Abstract
The principal finding of this study is that two drugs, alverine and benfluorex, used in vastly different clinical settings and previously unknown to share mechanistic or structural similarity, activated the nuclear receptor transcription factor HNF4α. Both were hits in a high-throughput screen for compounds that reversed the inhibitory effect of the fatty acid palmitate on human insulin promoter activity. Alverine is used in the treatment of irritable bowel syndrome, while benfluorex (Mediator) was used to treat hyperlipidemia and type II diabetes. Benfluorex was withdrawn from the market recently because of serious cardiovascular side effects related to fenfluramine-like activity. Strikingly, alverine and benfluorex have a previously unrecognized structural similarity, consistent with a common mechanism of action. Gene expression and biochemical studies revealed that they both activate HNF4α. This novel mechanism of action should lead to a reinterpretation of previous studies with these drugs and suggests a path towards the development of therapies for diseases such as inflammatory bowel and diabetes that may respond to HNF4α activators.
INTRODUCTION
A key element in the pathogenesis of type II diabetes is β-cell dysfunction and loss, resulting in deterioration in insulin secretion over time1. A major factor leading to β-cell dysfunction is thought to be high levels of circulating lipids, including free fatty acids. Chronic exposure of pancreatic β-cells to fatty acids inhibits β-cell function in vitro and in vivo and can lead to β-cell apoptosis2. Recent data suggest that free fatty acid inhibition of insulin gene expression is an early functional defect that may contribute to β-cell failure in type 2 diabetes3. Thus, β-cells exposed to elevated fatty acids represent an in vitro model of some important aspects of type 2 diabetes,
Given the central role of fatty acids in the pathogenesis of type II diabetes, compounds that prevent β-cell failure in the face of lipotoxic stress from fatty acids hold promise as potential drugs. One approach to the discovery and development of such drugs is high throughput screening (HTS) with cells cultured in the presence of fatty acids to detect compounds that antagonize lipotoxic effects on those cells. Previously, we performed HTS for compounds that modulate the insulin promoter using a novel human islet cell line, T6PNE, derived from human fetal islets4.
In one case, we found a previously unrecognized activity of antipsychotics in activating the TGFβ pathway effector SMAD34b, c. Another screen yielded a potent antagonist of the nuclear receptor transcription factor hepatic nuclear factor 4 alpha (HNF4α)4a. HNF4α is a central regulator of gene expression in cell types that play a critical role in metabolic homeostasis, including pancreatic β-cells, where it plays a critical role in insulin gene expression and secretion and is mutated in a monogenic form of diabetes, MODY15.
To search for compounds that reversed the inhibitory effects of fatty acids on the insulin promoter, we adapted the T6PNE insulin promoter assay, priming the assay with the fatty acid palmitate to inhibit insulin promoter activity. A screen of a library of known drugs for those that increased insulin promoter activity in the presence of palmitate yielded as hits the drugs alverine and benfluorex. In light of the fact that these two drugs are used clinically for completely different purposes and were believed to have distinct mechanisms of action, we were surprised to find that they have a high degree of structural similarity. Given that similarity and the fact that they were hits in the same assay, we hypothesized that they might be acting on a common target. Here, we report that alverine and benfluorex act as HNF4α activators. This finding should lead to a reinterpretation of previous studies of these drugs, as well as open the door to new therapeutic opportunities based on this novel mechanism of action.
RESULTS AND DISCUSSION
Screen of a library of known drugs for those that reverse insulin promoter repression by fatty acids
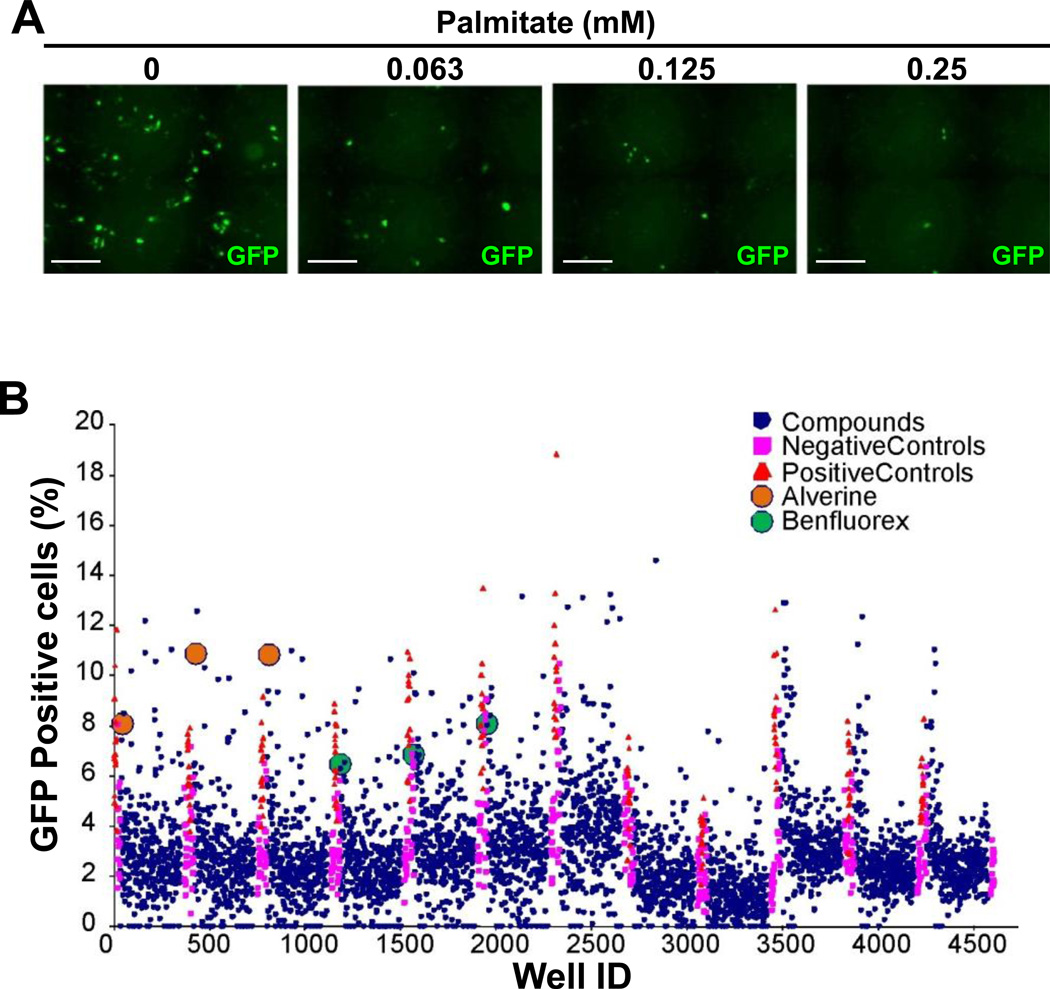
Previously, we found that fatty acids repressed the human insulin promoter in T6PNE cells4a. This offered the opportunity to adapt the T6PNE insulin promoter assay4c to detect compounds that reversed the effect of fatty acids on the insulin promoter. To that end, we developed a dose response curve of the human insulin promoter-GFP transgene in T6PNE cells to palmitate. Consistent with our previous results demonstrating that palmitate dose-dependently inhibited the endogenous human insulin promoter in T6PNE cells4a, it repressed the transgene as well (Figure 1A).
Figure 1. Screen of a library of known drugs for those that reverse insulin promoter repression by fatty acids.
A) Palmitate inhibited insulin promoter-GFP activity in T6PNE cells. T6PNE cells were cultured in the presence of 1µM tamoxifen and the indicated concentration of palmitate. GFP-positive cells, indicating expression from the human insulin promoter-GFP transgene introduced by lentivirus-mediated gene transfer, were visualized by fluorescence microscopy after two days. Cells were then harvested for RNA isolation and determination of insulin mRNA level by quantitative RT-PCR30. Palmitate induced a dose-dependent decrease in the number of GFP-positive cells. Scale bars=200µm. B) Alverine and Benfluorex were hits in a lipotoxicity reversal screen. T6PNE cells were plated in 384 well plates in the presence of 1µM tamoxifen, 0.03 mM palmitate and compounds from the NIH/JDRF library of known drugs4c and was conducted in triplicate because of substantial assay variability. After 48 hours, cells were fixed and read on a high throughput microscopy system. Effects of compounds on the exogenous insulin promoter in T6PNE is reported as percent GFP+ cells, as determined by imaging the green channel and normalizing to the total number of cells per well. The drugs benfluorex (green dot) and alverine (orange dot) were consistent at activating insulin promoter activity. Positive controls (orange triangle) were 0mM palmitate and negative controls were vehicle (DMSO, magenta rectangle) with no compound.
Based on the dose-response curve, we conducted a screen of T6PNE cells in the presence of 0.03mM palmitate. A screen of the NIH/JDRF library of known drugs4c for those that increased the number of GFP-positive cells in the presence of palmitate was conducted in triplicate. Benfluorex and alverine citrate consistently activated insulin promoter activity as measured by an increased number of GFP-positive cells (Figure 1B). Both drugs passed confirmatory assays, including increasing the number of GFP-positive cells in a dose-responsive manner (Figure 2A) and increasing the level of endogenous insulin mRNA (Figure 2B). Neither had estrogenic activity, which causes false-positive results in our assay4c.
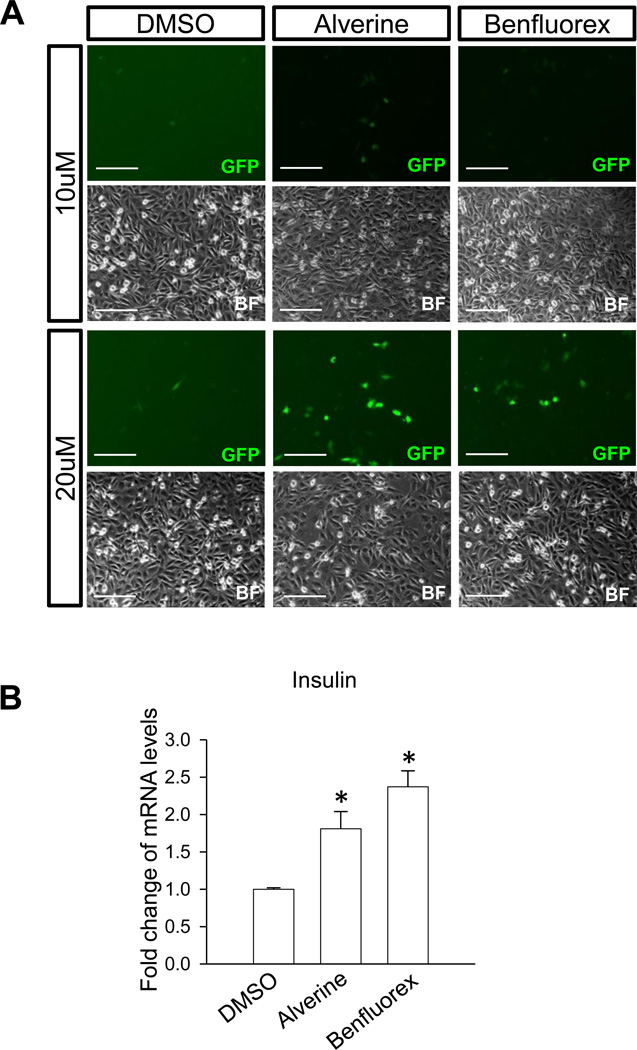
Figure 2. Alverine and Benfluorex activated the human insulin promoter.
A) Alverine and benfluorex activated the insulin promoter-GFP transgene. To confirm that alverine and benfluorex activated the insulin promoter-GFP transgene, they were added to T6PNE cells at 10 and 20µM for 3 days in the presence of 0.5µM tamoxifen. Activation was dose-dependent as seen by the number of GFP-positive cells (green GFP and corresponding bright field (BF) images). Scale bars=200µm. B) Alverine and benfluorex activated the endogenous human insulin promoter. Drugs were added to T6PNE cells (20µM for 2 days) followed by harvest of RNA for quantitative RT-PCR. Values represent the mean ± SEM, *p<0.05, n=3 biological replicates.
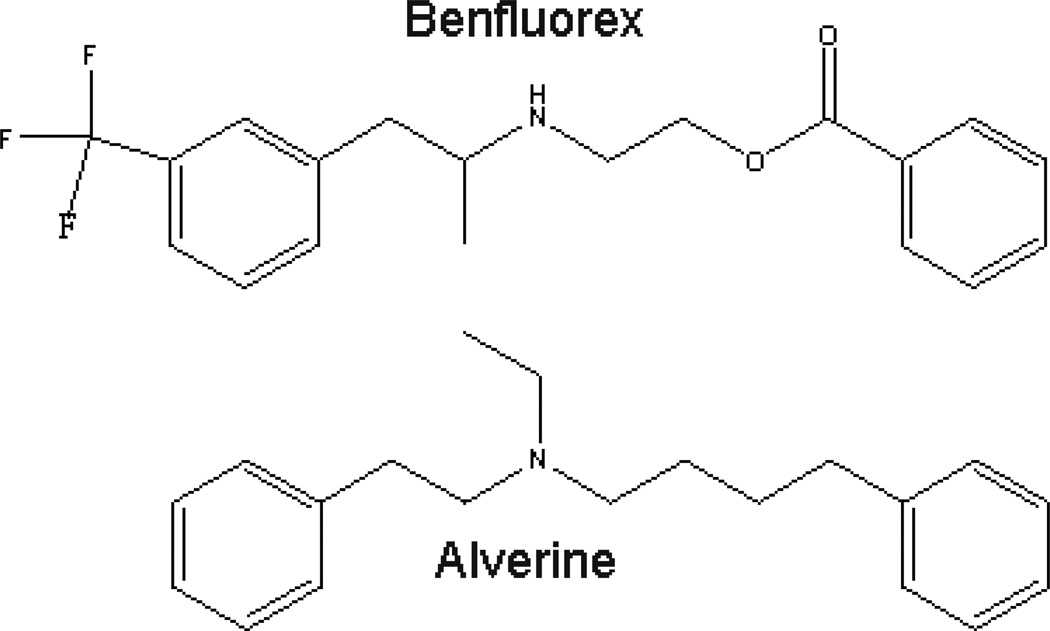
The fact that benfluorex was positive in our assay was gratifying, as it was used in the treatment of hyperlipidemia and type II diabetes6. Alverine is an antispasmodic used in the treatment of irritable bowel syndrome7, with no known connection to diabetes or the effects of fatty acids. Strikingly, an examination of the structures of benfluorex and alverine revealed substantial similarity between the two compounds (Figure 3), a finding that has not been reported previously.
Figure 3. Structures of alverine and benfluorex.
Alverine was first described in 1939 as having been synthesized as a synthetic analog of papaverine, a smooth muscle relaxant8. It has been used since then as an antispasmodic in the treatment of irritable bowel syndrome, a poorly defined condition that can present with diarrhea or constipation7. Therapeutic benefit in that condition has been variable7, 9. Alverine has not been well studied for its mechanism of action10, although it has been described as an antagonist of the 5-hydroxytryptamine 1A (5-HT1A) receptor11.
Benfluorex (CAS 23602-78-0) was described in a French patent issued in 196912 as a synthetic an analog of fenfluramine12. It came into clinical use as a therapy for type II diabetes, where it was found to improve lipid profiles and to reduce the hemoglobin A1C13. However the mechanism by which it exerted those effects was never studied carefully. Fenfluramine is a potent agonist of serotonin 5-HT2 receptors14, and gained notoriety for being part of the diet drug Phen-Fen. As a result, a common assumption was that the mechanism of action of benfluorex was through effects on weight15. Fenfluramine was found to cause severe cardiopulmonary side effects, including valvular heart disease, leading to its withdrawal from the market in the USA and elsewhere in 199716. In 2006, benfluorex was recognized as causing the same problem15, 17, leading to its withdrawal from the market in Europe amidst a high degree of controversy because of the long delay between the recognition of side effects from fenfluramine and the withdrawal of benfluorex from the market18.
Alverine and Benfluorex are HNF4α activators
Previous studies of benfluorex and alverine indicated that they had distinct mechanisms of action10–11, 19. However, given that the compounds were structurally similar, we reasoned that a previously unrecognized but common target of the two drugs might be responsible for their effect in our assay.
Determining the precise molecular target of HTS hits from phenotypic assays is notoriously difficult20. Previously, we used a cheminformatic approach to discover that a small molecule from a diverse chemical library used to screen the T6PNE insulin promoter assay was a potent antagonist of HNF4α4a. The natural ligands for HNF4α are thought to be fatty acids21, and we found them to act as HNF4α antagonists4a. Thus, we hypothesized that alverine and benfluorex, which increase insulin promoter activity in the face of added fatty acids, might be acting as HNF4α agonists.
As an initial test of that hypothesis, we determined whether alverine and benfluorex increased the expression of HNF4α mRNA, as we found previously that potent HNF4α antagonists inhibited HNF4α expression through a well-known positive feedback loop22. Consistent with their being HNF4α activators, both alverine and benfluorex stimulated HNF4α expression (Figure 4).
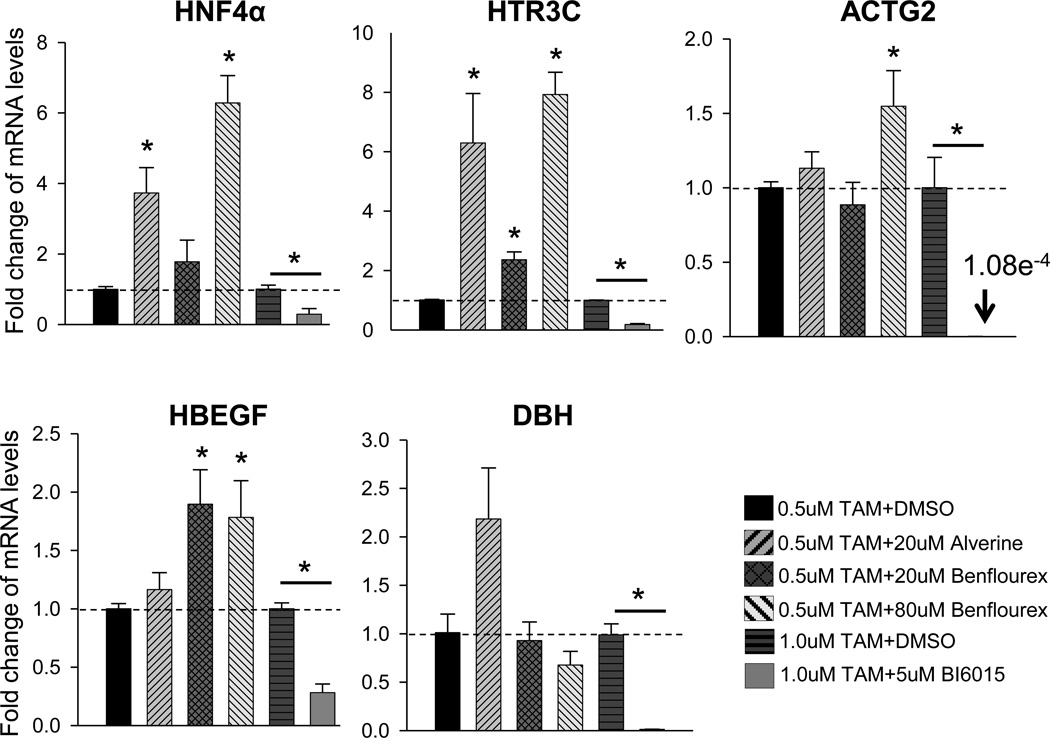
Figure 4. Alverine and Benfluorex are HNF4α activators.
T6PNE cells were treated with 0.5µM or 1µM tamoxifen and either 20µM alverine, 20µM, 80µM benfluorex for 3 days and 5µM BI6015 for 2 days or vehicle (DMSO). mRNA levels were normalized to 18S rRNA. BI6015 repressed genes selected from previously published microarray from HNF4α antagonist (Gene Expression Omnibus database-GSE33432) compared to DMSO. Values represent the mean ± SEM, 0.5µM tamoxifen with DMSO vs alverine, benfluorex and 1.0µM tamoxifen with DMSO vs. BI6015, *p<0.05, n=3 biological replicates.
As a further test of whether alverine and benfluorex acted on HNF4α, we tested whether they affected genes that were repressed by a synthetic HNF4α antagonist, BIM5078, that was discovered by us as a potent repressor of insulin gene expression4a. Genes were selected from those that were most repressed by BIM5078 in T6PNE cells4a (Gene Expression Omnibus database-GSE33432). Consistent with our previous results, all of the genes were strongly repressed by BI6015, a more potent and specific HNF4α antagonist than BIM5078, to which it is highly structurally related4a.
HTR3C, ACTG2, and HBEGF mRNA responded to alverine, benfluorex, or both, while DBH was not significantly affected by either (Figure 4). Given that HNF4α has a high level of basal activity, it is not unexpected that some genes that are repressed by an HNF4α antagonist will respond to a much lesser extent, or perhaps not at all, to an agonist, depending on the extent to which the maximal response of the gene to HNF4α has already been achieved in that cell. Consequently, the response of the genes tested is consistent with what is expected for an HNF4α activator.
Effect on HNF4α structure
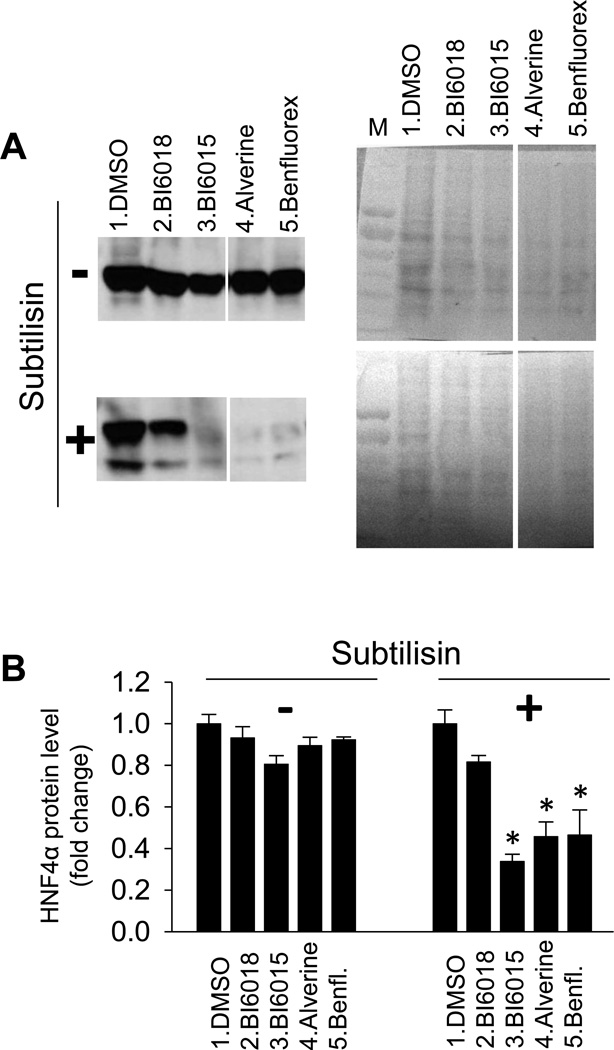
Because HNF4α ligand exchange is extremely inefficient in vitro21a, we have used a protease sensitivity assay termed drug affinity target responsive stability (DARTS)23, to determine whether a compound interacts directly with a putative target. With DARTS, the effect of a putative ligand on protein structure is detected by a change in the sensitivity of the protein to proteolytic cleave. Previously, we used this technique to show that novel synthetic HNF4α antagonists interacted with HNF4α4a. Here, we used it to ascertain whether alverine and benfluorex induced a conformational change in HNF4α, suggesting direct interaction. BI6015, alverine, and benfluorex each altered HNF4α protease sensitivity, while the inactive control compound BI60184a did not (Figure 5).
Figure 5. Alverine and Benfluorex altered proteolytic sensitivity in HNF4α protein.
A) For the DARTS assay, HepG2 cells were treated with DMSO (lane 1), BI6018 (lane 2), BI6015 (lane 3), Alverine (lane 4) or Benfluorex (lane 5) at a concentration of 20µM or 40µM for 16hr. Total cell protein was extracted and each sample was split into two aliquots for proteolysis without (−) or with (+) subtilisin (A, left panels) and blotted with HNF4α antibody. After detection of HNF4α, the membrane was stained with Ponceau S (A, right panels) as a control to ensure that the compounds did not induce nonspecific proteolysis (Lane M-MW markers). All compounds were run on the same gel. A lane from a compound unrelated to the studies reported here was deleted, accounting for the gap between lanes 3 and 4. B) Western blots were quantified using ImageJ software, demonstrating a statistically significant effect of BI6015, alverine, benfluorex on subtilisin sensitivity. Values represent the mean ± SE of 3 biological replicates, *p<0.05.
The beneficial effect of benfluorex and the well-known role of HNF4α in diabetes5d, 24 raises the possibility that its mechanism of action in that disease might have been through effects on HNF4α rather than or in addition to activation of serotonin receptors. HNF4α plays an important role in diabetes pathogenesis, being mutated in a monogenic form of diabetes, MODY15e, and playing a critical role in hepatocytes and β-cells, two of the major cell types involved in diabetes5c, d, 25. Our recent finding that fatty acids act as HNF4α antagonists4a suggests that reversing the deleterious effects of fatty acids on HNF4α by administration of an HNF4α activator may be beneficial as a therapy for type II diabetes. However, neither alverine, which is poorly absorbed systemically, nor benfluorex, which causes valvular heart disease, is suitable. Improved HNF4α agonists must be developed to test that hypothesis.
Benfluorex is metabolized by cleavage of an ester moiety into fenfluramine but alverine lacks the ester linkage. Alverine is poorly absorbed through the gastrointestinal tract, limiting its systemic effects26. Thus, it is well suited for applications where enhancement of HNF4α activity in the intestine might be of value, such as inflammatory bowel disease, a disease state in which HNF4α plays an important role27. A subset of patients with irritable bowel syndrome, the current indication for alverine, have an inflammatory component to their disease28, and some overlap between irritable bowel syndrome and inflammatory bowel disease appears to exist29. To date, alverine has not been tested in inflammatory bowel disease.
In conclusion, the finding that benfluorex and alverine activate the nuclear receptor transcription factor HNF4α opens the door to a reinterpretation of their previously noted clinical effects, as well as to a potential for new therapeutic applications.
METHODS
Compound library screening in T6PNE
The properties of the T6PNE cells and their use in HTS was described previously4a. The previously described assay was modified as follows: T6PNE cells were seeded at 2,000 cells per well in 384-well tissue culture plates (Greiner Bio-One) in the presence of 1µM tamoxifen and 0.03mM palmitate. Compound addition (active compound in DMSO or vehicle alone) occurred 24 hours after tamoxifen administration with the BiomekFX (Beckman Coulter). Forty-eight hours after compound addition, cells were fixed in 4% paraformaldehyde (USbio) and stained with DAPI (0.167 µg/ml, Invitrogen). Blue (DAPI) and green (human insulin promoter driving GFP) channels were imaged using the GE/Amersham InCell 1000 high-throughput microscopy system. Images were processed (Cytoshop, Beckman Coulter) then a MATLAB algorithm was used to calculate the percentage of cells containing GFP levels greater than a threshold of 1.5 fold over baseline (%GFP+ cells), Values were reported as fold change in %GFP + cells over vehicle (DMSO, Sigma-Aldrich).
Cell culture and chemical treatment
T6PNE cells were maintained in RPMI (5.5mM glucose, Hyclone) supplemented with 10% fetal bovine serum (FBS, Hyclone) and 1% penicillin-streptomycin (pen-strep, Gibco) and grown in 5% CO2, 37°C. To induce E47 activity, 0.5µM or 1µM tamoxifen (Sigma-Aldrich) was added to culture media. HepG2 cells were cultured in DMEM (high glucose, Hyclone) supplemented with 10% FBS and 1% pen-strep and grown at 5% CO2, 37°C on collagen plates (BD Bio Coat). DMSO or 20µM alverine (Sigma-Aldrich), 20µM, 80µM benfluorex (Sigma-Aldrich) for 3 days and DMSO or 5µM BI6015 were added to T6PNE cells for 2days.
QPCR
RNA was purified using RNeasy Kits (Qiagen), then converted to cDNA using the qScript cDNA SuperMix (Quanta BioSciences). Q-PCR was conducted on cDNA corresponding to 2µg of RNA using an Opticon Real-Time System (MJ Research) and QPCR SuperMix (BioPioneer). All mRNA values were normalized to 18S rRNA values and are expressed as fold changes over vehicle-treated control.
DARTS assay
HepG2 cells were treated with DMSO, BI6018, BI6015, Alverine or Benfluorex at a concentration of 20µM or 40µM for 16hr.Total cell protein was extracted, measured by BCA protein assay (Thermo scientific).Each sample was split into two aliquots for proteolysis without (−) or with(+) Subtilisin (Sigma-Aldrich). Twenty ug of cell lysate was incubated with or without protease (20ng/ml subtilisin) for 35 minutes at room temperature. Western blot was then performed with primary anti-HNF4α polyclonal antibody (1:1000 dilution, Santa Cruz, 54kDa) and secondary HRP conjugated anti-goat IgG (1:2000 dilution, Jackson Immuno), detected with chemiluminescence ECL kit (Thermo Scientific). After detection, membrane was stained with Ponceau S solution (Sigma-Aldrich, M : protein ladder).
Statistical analysis
Data are presented as mean ± SEM of three or more independent cultures. Statistical significance was assessed using two-tailed unpaired Student's t-test.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Hud Freeze, Margaret Dunbar, and Anthony Pinkerton for helpful discussions. This work was supported by the Sanford Children’s Health Research Center, BetaBat (in the Framework Program 7 of the European Community), and the Kenneth Rainin Foundation.
Footnotes
AUTHOR CONTRIBUTIONS
S-HL, SA, AK, TC carried out experiments. FL, S-HL, SA, AK, and TC designed experiments. S-HL and FL analyzed the data and wrote the manuscript.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
REFERENCES
- 1.Porte D, Jr, Kahn SE. beta-cell dysfunction and failure in type 2 diabetes: potential mechanisms. Diabetes. 2001;50(Suppl 1):S160–S163. doi: 10.2337/diabetes.50.2007.s160. [DOI] [PubMed] [Google Scholar]
- 2.(a) Poitout V. Glucolipotoxicity of the pancreatic beta-cell: myth or reality? Biochem Soc Trans. 2008;36(Pt 5):901–904. doi: 10.1042/BST0360901. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Unger RH. Lipotoxicity in the pathogenesis of obesity-dependent NIDDM. Genetic and clinical implications. Diabetes. 1995;44(8):863–870. doi: 10.2337/diab.44.8.863. [DOI] [PubMed] [Google Scholar]; (c) Maedler K, Spinas GA, Dyntar D, Moritz W, Kaiser N, Donath MY. Distinct effects of saturated and monounsaturated fatty acids on beta-cell turnover and function. Diabetes. 2001;50(1):69–76. doi: 10.2337/diabetes.50.1.69. [DOI] [PubMed] [Google Scholar]
- 3.(a) Hagman DK, Hays LB, Parazzoli SD, Poitout V. Palmitate inhibits insulin gene expression by altering PDX-1 nuclear localization and reducing MafA expression in isolated rat islets of Langerhans. J Biol Chem. 2005;280(37):32413–32418. doi: 10.1074/jbc.M506000200. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Kelpe CL, Moore PC, Parazzoli SD, Wicksteed B, Rhodes CJ, Poitout V. Palmitate inhibition of insulin gene expression is mediated at the transcriptional level via ceramide synthesis. J Biol Chem. 2003;278(32):30015–30021. doi: 10.1074/jbc.M302548200. [DOI] [PubMed] [Google Scholar]; (c) Jacqueminet S, Briaud I, Rouault C, Reach G, Poitout V. Inhibition of insulin gene expression by long-term exposure of pancreatic beta cells to palmitate is dependent on the presence of a stimulatory glucose concentration. Metabolism. 2000;49(4):532–536. doi: 10.1016/s0026-0495(00)80021-9. [DOI] [PubMed] [Google Scholar]
- 4.(a) Kiselyuk A, Lee SH, Farber-Katz S, Zhang M, Athavankar S, Cohen T, Pinkerton AB, Ye M, Bushway P, Richardson AD, Hostetler HA, Rodriguez-Lee M, Huang L, Spangler B, Smith L, Higginbotham J, Cashman J, Freeze H, Itkin-Ansari P, Dawson MI, Schroeder F, Cang Y, Mercola M, Levine F. HNF4alpha antagonists discovered by a high-throughput screen for modulators of the human insulin promoter. Chem Biol. 2012;19(7):806–818. doi: 10.1016/j.chembiol.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Cohen T, Sundaresh S, Levine F. Antipsychotics activate the TGFbeta pathway effector SMAD3. Mol Psychiatry. 2012 doi: 10.1038/mp.2011.186. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Kiselyuk A, Farber-Katz S, Cohen T, Lee SH, Geron I, Azimi B, Heynen-Genel S, Singer O, Price J, Mercola M, Itkin-Ansari P, Levine F. Phenothiazine neuroleptics signal to the human insulin promoter as revealed by a novel high-throughput screen. J Biomol Screen. 2010;15(6):663–670. doi: 10.1177/1087057110372257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.(a) Eeckhoute J, Briche I, Kurowska M, Formstecher P, Laine B. Hepatocyte nuclear factor 4 alpha ligand binding and F domains mediate interaction and transcriptional synergy with the pancreatic islet LIM HD transcription factor Isl1. Journal of molecular biology. 2006;364(4):567–581. doi: 10.1016/j.jmb.2006.07.096. [DOI] [PubMed] [Google Scholar]; (b) Bartoov-Shifman R, Hertz R, Wang H, Wollheim CB, Bar-Tana J, Walker MD. Activation of the insulin gene promoter through a direct effect of hepatocyte nuclear factor 4 alpha. J Biol Chem. 2002;277(29):25914–25919. doi: 10.1074/jbc.M201582200. [DOI] [PubMed] [Google Scholar]; (c) Gupta RK, Vatamaniuk MZ, Lee CS, Flaschen RC, Fulmer JT, Matschinsky FM, Duncan SA, Kaestner KH. The MODY1 gene HNF-4alpha regulates selected genes involved in insulin secretion. J Clin Invest. 2005;115(4):1006–1015. doi: 10.1172/JCI200522365. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Stoffel M, Duncan SA. The maturity-onset diabetes of the young (MODY1) transcription factor HNF4alpha regulates expression of genes required for glucose transport and metabolism. Proc Natl Acad Sci U S A. 1997;94(24):13209–13214. doi: 10.1073/pnas.94.24.13209. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Yamagata K, Furuta H, Oda N, Kaisaki PJ, Menzel S, Cox NJ, Fajans SS, Signorini S, Stoffel M, Bell GI. Mutations in the hepatocyte nuclear factor-4alpha gene in maturity-onset diabetes of the young (MODY1) Nature. 1996;384(6608):458–460. doi: 10.1038/384458a0. [DOI] [PubMed] [Google Scholar]
- 6.Moulin P, Andre M, Alawi H, Dos Santos LC, Khalid AK, Koev D, Moore R, Serban V, Picandet B, Francillard M. Efficacy of benfluorex in combination with sulfonylurea in type 2 diabetic patients: an 18 to 34-week, open-label, extension period. Diabetes and Metabolism. 2009;35(1):64–70. doi: 10.1016/j.diabet.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Wittmann T, Paradowski L, Ducrotte P, Bueno L, Andro Delestrain MC. Clinical trial: the efficacy of alverine citrate/simeticone combination on abdominal pain/discomfort in irritable bowel syndrome--a randomized, double-blind, placebo-controlled study. Aliment Pharmacol Ther. 2010;31(6):615–624. doi: 10.1111/j.1365-2036.2009.04216.x. [DOI] [PubMed] [Google Scholar]
- 8.(a) Buth W, Kulz F, Rosemund KW. Über Synthesen spasmolytisch wirkendender Stoffe. Ber. Dtsch. Chem. Ges. B. 1939;72:19–28. [Google Scholar]; (b) Kulz F, Rosenmund KW, Kayser E, Schwarzhaupt O, Sommer H. Über Synthesen spasmolytisch wirkender Stoffe. II. Mitteilung. Berichte der deutschen chemischen Gesellschaft (A and B Series) 1939;72(12):2161–2167. [Google Scholar]
- 9.Mitchell SA, Mee AS, Smith GD, Palmer KR, Chapman RW. Alverine citrate fails to relieve the symptoms of irritable bowel syndrome: results of a double-blind, randomized, placebo-controlled trial. Aliment Pharmacol Ther. 2002;16(6):1187–1195. doi: 10.1046/j.1365-2036.2002.01277.x. [DOI] [PubMed] [Google Scholar]
- 10.Hayase M, Hashitani H, Suzuki H, Kohri K, Brading AF. Evolving mechanisms of action of alverine citrate on phasic smooth muscles. Br J Pharmacol. 2007;152(8):1228–1238. doi: 10.1038/sj.bjp.0707496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coelho AM, Jacob L, Fioramonti J, Bueno L. Rectal antinociceptive properties of alverine citrate are linked to antagonism at the 5-HT1A receptor subtype. J Pharm Pharmacol. 2001;53(10):1419–1426. doi: 10.1211/0022357011777783. [DOI] [PubMed] [Google Scholar]
- 12.Beregi L, Pierre H, Le Douarec JC. Trifluoromethyl derivatives of phenyl amino propanes as anorexiants, analgesics, anticonvulsants, or lipid metabolism regulators. 1969 2029385. [Google Scholar]
- 13.(a) Brindley DN. Mechanisms for the effects of benfluorex on the obese-diabetic-dyslipidemic syndrome. Diabetes/metabolism reviews. 1993;9 Suppl 1:51S–56S. doi: 10.1002/dmr.5610090509. [DOI] [PubMed] [Google Scholar]; (b) Moulin P, Andre M, Alawi H, dos Santos LC, Khalid AK, Koev D, Moore R, Serban V, Picandet B, Francillard M. Efficacy of benfluorex in combination with sulfonylurea in type 2 diabetic patients: an 18-week, randomized, double-blind study. Diabetes Care. 2006;29(3):515–520. doi: 10.2337/diacare.29.03.06.dc05-1439. [DOI] [PubMed] [Google Scholar]
- 14.Porter RH, Benwell KR, Lamb H, Malcolm CS, Allen NH, Revell DF, Adams DR, Sheardown MJ. Functional characterization of agonists at recombinant human 5-HT2A, 5-HT2B and 5-HT2C receptors in CHO-K1 cells. Br J Pharmacol. 1999;128(1):13–20. doi: 10.1038/sj.bjp.0702751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boutet K, Frachon I, Jobic Y, Gut-Gobert C, Leroyer C, Carlhant-Kowalski D, Sitbon O, Simonneau G, Humbert M. Fenfluramine-like cardiovascular side-effects of benfluorex. European Respiratory Journal. 2009;33(3):684–688. doi: 10.1183/09031936.00086308. [DOI] [PubMed] [Google Scholar]
- 16.Connolly HM, Crary JL, McGoon MD, Hensrud DD, Edwards BS, Edwards WD, Schaff HV. Valvular heart disease associated with fenfluramine-phentermine. N Engl J Med. 1997;337(9):581–588. doi: 10.1056/NEJM199708283370901. [DOI] [PubMed] [Google Scholar]
- 17.Noize P, Sauer M, Bruneval P, Moreau M, Pathak A, Bagheri H, Montastruc JL. Valvular heart disease in a patient taking benfluorex. Fundamental and Clinical Pharmacology. 2006;20(6):577–578. doi: 10.1111/j.1472-8206.2006.00441.x. [DOI] [PubMed] [Google Scholar]
- 18.(a) Fournier A, Zureik M. Estimate of deaths due to valvular insufficiency attributable to the use of benfluorex in France. Pharmacoepidemiol Drug Saf. 2012;21(4):343–351. doi: 10.1002/pds.3213. [DOI] [PubMed] [Google Scholar]; (b) Lessons from the Mediator scandal. Prescrire Int. 2011;20(122):284. [PubMed] [Google Scholar]; (c) Kmietowicz Z. European drug regulator is being investigated by fraud agency. BMJ. 2011;343:d7283. doi: 10.1136/bmj.d7283. [DOI] [PubMed] [Google Scholar]
- 19.(a) Ravel D, Laudignon N. Research prospects with benfluorex. Journal of Diabetes and its Complications. 1996;10(5):246–254. doi: 10.1016/1056-8727(96)00045-1. [DOI] [PubMed] [Google Scholar]; (b) Kohl C, Ravel D, Girard J, Pegorier JP. Effects of benfluorex on fatty acid and glucose metabolism in isolated rat hepatocytes: from metabolic fluxes to gene expression. Diabetes. 2002;51(8):2363–2368. doi: 10.2337/diabetes.51.8.2363. [DOI] [PubMed] [Google Scholar]
- 20.Mayr LM, Bojanic D. Novel trends in high-throughput screening. Curr Opin Pharmacol. 2009;9(5):580–588. doi: 10.1016/j.coph.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 21.(a) Dhe-Paganon S, Duda K, Iwamoto M, Chi YI, Shoelson SE. Crystal structure of the HNF4 alpha ligand binding domain in complex with endogenous fatty acid ligand. J Biol Chem. 2002;277(41):37973–37976. doi: 10.1074/jbc.C200420200. [DOI] [PubMed] [Google Scholar]; (b) Yuan X, Ta TC, Lin M, Evans JR, Dong Y, Bolotin E, Sherman MA, Forman BM, Sladek FM. Identification of an endogenous ligand bound to a native orphan nuclear receptor. PLoS One. 2009;4(5):e5609. doi: 10.1371/journal.pone.0005609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bailly A, Torres-Padilla ME, Tinel AP, Weiss MC. An enhancer element 6 kb upstream of the mouse HNF4alpha1 promoter is activated by glucocorticoids and liver-enriched transcription factors. Nucleic Acids Research. 2001;29(17):3495–3505. doi: 10.1093/nar/29.17.3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lomenick B, Hao R, Jonai N, Chin RM, Aghajan M, Warburton S, Wang J, Wu RP, Gomez F, Loo JA, Wohlschlegel JA, Vondriska TM, Pelletier J, Herschman HR, Clardy J, Clarke CF, Huang J. Target identification using drug affinity responsive target stability (DARTS) Proc Natl Acad Sci U S A. 2009;106(51):21984–21989. doi: 10.1073/pnas.0910040106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.(a) Stanger BZ. HNF4A and diabetes: injury before insult? Diabetes. 2008;57(6):1461–1462. doi: 10.2337/db08-0454. [DOI] [PubMed] [Google Scholar]; (b) Wang H, Maechler P, Antinozzi PA, Hagenfeldt KA, Wollheim CB. Hepatocyte nuclear factor 4alpha regulates the expression of pancreatic beta-cell genes implicated in glucose metabolism and nutrient-induced insulin secretion. Journal of Biological Chemistry. 2000;275(46):35953–35959. doi: 10.1074/jbc.M006612200. [DOI] [PubMed] [Google Scholar]
- 25.(a) Odom DT, Zizlsperger N, Gordon DB, Bell GW, Rinaldi NJ, Murray HL, Volkert TL, Schreiber J, Rolfe PA, Gifford DK, Fraenkel E, Bell GI, Young RA. Control of pancreas and liver gene expression by HNF transcription factors. Science. 2004;303(5662):1378–1381. doi: 10.1126/science.1089769. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Hayhurst GP, Lee YH, Lambert G, Ward JM, Gonzalez FJ. Hepatocyte nuclear factor 4alpha (nuclear receptor 2A1) is essential for maintenance of hepatic gene expression and lipid homeostasis. Mol Cell Biol. 2001;21(4):1393–1403. doi: 10.1128/MCB.21.4.1393-1403.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Limited N. UK Licence No: PL20620/0037-8. Medicines and Healthcare Products Regulatory Agency; 2009. Public Assessment Report, Decentralized Procedure, Alverine Citrate 60mg & 120mg Capsules. Vol. UK/H/1361/001-002/DC. [Google Scholar]
- 27.(a) van Sommeren S, Visschedijk MC, Festen EA, de Jong DJ, Ponsioen CY, Wijmenga C, Weersma RK. HNF4alpha and CDH1 are associated with ulcerative colitis in a Dutch cohort. Inflamm Bowel Dis. 2011;17(8):1714–1718. doi: 10.1002/ibd.21541. [DOI] [PubMed] [Google Scholar]; (b) Ahn SH, Shah YM, Inoue J, Morimura K, Kim I, Yim S, Lambert G, Kurotani R, Nagashima K, Gonzalez FJ, Inoue Y. Hepatocyte nuclear factor 4alpha in the intestinal epithelial cells protects against inflammatory bowel disease. Inflamm Bowel Dis. 2008;14(7):908–920. doi: 10.1002/ibd.20413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.(a) Liebregts T, Adam B, Bredack C, Roth A, Heinzel S, Lester S, Downie-Doyle S, Smith E, Drew P, Talley NJ, Holtmann G. Immune activation in patients with irritable bowel syndrome. Gastroenterology. 2007;132(3):913–920. doi: 10.1053/j.gastro.2007.01.046. [DOI] [PubMed] [Google Scholar]; (b) Akiho H, Ihara E, Nakamura K. Low-grade inflammation plays a pivotal role in gastrointestinal dysfunction in irritable bowel syndrome. World J Gastrointest Pathophysiol. 2010;1(3):97–105. doi: 10.4291/wjgp.v1.i3.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Halpin SJ, Ford AC. Prevalence of symptoms meeting criteria for irritable bowel syndrome in inflammatory bowel disease: systematic review and meta-analysis. Am J Gastroenterol. 2012;107(10):1474–1482. doi: 10.1038/ajg.2012.260. [DOI] [PubMed] [Google Scholar]
- 30.Ball AJ, Abrahamsson AE, Tyrberg B, Itkin-Ansari P, Levine F. HES6 reverses nuclear reprogramming of insulin-producing cells following cell fusion. Biochem Biophys Res Commun. 2007;355(2):331–337. doi: 10.1016/j.bbrc.2007.01.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.