Abstract
Background
A possible association between nonalcoholic fatty liver disease (NAFLD) and hypothyroidism has been suggested. Possible explanations for this association are the recognized links between hypothyroidism and various elements of the metabolic syndrome which is often present in NAFLD. To further explore this association, we determined the prevalence of hypothyroidism in a cohort of patients with NAFLD and analyzed the potential factors associated with hypothyroidism in this patient population.
Methods
Two hundred and forty six patients with biopsy proven NAFLD attending hepatology clinics at the Cleveland Clinic between October 2006 to June 2009 and 430 age, gender, race and BMI matched control subjects seen in the general internal medicine clinic were included. Patients with a clinical diagnosis of hypothyroidism who were on thyroid replacement therapy were considered to be hypothyroid.
Results
Hypothyroidism was more frequent among patients with NAFLD (21%vs 9.5%.; P<0.01) compared to controls and was higher in NASH patients than NAFLD patients without NASH (25% vs 12.8%, P=0.03). Subjects with hypothyroidism were 2.1 (95% CI: 1.1, 3.9,P=0.02)) and 3.8 (95% CI:2,6.9, P<0.001) times more likely to have NAFLD and NASH respectively. By Multivariate analysis, female gender (P<0.001) and increased BMI (P=.03) were associated with hypothyroidism. NAFLD subjects who reported mild alcohol consumption were less likely to have hypothyroidism compared to those who reported complete abstinence (OR 0.37, P=0.008).
Conclusions
A higher prevalence of hypothyroidism was demonstrated in patients with NAFLD compared to controls. Patients with hypothyroidism were more likely to have NASH. Among subjects with NALFD, female gender, increased BMI and history of abstinence from alcohol were associated with hypothyroidism. Further studies are needed in order to confirm and better characterized these findings as well as the described associations and their pathogenesis.
Keywords: Fatty liver, Non alcoholic steatohepatitis, Hypothyroidism, Insulin resistance
Introduction
Non alcoholic fatty liver disease (NAFLD) is a chronic liver disease with a histological spectrum ranging from steatosis alone to non alcoholic steatohepatitis (NASH), the latter having an increased risk for progression to cirrhosis. The prevalence of NAFLD in adults has been reported to be as high as 33% making it the most common cause of chronic liver disease in the United States(1).Thyroid dysfunction especially hypothyroidism has been associated with insulin resistance(2, 3), dyslipidemia(4, 5) and obesity (6, 7)all of which are important components of the metabolic syndrome.
Recent data suggest that hypothyroidism may be associated with NAFLD (8). However, clinical data supporting this association are incomplete and the pathophysiology underlying this k association remains unclear. Additional information is needed to confirm and better characterize the proposed association between NAFLD and hypothyroidism. Therefore, we conducted a case control study to assess the prevalence of hypothyroidism in consecutive NAFLD patients compared to matched controls while assessing potential factors that could be associated with hypothyroidism in this patient population.
Methods and Design
Study design and Patient population
The study population consisted of 246 adult patients individuals at least ≥18 years of age with biopsy proven non alcoholic fatty liver disease (NAFLD) seen in the hepatology outpatient clinic of the Cleveland Clinic Foundation in Cleveland, Ohio between October 2006 and June 2009. Out of the study population 233 NAFLD cases were matched by age, gender, race and BMI with 430 controls without any evidence of chronic liver disease attending the general medicine outpatient clinics of Cleveland Clinic Foundation. The controls were identified using the e-Cleveland clinic electronic medical record system. Records of all cases and controls were reviewed by a single physician (MP) and relevant data were abstracted.
Exclusion criteria included subjects with significant alcohol use (>14 drinks per week in males or 7 drink/day in females), or those with any laboratory or clinical evidence to suggest certain or probable underlying chronic liver disease including viral hepatitis, hemochromatosis, autoimmune hepatitis, Wilson’s disease, alpha1 antitrypsin disease or chronic cholestatic liver disease.
Liver biopsy specimens were reviewed by a single experienced liver pathologist. The histological grading and staging for NAFLD were performed using the NASH Clinical Research Network validated histological scoring system (9)
Definitions
Subjects were defined as having “hypothyroidism” if they carried a clinical diagnosis of hypothyroidism and were on thyroid replacement therapy.
Controls were required to have normal liver tests (ALT ≤45 IU/L, AST ≤40 IU/L, bilirubin ≤1.5 mg/dl and alkaline phosphatase ≤150 IU/L), and have absence of any acute or chronic liver disease, and the absence of fatty liver on at least one radiographic imaging study.
Ethical consideration
This study was designed as a retrospective case-control study and was conducted in accordance with the ethical guidelines of the Helsinki declaration and approved by the Institutional Review Board at the Cleveland clinic, Cleveland, Ohio.
Statistical analysis
Descriptive statistics were computed for all factors. Mean and standard deviations were calculated for continuous variables and frequencies and percentages for categorical variables. A p<0.05 was considered statistically significant. SAS version 9.2 software (The SAS Institute, Cary, NC) and R version 2.10.1 software (The R Foundation for Statistical Computing, Vienna, Austria) were used to perform all analyses.
Propensity score matching was used to match NAFLD subjects to controls without liver disease. A propensity score (PS) was created using age, gender, ethnicity and BMI and up to 2 control subjects were matched to each NAFLD patient within a caliper of PS ± 0.03 using the greedy algorithm. Out of a pool of 246 NAFLD patients and 1455 controls, a total of 197 NAFLD subjects were matched 1:2 and an additional 36 were matched 1:1. Conditional logistic regression was used to compare the 2 groups.
All 246 NAFLD patients were used to assess prevalence of hypothyroidism in subjects with NAFLD and what factors were associated with hypothyrodism in these patients. Student’s t-tests or Wilcoxon rank sum tests were used to evaluate associations between continuous variables and presence of hypothyroidism, Pearson’s chi-square were used for categorical variables and Mantel-Haenzel tests for steatosis, fibrosis, inflammation and ballooning In addition, a multivariable logistic regression analysis was performed to evaluate factors associated with presence of hypothyroidism. An automated stepwise variable selection was performed on 1,000 bootstrap samples to choose the final model; variables with more than 10% missing values were not considered for inclusion and factors that appeared in ≥30% of replications were kept in the final model.
Results
Table 1 summarizes clinical and demographical information of the subjects. The two groups were matched for age, BMI, gender and ethnicity. The mean age among NAFLD cases was 50.4 years, 56.2% were females and the the mean BMI was 35.7 kg/m2. There was no difference in ethnicity between the two groups. Diabetes mellitus, hypertension and hyperlipidemia were more frequent in the NAFLD group (P<0.001) compared to controls. In addition, the mean levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphtase and thyroid stimulating harmone (TSH) were higher in NAFLD compared to controls (P<0.01).
Table 1.
Demographic and Clinical Characteristics: NAFLD vs. Controls
| Factor | NAFLD (N=233) |
Controls (N=430) |
P |
|---|---|---|---|
| Age | 50.4 (11.1) | 51.0 (14.1) | 0.76 |
| BMI (kg/m2) | 35.7 (8.6) | 34.7 (8.1) | 0.86 |
| Female | 131 (56.2) | 243 (56.5) | 0.86 |
| Ethnicity | 0.57 | ||
| Caucasian | 219 (94) | 401 (93.3) | |
| African-American | 8 (3.4) | 20 (4.6) | |
| Hispanic | 3 (1.3) | 3 (0.7) | |
| Other | 3 (1.3) | 6 (1.4) | |
| DM † | 99 (42.7) | 95 (22.1) | <0.001 |
| HTN † | 137 (58.8) | 188 (43.6) | <0.001 |
| Hyperlipidemia † | 174 (86.1) | 183 (42.6) | <0.001 |
| Hypothyroidism | 51 (21.8) | 41 (9.5) | <0.001 |
| AST† (IU/L) | 41.5 | 21.0 | <0.001 |
| ALT† (IU/L) | 48.0 | 19.0 | <0.001 |
| ALP† (IU/L) | 80.0 | 76.0 | 0.001 |
| Bilirubin† (mg/dL) | 0.8 | 0.8 | 0.97 |
| TSH† (mU/L) | 2.2 | 1.7 | 0.013 |
| Hypothyroid | 49 (21) | 41 (9.5) | <0.001 |
AST-Aspartate aminotransferase, ALT- Alanine aminotransferase, ALP- Alkaline phosphtase
Values presented as N(%) for gender, ethnicity, diabetes, HTN, hyperlipidemia and hypothyoidism and Mean (SD). p-values correspond to univariable conditional logistic regression analyisis to account for matching.
Data not available for all subjects. Diabetes n=664; HTN n=664; Hyperlipidemia n=633; Bilirubin n=558; AST n=562; ALT n=567; Albumin n=559; INR n=404; PT n=404; Cholesterol n=461; TG n=462; HDL n=458; LDL n=448; ALP n=547; TSH n=392.
Prevalence of hypothyroidism in NAFLD
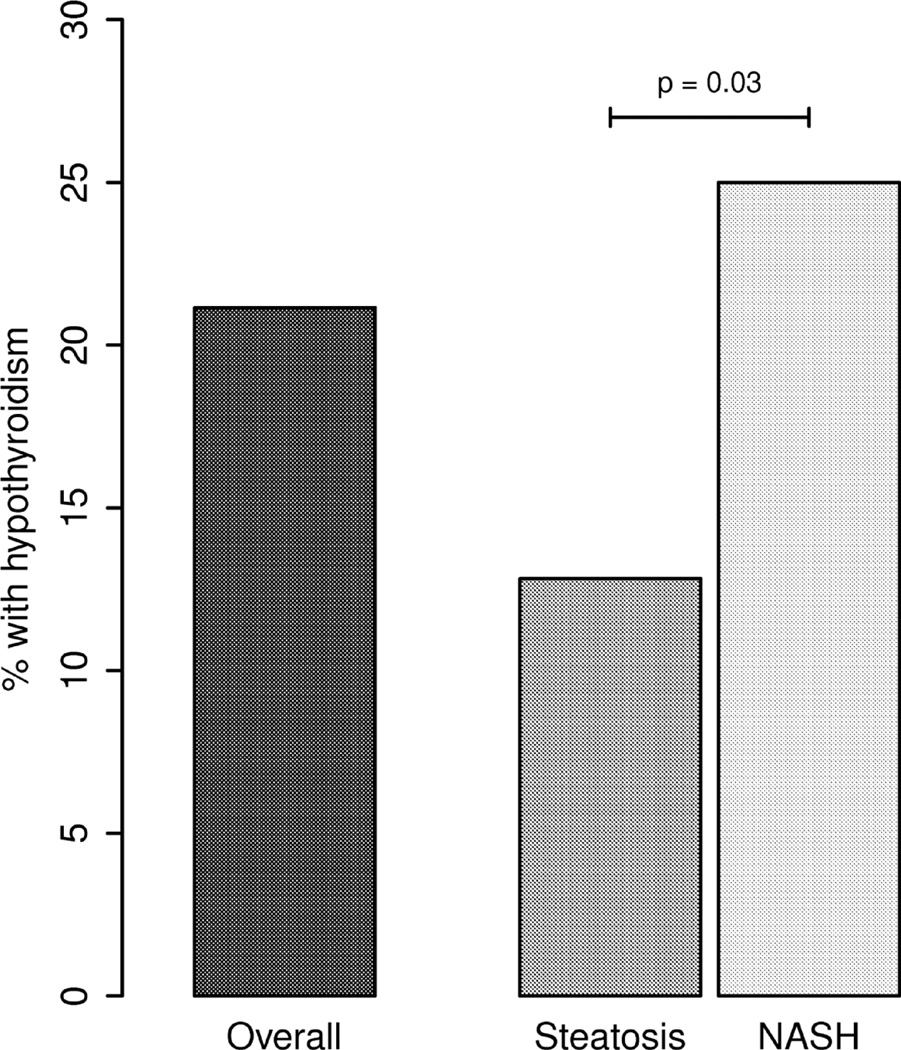
The prevalence of hypothyroidism was higher among patients with NAFLD (Figure.1) compared to the control group (21.1 % vs 9.5%, P<0.001) among subjects with NAFLD. Hypothyroidism was also more common in patients with NASH when compared to those with No NASH (25 % vs 12.8%, P=0.03) (figure 2). In multivariate analysis subjects with hypothyroidism were 2.1 (95% CI:1.1, 3.9) and 3.8 (95% CI:2,6.9) times more likely to have NAFLD and NASH respectively. After adjusting for diabetes mellitus, dyslipidemia and hypertension, this association remained statistically significant.
Figure 1.
The prevalence of hypothyroidism in subjects with NAFLD vs Controls from general population.
Figure 2.
Shows the over all prevalence of hypothyroidism in NAFLD and the distribution of NASH vs No NASH.
Univariable analysis
In the univariable analysis (Table 2), NAFLD patients with hypothyroidism were more likely to be females, have an older age and higher BMI compared to NAFLD patients without hypothyroidism. A higher proportion of patients with hypothyroidism had lower ALT levels. As expected hypothyroid patients had higher TSH compared to those without the disease. Steatohepatitis was more prevalent in patients with hypothyroidism compared to those without the disorder (P=0.03). Additionally, hypothyroidism was more frequent in NAFLD patients who reported no alcohol consumption compared to those who reported mild to moderate alcohol consumption.
Table 2.
Univariable analysis of factors associated with hypothyroidism in NAFLD
| Factor | Hypothyroidism (N=52) |
No Hypothyroidism (N=194) |
P Value |
|---|---|---|---|
| Female | 46 (88.5) | 94 (48.5) | <0.001 |
| Age | 53.5 (11.2) | 49.5 (10.6) | 0.035 |
| BMI (kg/m2) | 39.5 (11.8) | 35.4 (8.5) | 0.023 |
| Alcohol use† | 13 (25.0) | 106 (54.9) | <0.001 |
| ALT† (IU/L) | 37 (23,73) | 49.0 (30,88.0) | 0.045 |
| TSH† (mU/L) | 3.1 (1.8,4.8) | 2.0 (1.4,3.0) | 0.008 |
| DM † | 26 (50) | 84 (43.5) | 0.4 |
| HTN † | 30 (57.7) | 116 (60.1) | 0.7 |
| Hyperlipidemia † | 39 (86.7) | 147 (86.5) | 0.97 |
| Metabolic Syndrome | 36 (80.0) | 131 (73.6) | 0.38 |
| Ferritin† | 133 (87,285) | 170 (72.0,276.5) | 0.76 |
| NASH | 42 (80.8) | 126 (65.0) | 0.03 |
Values presented as Mean (SD) or Median (P25, P75) for continuous factors and N (%) for categorical factors
p-values correspond to Student's t-test or Wilcoxon rank sum tests for continuous factors, Fisher's Exact tests for ethnicity and INR>1, Mantel-Haenszel chi-square for histology scores and Pearson's chi-square for all other categorical factors.
Data not available for all subjects. EtOH n=245; HTN n=245; DM n=245; MS n=223; Hyperlipidemia n=215; ALT n=237; Ferritin n=169; TSH n=142
Multivariable analysis
Table 3 represents the results of the multivariate logistic regression analysis. Female gender (OR, 5.9; P< 0.001), higher BMI (OR,1.04; P=0.03) were associated with increased risk of hypothyroidism in NAFLD. In contrast, mild to moderate alcohol consumption was associated with a reduced risk of developing hypothyroidism compared to those who reported complete abstinence (OR, 0.31; P<.001). Though patients with hypothyroidism had increased risk of NASH it was not statistically significant. (OR 2; P=0.08).
Table 3.
Multivariable analysis of factors associated with hypothyroidism in NAFLD
| Predictive Variable | OR (95% CI) | P value |
|---|---|---|
| Female | 5.9 (2.3, 14.8) | <0.001 |
| Alcohol use * | 0.3 (0.18, 0.77) | <0.008 |
| BMI(kg/m2) | 3.3 (1.08, 9.8) | 0.03 |
| NASH# | 2.8 (0.908, 4.6) | 0.08 |
CI: confidence interval
Use of moderate alcohol has a protective effect.
Histological diagnosis of NASH.
Discussion
The prevalence of hypothyroidism in the united states is 3.7% as observed in the National Health and Nutritional Examination Survey (NHANES) conducted between 1999 and 2002 (10). Other studies reported the prevalence of subclinical and overt hypothyroidism to be 4%–10% and 0.3%–5% in the general population; respectively and 5% in the geriatric population (10–14). We found an increased prevalence of hypothyroidism among patients with NAFLD compared to age, gender, race and BMI matched controls without known chronic liver disease with the prevalence being highest in subjects with NASH. Two previous studies with smaller sample sizes and incomplete histology reported prevalence rates of 15% and 20% for hypothyroidism in NAFLD(8, 15). Our study included a large sample size with liver histology read by a single pathologist for all the patients. We also controlled for known factors associated with hypothyroidism (age, gender, ethnicity and BMI). These current findings confirm an association between the presence of hypothyroidism and NAFLD.
Although a role of hypothyroidism in the pathogenesis of non alcoholic fatty liver disease has not been established, a number of possible mechanisms could be involved. Hypothyroidism has been associated with insulin resistance (2, 3, 16), dyslipidemia(4, 5) and obesity(6, 7); all of which are important components of the metabolic syndrome. In addition, hypothyroidism is also associated with the metabolic syndrome (17), which plays an important role in the development of NAFLD (18).
Insulin resistance in the setting of hypothyroidism has been documented (2)and is associated with decreased responsiveness of glucose uptake in muscle and adipose tissue to insulin, as well as decreased glycogen synthesis in skeletal muscle in both animal and human studies(2, 3, 16, 19, 20). These effects were alleviated by thyroid replacement (3). Hypothyroidism is also more common in patients with diabetes than in the general population(21). If hypothyroidism enhances the degree of insulin resistance in NAFLD patients, it may increase the already elevated lipolysis and free fatty acid delivery to the liver and thereby accelerate liver injury in NAFLD (22).
There is also an increased prevalence of hypothyroidism in the obese population as compared to the general population (7) with the prevalence of hypothyroidism being 10%–20% in obesity(6, 7). On average hypothyroid patients weigh 15 – 30% more than during euthyroid state(23). Leptin, and adipocytokine that affects thermagenesis and appetite and is an indicator of body fat content, may have a possible role in hypothyroidism and obesity(24). Hypothyroidism patients have increased levels of leptin (25) which increases collagen production and insulin resistance in the liver (26, 27). Furthermore hypothyroidism can also increase risk of hypertension(28). Possible mechanisms responsible for hypertension in individuals with hypothyroidism include increased peripheral vascular resistance and arterial stiffness respectively(29); abnormalities that occur in NAFLD patients(30).
Up to 90% of hypothyroid patients have abnormal lipid values (5). While hypothyroidism primarily causes elevation in cholesterol and low density lipoproteins, it also affects the synthesis, mobilization and degradation of all aspects of lipid metabolism(4, 31). There is evidence of increased triglyceride levels in hypothyroid subjects due to increased estertification of hepatic fatty acids and decreased lipoprotein lipase activity (4)and there is some evidence of low HDL levels in these subjects(4, 5). It is possible that hypothyroidism can be contributing to the dyslipidemia in NAFLD which is a common abnormality in these patients (32). The antisteatotic and triglyceride reducing effects of a liver- selective thyroid receptor (TR) agonist on livers of animal models with fatty liver have been described (33).Therefore hypothyroidism may exacerbate the preexisting lipid abnormalities in NAFLD.
On multivariate analysis females with NAFLD were at a higher risk of hypothyroidism compared to male cases. This gender difference in hypothyroidism has been well described (11–13). A novel observation in this study was the apparent protective effect of mild alcohol intake on hypothyroidism among NAFLD cases. While excessive alcohol consumption is known to suppress peripheral thyroid metabolism in patients with alcoholism and especially those with alcoholic cirrhosis independent of the liver damage(34, 35), we did not find any literature that described the association of alcohol consumption and thyroid disease in the general population. It is unclear why abstinence from alcohol compared to those who drank in moderation resulted in increased hypothyroidism in NAFLD. However, alcohol consumption has been shown to decrease thyroid volume and as well as the prevalence of goiter suggesting a possible protective effect of alcohol on the thyroid gland(36). In addition there is some evidence of potential protective role of alcohol in thyroid cancer (37). Similar benefits of alcohol in cardiovascular morbidity and mortality insulin resistance, type 2 diabetes mellitus and HDL have been demonstrated(38, 39).
Markers of oxidative stress including reactive oxygen species and markers of lipid peroxidation have been reported in patients with hypothyroidism (40, 41); abnormalities that also occur in NASH patients to a greater extent than other forms of NAFLD (42). This may explain, in part, the mechanism for the increased presence of hypothyroidism in NASH patients. Recently, mitochondrial dysfunction has been implicated in pathogenesis of NASH (43). This of particular interest since thyroid dysfunction alters mitochondrial respiration (44). Mitochondria abnormalitities and dysfunction in skeletal muscle and alteration of cardiolipin (an important phospholipid in the inner membrane of mitochondria) have been described in hypothyroidism (45, 46).
There were a number of limitations to our study. The retrospective study design cannot define the time line between the development of hypothyroidism and that of fatty liver. This limits the ability to establish a temporal causality between these two factors. Data regarding the time of diagnosis of hypothyroidism as well as accurate results of thyroid function tests were not available. Similar to earlier studies, we had to use thyroid replacement therapy as a surrogate for diagnosis.
Even though we did not study the prevalence of hypothyroidism among other types of chronic liver disease, our data indicated that the prevalence of hypothyroidism is higher than other chronic liver diseases such as primary biliary cirrhosis (PBC), primary sclerosing cholangitis (PSE) and viral hepatitis with prevalence ratses of 10%, 6% and 7.3% respectively(8, 15)
Hepatocellular carcinoma (HCC) is known to occur in patients with cirrhosis including those related to NASH. Interestingly, the presence of hypothyroidism in patient with cirrhosis has been associated with an increased risk of HCC (47).The prevalence of hypothyroidism in HCC is 11.7%. Therefore it may be important to identify those patients with NASH cirrhosis who have hypothyroidism which would put them at a higher risk for developing HCC.
Based on available data, it appears the independent affects of hypothyroidism on insulin resistance, dyslipidemia and BMI may exacerbate these same pre-existing abnormalities in NAFLD resulting in the high prevalence of NASH in NAFLD patients with hypothyrodism. In summary, our study indicates that patients with NAFLD have a higher prevalence of hypothyroidism when compared to a matched control population and is higher among those with NASH compared to no NASH. In addition moderate alcohol use appears to have a protective effect for developing hypothyroidism in NAFLD. The high prevalence of this disorder among NAFLD patients, suggests that hypothyroidism may identify a subgroup of patients in the general population who may benefit from screening for the presence of fatty liver disease.
Footnotes
Results of this study were partially presented at the ACG 2009 meeting, San Diego, CA, USA
References
- 1.Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, et al. Prevalence of hepatic steatosis in an urban population in the united states: Impact of ethnicity. Hepatology. 2004;40(6):1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 2.Dimitriadis G, Mitrou P, Lambadiari V, Boutati E, Maratou E, Panagiotakos DB, Koukkou E, et al. Insulin action in adipose tissue and muscle in hypothyroidism. J Clin Endocrinol Metab. 2006;91(12):4930–4937. doi: 10.1210/jc.2006-0478. [DOI] [PubMed] [Google Scholar]
- 3.Rochon C, Tauveron I, Dejax C, Benoit P, Capitan P, Fabricio A, Berry C, et al. Response of glucose disposal to hyperinsulinaemia in human hypothyroidism and hyperthyroidism. Clin Sci (Lond) 2003;104(1):7–15. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- 4.Pucci E, Chiovato L, Pinchera A. Thyroid and lipid metabolism. Int J Obes Relat Metab Disord. 2000;24(Suppl 2):S109–S112. doi: 10.1038/sj.ijo.0801292. [DOI] [PubMed] [Google Scholar]
- 5.O'Brien T, Dinneen SF, O'Brien PC, Palumbo PJ. Hyperlipidemia in patients with primary and secondary hypothyroidism. Mayo Clin Proc. 1993;68(9):860–866. doi: 10.1016/s0025-6196(12)60694-6. [DOI] [PubMed] [Google Scholar]
- 6.Michalaki MA, Vagenakis AG, Leonardou AS, Argentou MN, Habeos IG, Makri MG, Psyrogiannis AI, et al. Thyroid function in humans with morbid obesity. Thyroid. 2006;16(1):73–78. doi: 10.1089/thy.2006.16.73. [DOI] [PubMed] [Google Scholar]
- 7.Raftopoulos Y, Gagne DJ, Papasavas P, Hayetian F, Maurer J, Bononi P, Caushaj PF. Improvement of hypothyroidism after laparoscopic roux-en-Y gastric bypass for morbid obesity. Obes Surg. 2004;14(4):509–513. doi: 10.1381/096089204323013514. [DOI] [PubMed] [Google Scholar]
- 8.Liangpunsakul S, Chalasani N. Is hypothyroidism a risk factor for non-alcoholic steatohepatitis? J Clin Gastroenterol. 2003;37(4):340–343. doi: 10.1097/00004836-200310000-00014. [DOI] [PubMed] [Google Scholar]
- 9.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41(6):1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 10.Aoki Y, Belin RM, Clickner R, Jeffries R, Phillips L, Mahaffey KR. Serum TSH and total T4 in the united states population and their association with participant characteristics: National health and nutrition examination survey (NHANES 1999–2002) Thyroid. 2007;17(12):1211–1223. doi: 10.1089/thy.2006.0235. [DOI] [PubMed] [Google Scholar]
- 11.Bindels AJ, Westendorp RG, Frolich M, Seidell JC, Blokstra A, Smelt AH. The prevalence of subclinical hypothyroidism at different total plasma cholesterol levels in middle aged men and women: A need for case-finding? Clin Endocrinol (Oxf) 1999;50(2):217–220. doi: 10.1046/j.1365-2265.1999.00638.x. [DOI] [PubMed] [Google Scholar]
- 12.Canaris GJ, Manowitz NR, Mayor G, Ridgway EC. The colorado thyroid disease prevalence study. Arch Intern Med. 2000;160(4):526–534. doi: 10.1001/archinte.160.4.526. [DOI] [PubMed] [Google Scholar]
- 13.Bjoro T, Holmen J, Kruger O, Midthjell K, Hunstad K, Schreiner T, Sandnes L, et al. Prevalence of thyroid disease, thyroid dysfunction and thyroid peroxidase antibodies in a large, unselected population. the health study of nord-trondelag (HUNT) Eur J Endocrinol. 2000;143(5):639–647. doi: 10.1530/eje.0.1430639. [DOI] [PubMed] [Google Scholar]
- 14.Ayala C, Cozar MV, Rodriguez JR, Silva H, Pereira JL, Garcia-Luna PP. Subclinical thyroid disease in institutionalised healthy geriatric population. Med Clin (Barc) 2001;117(14):534–535. doi: 10.1016/s0025-7753(01)72169-0. [DOI] [PubMed] [Google Scholar]
- 15.Silveira MG, Mendes FD, Diehl NN, Enders FT, Lindor KD. Thyroid dysfunction in primary biliary cirrhosis, primary sclerosing cholangitis and non-alcoholic fatty liver disease. Liver Int. 2009;29(7):1094–1100. doi: 10.1111/j.1478-3231.2009.02003.x. [DOI] [PubMed] [Google Scholar]
- 16.Kosovskii MI, Katkova SP, Mirakhmedov MM, Rakhimdzhanov RT. Insulin resistance in experimental hypo- and hyperthyroidism. Probl Endokrinol (Mosk) 1989;35(3):50–54. [PubMed] [Google Scholar]
- 17.Shantha GP, Kumar AA, Jeyachandran V, Rajamanickam D, Rajkumar K, Salim S, Subramanian KK, et al. Association between primary hypothyroidism and metabolic syndrome and the role of C reactive protein: A cross-sectional study from south india. Thyroid Res. 2009;2(1):2. doi: 10.1186/1756-6614-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marchesini G, Brizi M, Bianchi G, Tomassetti S, Bugianesi E, Lenzi M, McCullough AJ, et al. Nonalcoholic fatty liver disease: A feature of the metabolic syndrome. Diabetes. 2001;50(8):1844–1850. doi: 10.2337/diabetes.50.8.1844. [DOI] [PubMed] [Google Scholar]
- 19.Dimitriadis G, Parry-Billings M, Bevan S, Leighton B, Krause U, Piva T, Tegos K, et al. The effects of insulin on transport and metabolism of glucose in skeletal muscle from hyperthyroid and hypothyroid rats. Eur J Clin Invest. 1997;27(6):475–483. doi: 10.1046/j.1365-2362.1997.1380688.x. [DOI] [PubMed] [Google Scholar]
- 20.Dimitriadis GD, Leighton B, Parry-Billings M, West D, Newsholme EA. Effects of hypothyroidism on the sensitivity of glycolysis and glycogen synthesis to insulin in the soleus muscle of the rat. Biochem J. 1989;257(2):369–373. doi: 10.1042/bj2570369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smithson MJ. Screening for thyroid dysfunction in a community population of diabetic patients. Diabet Med. 1998;15(2):148–150. doi: 10.1002/(SICI)1096-9136(199802)15:2<148::AID-DIA540>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 22.Bugianesi E, McCullough AJ, Marchesini G. Insulin resistance: A metabolic pathway to chronic liver disease. Hepatology. 2005;42(5):987–1000. doi: 10.1002/hep.20920. [DOI] [PubMed] [Google Scholar]
- 23.Krotkiewski M. Thyroid hormones and treatment of obesity. Int J Obes Relat Metab Disord. 2000;24(Suppl 2):S116–S119. doi: 10.1038/sj.ijo.0801294. [DOI] [PubMed] [Google Scholar]
- 24.Vettor R. The metabolic actions of thyroid hormone and leptin: A mandatory interplay or not? Diabetologia. 2005;48(4):621–623. doi: 10.1007/s00125-005-1703-9. [DOI] [PubMed] [Google Scholar]
- 25.Leonhardt U, Ritzel U, Schafer G, Becker W, Ramadori G. Serum leptin levels in hypo- and hyperthyroidism. J Endocrinol. 1998;157(1):75–79. doi: 10.1677/joe.0.1570075. [DOI] [PubMed] [Google Scholar]
- 26.Saxena NK, Ikeda K, Rockey DC, Friedman SL, Anania FA. Leptin in hepatic fibrosis: Evidence for increased collagen production in stellate cells and lean littermates of ob/ob mice. Hepatology. 2002;35(4):762–771. doi: 10.1053/jhep.2002.32029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Benomar Y, Wetzler S, Larue-Achagiotis C, Djiane J, Tome D, Taouis M. In vivo leptin infusion impairs insulin and leptin signalling in liver and hypothalamus. Mol Cell Endocrinol. 2005;242(1–2):59–66. doi: 10.1016/j.mce.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 28.Luboshitzky R, Aviv A, Herer P, Lavie L. Risk factors for cardiovascular disease in women with subclinical hypothyroidism. Thyroid. 2002;12(5):421–425. doi: 10.1089/105072502760043512. [DOI] [PubMed] [Google Scholar]
- 29.Obuobie K, Smith J, Evans LM, John R, Davies JS, Lazarus JH. Increased central arterial stiffness in hypothyroidism. J Clin Endocrinol Metab. 2002;87(10):4662–4666. doi: 10.1210/jc.2002-020493. [DOI] [PubMed] [Google Scholar]
- 30.Villanova N, Moscatiello S, Ramilli S, Bugianesi E, Magalotti D, Vanni E, Zoli M, et al. Endothelial dysfunction and cardiovascular risk profile in nonalcoholic fatty liver disease. Hepatology. 2005;42(2):473–480. doi: 10.1002/hep.20781. [DOI] [PubMed] [Google Scholar]
- 31.Pearce EN. Hypothyroidism and dyslipidemia: Modern concepts and approaches. Curr Cardiol Rep. 2004;6(6):451–456. doi: 10.1007/s11886-004-0054-3. [DOI] [PubMed] [Google Scholar]
- 32.Toledo FG, Sniderman AD, Kelley DE. Influence of hepatic steatosis (fatty liver) on severity and composition of dyslipidemia in type 2 diabetes. Diabetes Care. 2006;29(8):1845–1850. doi: 10.2337/dc06-0455. [DOI] [PubMed] [Google Scholar]
- 33.Cable EE, Finn PD, Stebbins JW, Hou J, Ito BR, van Poelje PD, Linemeyer DL, et al. Reduction of hepatic steatosis in rats and mice after treatment with a liver-targeted thyroid hormone receptor agonist. Hepatology. 2009;49(2):407–417. doi: 10.1002/hep.22572. [DOI] [PubMed] [Google Scholar]
- 34.Hermann D, Heinz A, Mann K. Dysregulation of the hypothalamic-pituitary-thyroid axis in alcoholism. Addiction. 2002;97(11):1369–1381. doi: 10.1046/j.1360-0443.2002.00200.x. [DOI] [PubMed] [Google Scholar]
- 35.Hegedus L, Rasmussen N, Ravn V, Kastrup J, Krogsgaard K, Aldershvile J. Independent effects of liver disease and chronic alcoholism on thyroid function and size: The possibility of a toxic effect of alcohol on the thyroid gland. Metabolism. 1988;37(3):229–233. doi: 10.1016/0026-0495(88)90100-x. [DOI] [PubMed] [Google Scholar]
- 36.Knudsen N, Bulow I, Laurberg P, Perrild H, Ovesen L, Jorgensen T. Alcohol consumption is associated with reduced prevalence of goitre and solitary thyroid nodules. Clin Endocrinol (Oxf) 2001;55(1):41–46. doi: 10.1046/j.1365-2265.2001.01325.x. [DOI] [PubMed] [Google Scholar]
- 37.Allen NE, Beral V, Casabonne D, Kan SW, Reeves GK, Brown A, Green J, et al. Moderate alcohol intake and cancer incidence in women. J Natl Cancer Inst. 2009;101(5):296–305. doi: 10.1093/jnci/djn514. [DOI] [PubMed] [Google Scholar]
- 38.Facchini F, Chen YD, Reaven GM. Light-to-moderate alcohol intake is associated with enhanced insulin sensitivity. Diabetes Care. 1994;17(2):115–119. doi: 10.2337/diacare.17.2.115. [DOI] [PubMed] [Google Scholar]
- 39.Freiberg MS, Cabral HJ, Heeren TC, Vasan RS, Curtis Ellison R, Third National Health and Nutrition Examination Survey. Alcohol consumption and the prevalence of the metabolic syndrome in the US : A cross-sectional analysis of data from the third national health and nutrition examination survey. Diabetes Care. 2004;27(12):2954–2959. doi: 10.2337/diacare.27.12.2954. [DOI] [PubMed] [Google Scholar]
- 40.Nanda N, Bobby Z, Hamide A, Koner BC, Sridhar MG. Association between oxidative stress and coronary lipid risk factors in hypothyroid women is independent of body mass index. Metabolism. 2007;56(10):1350–1355. doi: 10.1016/j.metabol.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 41.Baskol G, Atmaca H, Tanriverdi F, Baskol M, Kocer D, Bayram F. Oxidative stress and enzymatic antioxidant status in patients with hypothyroidism before and after treatment. Exp Clin Endocrinol Diabetes. 2007;115(8):522–526. doi: 10.1055/s-2007-981457. [DOI] [PubMed] [Google Scholar]
- 42.Sanyal AJ, Campbell-Sargent C, Mirshahi F, Rizzo WB, Contos MJ, Sterling RK, Luketic VA, et al. Nonalcoholic steatohepatitis: Association of insulin resistance and mitochondrial abnormalities. Gastroenterology. 2001;120(5):1183–1192. doi: 10.1053/gast.2001.23256. [DOI] [PubMed] [Google Scholar]
- 43.Begriche K, Igoudjil A, Pessayre D, Fromenty B. Mitochondrial dysfunction in NASH: Causes, consequences and possible means to prevent it. Mitochondrion. 2006;6(1):1–28. doi: 10.1016/j.mito.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 44.Kvetny J, Wilms L, Pedersen PL, Larsen J. Subclinical hypothyroidism affects mitochondrial function. Horm Metab Res. 2010 doi: 10.1055/s-0030-1248261. [DOI] [PubMed] [Google Scholar]
- 45.Siciliano G, Monzani F, Manca ML, Tessa A, Caraccio N, Tozzi G, Piemonte F, et al. Human mitochondrial transcription factor A reduction and mitochondrial dysfunction in hashimoto's hypothyroid myopathy. Mol Med. 2002;8(6):326–333. [PMC free article] [PubMed] [Google Scholar]
- 46.Chicco AJ, Sparagna GC. Role of cardiolipin alterations in mitochondrial dysfunction and disease. Am J Physiol Cell Physiol. 2007;292(1):C33–C44. doi: 10.1152/ajpcell.00243.2006. [DOI] [PubMed] [Google Scholar]
- 47.Hassan MM, Kaseb A, Li D, Patt YZ, Vauthey JN, Thomas MB, Curley SA, et al. Association between hypothyroidism and hepatocellular carcinoma: A case-control study in the united states. Hepatology. 2009;49(5):1563–1570. doi: 10.1002/hep.22793. [DOI] [PMC free article] [PubMed] [Google Scholar]