joint infections in adults: results of an Emerging Infections Network survey. Int J Antimicrob Agents. 2013;41:272–7. doi: 10.1016/j.ijantimicag.2012.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dowell D, Polgreen PM, Beekmann SE, Workowski KA, Berman SM, Peterman TA. Dilemmas in the management of syphilis: a survey of infectious diseases experts. Clin Infect Dis. 2009;49:1526–9. doi: 10.1086/644737. [DOI] [PubMed] [Google Scholar]
- 19.Arnold EA, Hazelton P, Lane T, et al. A qualitative study of provider thoughts on implementing pre-exposure prophylaxis (PrEP) in clinical settings to prevent HIV infection. PLoS One. 2012;7:e40603. doi: 10.1371/journal.pone.0040603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tripathi A, Ogbuanu C, Monger M, Gibson JJ, Duffus WA. Preexposure prophylaxis for HIV infection: healthcare providers’ knowledge, perception, and willingness to adopt future implementation in the southern US. South Med J. 2012;105:199–206. doi: 10.1097/SMJ.0b013e31824f1a1b. [DOI] [PubMed] [Google Scholar]
- 21.Senn H, Wilton J, Sharma M, Fowler S, Tan D. Knowledge of and opinions on HIV pre-exposure prophylaxis among front-line service providers at Canadian AIDS Service Organizations. AIDS Res Hum Retroviruses. 2013;29:1183–9. doi: 10.1089/aid.2013.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Damme L, Corneli A, Ahmed K, et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2012;367:411–22. doi: 10.1056/NEJMoa1202614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hankins CA, Dybul MR. The promise of pre-exposure prophylaxis with antiretroviral drugs to prevent HIV transmission: a review. Curr Opin HIV AIDS. 2013;8:50–8. doi: 10.1097/COH.0b013e32835b809d. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.