Abstract
Large-size bone defects can severely compromise both aesthetics and musculoskeletal functions. Adipose-derived stem cells (ASCs)-based bone tissue engineering has recently become a promising treatment strategy for the above situation. As robust osteoinductive cytokines, bone morphogenetic proteins (BMPs) are commonly used to promote the osteogenesis of ASCs. In this process, BMP signaling plays a pivotal role. However, it remains ambiguous how the pleiotrophic BMPs are involved in the commitment of ASCs along osteogenesis instead of other lineages, such as adipogenesis. BMP receptor type-IB, extracellular signal-regulated kinase, and Wnt5a appear to be the main switches controlling the in vitro osteogenic commitment of ASCs. Tumor necrosis factor-alpha, an acute inflammatory cytokine, is reported to play an important role in mediating osteogenic commitment of ASCs in vivo. In addition, various active agents and methods have been used to enhance and accelerate the osteogenesis of ASCs through promoting BMP signaling. In this review, we summarize the current knowledge on the roles of BMPs and their signaling in the osteogenesis of ASCs in vitro and in vivo.
Introduction
Large-size bone defects, resulting from congenital nonunion, trauma, inflammation, and osteosarcoma resection, can severely compromise aesthetics and musculoskeletal functions. In the clinic, the autograft is regarded as the gold-standard treatment since it can simultaneously provide osteoconductive scaffolds, osteoinductive cytokines, and osteogenic cells. However, the use of autografts is limited by their low availability, donor-site pain, and morbidity.1 Therefore, various alternative grafts and techniques are being developed to facilitate osseous restoration in the clinic.
Adipose-derived stem cells (ASCs)-based bone tissue engineering appears to be very promising for the osseous restoration of large-size bone defects. ASCs can undergo rapid and efficient osteogenic differentiation both in vitro and in vivo.2–4 These properties confer human ASCs a promising application potential in the clinic. Several members of the bone morphogenetic protein (BMP) family are robust osteoinductive agents2,5,6 and have been shown to enhance and accelerate the osteogenesis of ASCs. Further, BMP signaling pathways also mediate the promoting effects of many drugs on the osteogenesis of ASCs. In this review, we summarize the current knowledge of the roles of BMPs and their signaling pathways in the osteogenesis of ASCs.
Adipose-Derived Stem Cells
The development of mesenchymal stem cell-based techniques has become a major research objective in the field of bone tissue engineering. Bone marrow was originally considered to be the main source of mesenchymal stem cells for such a purpose.7 However, the clinical use of bone marrow-derived stem cells (BMSCs) is associated with several disadvantages, such as donor-site pain and low cell output, which has led to continuous efforts to search for alternative sources of mesenchymal stem cells.
In 2001, Zuk et al., for the first time, demonstrated that human adipose tissue contains multipotent stem cells that can differentiate along different lineages, such as bone, cartilage, fat, and muscle.8 In 2004, the International Fat Applied Technology Society adopted “adipose-derived stem cells” (ASCs) as the official nomenclature for these multipotent stem cells harvested from adipose tissue.9 Unfortunately, hitherto, there are still no definitive surface markers for identifying ASCs, since marker expression changes during in vitro culture. In general, cells that express CD44, CD90, and CD105 and lack the hematopoietic lineage markers–CD34, CD45, and CD117 are recognized as ASCs.10,11 ASCs have several advantages over BMSCs: they are more easily accessible, have a 500-fold higher yield efficiency, and are associated with lower donor-site morbidity.12 In addition, the osteogenic capacity of ASCs is less affected by aging than that of BMSCs.13 Due to these properties, ASCs have become an attractive source of seed cells for bone tissue engineering.
BMPs and Their Signaling
The BMP family belongs to the superfamily of transforming growth factor-beta (TGF-β). The discovery of BMPs in the pioneering work by Urist in 196514 was a landmark in the development of bone tissue engineering. The classical role for BMPs was considered to be the induction of (ectopic) cartilage and bone formation.14 Several isotypes of BMPs have been demonstrated to play paramount roles in the osteogenic differentiation of various mesenchymal stem cells.2,15–17 Particularly, BMP-2 and BMP-7 have already been approved for clinical use in the United States, Europe, and Australia.18 Owing to continuous efforts over the last 50 years, BMPs are now recognized as a group of metabologens that constitute pivotal morphogenetic signals and orchestrate tissue architecture throughout the body.19 Consequently, it has been suggested to change their name from “bone morphogenetic proteins” to “body morphogenetic proteins.”18
BMPs play pleiotropic roles in promoting the differentiation of pluripotent stem cells along different lineages, for example, in osteogenesis,2 adipogenesis,20 and chondrogenesis.21 The cellular and therapeutic effects of BMPs are mediated by their downstream signaling pathways that are initiated by binding of BMPs to transmembrane serine/threonine kinase receptors. Subsequently, they trigger specific intracellular signaling pathways that control the transcription of specific target genes.22 Two types of BMP receptors exist: type I and type II. Type I receptors include activin receptor type-IA (ACTR-IA), BMP receptor type-IA (BMPR-IA), and BMP receptor type-IB (BMPR-IB). The type II receptors include BMP receptor type-II (BMPR-II), activin receptor type IIA (ACTR-IIA), and activin receptor type IIB (ACTR-IIB).23
BMPs can trigger two main downstream signaling pathways through binding to different receptor complexes: Smad-dependent and Smad-independent signaling pathways.22 Activated BMP receptors phosphorylate Smad1/5/8, which assembles into a complex with Smad4 and translocates to the nucleus, regulating the transcription of target genes, such as Runt-related transcription factor 2 (RUNX2) and osterix. In addition to Smad-dependent signaling, a series of Smad-independent downstream signaling pathways, including mitogen-activated protein kinase (MAPK) pathways such as p38, c-Jun N-terminal kinase (JNK), and extracellular signal-regulated kinase (ERK), are also activated. These pathways have essential roles in BMP-induced osteogenic events.24 On the other hand, BMP signaling is also crucial in inducing adipogenesis of pluripotent stem cells. Both Smad-dependent and Smad-independent signaling pathways participate in BMP-induced adipogenesis.20,25,26 Two key adipogenic transcription factors (CCAAT/enhancer binding protein [C/EBP] and peroxisome proliferator-activated receptor gamma [PPAR-γ]) can be subsequently upregulated by BMP signaling, which then leads to the specific activation of adipocyte-associated genes.25,26
The Roles of BMPs in the Osteogenesis of ASCs In Vitro and In Vivo
BMPs, as a group of pleiotrophic cytokines, play dynamic and pivotal roles in the osteogenic differentiation of ASCs.2,5,6 For the induction of osteogenesis of ASCs, BMPs are introduced through three ways: the administration of exogenous BMPs, gene technology, and the induction of endogenous BMPs.
Administration of exogenous BMPs
The ability of exogenous BMPs to promote in vitro osteogenic differentiation of ASCs is highly dependent on several factors, such as BMP type, concentration, differentiation medium, and administration time point.
BMP-2, BMP-6, and BMP-14 are highly associated with the osteogenic differentiation of ASCs in vitro.2,27,28 In contrast, BMP-7 is thought to contribute to both chondrogenesis29 and osteogenesis30 of ASCs. From the view of dose-effect, BMP-6 and BMP-14 exhibited a significantly higher efficiency than BMP-2.27,28 These results suggest that different types of BMPs induce the osteogenic differentiation of ASCs through distinct regulatory mechanisms. In addition, BMP-14 may simultaneously increase the expression of vascular epithelium growth factor (VEGF)—an angiogenic factor,28 which might contribute to more pronounced bone regeneration.
Concentration is one of the main modulating mechanisms for the effect of BMPs.31 This is also true for their effects on ASCs. For example, the transfection of BMP-4 significantly enhances the osteogenesis of ASCs,32,33 whereas BMP-4 at an extremely low concentration range (0.01–0.1 ng/mL) can increase the survival rate of ASCs and maintain their stemness and multipotency.34 Analogously, BMP-2 at a very high concentration range (300–500 ng/ml) can directly induce the osteogenic differentiation in vitro without the need for additional active agents.35,36 However, at an intermediate concentration range (5–200 ng/mL), the effects of BMP-2 remain ambiguous. BMP-2 appears to induce the osteogenic differentiation of ASCs only in the presence of osteogenic medium. It seems that the active agents, such as ascorbic acid and β-glycerophosphate in osteogenic medium, play crucial roles in committing the osteogenic differentiation of ASCs. In the presence of osteogenic medium, 50 ng/mL BMP-2 appears to be the minimum dose to obtain a significant difference in extracellular mineralization37; the optimal effect is obtained at approximately 100 ng/mL.6,37 The amount and type of active agents in osteogenic medium also influence the efficacy of the osteogenic differentiation of ASCs. For example, dexamethasone was one of the main components for the osteogenic differentiation of ASCs in the traditional osteogenic medium. However, a lack of efficacy and clinical biosafety may limit its clinical application.38–40 Vitamin D3 is a good alternative to dexamethasone.38 In addition, ascorbic acid induces collagen matrix formation, while β-glycerophosphate provides an organic phosphate source that supports mineral deposition during osteogenic differentiation. These latter two components form hydroxyapatite (HA)-containing mineral within the collagen matrix.41
Interestingly, administration time point has been recently shown to significantly influence the effects of BMPs on the osteogenic differentiation of ASCs. When vitamin D3 is constantly treated, BMP-2 is more efficacious to induce the osteogenesis of ASCs when treated for the last 7 days (8–14 days) than the first 7 days (1–7 days).42 It seems plausible that specific compositions in these media determine the differentiation direction of ASCs, and then BMPs can significantly both enhance and accelerate the process.
The possibility of coadministering BMP-2 with other cytokines to achieve synergistic effects has also been investigated. Retinoic acid (RA) can simultaneously promote the osteogenic differentiation and inhibit the adipogenic differentiation of murine ASCs. Coapplication of RA and BMP-2 is reported to synergistically promote the osteogenic differentiation of murine ASCs in vitro.43 In addition, combined treatment with BMP-2 and vitamin D3 can synergistically induce the osteogenic differentiation of human ASCs in vitro.42
Traditionally, in vitro pretreatment with osteogenic medium is necessary to commit the osteogenic differentiation of ASCs before transplantation in vivo. However, this method is not ideal because the long-term culture in vitro will increase the risks of contamination and the possibility of change in biological behavior of cells, and significantly compromise the application potential to some urgent clinical cases.44 Therefore, in vivo osteogenic induction in ASCs by BMPs has recently become an important research focus for ASCs-based bone tissue engineering. BMPs can be administrated by a simple subcutaneous injection for 1–3 days44 or preintegration into scaffolds.45 The latter has the advantage in aspects of cost-effectiveness and clinician-friendliness.
However, to exert their optimal osteoinductive efficacy, BMPs need to be gradually delivered to the target site, at a low level and in a sustained manner, instead of in a single high-dose burst.1 Therefore, many efforts have been performed to develop slow-delivery systems to significantly enhance the efficiency of BMPs in promoting the osteogenic differentiation of ASCs in vitro and in vivo. ASCs and the BMP-slow-delivery scaffolds synergistically enhance the osteogenic events. Composite scaffolds that comprise of organic and inorganic phases are especially promising for inducing osteogenesis of ASCs. For example, a HA/β-tricalcium phosphate (β-TCP) scaffold that can facilitate the sustained release of BMP-2 over a 20-day period has been shown to significantly augment the osteogenic differentiation of ASCs in vitro.46 In addition, many other scaffolds with similar properties have shown promise in this type of application, for example, a poly (DL-lactic-coglycolic acid) (PLGA) scaffold with a fibrin/hyaluronic acid coating47 and a gelatin/β-TCP scaffold.48 These slow-release systems can stimulate osteogenic differentiation, extracellular matrix deposition, maturation, and mineralization of ASCs.49 Further, besides the slowly released BMP-2, the scaffold biomaterial itself may also play a role in the osteogenic differentiation of ASCs in vitro.48 These benefits can be attributed to the interaction between BMP-2 and β-TCP: BMP-2 can increase the dissolution of β-TCP, while β-TCP can resorb BMP-2 from media and provide Ca2+ and PO43− that are needed for bone mineralization.45
Consistent with the findings of in vitro studies, a controlled-released system is thought to maximize the promoting effect of BMPs on the in vivo osteogenesis of ASCs. For example, a PLGA/HA composite scaffold is capable of releasing BMP-2 over a 4-week period in vitro and thereby stimulates bone regeneration following transplantation of undifferentiated human ASCs in vivo.49 This method avoids an in vitro culture period, and thus maximally favors the application potential of ASCs in clinic. The contribution of the transplanted human ASCs to newly formed bone is corroborated by the presence of human nuclear antigen-positive cells and the expression of specific human osteogenic proteins in the area of new bone formation in nude mice models.2,44,49,50
Gene technology
Over the past decade, BMPs and their signaling have also been introduced using gene technologies to induce the osteogenic differentiation of ASCs. These gene technologies include gene transfection of BMPs and the key BMP signaling components, and gene knockdown of the BMP antagonists (Fig. 1). Human ASCs transfected with BMP-2 have been shown to significantly promote bone formation in many animal models, including ectopic bone induction in mice,51–53 critical-size bone defects of rats,54,55 and spine fusion in rats.56 Further, cotransfection of RUNX2 or VEGF with BMP-2 is shown to enhance bone regeneration and accelerate the healing of segmental defects more effectively than BMP-2 alone in vivo.57,58 In addition to BMP-2, significant bone formation can also be achieved by transfection of ASCs with BMP-4,32,33 BMP-6,5 or BMP-7.59,60
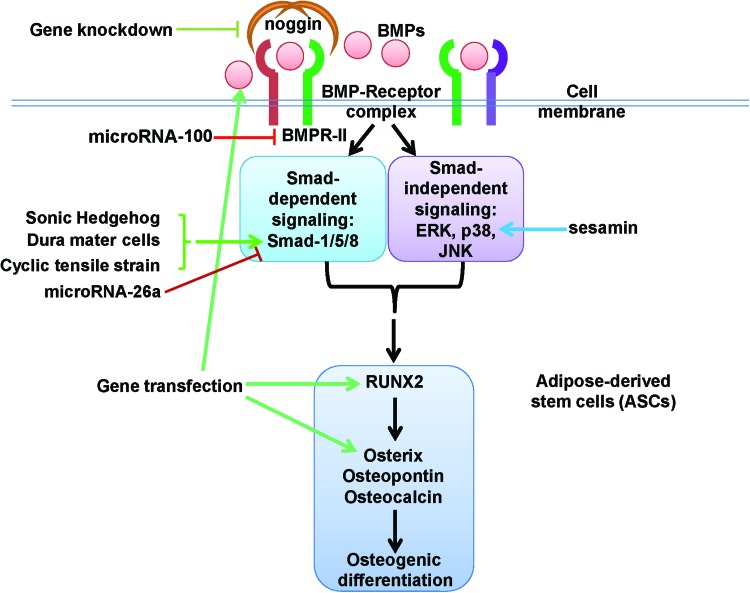
FIG. 1.
Schematic diagram depicting the modulation of osteogenesis of ASCs by different active agents through modulating BMP signaling pathways. ASCs, adipose-derived stem cells; BMP, bone morphogenetic protein; BMPR-II: BMP receptor type-II; ERK, extracellular signal-regulated kinase; JNK, Jun N-terminal kinase; RUNX2, Runt-related transcription factor 2; →, promotion; ⊣, inhibition. Color images available online at www.liebertpub.com/teb
On the other hand, the gene knockdown of noggin, one of the BMP antagonists, significantly upregulates BMP signaling and enhances BMP-2-induced osteogenic differentiation of ASCs in vitro and in vivo.2,61 Interestingly, noggin knockdown also increases angiogenesis, which is essential for bone formation.61 Moreover, simultaneous overexpression of BMP-2 and repression of noggin synergistically enhance the osteogenesis of ASCs.62
The induction of endogenous BMPs
Many active agents can upregulate endogenous BMPs (Fig. 2), which at least partially accounts for their promoting effect on the osteogenesis of ASCs. The expression of endogenous BMPs, such as BMP-2, BMP-4, and BMP-6, can be detected in osteogenic medium.3,37,38 The expression level of endogenous BMPs is also adopted as an important parameter to evaluate the potency of an osteogenic medium.3,38 The types of endogenous BMPs are also dependent on the types of active reagents in the osteogenic medium. For example, endogenous BMP-6 can be induced by both dexamethasone and vitamin D3, whereas BMP-2 is only detected in the presence of vitamin D3.38 These results suggest that the different active agents in osteogenic medium induce the osteogenic differentiation of ASCs through regulating BMPs and their signaling pathways.
FIG. 2.
Schematic diagram depicting the induction of endogeneous BMPs by different active agents for their promoting effects on the osteogenesis of ASCs. ICAT, inhibitor of β-catenin and TCF-4; Shh, sonic hedgehog; TGF-β1, transforming growth factor-β1. Color images available online at www.liebertpub.com/teb
In 1999, Mundy et al., for the first time, reported that statins could effectively stimulate bone formation in rodents, both in vitro and in vivo.63 Many subsequent studies confirmed that statins exert their osteoinductive effects through promoting BMP-2 expression.64 Among statins, simvastatin, an inhibitor of the competitive 3-hydroxy-3-methyl coenzyme A reductase, is considered to be the most potent inducer for the osteogenic differentiation of mesenchymal stem cells. We recently reported that simvastatin can enhance the osteogenesis of human ASCs in vitro and in vivo by significantly increasing the expression of mRNA encoding BMP-2, RUNX2, VEGF, and fibroblast growth factor-2.3 The upregulated BMP-2 seems to be one of the major mechanisms for the promoting effect of simvastatin on the osteogenesis of human ASCs.3 In ASCs, endogenous BMPs can also be induced by TGF-β1,65 sonic hedgehog (shh),66 inhibitor of β-catenin and TCF-4 (ICAT),67 and sesamin.68 These active agents may significantly contribute to a rapid significant progress of the ASC-based bone tissue engineering.
In addition to cytokines and active agents, mechanical loading, such as cyclic tensile strain, can modulate the osteogenic differentiation of ASCs via BMP-2 signaling.69 This mechanism may also account for the promotion of ASC osteogenesis by the application of an electromagnetic field.70
The Roles of BMP Signaling Pathway in the Osteogenesis of ASCs
The osteogenic differentiation of multipotent ASCs involves two main steps: commitment of ASCs along the osteogenic lineage and promotion of osteogenic differentiation. BMP signaling is repeatedly shown to play crucial but ambiguous roles in the osteogenesis of ASCs.57,62
The roles of BMP signaling in the commitment of ASCs to osteogenic differentiation
Multipotent mesenchymal stem cells express small amounts of both adipogenic factors, such as C/EBP and PPAR-γ, and osteogenic factors, such as RUNX2 and osterix. Factors of one lineage repress factors of the other lineage, thereby maintaining the undifferentiated state.71 Generally, the eventual fate of mesenchymal stem cells is considered to be controlled by the antagonistic balance between RUNX2 and PPAR-γ. For example, the activation of PPAR-γ can not only promote the adipogenesis, but also suppress the osteogenesis both by downregulating the expression and interfering with the transactivation ability of RUNX2.72 RUNX2−/− cells show enhanced adipocyte development,72 whereas PPAR-γ−/− cells fail to differentiate into adipocytes, but spontaneously differentiate into osteoblasts.73
Unlike BMSCs, ASCs show a native tendency to differentiate into adipogenic cells.33 For a single ASC, differentiation toward osteogenic or adipogenic lineages is mutually exclusive and antagonistic.74 Osteogenic commitment of ASCs requires both suppression of the adipogenic differentiation and enhancement of osteogenic differentiation. Interestingly, BMPs have been reported to promote both osteogenic and adipogenic differentiation of mesenchymal stem cells. This raises the question of whether BMPs can commit a certain differentiation direction of ASCs or not. It seemed that, in the most commonly used concentration range (5–250 ng/mL) and without additional osteogenic agents, BMPs cannot significantly commit ASCs to either osteogenesis or adipogenesis. One hypothesis is that BMP alone can activate and induce equivalent levels of osteogenic and adipogenic signaling. Both signaling antagonize each other through different signaling levels. The mutual suppression and inhibition between these two signaling result in a noncommitment stage.
The balance between osteogenic and adipogenic signaling can be disequilibrated by the addition of osteogenic agents, such as osteogenic medium and RA. The disequilibration may be mediated, at least partially, by three main switches: endogenous Wnt5a and ERK and changing the ratio of BMPR-IB/BMPR-IA (Fig. 3).
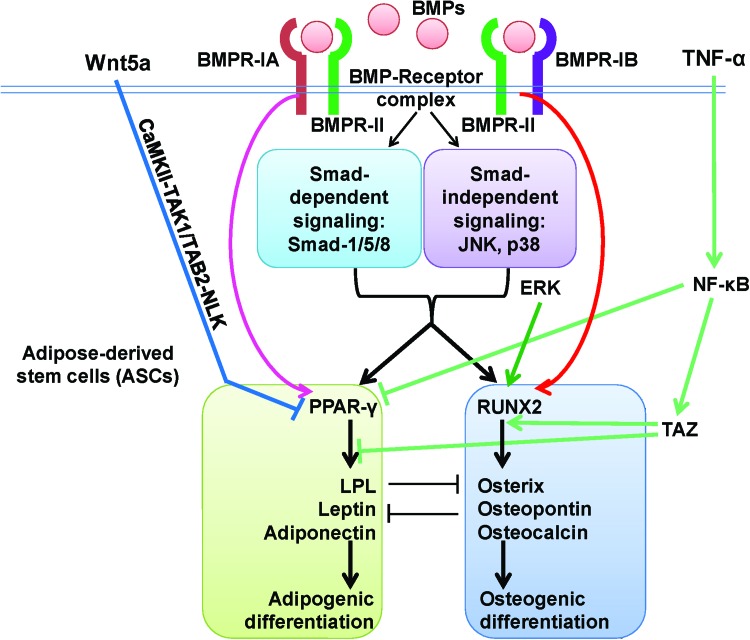
FIG. 3.
Schematic diagram depicting the signaling pathways of BMPs in ASCs and the main switches for the osteogenic commitment of ASCs. BMPR-IA, BMP receptor type-IA; BMPR-IB, BMP receptor type-IB; BMPR-II, BMP receptor type-II; CaMK-II, calcium/calmodulin-dependent kinase II; ERK, extracellular signal-regulated kinase; LPL, lipoprotein lipase; NF-κB, nuclear factor κB; NLK, Nemo-like kinase; PPAR-γ, peroxisome proliferator-activated receptor gamma; RUNX2, Runt-related transcription factor 2; TAB2, TGF-β-activated kinase 1/MAP3K7-binding protein 2; TAK1, transforming growth factor β-activated kinase-1; TAZ, transcriptional coactivator with PDZ-binding motif; TNF-α, tumor necrosis factor-alpha. →, promotion; ⊣, inhibition. Color images available online at www.liebertpub.com/teb
BMPR-IA and BMPR-IB exhibited versatile and divergent effects on the process of osteogenesis both in vitro and in vivo.75–77 Their exact functions are highly dependent on factors including cell type and differentiation stage.75 For mouse ASCs, signaling through BMPR-IB played a more significant role in osteogenic differentiation, whereas signaling through BMPR-IA seemed to be more relatively relevant to adipogenesis.43 Data from 2T3 mouse calvarial stem cells corroborated the distinct functions of these two BMP receptors.77 Therefore, the type of receptor and level of expression on stem cells can be a switch for their commitment. It is also important to note that RA signaling is indispensable for osteogenic differentiation in mouse ASCs,43 although this is not the case for human ASCs.78 Consequently, precautions must be taken when extrapolating data from mouse ASCs to humans.
ERK also appears to be a key switch of adipogenic and osteogenic differentiation of mesenchymal stem cells.79 Consistent with this finding, ERK is also important for the adipogenic and osteogenic commitment in ASCs.68,80–83 Inhibition of ERK by PD98059 blocks the expression of osteogenic differentiation-related proteins in a dose-dependent manner and switches ASCs to adipogenic differentiation.83 Further, ERK is indispensable for the effects of sesamin,81 akermanite,68 wedelolactone,82 and oncostatin M80 in promoting the osteogenic differentiation and inhibiting adipogenic differentiation of ASCs. However, how ERK is modulated during osteogenesis of ASCs remains to be elucidated.
Wnt signaling also has an important role in the osteogenic commitment of mesenchymal stem cells (Fig. 3). Wnt5a, a noncanonical Wnt ligand, promotes the osteogenesis by repressing PPAR-γ transactivation through CaMKII-TAK1/TAB2-NLK signaling cascade and the subsequent activation of the histone methyltransferase, SETDB1 (SET domain bifurcated 1).84,85 SETDB1 leads to the formation of a corepressor complex that inactivates PPAR-γ function through histone H3-K9 methylation. Thus, noncanonical Wnt5a has emerged as a fate determinant of mesenchymal stem cells through shifting from adipogenesis to osteogenesis.84,85 Exogenous Wnt5a induces osteogenic differentiation and downregulates PPAR-γ expression in human ASCs.86 However, the interaction between endogenous Wnt and BMP signaling during the osteogenesis of ASCs needs further elucidation.
In contrast with their ambiguous effects in vitro, BMPs have a more definite effect on promoting the in vivo osteogenesis of ASCs. Endogenous cytokines that are elevated during acute inflammation may also significantly facilitate the osteogenic commitment of ASCs in vivo (Fig. 3). Tumor necrosis factor-alpha (TNF-α) is one of the main cytokines responsible for acute inflammation.87 TNF-α activates nuclear factor-κB (NF-κB), which inhibits the transactivation of PPAR-γ through a physical association.85 NF-κB activation also leads to upregulation of TAZ (transcriptional coactivator with PDZ-binding motif), a coactivator of RUNX2-dependent transcription, and suppression of PPAR-γ-dependent transcription.88 Through these three pathways, TNF-α can help to commit the osteogenic differentiation of ASCs in in vivo microenvironments. The role of TNF-α in their osteogenic differentiation is supported by the finding that ASCs can be used to heal acute, but not chronic, calvarial defects in nude mice.89 TNF-α that occurs during acute inflammation may help to commit the osteogenesis of ASCs, leading to a much more definite effect of exogenous BMPs in inducing the osteogenesis of ASCs in vivo than that in vitro.
The roles of BMP signaling in the promotion of osteogenic differentiation of ASCs
BMP signaling plays a crucial role in the osteogenesis of ASCs. The upregulation of BMP signaling significantly enhances and accelerates the process. This is supported by the fact that the osteogenesis of ASCs positively correlates to the expression levels of BMPR-II, Smad-1/5, RUNX2, and osterix.4,90–94
Different active agents may modulate the osteogenesis of ASCs by targeting different genes and proteins for BMP signaling pathways (Fig. 1). For example, microRNA-26a inhibits osteogenic differentiation of human ASCs via targeting the Smad1 transcription factor.95 In addition, Shh promotes bone repair by ASCs, which is associated with upregulation of phospho-Smad1/5/8.66 Simvastatin promotion of ASC osteogenesis3 may be possibly mediated by the activation of Ras/Smad/ERK/BMP-2 pathway.96 Moreover, sesamin can stimulate the osteogenic differentiation of human ASCs with the upregulation of BMP-2, and further exert its effect via p38 and ERK1/2 MAPK signaling pathways.68
Conclusion
BMPs and their signaling pathways play paramount roles in the osteogenic differentiation of ASCs in vitro and in vivo. Various cytokines and gene technologies have been developed to promote the osteogenesis of ASCs through promoting BMPs-induced osteogenic signaling and suppressing adipogenesis. BMPs-ASCs-based bone tissue engineering is very promising for the repair of large-size bone defects.
Acknowledgments
This study was supported by a grant from the National Natural Science Foundation of China (2011/81170937 to Prof. Dr. Y. Zhou), and the Program for New Century Excellent Talents in University from Ministry of Education of China (NCET-11-0026).
Disclosure Statement
The authors indicate no potential conflicts of interest.
References
- 1.Sokolsky-Papkov M., Agashi K., Olaye A., Shakesheff K., and Domb A.J.Polymer carriers for drug delivery in tissue engineering. Adv Drug Del Rev 59,187, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Levi B., Hyun J.S., Nelson E.R., Li S., Montoro D.T., Wan D.C., Jia F.J., Glotzbach J.C., James A.W., Lee M., Huang M., Quarto N., Gurtner G.C., Wu J.C., and Longaker M.T.Nonintegrating knockdown and customized scaffold design enhances human adipose-derived stem cells in skeletal repair. Stem Cells 29,2018, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou Y., Ni Y., Liu Y., Zeng B., Xu Y., and Ge W.The role of simvastatin in the osteogenesis of injectable tissue-engineered bone based on human adipose-derived stromal cells and platelet-rich plasma. Biomaterials 31,5325, 2010 [DOI] [PubMed] [Google Scholar]
- 4.Ge W., Shi L., Zhou Y., Liu Y., Ma G.E., Jiang Y., Xu Y., Zhang X., and Feng H.Inhibition of osteogenic differentiation of human adipose-derived stromal cells by retinoblastoma binding protein 2 repression of RUNX2-activated transcription. Stem Cells 29,1112, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Sheyn D., Pelled G., Zilberman Y., Talasazan F., Frank J.M., Gazit D., and Gazit Z.Nonvirally engineered porcine adipose tissue-derived stem cells: use in posterior spinal fusion. Stem Cells 26,1056, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Prichard H.L., Reichert W., and Klitzman B.IFATS collection: adipose-derived stromal cells improve the foreign body response. Stem Cells 26,2691, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bianco P., and Robey P.G.Stem cells in tissue engineering. Nature 414,118, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Zuk P.A., Zhu M., Mizuno H., Huang J., Futrell J.W., Katz A.J., Benhaim P., Lorenz H.P., and Hedrick M.H.Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng 7,211, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Rada T., Reis R.L., and Gomes M.E.Adipose tissue-derived stem cells and their application in bone and cartilage tissue engineering. Tissue Eng Part B Rev 15,113, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Yoshimura K., Shigeura T., Matsumoto D., Sato T., Takaki Y., Aiba-Kojima E., Sato K., Inoue K., Nagase T., Koshima I., and Gonda K.Characterization of freshly isolated and cultured cells derived from the fatty and fluid portions of liposuction aspirates. J Cell Physiol 208,64, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Dominici M., Le Blanc K., Mueller I., Slaper-Cortenbach I., Marini F., Krause D., Deans R., Keating A., Prockop D., and Horwitz E.Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8,315, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Fraser J.K., Wulur I., Alfonso Z., and Hedrick M.H.Fat tissue: an underappreciated source of stem cells for biotechnology. Trends Biotechnol 24,150, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Chen H.T., Lee M.J., Chen C.H., Chuang S.C., Chang L.F., Ho M.L., Hung S.H., Fu Y.C., Wang Y.H., Wang H.I., Wang G.J., Kang L., and Chang J.K.Proliferation and differentiation potential of human adipose-derived mesenchymal stem cells isolated from elderly patients with osteoporotic fractures. J Cell Mol Med 16,582, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Urist M.R.Bone: formation by autoinduction. Science 150,893, 1965 [DOI] [PubMed] [Google Scholar]
- 15.Hanada K., Dennis J.E., and Caplan A.I.Stimulatory effects of basic fibroblast growth factor and bone morphogenetic protein-2 on osteogenic differentiation of rat bone marrow-derived mesenchymal stem cells. J Bone Miner Res 12,1606, 1997 [DOI] [PubMed] [Google Scholar]
- 16.Rui Y.F., Lui P.P., Ni M., Chan L.S., Lee Y.W., and Chan K.M.Mechanical loading increased BMP-2 expression which promoted osteogenic differentiation of tendon-derived stem cells. J Orthop Res 29,390, 2011 [DOI] [PubMed] [Google Scholar]
- 17.Xiang L., Liang C., Zhen-Yong K., Liang-Jun Y., and Zhong-Liang D.BMP9-induced osteogenetic differentiation and bone formation of muscle-derived stem cells. J Biomed Biotechnol 2012,610952, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reddi A.H.BMPs: from bone morphogenetic proteins to body morphogenetic proteins. Cytokine Growth Factor Rev 16,249, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Reddi A.H., and Reddi A.Bone morphogenetic proteins (BMPs): from morphogens to metabologens. Cytokine Growth Factor Rev 20,341, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Tseng Y.H., Kokkotou E., Schulz T.J., Huang T.L., Winnay J.N., Taniguchi C.M., Tran T.T., Suzuki R., Espinoza D.O., Yamamoto Y., Ahrens M.J., Dudley A.T., Norris A.W., Kulkarni R.N., and Kahn C.R.New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature 454,1000, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim H.J., and Im G.I.Combination of transforming growth factor-beta2 and bone morphogenetic protein 7 enhances chondrogenesis from adipose tissue-derived mesenchymal stem cells. Tissue Eng Part A 15,1543, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Derynck R., and Zhang Y.E.Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature 425,577, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Guo J., and Wu G.The signaling and functions of heterodimeric bone morphogenetic proteins. Cytokine Growth Factor Rev 23,61, 2012 [DOI] [PubMed] [Google Scholar]
- 24.Xiao G., Gopalakrishnan R., Jiang D., Reith E., Benson M.D., and Franceschi R.T.Bone morphogenetic proteins, extracellular matrix, and mitogen-activated protein kinase signaling pathways are required for osteoblast-specific gene expression and differentiation in MC3T3-E1 cells. J Bone Miner Res 17,101, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Hata K., Nishimura R., Ikeda F., Yamashita K., Matsubara T., Nokubi T., and Yoneda T.Differential roles of Smad1 and p38 kinase in regulation of peroxisome proliferator-activating receptor gamma during bone morphogenetic protein 2-induced adipogenesis. Mol Biol Cell 14,545, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang H., Song T.J., Li X., Hu L., He Q., Liu M., Lane M.D., and Tang Q.Q.BMP signaling pathway is required for commitment of C3H10T1/2 pluripotent stem cells to the adipocyte lineage. Proc Natl Acad Sci U S A 106,12670, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mizrahi O., Sheyn D., Tawackoli W., Kallai I., Oh A., Su S., Da X., Zarrini P., Cook-Wiens G., Gazit D., and Gazit Z.BMP-6 is more efficient in bone formation than BMP-2 when overexpressed in mesenchymal stem cells. Gene Ther 20,370, 2013 [DOI] [PubMed] [Google Scholar]
- 28.Zeng Q., Li X., Beck G., Balian G., and Shen F.H.Growth and differentiation factor-5 (GDF-5) stimulates osteogenic differentiation and increases vascular endothelial growth factor (VEGF) levels in fat-derived stromal cells in vitro. Bone 40,374, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Knippenberg M., Helder M.N., Zandieh D.B., Wuisman P.I., and Klein-Nulend J.Osteogenesis versus chondrogenesis by BMP-2 and BMP-7 in adipose stem cells. Biochem Biophys Res Commun 342,902, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Al-Salleeh F., Beatty M.W., Reinhardt R.A., Petro T.M., and Crouch L.Human osteogenic protein-1 induces osteogenic differentiation of adipose-derived stem cells harvested from mice. Arch Oral Biol 53,928, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Zheng Y., Wu G., Zhao J., Wang L., Sun P., and Gu Z.rhBMP2/7 heterodimer: an osteoblastogenesis inducer of not higher potency but lower effective concentration compared with rhBMP2 and rhBMP7 homodimers. Tissue Eng Part A 16,879, 2010 [DOI] [PubMed] [Google Scholar]
- 32.Lin L., Fu X., Zhang X., Chen L.X., Zhang J.Y., Yu C.L., Ma K.T., and Zhou C.Y.Rat adipose-derived stromal cells expressing BMP4 induce ectopic bone formation in vitro and in vivo. Acta Pharmacol Sin 27,1608, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Lin L., Shen Q., Wei X., Hou Y., Xue T., Fu X., Duan X., and Yu C.Comparison of osteogenic potentials of BMP4 transduced stem cells from autologous bone marrow and fat tissue in a rabbit model of calvarial defects. Calcif Tissue Int 85,55, 2009 [DOI] [PubMed] [Google Scholar]
- 34.Vicente L.M.A., Vazquez G.M.N., Entrena A., Olmedillas L.S., Garcia-Arranz M., Garcia-Olmo D., and Zapata A.Low doses of bone morphogenetic protein 4 increase the survival of human adipose-derived stem cells maintaining their stemness and multipotency. Stem Cells Dev 20,1011, 2011 [DOI] [PubMed] [Google Scholar]
- 35.Mehrkens A., Saxer F., Guven S., Hoffmann W., Muller A.M., Jakob M., Weber F.E., Martin I., and Scherberich A.Intraoperative engineering of osteogenic grafts combining freshly harvested, human adipose-derived cells and physiological doses of bone morphogenetic protein-2. Eur Cell Mater 24,308, 2012 [DOI] [PubMed] [Google Scholar]
- 36.Shiraishi T., Sumita Y., Wakamastu Y., Nagai K., and Asahina I.Formation of engineered bone with adipose stromal cells from buccal fat pad. J Dent Res 91,592, 2012 [DOI] [PubMed] [Google Scholar]
- 37.Panetta N.J., Gupta D.M., Lee J.K., Wan D.C., Commons G.W., and Longaker M.T.Human adipose-derived stromal cells respond to and elaborate bone morphogenetic protein-2 during in vitro osteogenic differentiation. Plast Reconstr Surg 125,483, 2010 [DOI] [PubMed] [Google Scholar]
- 38.Zhou Y.S., Liu Y.S., and Tan J.G.Is 1, 25-dihydroxyvitamin D3 an ideal substitute for dexamethasone for inducing osteogenic differentiation of human adipose tissue-derived stromal cells in vitro? Chin Med J (Engl) 119,1278, 2006 [PubMed] [Google Scholar]
- 39.Malladi P., Xu Y., Yang G.P., and Longaker M.T.Functions of vitamin D, retinoic acid, and dexamethasone in mouse adipose-derived mesenchymal cells. Tissue Eng 12,2031, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Zuk P.A., Zhu M., Ashjian P., De Ugarte D.A., Huang J.I., Mizuno H., Alfonso Z.C., Fraser J.K., Benhaim P., and Hedrick M.H.Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell 13,4279, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beck G.R., Jr., Zerler B., and Moran E.Phosphate is a specific signal for induction of osteopontin gene expression. Proc Natl Acad Sci U S A 97,8352, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Song I., Kim B.S., Kim C.S., and Im G.I.Effects of BMP-2 and vitamin D3 on the osteogenic differentiation of adipose stem cells. Biochem Biophys Res Commun 408,126, 2011 [DOI] [PubMed] [Google Scholar]
- 43.Wan D.C., Shi Y.Y., Nacamuli R.P., Quarto N., Lyons K.M., and Longaker M.T.Osteogenic differentiation of mouse adipose-derived adult stromal cells requires retinoic acid and bone morphogenetic protein receptor type IB signaling. Proc Natl Acad Sci U S A 103,12335, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Levi B., James A.W., Nelson E.R., Vistnes D., Wu B., Lee M., Gupta A., and Longaker M.T.Human adipose derived stromal cells heal critical size mouse calvarial defects. PLoS One 5,e11177, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.E L.L., Xu L.L., Wu X., Wang D.S., Lv Y., Wang J.Z., and Liu H.C.The interactions between rat-adipose-derived stromal cells, recombinant human bone morphogenetic protein-2, and beta-tricalcium phosphate play an important role in bone tissue engineering. Tissue Eng Part A 16,2927, 2010 [DOI] [PubMed] [Google Scholar]
- 46.Szpalski C., Nguyen P.D., Cretiu V.C.E., Chesnoiu-Matei I., Ricci J.L., Clark E., Smay J.E., and Warren S.M.Bony engineering using time-release porous scaffolds to provide sustained growth factor delivery. J Craniofac Surg 23,638, 2012 [DOI] [PubMed] [Google Scholar]
- 47.Kang S.W., Kim J.S., Park K.S., Cha B.H., Shim J.H., Kim J.Y., Cho D.W., Rhie J.W., and Lee S.H.Surface modification with fibrin/hyaluronic acid hydrogel on solid-free form-based scaffolds followed by BMP-2 loading to enhance bone regeneration. Bone 48,298, 2011 [DOI] [PubMed] [Google Scholar]
- 48.Weinand C., Nabili A., Khumar M., Dunn J.R., Ramella-Roman J., Jeng J.C., Jordan M.H., and Tabata Y.Factors of osteogenesis influencing various human stem cells on third-generation gelatin/beta-tricalcium phosphate scaffold material. Rejuvenation Res 14,185, 2011 [DOI] [PubMed] [Google Scholar]
- 49.Jeon O., Rhie J.W., Kwon I.K., Kim J.H., Kim B.S., and Lee S.H.In vivo bone formation following transplantation of human adipose-derived stromal cells that are not differentiated osteogenically. Tissue Eng Part A 14,1285, 2008 [DOI] [PubMed] [Google Scholar]
- 50.Cowan C.M., Shi Y.Y., Aalami O.O., Chou Y.F., Mari C., Thomas R., Quarto N., Contag C.H., Wu B., and Longaker M.T.Adipose-derived adult stromal cells heal critical-size mouse calvarial defects. Nat Biotechnol 22,560, 2004 [DOI] [PubMed] [Google Scholar]
- 51.Dragoo J.L., Choi J.Y., Lieberman J.R., Huang J., Zuk P.A., Zhang J., Hedrick M.H., and Benhaim P.Bone induction by BMP-2 transduced stem cells derived from human fat. J Orthop Res 21,622, 2003 [DOI] [PubMed] [Google Scholar]
- 52.Dragoo J.L., Samimi B., Zhu M., Hame S.L., Thomas B.J., Lieberman J.R., Hedrick M.H., and Benhaim P.Tissue-engineered cartilage and bone using stem cells from human infrapatellar fat pads. J Bone Joint Surg Br 85,740, 2003 [PubMed] [Google Scholar]
- 53.Dragoo J.L., Lieberman J.R., Lee R.S., Deugarte D.A., Lee Y., Zuk P.A., Hedrick M.H., and Benhaim P.Tissue-engineered bone from BMP-2-transduced stem cells derived from human fat. Plast Reconstr Surg 115,1665, 2005 [DOI] [PubMed] [Google Scholar]
- 54.Peterson B., Zhang J., Iglesias R., Kabo M., Hedrick M., Benhaim P., and Lieberman J.R.Healing of critically sized femoral defects, using genetically modified mesenchymal stem cells from human adipose tissue. Tissue Eng 11,120, 2005 [DOI] [PubMed] [Google Scholar]
- 55.Lin Y., Tang W., Wu L., Jing W., Li X., Wu Y., Liu L., Long J., and Tian W.Bone regeneration by BMP-2 enhanced adipose stem cells loading on alginate gel. Histochem Cell Biol 129,203, 2008 [DOI] [PubMed] [Google Scholar]
- 56.Hsu W.K., Wang J.C., Liu N.Q., Krenek L., Zuk P.A., Hedrick M.H., Benhaim P., and Lieberman J.R.Stem cells from human fat as cellular delivery vehicles in an athymic rat posterolateral spine fusion model. J Bone Joint Surg Am 90,1043, 2008 [DOI] [PubMed] [Google Scholar]
- 57.Lee S.J., Kang S.W., Do H.J., Han I., Shin D.A., Kim J.H., and Lee S.H.Enhancement of bone regeneration by gene delivery of BMP2/Runx2 bicistronic vector into adipose-derived stromal cells. Biomaterials 31,5652, 2010 [DOI] [PubMed] [Google Scholar]
- 58.Lin C.Y., Lin K.J., Kao C.Y., Chen M.C., Lo W.H., Yen T.C., Chang Y.H., and Hu Y.C.The role of adipose-derived stem cells engineered with the persistently expressing hybrid baculovirus in the healing of massive bone defects. Biomaterials 32,6505, 2011 [DOI] [PubMed] [Google Scholar]
- 59.Yang M., Ma Q.J., Dang G.T., Ma K., Chen P., and Zhou C.Y.In vitro and in vivo induction of bone formation based on ex vivo gene therapy using rat adipose-derived adult stem cells expressing BMP-7. Cytotherapy 7,273, 2005 [DOI] [PubMed] [Google Scholar]
- 60.Kang Y., Liao W.M., Yuan Z.H., Sheng P.Y., Zhang L.J., Yuan X.W., and Lei L.In vitro and in vivo induction of bone formation based on adeno-associated virus-mediated BMP-7 gene therapy using human adipose-derived mesenchymal stem cells. Acta Pharmacol Sin 28,839, 2007 [DOI] [PubMed] [Google Scholar]
- 61.Levi B., Nelson E.R., Hyun J.S., Glotzbach J.P., Li S., Nauta A., Montoro D.T., Lee M., Commons G.C., Hu S., Wu J.C., Gurtner G.C., and Longaker M.T.Enhancement of human adipose-derived stromal cell angiogenesis through knockdown of a BMP-2 inhibitor. Plast Reconstr Surg 129,53, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ramasubramanian A., Shiigi S., Lee G.K., and Yang F.Non-viral delivery of inductive and suppressive genes to adipose-derived stem cells for osteogenic differentiation. Pharm Res 28,1328, 2011 [DOI] [PubMed] [Google Scholar]
- 63.Mundy G., Garrett R., Harris S., Chan J., Chen D., Rossini G., Boyce B., Zhao M., and Gutierrez G.Stimulation of bone formation in vitro and in rodents by statins. Science 286,1946, 1999 [DOI] [PubMed] [Google Scholar]
- 64.Maeda T., Matsunuma A., Kawane T., and Horiuchi N.Simvastatin promotes osteoblast differentiation and mineralization in MC3T3-E1 cells. Biochem Biophys Res Commun 280,874, 2001 [DOI] [PubMed] [Google Scholar]
- 65.Levi B., James A.W., Xu Y., Commons G.W., and Longaker M.T.Divergent modulation of adipose-derived stromal cell differentiation by TGF-beta1 based on species of derivation. Plast Reconstr Surg 126,412, 2010 [DOI] [PubMed] [Google Scholar]
- 66.James A.W., Leucht P., Levi B., Carre A.L., Xu Y., Helms J.A., and Longaker M.T.Sonic Hedgehog influences the balance of osteogenesis and adipogenesis in mouse adipose-derived stromal cells. Tissue Eng Part A 16,2605, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim Y.J., Kim J.T., Bae Y.C., Suh K.T., and Jung J.S.ICAT participates in proliferation and osteogenic differentiation of human adipose tissue-derived mesenchymal stem cell. Life Sci 83,851, 2008 [DOI] [PubMed] [Google Scholar]
- 68.Wanachewin O., Boonmaleerat K., Pothacharoen P., Reutrakul V., and Kongtawelert P.Sesamin stimulates osteoblast differentiation through p38 and ERK1/2 MAPK signaling pathways. BMC Complement Altern Med 12,71, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang X., Gong P., Lin Y., Zhang L., Li X., Yuan Q., Tan Z., Wang Y., Man Y., and Tang H.Cyclic tensile stretch modulates osteogenic differentiation of adipose-derived stem cells via the BMP-2 pathway. Arch Med Sci 6,152, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kang K.S., Hong J.M., Seol Y.J., Rhie J.W., Jeong Y.H., and Cho D.W.Short-term evaluation of electromagnetic field pretreatment of adipose-derived stem cells to improve bone healing. J Tissue Eng Regen Med 2012. [Epub ahead of print]; DOI: 10.1002/term.1664 [DOI] [PubMed] [Google Scholar]
- 71.Rosen E.D., and MacDougald O.A.Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol 7,885, 2006 [DOI] [PubMed] [Google Scholar]
- 72.Jeon M.J., Kim J.A., Kwon S.H., Kim S.W., Park K.S., Park S.W., Kim S.Y., and Shin C.S.Activation of peroxisome proliferator-activated receptor-gamma inhibits the Runx2-mediated transcription of osteocalcin in osteoblasts. J Biol Chem 278,23270, 2003 [DOI] [PubMed] [Google Scholar]
- 73.Akune T., Ohba S., Kamekura S., Yamaguchi M., Chung U.I., Kubota N., Terauchi Y., Harada Y., Azuma Y., Nakamura K., Kadowaki T., and Kawaguchi H.PPARgamma insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. J Clin Invest 113,846, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lin Y.F., Jing W., Wu L., Li X.Y., Wu Y., Liu L., Tang W., Long J., Tian W.D., and Mo X.M.Identification of osteo-adipo progenitor cells in fat tissue. Cell Prolif 41,803, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mishina Y., Starbuck M.W., Gentile M.A., Fukuda T., Kasparcova V., Seedor J.G., Hanks M.C., Amling M., Pinero G.J., Harada S., and Behringer R.R.Bone morphogenetic protein type IA receptor signaling regulates postnatal osteoblast function and bone remodeling. J Biol Chem 279,27560, 2004 [DOI] [PubMed] [Google Scholar]
- 76.Kaps C., Hoffmann A., Zilberman Y., Pelled G., Haupl T., Sittinger M., Burmester G., Gazit D., and Gross G.Distinct roles of BMP receptors type IA and IB in osteo-/chondrogenic differentiation in mesenchymal progenitors (C3H10T1/2). Biofactors 20,71, 2004 [DOI] [PubMed] [Google Scholar]
- 77.Chen D., Ji X., Harris M.A., Feng J.Q., Karsenty G., Celeste A.J., Rosen V., Mundy G.R., and Harris S.E.Differential roles for bone morphogenetic protein (BMP) receptor type IB and IA in differentiation and specification of mesenchymal precursor cells to osteoblast and adipocyte lineages. J Cell Biol 142,295, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Levi B., and Longaker M.T.Concise review: adipose-derived stromal cells for skeletal regenerative medicine. Stem Cells 29,576, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jaiswal R.K., Jaiswal N., Bruder S.P., Mbalaviele G., Marshak D.R., and Pittenger M.F.Adult human mesenchymal stem cell differentiation to the osteogenic or adipogenic lineage is regulated by mitogen-activated protein kinase. J Biol Chem 275,9645, 2000 [DOI] [PubMed] [Google Scholar]
- 80.Song H.Y., Jeon E.S., Kim J.I., Jung J.S., and Kim J.H.Oncostatin M promotes osteogenesis and suppresses adipogenic differentiation of human adipose tissue-derived mesenchymal stem cells. J Cell Biochem 101,1238, 2007 [DOI] [PubMed] [Google Scholar]
- 81.Gu H., Guo F., Zhou X., Gong L., Zhang Y., Zhai W., Chen L., Cen L., Yin S., Chang J., and Cui L.The stimulation of osteogenic differentiation of human adipose-derived stem cells by ionic products from akermanite dissolution via activation of the ERK pathway. Biomaterials 32,7023, 2011 [DOI] [PubMed] [Google Scholar]
- 82.Lim S., Jang H.J., Park E.H., Kim J.K., Kim J.M., Kim E.K., Yea K., Kim Y.H., Lee-Kwon W., Ryu S.H., and Suh P.G.Wedelolactone inhibits adipogenesis through the ERK pathway in human adipose tissue-derived mesenchymal stem cells. J Cell Biochem 113,3436, 2012 [DOI] [PubMed] [Google Scholar]
- 83.Liu Q., Cen L., Zhou H., Yin S., Liu G., Liu W., Cao Y., and Cui L.The role of the extracellular signal-related kinase signaling pathway in osteogenic differentiation of human adipose-derived stem cells and in adipogenic transition initiated by dexamethasone. Tissue Eng Part A 15,3487, 2009 [DOI] [PubMed] [Google Scholar]
- 84.Takada I., Mihara M., Suzawa M., Ohtake F., Kobayashi S., Igarashi M., Youn M.Y., Takeyama K., Nakamura T., Mezaki Y., Takezawa S., Yogiashi Y., Kitagawa H., Yamada G., Takada S., Minami Y., Shibuya H., Matsumoto K., and Kato S.A histone lysine methyltransferase activated by non-canonical Wnt signalling suppresses PPAR-gamma transactivation. Nat Cell Biol 9,1273, 2007 [DOI] [PubMed] [Google Scholar]
- 85.Takada I., Kouzmenko A.P., and Kato S.Molecular switching of osteoblastogenesis versus adipogenesis: implications for targeted therapies. Expert Opin Ther Targets 13,593, 2009 [DOI] [PubMed] [Google Scholar]
- 86.Santos A., Bakker A.D., de Blieck-Hogervorst J.M., and Klein-Nulend J.WNT5A induces osteogenic differentiation of human adipose stem cells via rho-associated kinase ROCK. Cytotherapy 12,924, 2010 [DOI] [PubMed] [Google Scholar]
- 87.Locksley R.M., Killeen N., and Lenardo M.J.The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell 104,487, 2001 [DOI] [PubMed] [Google Scholar]
- 88.Cho H.H., Shin K.K., Kim Y.J., Song J.S., Kim J.M., Bae Y.C., Kim C.D., and Jung J.S.NF-kappaB activation stimulates osteogenic differentiation of mesenchymal stem cells derived from human adipose tissue by increasing TAZ expression. J Cell Physiol 223,168, 2010 [DOI] [PubMed] [Google Scholar]
- 89.Levi B., James A.W., Nelson E.R., Peng M., Wan D.C., Commons G.W., Lee M., Wu B., and Longaker M.T.Acute skeletal injury is necessary for human adipose-derived stromal cell-mediated calvarial regeneration. Plast Reconstr Surg 127,1118, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zeng Y., Qu X., Li H., Huang S., Wang S., Xu Q., Lin R., Han Q., Li J., and Zhao R.C.MicroRNA-100 regulates osteogenic differentiation of human adipose-derived mesenchymal stem cells by targeting BMPR2. FEBS Lett 586,2375, 2012 [DOI] [PubMed] [Google Scholar]
- 91.Zhang X., Yang M., Lin L., Chen P., Ma K.T., Zhou C.Y., and Ao Y.F.Runx2 overexpression enhances osteoblastic differentiation and mineralization in adipose—derived stem cells in vitro and in vivo. Calcif Tissue Int 79,169, 2006 [DOI] [PubMed] [Google Scholar]
- 92.Levi B., Nelson E.R., Li S., James A.W., Hyun J.S., Montoro D.T., Lee M., Glotzbach J.P., Commons G.W., and Longaker M.T.Dura mater stimulates human adipose-derived stromal cells to undergo bone formation in mouse calvarial defects. Stem Cells 29,1241, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wu L., Wu Y., Lin Y., Jing W., Nie X., Qiao J., Liu L., Tang W., and Tian W.Osteogenic differentiation of adipose derived stem cells promoted by overexpression of osterix. Mol Cell Biochem 301,83, 2007 [DOI] [PubMed] [Google Scholar]
- 94.Lee J.S., Lee J.M., and Im G.I.Electroporation-mediated transfer of Runx2 and Osterix genes to enhance osteogenesis of adipose stem cells. Biomaterials 32,760, 2011 [DOI] [PubMed] [Google Scholar]
- 95.Luzi E., Marini F., Sala S.C., Tognarini I., Galli G., and Brandi M.L.Osteogenic differentiation of human adipose tissue-derived stem cells is modulated by the miR-26a targeting of the SMAD1 transcription factor. J Bone Miner Res 23,287, 2008 [DOI] [PubMed] [Google Scholar]
- 96.Chen P.Y., Sun J.S., Tsuang Y.H., Chen M.H., Weng P.W., and Lin F.H.Simvastatin promotes osteoblast viability and differentiation via Ras/Smad/Erk/BMP-2 signaling pathway. Nutr Res 30,191, 2010 [DOI] [PubMed] [Google Scholar]