Abstract
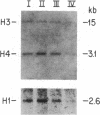
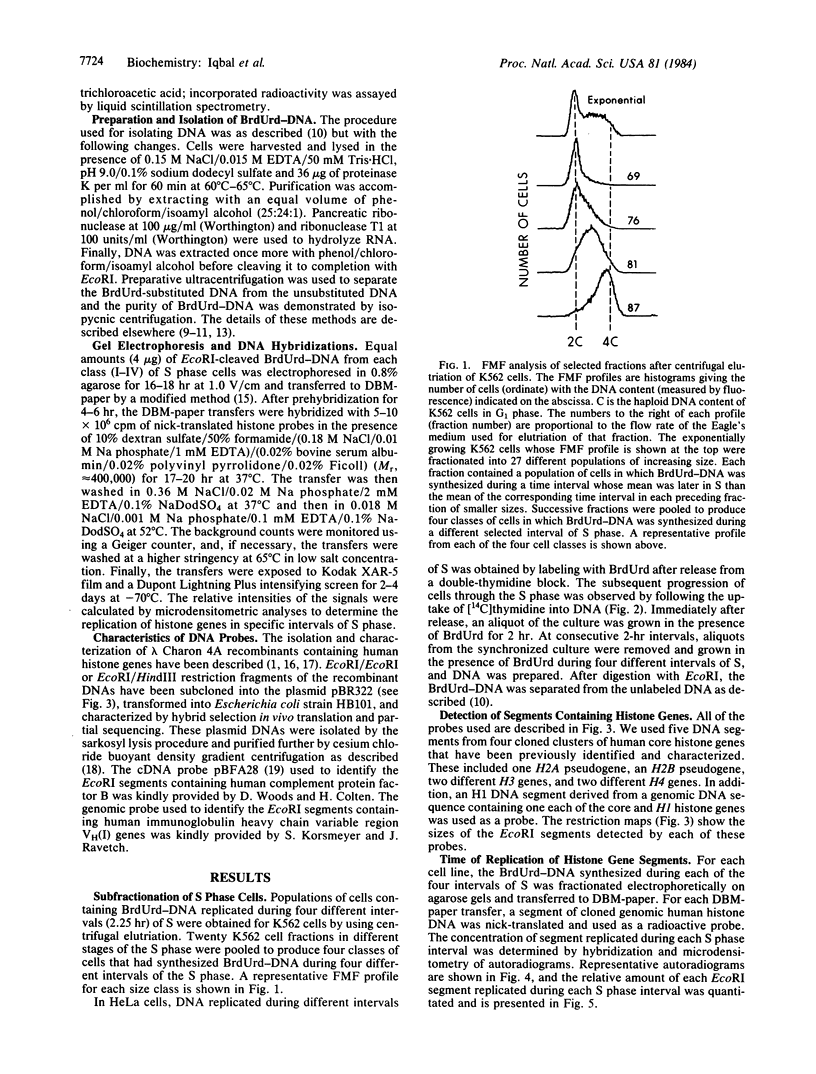
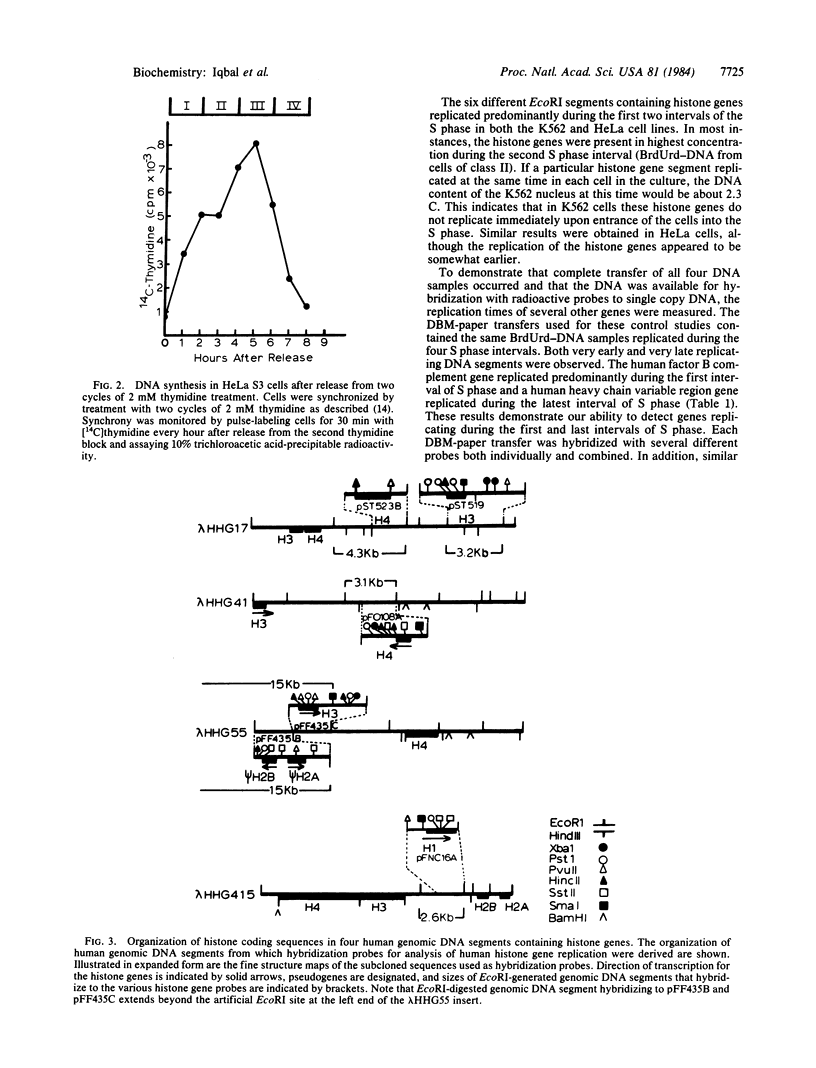
Cells of the K562 human erythroleukemia cell line were obtained in different stages of the cell cycle by centrifugal elutriation. The cells had been previously labeled for 2 hr with BrdUrd so that BrdUrd-DNA synthesized during four different selected intervals of the S phase could be isolated. This DNA was used to determine the temporal replication during S phase of EcoRI segments containing histone genes. Cloned human genomic segments containing the core histone genes (H2A, H2B, H3, and H4), H2A and H2B pseudogenes, and the H1 gene were prepared. The genomic inserts were excised from these plasmids, nick-translated, and used as hybridization probes. The results with different probes compared on the same and on independently prepared DBM-paper transfers indicate that all of these histone genes replicate during the first half of the S phase. These genes were not among the earliest to replicate in the K562 cell line. Similar studies were carried out with HeLa cells in which EcoRI segments containing the H4 histone and H2A and H2B pseudogenes were found to replicate during the first half of the S phase. These histone genes replicate during the interval of the S phase when histone mRNA appears in the cytoplasm at the maximal rate. The possible relationship between these events is discussed.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alterman R. B., Ganguly S., Schulze D. H., Marzluff W. F., Schildkraut C. L., Skoultchi A. I. Cell cycle regulation of mouse H3 histone mRNA metabolism. Mol Cell Biol. 1984 Jan;4(1):123–132. doi: 10.1128/mcb.4.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balazs I., Brown E. H., Schildkraut C. L. The temporal order of replication of some DNA cistrons. Cold Spring Harb Symp Quant Biol. 1974;38:239–245. doi: 10.1101/sqb.1974.038.01.027. [DOI] [PubMed] [Google Scholar]
- Baumbach L. L., Marashi F., Plumb M., Stein G., Stein J. Inhibition of DNA replication coordinately reduces cellular levels of core and H1 histone mRNAs: requirement for protein synthesis. Biochemistry. 1984 Apr 10;23(8):1618–1625. doi: 10.1021/bi00303a006. [DOI] [PubMed] [Google Scholar]
- Braunstein J. D., Schulze D., DelGiudice T., Furst A., Schildkraut C. L. The temporal order of replication of murine immunoglobulin heavy chain constant region sequences corresponds to their linear order in the genome. Nucleic Acids Res. 1982 Nov 11;10(21):6887–6902. doi: 10.1093/nar/10.21.6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown E. H., Schildkraut C. L. Perturbation of growth and differentiation of Friend murine erythroleukemia cells by 5-bromodeoxyuridine incorporation in early S phase. J Cell Physiol. 1979 May;99(2):261–278. doi: 10.1002/jcp.1040990213. [DOI] [PubMed] [Google Scholar]
- Calza R. E., Eckhardt L. A., DelGiudice T., Schildkraut C. L. Changes in gene position are accompanied by a change in time of replication. Cell. 1984 Mar;36(3):689–696. doi: 10.1016/0092-8674(84)90349-0. [DOI] [PubMed] [Google Scholar]
- Carozzi N., Marashi F., Plumb M., Zimmerman S., Zimmerman A., Coles L. S., Wells J. R., Stein G., Stein J. Clustering of human H1 and core histone genes. Science. 1984 Jun 8;224(4653):1115–1117. doi: 10.1126/science.6719136. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Properties of a supercoiled deoxyribonucleic acid-protein relaxation complex and strand specificity of the relaxation event. Biochemistry. 1970 Oct 27;9(22):4428–4440. doi: 10.1021/bi00824a026. [DOI] [PubMed] [Google Scholar]
- Furst A., Brown E. H., Braunstein J. D., Schildkraut C. L. alpha-Globulin sequences are located in a region of early-replicating DNA in murine erythroleukemia cells. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1023–1027. doi: 10.1073/pnas.78.2.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman M. A., Holmquist G. P., Gray M. C., Caston L. A., Nag A. Replication timing of genes and middle repetitive sequences. Science. 1984 May 18;224(4650):686–692. doi: 10.1126/science.6719109. [DOI] [PubMed] [Google Scholar]
- Heintz N., Sive H. L., Roeder R. G. Regulation of human histone gene expression: kinetics of accumulation and changes in the rate of synthesis and in the half-lives of individual histone mRNAs during the HeLa cell cycle. Mol Cell Biol. 1983 Apr;3(4):539–550. doi: 10.1128/mcb.3.4.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hereford L., Bromley S., Osley M. A. Periodic transcription of yeast histone genes. Cell. 1982 Aug;30(1):305–310. doi: 10.1016/0092-8674(82)90036-8. [DOI] [PubMed] [Google Scholar]
- Lennox R. W., Cohen L. H. The histone H1 complements of dividing and nondividing cells of the mouse. J Biol Chem. 1983 Jan 10;258(1):262–268. [PubMed] [Google Scholar]
- Osley M. A., Hereford L. M. Yeast histone genes show dosage compensation. Cell. 1981 May;24(2):377–384. doi: 10.1016/0092-8674(81)90327-5. [DOI] [PubMed] [Google Scholar]
- Plumb M., Stein J., Stein G. Coordinate regulation of multiple histone mRNAs during the cell cycle in HeLa cells. Nucleic Acids Res. 1983 Apr 25;11(8):2391–2410. doi: 10.1093/nar/11.8.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra F., Lichtler A., Marashi F., Rickles R., Van Dyke T., Clark S., Wells J., Stein G., Stein J. Organization of human histone genes. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1795–1799. doi: 10.1073/pnas.79.6.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein G. S., Borun T. W. The synthesis of acidic chromosomal proteins during the cell cycle of HeLa S-3 cells. I. The accelerated accumulation of acidic residual nuclear protein before the initiation of DNA replication. J Cell Biol. 1972 Feb;52(2):292–307. doi: 10.1083/jcb.52.2.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods D. E., Markham A. F., Ricker A. T., Goldberger G., Colten H. R. Isolation of cDNA clones for the human complement protein factor B, a class III major histocompatibility complex gene product. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5661–5665. doi: 10.1073/pnas.79.18.5661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu R. S., Tsai S., Bonner W. M. Patterns of histone variant synthesis can distinguish G0 from G1 cells. Cell. 1982 Dec;31(2 Pt 1):367–374. doi: 10.1016/0092-8674(82)90130-1. [DOI] [PubMed] [Google Scholar]