Abstract
Purpose
Anisometropia shows an exponential increase in prevalence with increasing age based on cross-sectional studies. The purpose of this study was to evaluate longitudinal changes in anisometropia in all refractive components in older observers and to assess the influence of early cataract development.
Methods
Refractive error was assessed at two time points separated by ~12 years in 118 older observers (ages 67.1 and 79.3 years at the two test times). Anisometropia defined as ≥1.00 D was calculated for all refractive components. The subjects had intact ocular lenses in both eyes throughout the study. Lens evaluations were performed at the second test using LOCS III.
Results
All refractive components approximately doubled in prevalence of anisometropia. Spherical equivalent anisometropia changed from 16.1% to 32.2%. Similar changes were found for spherical error (17% to 38.1%), primary astigmatism (7.6% to 17.8%) and oblique astigmatism (14.4% to 29.7%). Many who did not have anisometropia at the first visit subsequently developed anisometropia (for ex. 26.3% for spherical error and 22.9% for oblique cylinder). The onset of anisometropia occurred at all ages within the studied age range with no particular preference for any one age. A small number lost anisometropia over time. Individual comparisons of refractive error changes in the two eyes in combination with nuclear lens changes showed that early changes in nuclear sclerosis in the two eyes could account for a large proportion of anisometropia (~40%) but unequal hyperopic shift in the spherical component in the two eyes was the primary cause of the anisometropia.
Conclusions
Anisometropia is at least 10 times more common in the elderly than in children and anisometropia develops in all refractive components in the oldest observers. Clinicians need to be aware of this common condition that could lead to binocular vision problems and potentially cause falls in the elderly.
Keywords: anisometropia, longitudinal, old age, refractive components, nuclear cataract, primary cylinder, oblique cylinder
Human refractive error undergoes significant change throughout the lifespan. Even after adulthood is reached, refractive error changes. Cross sectional studies have found an increase in hyperopia or decrease in myopia with increasing age followed by myopia in some studies in the very old in association with development of cataracts1–5 see Hyman, 2007 for review.6
Kempen et al. (2004)7 estimated the prevalence of refractive error by pooling the data from several large population surveys in the US and Western Europe involving 29,000 or more adult observers over the age of 40 years. Refractive error was characterized using the eye with the highest error (spherical equivalent of +3.00D or more, −1.00 or more and −5.00 or more). For white observers, the prevalence of spherical equivalent of +3.00D or more increased from about 3.6% in the 40–49 age range to about 25% in the over 80 age group-a factor of about 7 increase in prevalence of significant hyperopia. This is a surprisingly high percentage considering that this is the spherical equivalent and astigmatism increases significantly with age, which would decrease the hyperopic spherical equivalent. In our own previously published data in 569 older observers, the prevalence of equivalent sphere of +3.00D or more increased from 6.1% in the youngest group (59–65 years) to 10.4% for those over 80 years.8
In the Kempen et al. study7, spherical equivalent myopia of 1 D or more decreased significantly from about 41% in the 40–49 age group (combining men and women) to about 19% in those over 80 years (a factor of 2 decrease in prevalence). Our prior data show similar changes from 30.7% to 18.4% from the youngest group to those over 80 years8. In the Kempen et al. study7, the rate of myopia for the oldest group was higher than for middle age groups suggesting a quadratic shape to the prevalence of myopia of 1D or more vs. age. The prevalence of high myopia (−5.00 D or more) decreased slowly with age. Rates for men and women were similar for whites and the rates for whites were quite different from those for blacks and Hispanics. The biggest differences were seen for black men who showed a much lower prevalence of hyperopia of +3.00D or more and who also showed a more significant decrease in the prevalence of myopia of −1.00D or more from 22.5% for the youngest group to 2.8% for the oldest group. Substantial gender and race differences in refractive error changes with age were present.
The Beaver Dam Study9 presented group longitudinal changes in equivalent sphere over a period of 10 years and found that younger people became more hyperopic while older people became more myopic. The size of the group changes were fairly small with changes of +0.54D for those 43–59 years, −0.03D for those 60–69 years and −0.41D for those 70 years and over at baseline. They also found a birth cohort effect: when comparing people of the same age across examinations, those born in more recent years had more myopia than those born in earlier years. Astigmatism and anisometropia were not addressed. The Blue Mountains Eye study came to similar conclusions regarding hyperopia and myopia.4 In addition, they showed a 10% increase in against-the rule astigmatism over the 10-year time span.
Another manuscript from the Blue Mountains study in a large group of Australian participants10 demonstrated an increase in prevalence and severity of anisometropia (≥ 1D in spherical equivalent) with increasing age and also reported associations between anisometropia, cataract and higher refractive errors. Similar findings have been reported in adult Chinese observers.11
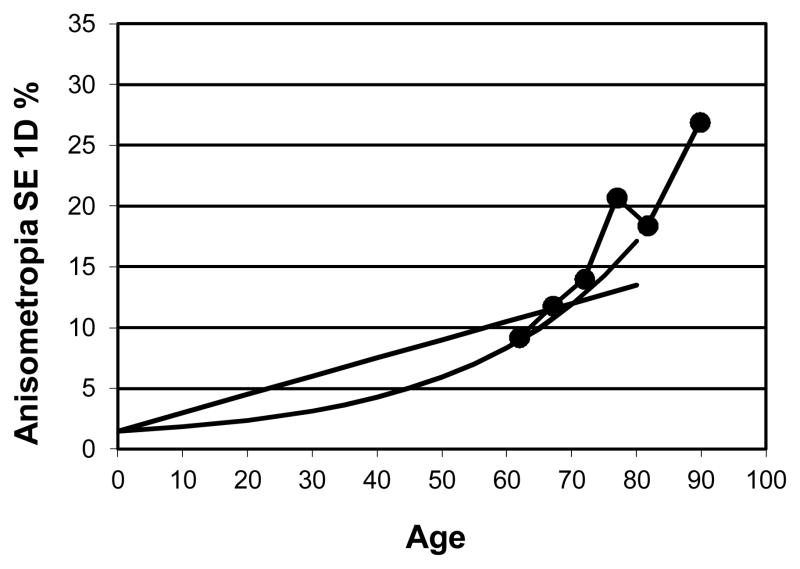
Weale’s summary paper12 presented convincing evidence of an increase in anisometropia (≥ 1D in spherical equivalent) with increasing age using cross-sectional data from many different studies in different countries. The prevalence ranged from less than a few percent in infants to about 7–10% at age 40–50 years and at least 15% or much more at age 80 years depending on the study. Weale found that a linear function fit the data as well as an exponential function with age. His fitted functions are shown in Figure 1, which shows the prevalence of anisometropia of 1D or more for the spherical equivalent refractive error as a function of age. The addition of our cross sectional data from the SKI study (569 observers with intact lenses8) in Figure 1 clearly shows that an exponential function is a better fit to the data of our older observers than a linear function. Our cross-sectional results8 showed an increase in the percentage of subjects with anisometropia for spherical equivalent (≥ 1D) from 10% in those under 65 years of age to 25% in subjects over 85 years of age.
Figure 1.
Linear and exponential model of the prevalence of 1D of anisometropia (spherical equivalent) from Weale12 as a function of age. The data points show the percentage of anisometropia for the participants in a subgroup of the SKI study N=569.
In order to evaluate what refractive components are responsible for this large increase in anisometropia in the oldest old, we present individual longitudinal refractive data on anisometropia for 118 older observers for spherical equivalent, spherical error, primary and oblique astigmatism over a period of 12.2 years on average.
METHODS
Subjects
The SKI Study is a longitudinal study in Marin County, CA evaluating changes in vision function and visual performance in the elderly. The initial population was randomly selected (N=900) and a total of 4 follow-up tests have been completed over a period of 15.4 years.8, 13–15 No exclusion criteria were used other than age (must be 55 years and older). At the subsequent follow-up visits 596, 452 and 254 and 190 of the original participants were re-tested. The participants selected for this evaluation of anisometropia were a subset of the SKI study population. Participants who had medical records which included refraction at the baseline visit and who completed three follow-up visits in the SKI study were included. Only subjects with intact ocular lenses in both eyes throughout the entire period were included. A total of 118 people were included. The average age for the 118 people at the two assessment times was 67.1 (SD 6.0) and 79.3 (SD 4.9) with 12.2 years between tests on average. Subjective refractive error was collected from medical records the first time of testing. At the 2nd time, the refractive error was determined using subjective refraction. The same examiner conducted all the subjective refractions.
One examiner graded all lenses at the 2nd visit for nuclear, cortical cataract and posterior subcapsular cataract using the LOCS III system at the slitlamp.16 None of the participants had nuclear cataract scores greater than 2.0, which means that they would not be characterized as having nuclear cataract according to common use of this scale.17–18
This research followed the tenets of the Declaration of Helsinki. Informed consent was obtained from the subjects after explanation of the nature and possible consequences of the study. This research was approved by the appropriate institutional review board (IRB).
Analysis of Refractive Components
Anisometropia was calculated for equivalent sphere, spherical error and primary astigmatism (with the rule or against the rule astigmatism (minus cylinder axis 180 or 90) and oblique astigmatism. Vector analysis was used to determine the primary and oblique astigmatism.19–20 Primary astigmatism was calculated as minus cylinder power x cosine (2 x axis0). The formula for the oblique astigmatism was (minus cylinder power x sin (2 x axis0). Anisometropia was defined as a difference between the two eyes of at least 1.00D.
RESULTS
Table 1 shows the distribution of anisometropia in the population at the two test times for equivalent sphere, spherical error, primary astigmatism (with the rule or against the rule astigmatism (minus cylinder axis 180 or 90) and oblique astigmatism. Anisometropia for equivalent sphere of 1.00 D or more increased from 16.1% to 32.2% over the ~12 year period while anisometropia of the spherical error increased from 17.0% to 38.1% and primary astigmatism anisometropia increased from 7.6% to 17.8%. Oblique astigmatism also increased from 14.4% to 29.7%. In short, the prevalence approximately doubled for each component of anisometropia. The incidence of anisometropia is also listed in the table (the percentage of people who did not have anisometropia the 1st time but who developed it by the 2nd time). The difference between the sum of the prevalence at the 1st time and the incidence values compared to the prevalence at the 2nd time is caused by people who had anisometropia at the first time only.
Table 1.
Changes in prevalence of anisometropia in refractive components over ~12 year period and incidence of anisometropia of 1.00D or more.
| Diopters of anisometropia | 0.00–0.99D | 1.00–1.99D | 2.00D+ | All 1.00D+ | Incidence 1.00D+ | |
|---|---|---|---|---|---|---|
| Equivalent Sphere | 1st time | 83.9% | 10.2% | 5.9% | 16.1% | 21.2% |
| 2nd time | 67.8% | 19.5% | 12.7% | 32.2% | ||
|
| ||||||
| Spherical Error | 1st time | 83.0% | 11.9% | 5.1% | 17.0% | 26.3% |
| 2nd time | 61.9% | 24.6% | 13.5% | 38.1% | ||
|
| ||||||
| Primary Astigmatism | 1st time | 92.4% | 6.8% | 0.8% | 7.6% | 12.7% |
| 2nd time | 82.2% | 13.6% | 4.2% | 17.8% | ||
|
| ||||||
| Oblique Astigmatism | 1st time | 85.6% | 11.9% | 2.5% | 14.4% | 22.9% |
| 2nd time | 70.3% | 21.2% | 8.5% | 29.7% | ||
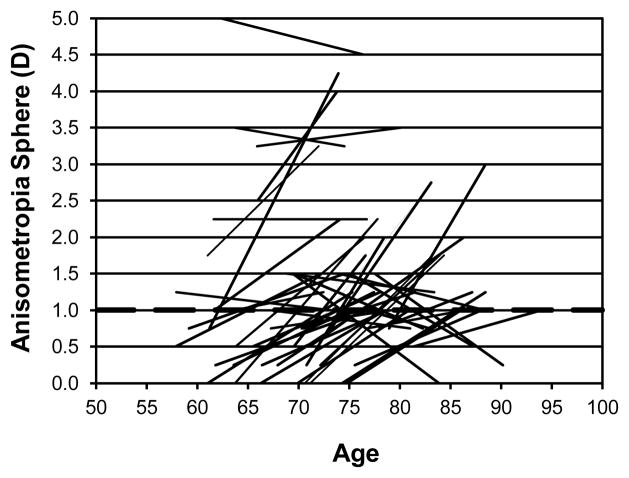
Figure 2 shows the individual changes in anisometropia for equivalent sphere for those 44 participants who showed at least 1 D of anisometropia for equivalent sphere at either the first or second visit (or both) as a function of age (years). The first data point is plotted at the age at the time of the refraction. A line connects the two data points for each observer but of course we are not implying a linear change in refraction. The most common pattern is an increase in anisometropia from less than 1 D to more than 1 D but a small percentage of participants also lost large amounts of anisometropia and some who already had significant amounts of anisometropia developed more. For example, one observer at ~79 years of age had 1.5 D of anisometropia which increased to 4.25 D at age ~88.5. Onset of anisometropia occurred over the studied age span with no particular concentration at any one age. The average time between visits was ~ 12 years.
Figure 2.
Individual changes in anisometropia for equivalent sphere for those 44 participants who showed at least 1D of anisometropia for equivalent sphere at either the first or second visit (or both) are shown as a function of age (years). The dashed horizontal line indicates 1D of anisometropia.
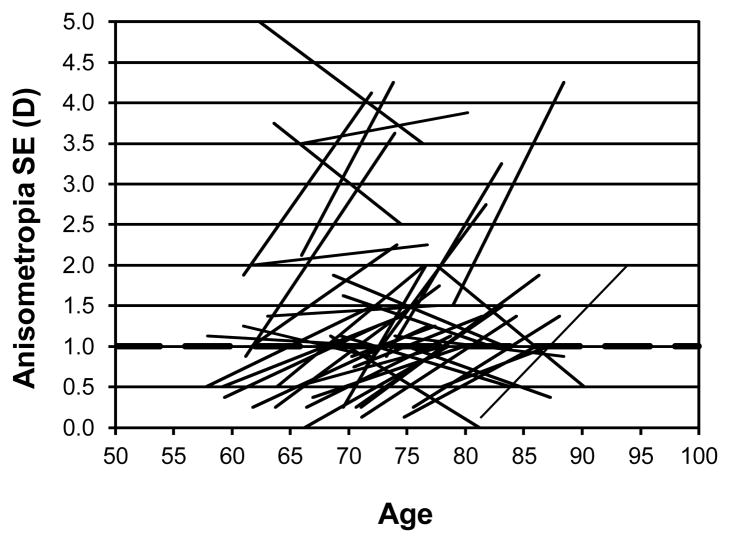
Figure 3 shows the individual changes with age in anisometropia for spherical error for those 51 participants who showed at least 1 D of anisometropia for spherical error at either the first or second visit (or both). The pattern is similar to that for equivalent sphere with most people who develop anisometropia having no difference in spherical error at the first visit. A small number lose their significant anisometropia while a handful of others maintain theirs.
Figure 3.
Individual changes in anisometropia for spherical error for those 51 participants who showed at least 1 D of anisometropia for spherical error at either the first or second visit (or both) are shown as a function of age (years). The dashed horizontal line indicates 1D of anisometropia.
There is no relationship between spherical error (RE) 1st time and change in anisometropia for sphere (r=0.16, p=0.09) and no statistically significant relationship between change in anisometropia for sphere with age (r=0.02, p=0.86). There is also no relation between equivalent sphere (RE) 1st time and change/decade in anisometropia for equivalent sphere (r=0.11, p=0.22).
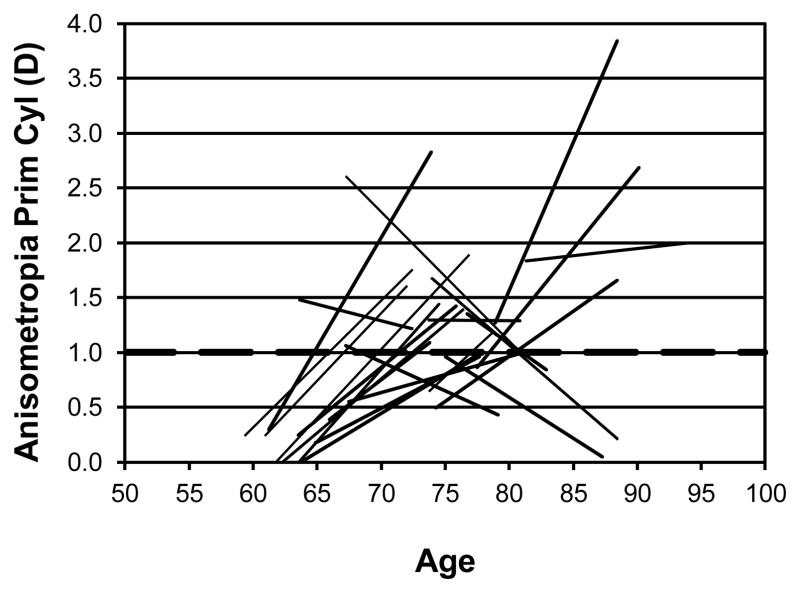
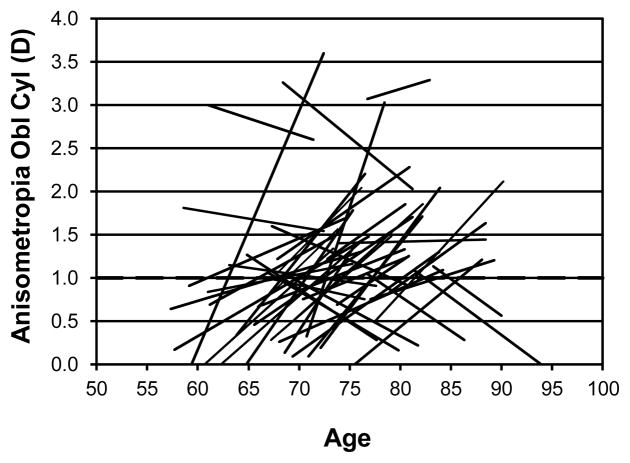
In addition to the changes in the spherical component, there are also changes in the degree of anisometropia for primary cylinder and considerable changes in oblique cylinder. Figure 4 shows the individual changes for primary cylinder (axis 180 or 90). 23 participants had 1D of anisometropia in the primary cylindrical component at either or both testing times. The development of primary cylinder anisometropia occurred primarily in the younger age groups while several participants lost their primary cylinder. Figure 5 shows the individual changes in oblique cylinder. 45 participants had 1D of anisometropia of oblique astigmatism at either or both times. The pattern is similar to the primary cylinder with most people developing anisometropia of the oblique cylinder but some also lose the anisometropia of the oblique component that they had. The development of the anisometropia of the oblique cylinder component occurred over a wider range of ages than for the primary cylinder anisometropia.
Figure 4.
Individual changes in anisometropia for primary cylinder (axis 90 or 180) for those 23 participants who showed at least 1D of anisometropia for primary cylinder at either the first or second visit (or both) are shown as a function of age (years). The dashed horizontal line indicates 1D of anisometropia.
Figure 5.
Individual changes in anisometropia for oblique cylinder for those 45 participants who showed at least 1D of anisometropia for oblique cylinder at either the first or second visit (or both) are shown as a function of age (years). The dashed horizontal line indicates 1D of anisometropia.
It has long been reported that nuclear sclerosis is associated with development of myopia and many modern population studies find large refractive changes due to lens changes but there are also studies that disagree.4, 6, 17
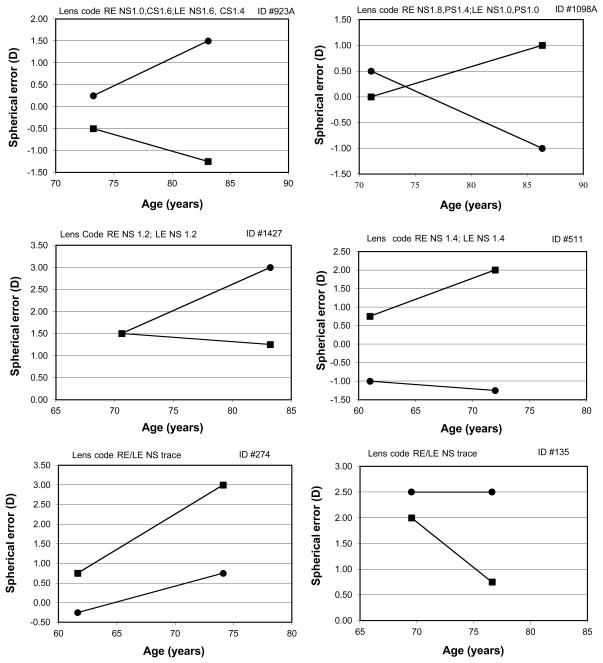
Figure 6 shows some examples of refractive changes in individuals with the addition of quantification of lens changes. The figure shows the spherical error for both eyes as a function of age. The lenses were graded at the slitlamp using LOCS III.16 The lens score is noted on the graph for each individual. In some cases, it is clear that lens changes are likely to be responsible for the increase in anisometropia.
Figure 6.
Individual examples of spherical error in the two eyes for the two test times. The individual lens codes for the two eyes are shown for each participant.
For example, subject #923A (top left) has less than 1 D of anisometropia at the first visit. This subject has considerably more nuclear sclerosis in the left eye (1.6 vs. 1.0) at the 2nd visit and this eye has undergone a myopic shift while the right eye, which has more cortical spoking, changed in the hyperopic direction resulting in 2.75D of spherical anisometropia at the age of 83 years. Subject #1098A (top right) also showed a 1.5 D myopic shift for the eye with the larger amount of nuclear sclerosis (1.8 vs. 1.0). The left eye had minor lens changes and increased in hyperopia by 1.0D.
The middle panels show examples where each eye has the same lens code and the refraction changed in the hyperopic direction in one eye while the other eye remained stable. For example, subject #511 (middle right) had significant anisometropia of 1.75D at the first visit, which increased to 3.25D at the 2nd visit. Clearly myopia induced by lenticular sclerosis is not the reason for the significant anisometropia in this case since the lens code is the same in each eye. The bottom panels of Figure 6 show two participants with no discernable lens changes who also developed significant anisometropia.
Individual analysis of the refractive changes and the lens scores indicated that unequal development of nuclear sclerosis in the two eyes could explain the spherical error anisometropia in about 40% of subjects who developed 1D or more of anisometropia, but in the remaining subjects, other factors must be responsible for these changes in the spherical error. When spherical equivalent is used, many people developed anisometropia from changes in the power and/or axis of the cylinder components.
DISCUSSION
Increased prevalence of anisometropia for spherical equivalent refractive error with increasing age after reaching adulthood has been reported by many different studies.3, 10–11, 21–23 In our own cross-sectional study8, more than 25% of the participants in the oldest age group (85 years and older) showed anisometropia of 1D or more for spherical equivalent. This is a surprisingly high proportion of the oldest old. Every component of the refractive error showed similar trends; anisometropia of the sphere component of 43% and primary cylinder anisometropia of more than 25%.
The current longitudinal study showed that 16.1% had anisometropia for equivalent sphere at the first visit (age 67.1 years), which nearly doubled at the second visit on average 12 years later (to 32.2%). In the longitudinal data presented here, all components of the refractive error showed a near doubling at the second visit. The incidence of anisometropia ranged from a low of 12.7% for primary cylinder to a high of 26.3% for spherical error (see Table 1). Figures 2–5 show that the onset of anisometropia occurred at all ages within the studied age range with no particular preference for any one age.
Since more people have anisometropia in the oblique cylinder component rather than the primary cylinder component (see Figs 4, 5), this suggests that axis changes from with-the rule to against-the rule with age may be responsible if the axis is changing faster in one eye than the other. It is well known that the astigmatic axis changes from with the rule (minus cylinder axis 180) to against the rule (minus cylinder axis 90) with increasing age.1, 8, 24
Table 2 shows a hypothetical representation of what would happen to the anisometropia for primary and oblique cylinder if the power remains constant but there are unequal axis changes in the two eyes. If one eye changed axis from 180 to 90 deg but the other eye only changed from 180 to 60 deg, both cylinder components would develop anisometropia. Clearly, differential changes in axis alone without any change in power are sufficient to produce significant anisometropia in the cylinder components.
Table 2.
Example of anisometropia produced by only unequal changes in cylinder axis in the two eyes without a power change.
| Refractive Error | Right Eye | Left Eye | Primary Cylinder RE | Primary Cylinder LE | Aniso. Primary Cylinder | Oblique Cylinder RE | Oblique Cylinder LE | Aniso. Oblique Cylinder |
|---|---|---|---|---|---|---|---|---|
| First time | Plano -2.00X180 | Plano-2.00x180 | 2.00D | 2.00D | 0.00D | 0.00D | 0.00D | 0.00D |
| Second time | Plano -2.00x060 | Plano-2.00x090 | −1.00D | −2.00D | 1.00D | 1.73D | 0.00D | 1.73D |
Causes of anisometropia include changes in sphere and/or cylinder power through lens or corneal changes, unequal axis changes in the cylinder component, unequal cataract development in the two eyes and other factors. The most common cause of increase in anisometropia in this group was unequal increase in hyperopic spherical error in the two eyes.
Some participants lost anisometropia: of the three people whose spherical anisometropia declined the most, the cause for all of them was differential nuclear sclerosis in the two eyes leading to a different decrease in hyperopia in the two eyes. For the four people who showed the largest declines in spherical equivalent anisometropia, the cause for all of them was development of significant cylindrical error in one or both eyes.
Brown and Hill, 198725 showed that there is a myopic shift in refraction prior to the onset of significant nuclear cataract. In that study, no standardized evaluation of the cataracts were done-“ patients were assigned to the cataract group when they had cataract visible by slit-lamp examination in the undilated pupil and at least minimal interference with visual acuity 6/7.5 or worse after refractive correction”. Later population studies with quantified lens evaluations have established that myopia is associated with nuclear cataracts.26–28 For example, the Blue Mountains Eye study17 (N=3654) found that people without nuclear cataract showed an annual mean hyperopic shift of 0.05D while this hyperopic shift disappeared in people with nuclear cataract. In this large population, a myopic shift only occurred in people with nuclear opacities of level 4 and higher in the Wisconsin grading system. Anisometropia has also been found to be associated with cataract (and increasing ametropia).10 In our limited study, unequal lens grading in the two eyes associated with increase in myopia in one eye was found in about 40% of the subjects who developed anisometropia at the second time.
It should be noted that no one in our study had a nuclear sclerosis score of more than 2.0, which means that their lenses were relatively clear and in many other studies, they would not have been classified as having nuclear cataract.17, 29–30
Weale12 argued that the increase in anisometropia occurs significantly earlier than the onset of cataracts. He argued that the prevalence of cataract in the age group 40–50 years is about 1% while the prevalence of anisometropia is an order of magnitude greater thus suggesting that development of cataract cannot account for the anisometropia. He based his estimates of cataract on the study of Leske et al.31 who defined cataract using LOCS grading system as N1 or N2 nuclear sclerosis or C1 or C2 cortical changes. This criterion (which has much lower lens grade labeled as cataract than more recent studies) would thus generate a high prevalence of cataract in the population. The prevalence of cataract in the US in the 40–49 year age group has subsequently been estimated at 2.5%.32 Conceivably, the percentage of anisometropia ascribed to nuclear sclerotic lens changes could increase with age.
Hyperopia increases with age have been documented for a long time1, 33 (see Kempen et al., 20047 for a summary and consolidation of newer population studies). The cause of increased hyperopia with age is debated. The lens increases in thickness and moves closer to the cornea with age, which should produce myopia; the cornea does not decrease in power with age and the axial length does not decrease which would potentially produce hyperopia; instead a change in the gradient of index of refraction of the lens has been suggested as the primary cause of the age-related hyperopia.34–35
CONCLUSIONS
This study has confirmed that anisometropia is a common finding among the elderly and that the function describing anisometropia with age is likely exponential in shape. Many who did not have anisometropia at the first visit subsequently developed significant anisometropia. Even though the presence of unequal amounts of nuclear cataract development in the two eyes can explain a significant portion of the anisometropia, the primary cause was unequal hyperopic shift in the spherical component of the refractive error, which also implicates the lens. Whatever the cause of the increase in anisometropia with aging, the fact that significant anisometropia is at least 10 times more common in those over 75 years of age than in children needs to be clearly emphasized to clinicians. The prevalence of anisometropia in US children is between 2 and 4% for spherical equivalent 36–39 while our data for those near 80 years of age shows 32% with 1.00D or more anisometropia. Uncorrected anisometropia is likely to lead to disturbances in binocular vision and stereopsis, which in turn may contribute to falls in the elderly.40–42 The importance of appropriate refractive error correction in the elderly cannot be over-emphasized.
Acknowledgments
This study was supported by National Eye Institute, National Institutes of Health grant EY09588 to JAB and the Smith-Kettlewell Eye Research Institute. Presented, in part, at the annual meeting of the Association for Research in Vision and Ophthalmology (ARVO), May 2011, Ft. Lauderdale, Florida.
References
- 1.Hirsch MJ. Refractive changes with age. In: Hirsch MJ, Wick RE, editors. Vision of the Aging Patient. Philadelphia: Chilton; 1960. pp. 63–82. [Google Scholar]
- 2.Wang Q, Klein BE, Klein R, Moss SE. Refractive status in the Beaver Dam Eye Study. Invest Ophthalmol Vis Sci. 1994;35:4344–7. [PubMed] [Google Scholar]
- 3.Attebo K, Ivers RQ, Mitchell P. Refractive errors in an older population: the Blue Mountains Eye Study. Ophthalmology. 1999;106:1066–72. doi: 10.1016/S0161-6420(99)90251-8. [DOI] [PubMed] [Google Scholar]
- 4.Fotedar R, Mitchell P, Burlutsky G, Wang JJ. Relationship of 10-year change in refraction to nuclear cataract and axial length findings from an older population. Ophthalmology. 2008;115:1273–8. 8 e1. doi: 10.1016/j.ophtha.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 5.Bertelsen G, Erke MG, von Hanno T, Mathiesen EB, Peto T, Sjølie AK, Njølstad I. The Tromsø Eye Study: study design, methodology and results on visual acuity and refractive errors. Acta Ophthalmol. 2012 Sep 11; doi: 10.1111/j.1755-3768.2012.02511.x. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 6.Hyman L. Myopic and hyperopic refractive error in adults: an overview. Ophthalmic Epidemiol. 2007;14:192–7. doi: 10.1080/09286580701535517. [DOI] [PubMed] [Google Scholar]
- 7.Kempen JH, Mitchell P, Lee KE, Tielsch JM, Broman AT, Taylor HR, Ikram MK, Congdon NG, O'Colmain BJ. The prevalence of refractive errors among adults in the United States, Western Europe, and Australia. The Eye Disease Prevalence Research Group. Arch Ophthalmol. 2004;122:495–505. doi: 10.1001/archopht.122.4.495. [DOI] [PubMed] [Google Scholar]
- 8.Haegerstrom-Portnoy G, Schneck ME, Brabyn JA, Lott LA. Development of refractive errors into old age. Optom Vis Sci. 2002;79:643–9. doi: 10.1097/00006324-200210000-00010. [DOI] [PubMed] [Google Scholar]
- 9.Lee KE, Klein BE, Klein R, Wong TY. Changes in refraction over 10 years in an adult population: the Beaver Dam Eye study. Invest Ophthalmol Vis Sci. 2002;43:2566–71. [PubMed] [Google Scholar]
- 10.Guzowski M, Fraser-Bell S, Rochtchina E, Wang JJ, Mitchell P. Asymmetric refraction in an older population: the Blue Mountains Eye Study. Am J Ophthalmol. 2003;136:551–3. doi: 10.1016/s0002-9394(03)00246-0. [DOI] [PubMed] [Google Scholar]
- 11.Xu L, Li J, Cui T, Hu A, Fan G, Zhang R, Yang H, Sun B, Jonas JB. Refractive error in urban and rural adult Chinese in Beijing. Ophthalmology. 2005;112:1676–83. doi: 10.1016/j.ophtha.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 12.Weale RA. On the age-related prevalence of anisometropia. Ophthalmic Res. 2002;34:389–92. doi: 10.1159/000067040. [DOI] [PubMed] [Google Scholar]
- 13.Haegerstrom-Portnoy G, Schneck ME, Brabyn JA. Seeing into old age: vision function beyond acuity. Optom Vis Sci. 1999;76:141–58. doi: 10.1097/00006324-199903000-00014. [DOI] [PubMed] [Google Scholar]
- 14.Haegerstrom-Portnoy G. The Glenn A. Fry Award Lecture 2003: Vision in elders--summary of findings of the SKI study. Optom Vis Sci. 2005;82:87–93. doi: 10.1097/01.opx.0000153162.05903.4c. [DOI] [PubMed] [Google Scholar]
- 15.Brabyn J, Schneck M, Haegerstrom-Portnoy G, Lott L. The Smith-Kettlewell Institute (SKI) longitudinal study of vision function and its impact among the elderly: an overview. Optom Vis Sci. 2001;78:264–9. doi: 10.1097/00006324-200105000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Chylack LT, Jr, Wolfe JK, Singer DM, Leske MC, Bullimore MA, Bailey IL, Friend J, McCarthy D, Wu SY. The Lens Opacities Classification System III. The Longitudinal Study of Cataract Study Group. Arch Ophthalmol. 1993;111:831–6. doi: 10.1001/archopht.1993.01090060119035. [DOI] [PubMed] [Google Scholar]
- 17.Samarawickrama C, Wang JJ, Burlutsky G, Tan AG, Mitchell P. Nuclear cataract and myopic shift in refraction. Am J Ophthalmol. 2007;144:457–9. doi: 10.1016/j.ajo.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 18.Tan AC, Wang JJ, Lamoureux EL, Wong W, Mitchell P, Li J, Tan AG, Wong TY. Cataract prevalence varies substantially with assessment systems: comparison of clinical and photographic grading in a population-based study. Ophthalmic Epidemiol. 2011;18:164–70. doi: 10.3109/09286586.2011.594205. [DOI] [PubMed] [Google Scholar]
- 19.Humphrey WE. Automatic retinoscopy: the Humphrey Vision Analyzer. Optician. 1977;173:7–17. [Google Scholar]
- 20.Thibos LN, Wheeler W, Horner D. Power vectors: an application of Fourier analysis to the description and statistical analysis of refractive error. Optom Vis Sci. 1997;74:367–75. doi: 10.1097/00006324-199706000-00019. [DOI] [PubMed] [Google Scholar]
- 21.Fledelius HC. Prevalences of astigmatism and anisometropia in adult danes. With reference to presbyopes' possible use of supermarket standard glasses. Acta Ophthalmol (Copenh) 1984;62:391–400. doi: 10.1111/j.1755-3768.1984.tb08419.x. [DOI] [PubMed] [Google Scholar]
- 22.Lavery JR, Gibson JM, Shaw DE, Rosenthal AR. Refraction and refractive errors in an elderly population. Ophthalmic Physiol Opt. 1988;8:394–6. doi: 10.1111/j.1475-1313.1988.tb01175.x. [DOI] [PubMed] [Google Scholar]
- 23.Qin XJ, Margrain TH, To CH, Bromham N, Guggenheim JA. Anisometropia is independently associated with both spherical and cylindrical ametropia. Invest Ophthalmol Vis Sci. 2005;46:4024–31. doi: 10.1167/iovs.05-0120. [DOI] [PubMed] [Google Scholar]
- 24.Gudmundsdottir E, Jonasson F, Jonsson V, Stefansson E, Sasaki H, Sasaki K. “With the rule” astigmatism is not the rule in the elderly. Reykjavik Eye Study: a population based study of refraction and visual acuity in citizens of Reykjavik 50 years and older”. Iceland-Japan Co-Working Study Groups. Acta Ophthalmol Scand. 2000;78:642–6. doi: 10.1034/j.1600-0420.2000.078006642.x. [DOI] [PubMed] [Google Scholar]
- 25.Brown NA, Hill AR. Cataract: the relation between myopia and cataract morphology. Br J Ophthalmol. 1987;71:405–14. doi: 10.1136/bjo.71.6.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang MA, Congdon NG, Bykhovskaya I, Munoz B, West SK. The association between myopia and various subtypes of lens opacity: SEE (Salisbury Eye Evaluation) project. Ophthalmology. 2005;112:1395–401. doi: 10.1016/j.ophtha.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 27.Wong TY, Foster PJ, Johnson GJ, Seah SK. Refractive errors, axial ocular dimensions, and age-related cataracts: the Tanjong Pagar survey. Invest Ophthalmol Vis Sci. 2003;44:1479–85. doi: 10.1167/iovs.02-0526. [DOI] [PubMed] [Google Scholar]
- 28.Panchapakesan J, Rochtchina E, Mitchell P. Myopic refractive shift caused by incident cataract: the Blue Mountains Eye Study. Ophthalmic Epidemiol. 2003;10:241–7. doi: 10.1076/opep.10.4.241.15911. [DOI] [PubMed] [Google Scholar]
- 29.Klein BE, Klein R, Lee KE, Gangnon RE. Incidence of age-related cataract over a 15-year interval the Beaver Dam Eye Study. Ophthalmology. 2008;115:477–82. doi: 10.1016/j.ophtha.2007.11.024. [DOI] [PubMed] [Google Scholar]
- 30.Chang JR, Koo E, Agron E, Hallak J, Clemons T, Azar D, Sperduto RD, Ferris FL, 3rd, Chew EY. Risk factors associated with incident cataracts and cataract surgery in the Age-related Eye Disease Study (AREDS): AREDS report number 32. Ophthalmology. 2011;118:2113–9. doi: 10.1016/j.ophtha.2011.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leske MC, Chylack LT, Jr, Wu SY The Lens Opacities Case-Control Study. Risk factors for cataract. The Lens Opacities Case-Control Study Group. Arch Ophthalmol. 1991;109:244–51. doi: 10.1001/archopht.1991.01080020090051. [DOI] [PubMed] [Google Scholar]
- 32.Congdon N, Vingerling JR, Klein BE, West S, Friedman DS, Kempen J, O'Colmain B, Wu SY, Taylor HR, Congdon N, Vingerling JR, Klein BE, West S, Friedman DS, Kempen J, O'Colmain B, Wu SY, Taylor HR. Prevalence of cataract and pseudophakia/aphakia among adults in the United States. Eye Diseases Prevalence Research Group. Arch Ophthalmol. 2004;122:487–94. doi: 10.1001/archopht.122.4.487. [DOI] [PubMed] [Google Scholar]
- 33.Slataper FJ. Age norms of refraction and vision. Arch Ophthalmol. 1950;43:466–81. [Google Scholar]
- 34.Smith G, Atchison DA, Pierscionek BK. Modeling the power of the aging human eye. J Opt Soc Am (A) 1992;9:2111–7. doi: 10.1364/josaa.9.002111. [DOI] [PubMed] [Google Scholar]
- 35.Atchison DA, Markwell EL, Kasthurirangan S, Pope JM, Smith G, Swann PG. Age-related changes in optical and biometric characteristics of emmetropic eyes. J Vis. 2008;8:29, 1–20. doi: 10.1167/8.4.29. [DOI] [PubMed] [Google Scholar]
- 36.Hirsch MJ. Anisometropia: a preliminary report of the Ojai Longitudinal Study. Am J Optom Arch Am Acad Optom. 1967;44:581–5. [PubMed] [Google Scholar]
- 37.Almeder LM, Peck LB, Howland HC. Prevalence of anisometropia in volunteer laboratory and school screening populations. Invest Ophthalmol Vis Sci. 1990;31:2448–55. [PubMed] [Google Scholar]
- 38.Borchert M, Tarczy-Hornoch K, Cotter SA, Liu N, Azen SP, Varma R. Anisometropia in Hispanic and african american infants and young children the multi-ethnic pediatric eye disease study. Ophthalmology. 2010;117:148–53. doi: 10.1016/j.ophtha.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deng L, Gwiazda JE. Anisometropia in children from infancy to 15 years. Invest Ophthalmol Vis Sci. 2012;53:3782–7. doi: 10.1167/iovs.11-8727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nevitt MC, Cummings SR, Kidd S, Black D. Risk factors for recurrent nonsyncopal falls. A prospective study. JAMA. 1989;261:2663–8. [PubMed] [Google Scholar]
- 41.Lord SR, Dayhew J. Visual risk factors for falls in older people. J Am Geriatr Soc. 2001;49:508–15. doi: 10.1046/j.1532-5415.2001.49107.x. [DOI] [PubMed] [Google Scholar]
- 42.Knudtson MD, Klein BE, Klein R. Biomarkers of aging and falling: the Beaver Dam eye study. Arch Gerontol Geriatr. 2009;49:22–6. doi: 10.1016/j.archger.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]