Abstract
Background
Peptic ulcer disease is a common cause of acute upper gastrointestinal hemorrhage. The aim of this study was to describe the endoscopic management of bleeding peptic ulcers in a large, U.S. multi-center endoscopic consortium with diverse practice settings.
Methods
Adult patients who underwent upper endoscopy (EGD) for hematemesis, melena or “suspected upper GI bleed” between 1/00–12/04 in the Clinical Outcomes Research Initiative (CORI) endoscopic database were screened for the finding of peptic ulcer. The ulcer stigmata, endoscopic therapy and the need for repeat EGD were compared across practice sites.
Results
Of 12,392 patients who underwent EGD for an upper gastrointestinal bleeding indication, 3,692 (30%) had at least one peptic ulcer (clean base 59.9%; flat pigmented spot 13.4%; active bleeding 10.7%; clot 7.2%; non-bleeding visible vessel (NBVV) 6.3%). Endoscopic therapy was applied to 93% of actively bleeding ulcers and 95% of NBVV. Repeat endoscopy was required in 7.3% of patients. Ulcers treated with injection monotherapy had the highest repeat EGD rates (12.2%) compared with contact thermal monotherapy (6.1%) and combination thermal/injection therapy (7.1%) (p=0.02). Immediate hemostasis rates were 88–97% across all therapeutic modalities. There was no statistical difference in hemostasis rates across therapy nor practice types.
Conclusion
In this multi-center consortium, initial hemostasis rates were high across therapy types and sites studied. Injection monotherapy was associated with the highest rates of repeat EGD, supporting guidelines that advise against its use in bleeding peptic ulcers.
Keywords: Peptic ulcer disease, endoscopy outcomes, CORI, practice variations, endoscopic therapy, repeat endoscopy
Introduction
Peptic ulcer disease (PUD) is a common cause of acute overt upper gastrointestinal hemorrhage (UGIH), accounting for 30–50% of all upper GI hemorrhage and represents a significant healthcare burden1. While approximately 80% of patients with non-variceal upper gastrointestinal hemorrhage will stop bleeding spontaneously, the remainder will continue to bleed or re-bleed. Clinical characteristics including patient age, co-morbid medical conditions, packed red blood cell transfusion requirements, use of non-steroidal anti-inflammatory drugs (NSAIDs), and ulcer size and location have been shown to be important determinants of patient outcomes from bleeding peptic ulcers2. Moreover, early endoscopy (within 24 hours of patient presentation) with endoscopic intervention decreases morbidity from bleeding peptic ulcer. Additionally, the endoscopic appearance of an ulcer (high-risk vs low-risk stigmata) provides additional helpful prognostic information on ulcer re-bleeding rates, need for urgent surgery, and mortality3–5.
Contemporary endoscopic treatments include injection therapy (e.g. saline, vasoconstrictors, sclerosing agents, tissue adhesives, or a combination thereof), thermal therapies (contact methods such as multipolar electrocoagulation and heater probe, and non-contact methods such as argon plasma coagulation), and mechanical therapy (endoscopic clips). Patients exhibiting bleeding ulcers with high-risk endoscopic stigmata (active bleeding, non-bleeding visible vessel) should undergo endoscopic hemostasis since this has been shown to reduce rates of further re-bleeding, need for surgery, and mortality compared with sham endoscopic therapy or medical therapy alone1, 5–9. Evidence-based consensus statements recommend combination therapy (commonly injection of a 1:10,000 admixture of epinephrine and saline followed by contact thermal therapy) as this has been shown superior to injection therapy alone8, 10–13 for the treatment of high risk ulcer stigmata. While there has been no demonstrated superiority of any particular hemostasis modality, epinephrine injection as monotherapy has been found to be inferior to combination therapy and contact thermal therapy alone and is therefore not recommended as definitive endoscopic therapy10, 12, 14–17.
The aim of this present study was to describe the endoscopic management of bleeding peptic ulcers in a large, multi-center national endoscopic consortium and to evaluate whether variations in practice exist among diverse gastrointestinal practices (academic, community/HMO and VA/Military). A secondary aim was to evaluate the impact of endoscopic therapy type on the need for repeat endoscopy.
Methods
Clinical Outcomes Research Initiative (CORI)
CORI was established in 1995 to study utilization and outcomes of endoscopy in diverse gastroenterology practice settings in the United States. All participating sites agree to use a standardized computerized report generator to create their endoscopic reports and comply with quality control requirements. One hundred percent of the sites’ CORI endoscopic data files are transmitted electronically on a weekly basis to a central data repository- the National Endoscopic Database (NED). Prior to transmission, all patient and physician identifiers are removed from the data file to protect both patient and physician confidentiality. The data then undergoes computerized quality control checks to identify missing fields. After quality control checks are completed, the data from all sites are merged in the NED for analysis. Site compliance is addressed annually; if a site fails to record more than 95% of endoscopic reports using CORI software, they are first given an opportunity to improve site compliance. Failure to do so may result in exclusion of site data from analysis; there is no pre-specified time frame for compliance. Multiple studies that have utilized CORI data have resulted in peer-reviewed publications5, 18–23.
Patient Inclusion and Exclusion Criteria
We identified all adult patients (≥ 18 years old) in the CORI database between January 1, 2000 and December 31, 2004, who underwent esophagogastroduodenoscopy (EGD) for the following indications: hematemesis, melena or “suspected upper GI bleed” (all available selections in the CORI indications menu). The ‘suspected upper GI bleed’ indication is based on an individual endoscopist’s suspicion for the diagnosis based on patient clinical history, physical examination, and laboratory data and does not reflect documentation of UGIH by nasogastric lavage or witnessed hematemesis or melena. If any additional indication was selected, the EGD was not included in this study. Hematochezia was initially considered in this study as an indication of interest but was subsequently withdrawn because of its primary association with lower gastrointestinal bleeding. Although a brisk upper gastrointestinal hemorrhage can present with hematochezia, the vast majority of patients presenting with hematochezia have a lower GI source (distal small bowel or colon) for their bleeding16.
In this present study, we identified and evaluated a subject’s index EGD followed by an analysis of any subsequent EGD (if indicated) within a 72-hour period following the index EGD.
Endoscopic Therapy
Ulcers that were discovered at EGD were further characterized by endoscopic stigmata type (high-risk stigmata: active bleeding, non-bleeding visible vessel, or adherent clot and low-risk stigmata: flat pigmented spot and clean base). We examined the endoscopic therapies that were employed on each stigmata type, specifically 1) monotherapy which included injection therapy alone (with epinephrine +/− saline), contact thermal therapy alone (e.g. multipolar electrocoagulation, heater probe), or mechanical therapy alone (e.g., endoscopic clips); 2) combination therapy (injection + thermal therapy), or 3) no endoscopic therapy. Due to the very limited number of cases identified that used mechanical therapy with hemoclips (n=7), this therapeutic modality was not subsequently included in the analyses. After an initial database search of the above, all endoscopic reports with findings of active bleeding, non-bleeding visible vessel, adherent clot and flat pigmented spot (FPS) (approximately 2,000 reports) were hand searched to capture additional data on ulcer descriptions, endoscopic therapies applied, and hemostasis outcomes that were provided by the endoscopist in the form of free text (rather than by CORI check box).
Primary Hemostasis and Practice Variation
Success of endoscopic therapy performed (primary hemostasis) on ulcers was defined as the endoscopist indicating “hemostasis successful” (an available CORI check box) or was clearly indicated by the endoscopist reporting in the free text as “bleeding stopped” or “no further bleeding.” Endoscopic therapies employed and initial hemostasis rates achieved were compared across practice types (academic, community/HMO, VA/Military) to assess for variations in endoscopic management.
Repeat Endoscopy
To assess ulcer re-bleeding, we examined all EGDs that were performed within 72 hours of the index EGD through a manual database search. A 72 hour time frame was chosen for repeat EGD as this time frame is generally considered the highest risk period for recurrent bleeding after primary hemostasis attempts. The indications for the repeat EGD were melena, hematemesis, suspected UGI bleeding or hematochezia. Repeat endoscopy performed outside a CORI affiliated site was not included in the study as we did not have access to such records. Evaluation of repeat endoscopy was not performed on cases that reported a clean-base ulcer at the index endoscopy.
Data Analysis
Stigmata types and endoscopic therapies employed were analyzed on a per-ulcer and per-patient basis. Comparisons of categorical data were performed using Pearson’s chi-square test of independence. An a priori determined p value of ≤ 0.05 was considered statistically significant. All analyses were performed using SAS software (SAS Institute, Inc., Cary, NC).
Results
During the study period 2000–2004, the CORI NED received 243,427 upper endoscopy reports from unique adult patients at 76 active practice sites in 26 states. The distribution of the practice sites was: community practice/HMO 71%, academic 16%, VA/Military 13%. A total of 12,392 patients received an EGD for an upper gastrointestinal bleeding indication. Of these, 3,692 patients (30%) had at least one documented ulcer (4,753 ulcers). Table 1 compares the demographics of the patients with documented peptic ulcers vs. those who had an EGD for any indication during the study time frame. Compared with patients who had an EGD for any indication, patients with documented ulcers were more likely to be male, even after excluding the VA sites from analysis. Ulcer patients were also more likely to be non-White minorities and older (mean age 65.2 vs. 57.8). Of the peptic ulcer patients, half underwent an EGD at a community/HMO setting and the majority (60.5%) were performed in an inpatient setting. Seventy-five percent of peptic ulcer patients had an American Society of Anesthesia (ASA) class of II or III, equating with a moderate severity of underlying medical co-morbidities.
Table 1.
Demographics of Patients with Ulcers vs All Patients who had an EGD 2000–2004
| Peptic Ulcer | All EGDs | |
|---|---|---|
| Total Patient | 3,692 | 243,427 |
| N (%) | N (%) | |
| Gender | ||
| Male | 2,584 (70.0%) | 120,186 (49.4%) |
| Female | 1,108 (30.0%) | 123,241 (50.6%) |
| Gender (exclude VA sites) | ||
| Male | 1,742 (61.7%) | 91,173 (43.0%) |
| Female | 1,082 (38.3%) | 120,657 (57.0%) |
| Race/Ethnicity | ||
| White Non-Hispanic | 2,631 (71.3%) | 189,796 (78.0%) |
| Black Non-Hispanic | 353 (9.6%) | 17,705 (7.3%) |
| Hispanic | 369 (10.0%) | 18,473 (7.6%) |
| Asian/Pacific Islander | 154 (4.2%) | 4,629 (1.9%) |
| Native American | 84 (2.3%) | 2,874 (1.2%) |
| Multi-racial | 6 (0.2%) | 412 (0.2%) |
| Unknown | 95 (2.6%) | 9,538 (3.9%) |
| Mean Age (yrs) | 65.2 | 57.8 |
| Site Type | ||
| Community/HMO | 1,852 (50.2%) | 172,972 (71.1%) |
| Academic | 972 (26.3%) | 38,858 (16.0%) |
| VA/Military | 868 (23.5%) | 31,597 (13.0%) |
Description of Ulcers and Endoscopic Therapy
A total of 4,753 ulcers were identified on EGD. The breakdown of these ulcers by description has been previously published5: clean base 2,847 (59.9%) flat pigmented spot 635 (13.4%), active bleeding 508 (10.7%), adherent clot 340 (7.2%), non-bleeding visible vessel 299 (6.3%), and unknown ulcer description 124 (2.6%) (Table 2). The distribution of ulcer stigma was similar across all practice site types.
Table 2.
Breakdown of Ulcer Stigmata
| Stigmata | N | % |
|---|---|---|
| Active Bleeding | 508 | 10.7% |
| Non Bleeding Visible Vessel | 299 | 6.3% |
| Adherent Clot | 340 | 7.2% |
| Flat Pigmented Spot | 635 | 13.4% |
| Clean base | 2,847 | 59.9% |
| Unknown | 124 | 2.6% |
| Total | 4,753 | 100% |
After excluding clean base ulcers from further analyses, 60% (1,075/1,782) of the remaining ulcers with bleeding stigmata (both high and low risk stigmata) received some form of endoscopic hemostasis therapy (Table 3). Five hundred and one (501, 28%) ulcers had monotherapy with either injection (n= 254), multipolar contact thermal therapy (n= 191) or heater probe (n= 56). Five hundred and seventy four (574, 32%) ulcers had combination therapy, either with injection + multipolar contact thermal (n= 423) or injection + heater probe (n= 151).
Table 3.
Breakdown of Endoscopic Therapy Instituted
| Combination | 574 | (32%) | N |
| Multipol/injection | 423 | ||
| Heater /injection | 151 | ||
| Monotherapy | 501 | (28%) | |
| Multipolar | 191 | ||
| Injection | 254 | ||
| Heater | 56 | ||
| No therapy | 707 | (40%) | |
| Total | 1,782 | (100%) | |
Therapies for specific ulcer stigmata
Ninety-three percent (93%, 476/508) of actively bleeding ulcers underwent some form of endoscopic hemostasis therapy [n= 271 (53%) combination, n= 205 (40%) monotherapy] (Table 4). When combination therapy was used, injection + multipolar contact thermal therapy was used two times more frequently than injection + heater probe therapy. When monotherapy was employed in the treatment of actively bleeding ulcers, injection-only was used in 119/205 (58%) and thermal only therapy in 86/205 (42%) of ulcer cases.
Table 4.
Ulcer Therapies by Ulcer Stigmata
| MONOTHERAPY | COMBINATION THERAPY | ||||||
|---|---|---|---|---|---|---|---|
| Multipol only | Heater only | Inject only | Multipol/inj | Heater/inj | NO Rx | TOTALS | |
| Bleeding Type | N(%) | N(%) | N(%) | N(%) | N(%) | N(%) | N |
| Active Bleeding | 72 (14%) | 14 (3%) | 119 (23%) | 191 (38%) | 80 (16%) | 32 (7%) | 508 |
| Non Bleeding Visible Vessel | 52 (17%) | 24 (8%) | 35 (12%) | 127 (43%) | 47 (16%) | 14 (5%) | 299 |
| Clot | 25 (7%) | 9 (3%) | 56 (17%) | 60 (18%) | 12 (4%) | 178 (52%) | 340 |
| Flat Pigmented Spot | 42 (7%) | 9 (1%) | 44 (7%) | 45 (7%) | 12 (2%) | 483 (76%) | 635 |
| Total | 191 (11%) | 56 (3%) | 254 (14%) | 423 (24%) | 151 (9%) | 707 (40%) | 1,782 |
Thirty-two (7%) actively bleeding ulcers received no apparent hemostasis therapy. Reasons reported for no therapy included 1) lack of backup support from surgery or interventional radiology 2) high-risk lesion that was considered by the endoscopist to be too large for safely performing endoscopic hemostasis; and 3) inability to appropriately position the endoscope to perform hemostasis. For the majority of cases (88%) in which an actively bleeding ulcer was not endoscopically treated, there was no clear justification reported by the treating endoscopist.
Ninety-five percent (285/299) of non-bleeding visible vessels (NBVV) were treated endoscopically [174 (58%) combination therapy, 111 (37%) monotherapy]. Injection + multipolar contact thermal combination therapy comprised 45% (127/285) of all therapies instituted, followed by multipolar contact thermal therapy alone (52/285, 18%), injection + heater probe (80/285, 16%), injection therapy alone (35/285, 12%) and heater probe alone (24/285, 8%) (Table 4). Injection therapy comprised 32% of all monotherapy applied.
Among the adherent clots, 48% (162/340) were endoscopically treated at initial EGD. Injection/multipolar and injection only were the two most frequent forms of therapy employed in treating adherent clots [60/162 (37%) and 56/162 (35%), respectively]. At least 57% (193/340) of the adherent clots were reported to be “irrigated” (yet the specific nature of endoscopic washing was not described) in an attempt to remove the clot with 45% (87/193) reporting success in clot removal. Once the clot was removed, the reported underlying ulcer stigmata were: active bleeding n= 31 (36%), non-bleeding visible vessel n= 43 (15%) and no active bleeding n= 13 (49%). Active bleeding and non-bleeding visible vessels were treated 97% of the time; despite not finding active bleeding (n= 13) under an adherent clot, 65% of these lesions were treated endoscopically.
Despite being considered to be low risk stigmata and not requiring endoscopic hemostasis, flat pigmented spots (FPS) had an endoscopic treatment rate of 24% (63/152).
No Endoscopic Hemostasis
After excluding clean base ulcers, of the remaining 1,782 ulcers (includes actively bleeding, NBVV, adherent clot and flat pigmented spot), 707 (40%) were not treated endoscopically. Flat pigmented spots comprised 481 (68%) of this total, followed by clot 177 (25%).
Primary Hemostasis
Our hemostasis data focused exclusively on actively bleeding ulcers on the index endoscopy. Primary hemostasis was denoted either by the endoscopists checking a box “hemostasis achieved” or specific wording in the free text (“bleeding ceased,” “no further bleeding,” etc). Reported primary hemostasis rates achieved were 88–97% across all ulcer therapies on index endoscopy (Table 5); there was no statistically significant difference in hemostatis rates across the therapies (p=0.32) nor across practice types (Academic, VA/Military, Community/HMO, 89–94%, p = 0.17).
Table 5.
Index EGD: Immediate Hemostasis Rates on Actively Bleeding Ulcers
| Therapy | N | Hemostasis rate | p-value |
|---|---|---|---|
| Multipolar only | 72 | 97% | 0.32 |
| Heater probe only | 14 | 93% | |
| Injection only | 119 | 88% | |
| Multipol/ injection | 191 | 91% | |
| Heater probe/ injection | 80 | 91% | |
| Total | 476 | 91% | |
Repeat Endoscopy
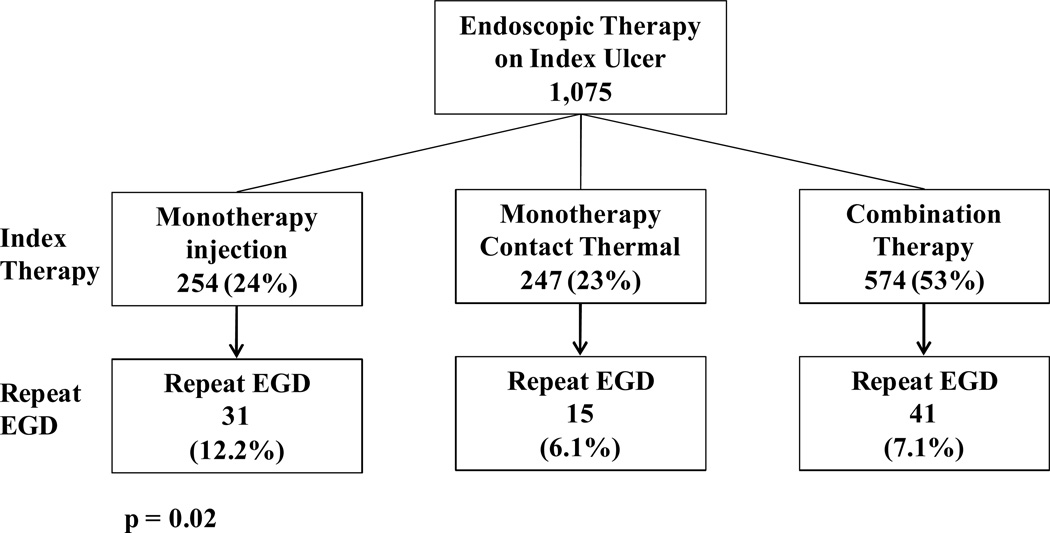
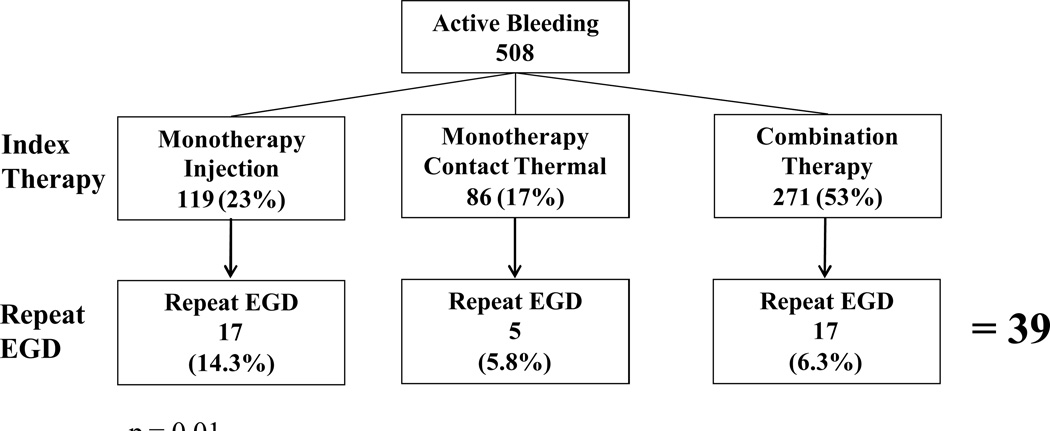
Of the 1,646 patients (1,782 ulcers) who had any active bleeding, NBVV, adherent clot or flat pigmented spot, 120 (7.3%) patients had a repeat EGD within 72 hours of index endoscopy. None of these patients had multiple ulcers documented on repeat EGD. Repeat endoscopy was performed for one of the bleeding indications previously described. Indications for repeat EGD were: “suspected UGI hemorrhage” 36.7%, hematemesis 30.0%, melena 27.5%, hematochezia 5.8%. The findings on repeat endoscopy are described in Table 6. Of the 120 patients who had a repeat endoscopy, 32.5% (39) had an active bleeding ulcer on index EGD, followed by NBVV 33 (27.5%), adherent clot 27 (22.5%), flat pigmented spot 21 (17.5%). 73% (87/120) of all repeat EGD patients had some form of endoscopic therapy during index EGD. Those ulcers treated at index EGD with monotherapy using injection alone had a significantly higher rate of repeat EGD at 12.2% compared with a repeat EGD rate of 6.1% and 7.1%, respectively for those treated with monotherapy using a contact thermal device or combination therapy (p = 0.02) (Figure 1). Of the 508 active bleeding ulcers on index EGD, 39 (7.7%) underwent a repeat EGD within 72 hours. Those active bleeding ulcers that were treated with injection alone at index EGD had a significantly higher rate of repeat EGD of 14.3%, compared with contact thermal therapy alone or combination therapy (5.8% and 6.3%, respectively, p = 0.01) (Figure 2). Of all actively bleeding ulcers found at the time of repeat EGD, 85% (28/33) had reported achievement of primary hemostasis at the time of index EGD; the remaining 5 patients had persistent bleeding despite endoscopic therapy and medical measures. Recurrent active bleeding (persistent active bleeding despite endoscopic treatment/an active bleeding ulcer on both EGDs) was seen in 18 patients (15% of all repeat EGDs). Of these 18 patients, 44% received monotherapy using injection at index EGD, 44% combination therapy, and 11% monotherapy with a contact thermal device. Eighty-three percent of recurrent active bleeding was treated with combination therapy.
Table 6.
Patients with Repeat EGD within 72 hours
| Index EGD | Repeat EGD | ||||
|---|---|---|---|---|---|
| Finding (n) | % of Index EGD |
# of patients who required repeat EGD |
% of Index EGD |
Finding (n) | |
| Active bleeding | 508 | 28.5% | 39 | 7.7% | 33 |
| NBVV | 299 | 16.8% | 33 | 11.0% | 15 |
| Adherent Clot | 340 | 19.1% | 27 | 7.9% | 12 |
| Flat Pigmented Spot | 635 | 35.6% | 21 | 3.3% | 20 |
| Clean Base | * | 40 | |||
| Total | 1,782 | 120 | 120 | ||
Finding of clean base ulcer on index GED was not subsequently evaluated
Per ulcer repeat EGD rate is 6.7% (120/1,782 ulcers), per patient rate is 7.3% (120/1,646 patients)
Figure 1.
Repeat EGD Rates Based on Type of Endoscopic Therapy on Index EGD
Figure 2.
Active Bleeding Ulcers: Repeat EGD Rates Based on Endoscopic Therapy on Index EGD
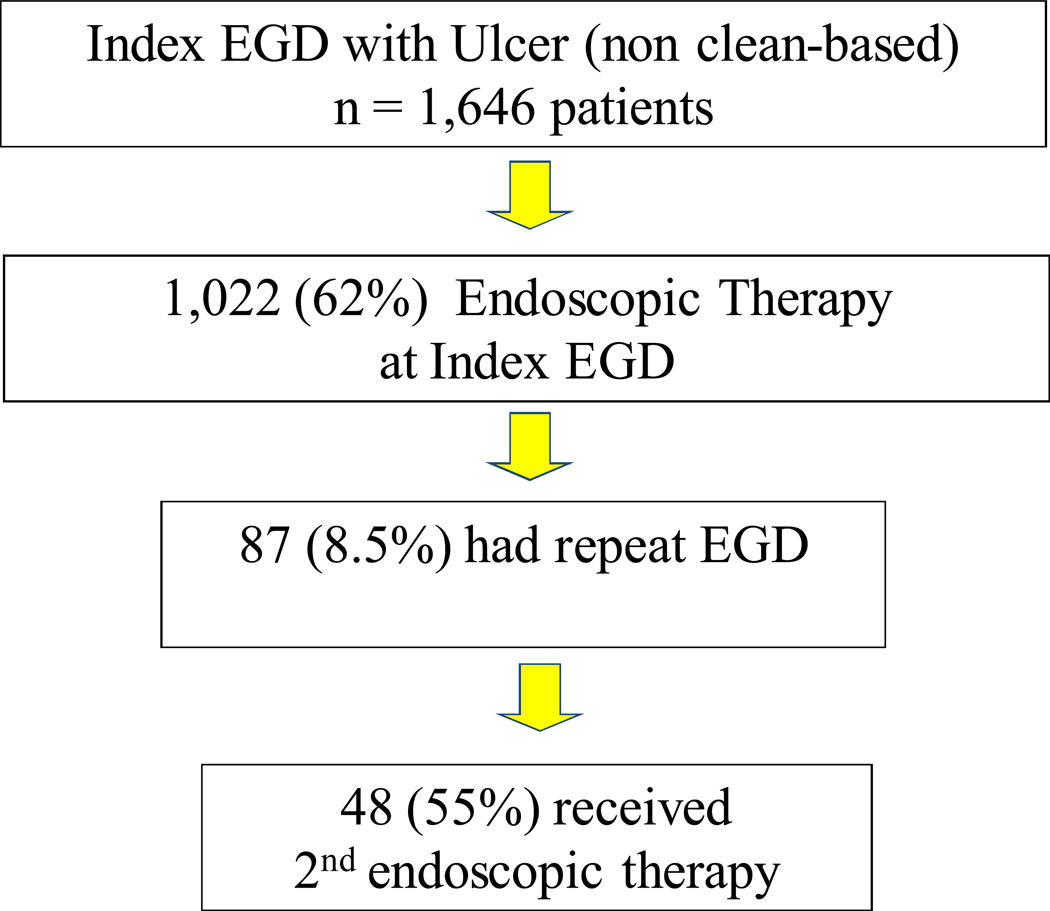
Repeat EGD data are summarized on a per patient basis in Figure 3.
Figure 3.
Repeat EGD: Per patient analysis
Practice Variations/Site Specifics
Across all practice site types, there was no difference in the utilization of neither specific endoscopic therapies nor whether the endoscopist chose to treat or not to treat specific ulcer stigmata (Table 7). Monotherapy was used 26–29% of the time across practice sites with combination therapy rates of 29–34% (p=0.17) and no-therapy rates were 35–40% across practice sites (p=0.07). With respect to active bleeding ulcers, VA/Military sites were significantly more likely to use combination therapy than Community/HMO or Academic sites (67% VA/Military vs 59% Academic and 52% Community/HMO, p=0.04). For NBVV, there was a similar trend for the VA to use more combination therapy than Community/HMO or Academic sites, but the difference was not statistically significant (p=0.10).
Table 7.
Endoscopic Therapy Utilization Across Site Types
| Monotherapy | Combination Therapy |
No Therapy | Total | ||
|---|---|---|---|---|---|
| Community/HMO | 29% | 29% | 42% | 893 | |
| Academic | 29% | 36% | 35% | 450 | |
| VA/Military | 26% | 34% | 40% | 439 | |
p = 0.17 for combination vs monotherapy
p=0.07 for no therapy
Discussion
This study examines the utilization of endoscopic treatment modalities for hemostasis of peptic ulcer bleeding in a large diverse multi-center U.S. consortium. We identified a peptic ulcer prevalence of 30%, with 60% of all ulcers at index endoscopy described as having a clean base. The observed peptic ulcer stigmata rates in the CORI data differ from previous studies; overall, the CORI sites reported fewer non bleeding visible vessels (6.4% compared with 22–25%)and more clean base ulcers (61.4% vs 32%) compared with prior studies1, 24–25. The observed difference in the types of ulcer stigmata as compared with previously published data may reflect the fact that other databases including the CURE and RUGBE data, are based on bleeding registries and therefore, maybe more likely to have a higher percentage of high risk stigmata whereas the CORI database represents endoscopies for all types of endoscopic indications. The CURE data may also be subject to referral bias. Additionally, the timing of endoscopy may influence the type of ulcer stigmata that are identified whereby institutions that have GI bleeding care pathways and/or GI bleeding teams that implement early endoscopy may favor finding higher risk endoscopic stigmata. The CORI database reflects what is observed in everyday clinical practice, rather than in a bleeding registry and therefore the reported stigmata rates may be more reflective of community GI clinical practice in the US.
The vast majority of actively bleeding ulcers and non-bleeding visible vessels were reported to be endoscopically treated in this present study (93% and 95% respectively). We found a relatively even distribution of monotherapy and combination therapy performed on actively bleeding ulcers and NBVV. Notably, monotherapy was performed on 37–40% of actively bleeding ulcers and NBVV with injection therapy alone comprising 23% of the therapy performed on active bleeding ulcers and 12% on NBVV (adherent clot 17%). It is encouraging that the vast majority (≥ 93%) of high-risk endoscopic lesions in this large community database received some method of endoscopic hemostasis as per an evidence-based international consensus statement and ASGE guidelines2, 6, 10 our data represents community practice based data, and therefore appears to reflect uptake of guidelines into clinical practice.
For the 7% of peptic ulcers with high-risk stigmata that did not receive endoscopic therapy, based on the available free text in the CORI report form, it appears that there were legitimate reasons for why these ulcers were left untreated (lesions deemed too large by the endoscopist or lack of availability of surgeons/interventional radiologists to provide up backup). Unfortunately, beyond this limited information, we have no further insight in to the endoscopist’s clinical decision making at the time of endoscopy and remains a limitation of any CORI and database study.
Despite the fact that the vast majority of high risk stigmata received some form of endoscopic hemostasis therapy, it appears that a significant number of these ulcer types may not be receiving optimal endoscopic treatment per published guidelines6, 10, 16 (37–40% of active bleeding ulcers and non- bleeding visible vessels were treated with monotherapy with injection alone instead of the recommended combination therapy or thermal therapy. Injection only was employed in 12–23% of high risk ulcer stigma cases. Subsequently, we found that ulcers treated with injection alone had a significantly higher rate of repeat EGD (12.2% overall, 14.3% for active bleeding ulcers), almost twice that of any other therapeutic modalities. This supports data that suggest that monotherapy with injection of epinephrine only (this includes epinephrine + saline combined) is inferior and inadequate as definitive endoscopic hemostasis {Gralnek, 2008 #8}. Recent data suggests that monotherapy in the form of contact thermal therapy may be comparable to combination therapy for the endoscopic treatment of high-risk ulcer stigmata26. In this study, despite deviation from guidelines, there appears to be no demonstrable effect on initial hemostasis rates at index endoscopy. Once again, given that our data is practice based, it likely reflects the uptake and implementation of practice guidelines.
We found that repeat endoscopy was performed on 7.3% (120/1,646) of patients, 60% (72/120) of whom had active bleeding or an adherent clot at index endoscopy (an additional 18%, 21 had NBVV at index EGD). On repeat exam, 60 (50%) had reported active bleeding, NBVV or adherent clot. 8.5% of those who had endoscopic therapy on index EGD had a repeat EGD. Of these, 48 (56%) received a second round of endoscopic therapy at repeat EGD. As stated, repeat EGD was two times more likely to be performed in those cases in which ulcers were treated with monotherapy with injection alone.
Despite some of these deviations from practice guidelines, primary hemostasis rates were similar across the three practice sites (89–94%, p=0.17) and across the therapeutic modalities used (87–88%, p=0.32). There was no apparent difference in whether or not endoscopic therapy was employed across sites, suggesting that there was no significant practice variation based on site type. The VA/Military sites utilized combination hemostasis therapy significantly more often than monotherapy (p=0.04) and there was a similar trend observed for NBVV (p= ns). These observed trends did not translate to a difference in hemostasis rates.
Interestingly, for unclear reasons, we observed some overuse of therapy as well: 1.2% of clean base ulcers and 24% of flat pigmented spots underwent some form of endoscopic hemostasis treatment. It is possible that endoscopists may have been concerned about misidentifying a flat pigmented spot for a non-bleeding visible vessel. Thus, erring on the side of caution, the endoscopist may have performed endoscopic hemostasis therapy.
This study raises some interesting findings on community reported endoscopic management of adherent clots. The management of clots is controversial and significant variability exists in their management16. Recent data suggests that we ought to consider adherent clots high-risk ulcer stigmata and perform endoscopic therapy consisting of clot removal and subsequent endoscopic treatment of any underlying high risk stigmata12, 27–28. Two randomized controlled trials on adherent clot management recommend adding clot to the high risk stigmata category11, 28–30. Re-bleeding rates after medical therapy were 34–35% compared with 0–4% for those treated endoscopically (injection + thermal contact therapy)11, 29. However, others have demonstrated good outcomes in patients with adherent clots treated with high-dose intravenous proton pump inhibitors alone8. In this present study, of the 340 adherent clots, 162 (48%), underwent some form of endoscopic therapy (monotherapy 90/340, 27%; injection only 56/340, 17%; combination therapy 72/340, 21%). Repeat EGD was performed for a CORI bleeding indication within 72 hours on 10% (33/340) of all adherent clots. 9.3% (15/162) of clots endoscopically treated at index EGD required repeat EGD compared with 10.1% (18/178) for clots that were not endoscopically treated on index EGD (p=0.69) suggesting that endoscopic therapy does not appear to influence the need for repeat EGD. However, when the clot was removed, high risk stigmata were discovered in 51% of cases. While not the primary focus of this study, given the ongoing controversy in the management of adherent clots, the CORI database and its large numbers of patients lends itself well to future studies in this area and may serve to help guide endoscopic management.
This study has several limitations. The endoscopic report is the sole source of data in this study. Therefore, clinical information beyond the endoscopic report is limited, including clinical correlation with the severity of the bleeding episode (e.g., Rockall Risk Score, Blatchford Score, anemia, hypotension, transfusion requirements) as has been done in prior studies. The current study cannot capture data on the use of proton pump inhibitors nor use of anticoagulants in this patient population as its documentation in the CORI endoscopic report is highly variable.
The information in the CORI database represents the input of the physician that performs the endoscopy and thus the use of check box notation and free text is variable. The use of free text further limits the efficiency of database queries as we discovered much valuable data stored in the free text that could otherwise have been overlooked. For example, the total number of bleeding ulcers identified in our study may actually be an underestimation as many procedures have several ulcers accounted for in the free text, despite only one ulcer box manually checked in the CORI software. Additionally, analysis of follow-up data in CORI is limited. Endoscopies that may have been performed for re-bleeding at non-CORI participating sites are not captured in our data and analysis. As a result, our repeat endoscopy data may be an “at least” figure as some patients may have sought care at a non-CORI participating site and would not have been captured in our database queries. Moreover, CORI is not a bleeding registry. Therefore, it is not entirely appropriate to compare these data to other bleeding databases that confirm UGIH objectively (CURE and RUGBE databases)1, 25. In our study, upper gastrointestinal hemorrhage was diagnosed based on the endoscopist’s suspicion to proceed with endoscopy based on the patient’s presenting symptoms, physical exam, and laboratory data. Our study uses repeat endoscopy within 72 hours if index endoscopy as a surrogate for ulcer re-bleeding which is limited by the indication provided for repeat endoscopy. Given the retrospective nature of this study, we cannot discern the true indication for repeat endoscopy beyond what was entered by the endoscopist (i.e clinical signs and symptoms of recurrent bleeding vs pre-arranged/ programmed second look endoscopy vs a second look endoscopy as reassurance that primary hemostasis was achieved). Such reliance on an endoscopist’s indication for endoscopy and assessment of findings (since there were no strict size or depth criteria for ulcers) may have possibly led to the inclusion of less severe bleeding into the study and therefore affect the rates of reported active bleeding and NBVV in our analysis. Our study may also have not captured massive upper gastrointestinal bleeding as we excluded the indication of hematochezia in our analyses. However, as previously stated, the majority of hematochezia is due to lower GI bleeding and if there was suspicion of an UGI bleed with hematochezia as the presentation, we expect that it would be captured under the indication “suspected UGI hemorrhage.” Finally, CORI sites are not necessarily a random sample of GI practices in the US and are susceptible to site selection bias.
Notwithstanding the above limitations, the CORI database remains unique in that it provides us with insight into how diverse GI practices are actually endoscopically managing peptic ulcer disease, in other words, how “real-life” endoscopy is being practiced in the United States. The large number of patients and endoscopies permits observation of management trends in clinical situations outside traditional academic centers and therefore, the CORI database is a powerful hypothesis-generating tool for future research studies.
Conclusions
In summary, in this large multi-center consortium, greater than 95% of high risk bleeding ulcer stigmata received some form of endoscopic therapy at index EGD, complying with current published consensus statements and guidelines2, 6, 10, 16. While a substantial portion of NBVV and actively bleeding ulcers did not receive combination endoscopic therapy, primary hemostasis rates were uniformly high across therapy types and sites studied. Despite endoscopic therapy on index EGD, 8.5% of patients had a repeat EGD for a bleeding indication, of which over half, required a second round of endoscopic therapy. Finally, monotherapy with injection alone had the highest rates of repeat EGD, supporting guidelines that advise against its use in bleeding peptic ulcers. Given these findings, future studies are needed to improve the outcomes of patients with peptic ulcer bleeding.
Acknowledgments
Grant Support: This project was supported with the funding from NIDDK UO1 CA 89389-01 and R33-DK61778-01. In addition, the practice network (Clinical Outcomes Research Initiative) has received support from the following entities to support the infrastructure of the practice based network: AstraZeneca, Bard International, Pentax USA, ProVation, Endosoft, GIVEN Imaging, and Ethicon. The commercial entities had no involvement in this research.
Dr. Lieberman is the executive director of CORI and Dr. Eisen is the executive co-director of CORI, a non-profit organization that receives funding from federal and industry sources.
Footnotes
This potential conflict of interest has been reviewed and managed by the OHSU Conflict of Interest in Research Committee.
References
- 1.Barkun A, Sabbah S, Enns R, et al. The Canadian Registry on Nonvariceal Upper Gastrointestinal Bleeding and Endoscopy (RUGBE): Endoscopic hemostasis and proton pump inhibition are associated with improved outcomes in a real-life setting. Am J Gastroenterol. 2004 Jul;99(7):1238–1246. doi: 10.1111/j.1572-0241.2004.30272.x. [DOI] [PubMed] [Google Scholar]
- 2.Barkun A, Bardou M, Marshall JK. Consensus recommendations for managing patients with nonvariceal upper gastrointestinal bleeding. Ann Intern Med. 2003 Nov 18;139(10):843–857. doi: 10.7326/0003-4819-139-10-200311180-00012. [DOI] [PubMed] [Google Scholar]
- 3.Forrest JA, Finlayson ND, Shearman DJ. Endoscopy in gastrointestinal bleeding. Lancet. 1974 Aug 17;2(7877):394–397. doi: 10.1016/s0140-6736(74)91770-x. [DOI] [PubMed] [Google Scholar]
- 4.Freeman ML, Cass OW, Peine CJ, Onstad GR. The non-bleeding visible vessel versus the sentinel clot: natural history and risk of rebleeding. Gastrointest Endosc. 1993 May-Jun;39(3):359–366. doi: 10.1016/s0016-5107(93)70106-6. [DOI] [PubMed] [Google Scholar]
- 5.Enestvedt BK, Gralnek IM, Mattek N, Lieberman DA, Eisen G. An evaluation of endoscopic indications and findings related to nonvariceal upper-GI hemorrhage in a large multicenter consortium. Gastrointest Endosc. 2008 Mar;67(3):422–429. doi: 10.1016/j.gie.2007.09.024. [DOI] [PubMed] [Google Scholar]
- 6.Adler DG, Leighton JA, Davila RE, et al. ASGE guideline: The role of endoscopy in acute non-variceal upper-GI hemorrhage. Gastrointest Endosc. 2004 Oct;60(4):497–504. doi: 10.1016/s0016-5107(04)01568-8. [DOI] [PubMed] [Google Scholar]
- 7.Sacks HS, Chalmers TC, Blum AL, Berrier J, Pagano D. Endoscopic hemostasis. An effective therapy for bleeding peptic ulcers. JAMA. 1990 Jul 25;264(4):494–499. doi: 10.1001/jama.264.4.494. [DOI] [PubMed] [Google Scholar]
- 8.Sung JJ, Chan FK, Lau JY, et al. The effect of endoscopic therapy in patients receiving omeprazole for bleeding ulcers with nonbleeding visible vessels or adherent clots: a randomized comparison. Ann Intern Med. 2003 Aug 19;139(4):237–243. doi: 10.7326/0003-4819-139-4-200308190-00005. [DOI] [PubMed] [Google Scholar]
- 9.Consensus conference: Therapeutic endoscopy and bleeding ulcers. JAMA. 1989 Sep 8;262(10):1369–1372. [PubMed] [Google Scholar]
- 10.Barkun AN, Bardou M, Kuipers EJ, et al. International consensus recommendations on the management of patients with nonvariceal upper gastrointestinal bleeding. Ann Intern Med. 2010 Jan 19;152(2):101–113. doi: 10.7326/0003-4819-152-2-201001190-00009. [DOI] [PubMed] [Google Scholar]
- 11.Bleau BL, Gostout CJ, Sherman KE, et al. Recurrent bleeding from peptic ulcer associated with adherent clot: a randomized study comparing endoscopic treatment with medical therapy. Gastrointest Endosc. 2002 Jul;56(1):1–6. doi: 10.1067/mge.2002.125365. [DOI] [PubMed] [Google Scholar]
- 12.Jensen DM, et al. Randomized controlled study of combination epinephrine injection and gold probe alone for hemostasis of actively bleeding peptic ulcers (abstract) Gastrointest Endosc. 2005;51(AB130) [Google Scholar]
- 13.Lau JY, Sung JJ, Lee KK, et al. Effect of intravenous omeprazole on recurrent bleeding after endoscopic treatment of bleeding peptic ulcers. N Engl J Med. 2000 Aug 3;343(5):310–316. doi: 10.1056/NEJM200008033430501. [DOI] [PubMed] [Google Scholar]
- 14.Bianco MA, Rotondano G, Marmo R, Piscopo R, Orsini L, Cipolletta L. Combined epinephrine and bipolar probe coagulation vs. bipolar probe coagulation alone for bleeding peptic ulcer: a randomized, controlled trial. Gastrointest Endosc. 2004 Dec;60(6):910–915. doi: 10.1016/s0016-5107(04)02232-1. [DOI] [PubMed] [Google Scholar]
- 15.Calvet X, Vergara M, Brullet E, Gisbert JP, Campo R. Addition of a second endoscopic treatment following epinephrine injection improves outcome in high-risk bleeding ulcers. Gastroenterology. 2004 Feb;126(2):441–450. doi: 10.1053/j.gastro.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 16.Gralnek IM, Barkun AN, Bardou M. Management of acute bleeding from a peptic ulcer. N Engl J Med. 2008 Aug 28;359(9):928–937. doi: 10.1056/NEJMra0706113. [DOI] [PubMed] [Google Scholar]
- 17.Park CH, Joo YE, Kim HS, Choi SK, Rew JS, Kim SJ. A prospective, randomized trial comparing mechanical methods of hemostasis plus epinephrine injection to epinephrine injection alone for bleeding peptic ulcer. Gastrointest Endosc. 2004 Aug;60(2):173–179. doi: 10.1016/s0016-5107(04)01570-6. [DOI] [PubMed] [Google Scholar]
- 18.Cram P, Fendrick AM, Inadomi J, Cowen ME, Carpenter D, Vijan S. The impact of a celebrity promotional campaign on the use of colon cancer screening: the Katie Couric effect. Arch Intern Med. 2003 Jul 14;163(13):1601–1605. doi: 10.1001/archinte.163.13.1601. [DOI] [PubMed] [Google Scholar]
- 19.Harewood GC, Lieberman DA. Colonoscopy practice patterns since introduction of medicare coverage for average-risk screening. Clin Gastroenterol Hepatol. 2004 Jan;2(1):72–77. doi: 10.1016/s1542-3565(03)00294-5. [DOI] [PubMed] [Google Scholar]
- 20.Lieberman D, Fennerty MB, Morris CD, Holub J, Eisen G, Sonnenberg A. Endoscopic evaluation of patients with dyspepsia: results from the national endoscopic data repository. Gastroenterology. 2004 Oct;127(4):1067–1075. doi: 10.1053/j.gastro.2004.07.060. [DOI] [PubMed] [Google Scholar]
- 21.Lieberman DA, Holub J, Eisen G, Kraemer D, Morris CD. Prevalence of polyps greater than 9 mm in a consortium of diverse clinical practice settings in the United States. Clin Gastroenterol Hepatol. 2005 Aug;3(8):798–805. doi: 10.1016/s1542-3565(05)00405-2. [DOI] [PubMed] [Google Scholar]
- 22.Lieberman DA, Holub J, Eisen G, Kraemer D, Morris CD. Utilization of colonoscopy in the United States: results from a national consortium. Gastrointest Endosc. 2005 Dec;62(6):875–883. doi: 10.1016/j.gie.2005.06.037. [DOI] [PubMed] [Google Scholar]
- 23.Lieberman DA, Holub JL, Moravec MD, Eisen GM, Peters D, Morris CD. Prevalence of colon polyps detected by colonoscopy screening in asymptomatic black and white patients. JAMA. 2008 Sep 24;300(12):1417–1422. doi: 10.1001/jama.300.12.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Katschinski B, Logan R, Davies J, Faulkner G, Pearson J, Langman M. Prognostic factors in upper gastrointestinal bleeding. Dig Dis Sci. 1994 Apr;39(4):706–712. doi: 10.1007/BF02087411. [DOI] [PubMed] [Google Scholar]
- 25.Savides TJ, Jensen DM. Therapeutic endoscopy for nonvariceal gastrointestinal bleeding. Gastroenterol Clin North Am. 2000 Jun;29(2):465–487. vii. doi: 10.1016/s0889-8553(05)70123-0. [DOI] [PubMed] [Google Scholar]
- 26.Soon MS, Wu SS, Chen YY, Fan CS, Lin OS. Monopolar coagulation versus conventional endoscopic treatment for high-risk peptic ulcer bleeding: a prospective, randomized study. Gastrointest Endosc. 2003 Sep;58(3):323–329. [PubMed] [Google Scholar]
- 27.Elmunzer BJ, Young SD, Inadomi JM, Schoenfeld P, Laine L. Systematic review of the predictors of recurrent hemorrhage after endoscopic hemostatic therapy for bleeding peptic ulcers. Am J Gastroenterol. 2008 Oct;103(10):2625–2632. doi: 10.1111/j.1572-0241.2008.02070.x. quiz 2633. [DOI] [PubMed] [Google Scholar]
- 28.Laine L. Systematic review of endoscopic therapy for ulcers with clots: Can a meta-analysis be misleading? Gastroenterology. 2005 Dec;129(6):2127. doi: 10.1053/j.gastro.2005.10.039. author reply 2127–2128. [DOI] [PubMed] [Google Scholar]
- 29.Jensen DM, Kovacs TO, Jutabha R, et al. Randomized trial of medical or endoscopic therapy to prevent recurrent ulcer hemorrhage in patients with adherent clots. Gastroenterology. 2002 Aug;123(2):407–413. doi: 10.1053/gast.2002.34782. [DOI] [PubMed] [Google Scholar]
- 30.Kahi CJ, Jensen DM, Sung JJ, et al. Endoscopic therapy versus medical therapy for bleeding peptic ulcer with adherent clot: a meta-analysis. Gastroenterology. 2005 Sep;129(3):855–862. doi: 10.1053/j.gastro.2005.06.070. [DOI] [PubMed] [Google Scholar]