Abstract
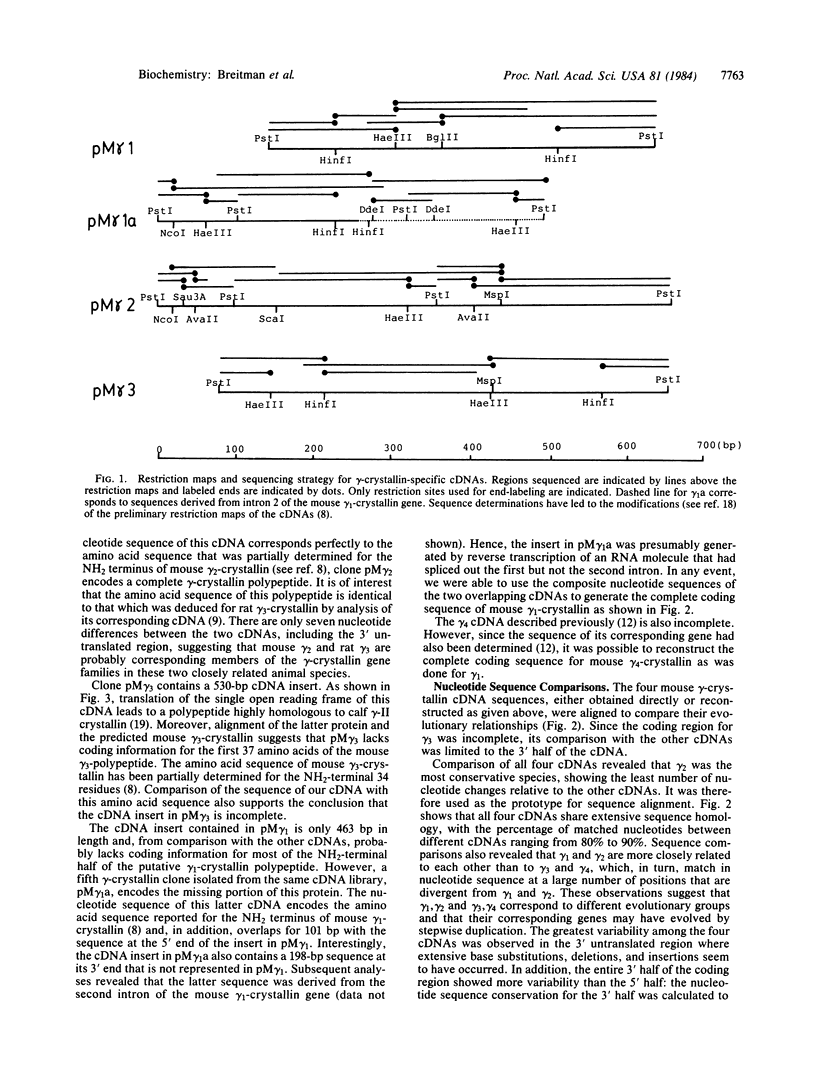
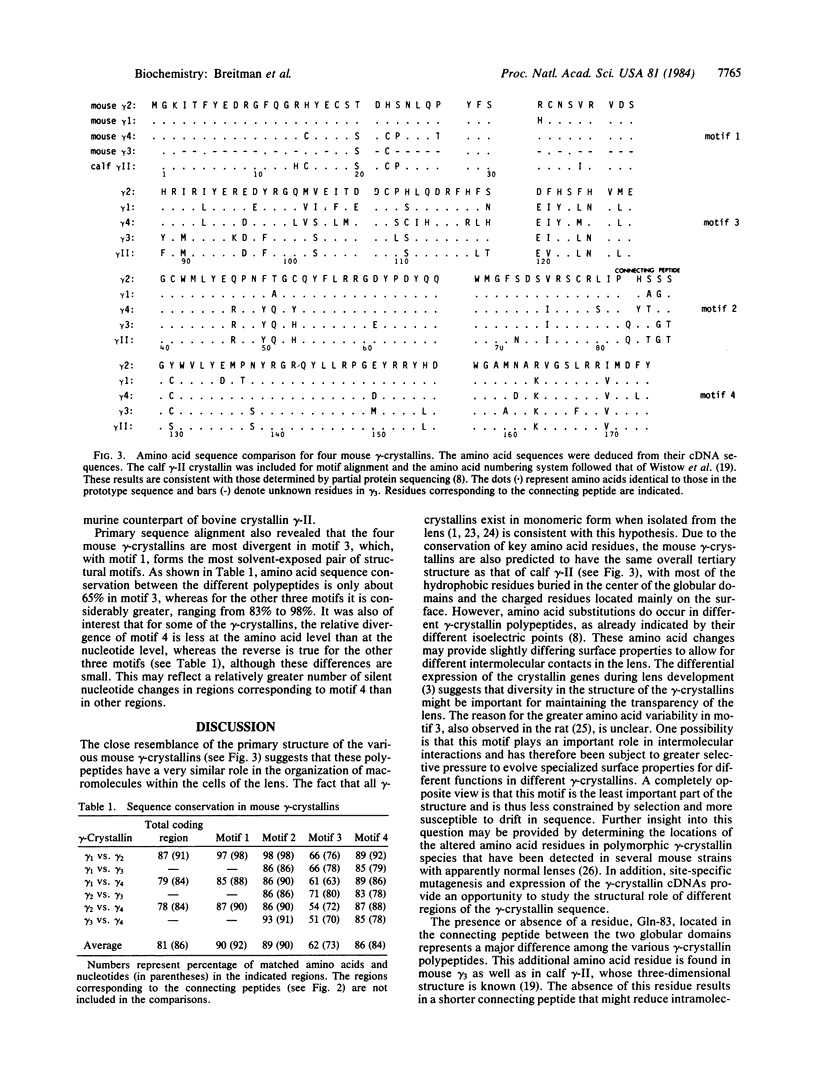
The heterogeneity inherent among gamma-crystallins of the mouse lens was investigated by sequence analysis of three gamma-crystallin-specific cDNAs. Comparison of the nucleotide sequence of these cDNAs and one previously reported by us revealed that the four gamma-cDNAs share 80-90% homology in nucleotide sequence. The entire 3' half of the coding region shows more variability than the 5' half, whereas the greatest variability is observed in the 3' untranslated region where numerous base substitutions, deletions, and insertions seem to have occurred. Alignment of the amino acid sequences of the four mouse gamma-crystallins according to the known four structural motifs of the major calf gamma-crystallin, gamma-II, suggests that all four mouse polypeptides are structurally very similar to calf gamma-II. However, most of the mouse polypeptides differ from gamma-II by the absence of one amino acid residue, resulting in a shorter connecting peptide between the two globular domains of the protein. Primary sequence alignment also revealed that the four mouse gamma-crystallins are most divergent in the third structural motif of the polypeptide. The significance of these differences in terms of the structure and function of the gamma-crystallins in the mouse lens is discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Askren C. C., Yu N. T., Kuck J. F., Jr Variation of the concentration of sulfhydryl along the visual axis of aging lenses by laser Raman optical dissection technique. Exp Eye Res. 1979 Dec;29(6):647–654. doi: 10.1016/0014-4835(79)90020-4. [DOI] [PubMed] [Google Scholar]
- Bhat S. P., Spector A. Complete nucleotide sequence of a cDNA derived from calf lens gamma-crystallin mRNA: presence of Alu I-like DNA sequences. DNA. 1984 Aug;3(4):287–295. doi: 10.1089/dna.1.1984.3.287. [DOI] [PubMed] [Google Scholar]
- Bloemendal H. The vertebrate eye lens. Science. 1977 Jul 8;197(4299):127–138. doi: 10.1126/science.877544. [DOI] [PubMed] [Google Scholar]
- Blundell T., Lindley P., Miller L., Moss D., Slingsby C., Tickle I., Turnell B., Wistow G. The molecular structure and stability of the eye lens: x-ray analysis of gamma-crystallin II. Nature. 1981 Feb 26;289(5800):771–777. doi: 10.1038/289771a0. [DOI] [PubMed] [Google Scholar]
- Croft L. R. Amino and carboxy terminal sequence of -crystallin, from haddock lens. Biochim Biophys Acta. 1973 Jan 25;295(1):174–177. doi: 10.1016/0005-2795(73)90085-8. [DOI] [PubMed] [Google Scholar]
- Dodemont H. J., Andreoli P. M., Moormann R. J., Ramaekers F. C., Schoenmakers J. G., Bloemendal H. Molecular cloning of mRNA sequences encoding rat lens crystallins. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5320–5324. doi: 10.1073/pnas.78.9.5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber A. T., Gold R. J. Comparative two-dimensional electrophoretic analysis of water soluble proteins from bovine and murine lenses. Exp Eye Res. 1982 Dec;35(6):585–596. doi: 10.1016/s0014-4835(82)80072-9. [DOI] [PubMed] [Google Scholar]
- Harding J. J., Dilley K. J. Structural proteins of the mammalian lens: a review with emphasis on changes in development, aging and cataract. Exp Eye Res. 1976 Jan;22(1):1–73. doi: 10.1016/0014-4835(76)90033-6. [DOI] [PubMed] [Google Scholar]
- Kuck J. F., Yu N. T., Askren C. C. Total sulfhydryl by raman spectroscopy in the intact lens of several species: variations in the nucleus and along the optical axis during aging. Exp Eye Res. 1982 Jan;34(1):23–37. doi: 10.1016/0014-4835(82)90005-7. [DOI] [PubMed] [Google Scholar]
- Lok S., Tsui L. C., Shinohara T., Piatigorsky J., Gold R., Breitman M. Analysis of the mouse gamma-crystallin gene family: assignment of multiple cDNAs to discrete genomic sequences and characterization of a representative gene. Nucleic Acids Res. 1984 Jun 11;12(11):4517–4529. doi: 10.1093/nar/12.11.4517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Moormann R. J., Jongbloed R., Schoenmakers J. G. Isolation and characterization of beta- and gamma-crystallin genes from rat genomic cosmid libraries. Gene. 1984 Jul-Aug;29(1-2):1–9. doi: 10.1016/0378-1119(84)90159-8. [DOI] [PubMed] [Google Scholar]
- Moormann R. J., den Dunnen J. T., Bloemendal H., Schoenmakers J. G. Extensive intragenic sequence homology in two distinct rat lens gamma-crystallin cDNAs suggests duplications of a primordial gene. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6876–6880. doi: 10.1073/pnas.79.22.6876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moormann R. J., den Dunnen J. T., Mulleners L., Andreoli P., Bloemendal H., Schoenmakers J. G. Strict co-linearity of genetic and protein folding domains in an intragenically duplicated rat lens gamma-crystallin gene. J Mol Biol. 1983 Dec 25;171(4):353–368. doi: 10.1016/0022-2836(83)90034-7. [DOI] [PubMed] [Google Scholar]
- Piatigorsky J. Lens differentiation in vertebrates. A review of cellular and molecular features. Differentiation. 1981;19(3):134–153. doi: 10.1111/j.1432-0436.1981.tb01141.x. [DOI] [PubMed] [Google Scholar]
- Piatigorsky J., Nickerson J. M., King C. R., Inana G., Hejtmancik J. F., Hawkins J. W., Borras T., Shinohara T., Wistow G., Norman B. Crystallin genes: templates for lens transparency. Ciba Found Symp. 1984;106:191–207. doi: 10.1002/9780470720875.ch11. [DOI] [PubMed] [Google Scholar]
- Ringens P. J., Hoenders H. J., Bloemendal H. Protein distribution and characterization in the prenatal and postnatal human lens. Exp Eye Res. 1982 May;34(5):815–823. doi: 10.1016/s0014-4835(82)80041-9. [DOI] [PubMed] [Google Scholar]
- Schoenmakers J. G., den Dunnen J. T., Moormann R. J., Jongbloed R., van Leen R. W., Lubsen N. H. The crystallin gene families. Ciba Found Symp. 1984;106:208–218. doi: 10.1002/9780470720875.ch12. [DOI] [PubMed] [Google Scholar]
- Shinohara T., Robinson E. A., Appella E., Piatigorsky J. Multiple gamma-crystallins of the mouse lens: fractionation of mRNAs by cDNA cloning. Proc Natl Acad Sci U S A. 1982 May;79(9):2783–2787. doi: 10.1073/pnas.79.9.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skow L. C. Location of a gene controlling electrophoretic variation in mouse gamma-crystallins. Exp Eye Res. 1982 Apr;34(4):509–516. doi: 10.1016/0014-4835(82)90023-9. [DOI] [PubMed] [Google Scholar]
- Slingsby C., Croft L. R. Microheterogeneity at the C-terminus of gamma-crystallin fraction IVb. Biochem J. 1972 Apr;127(3):609–610. doi: 10.1042/bj1270609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slingsby C., Croft L. R. Structural studies on calf lens gamma-crystallin fraction IV: a comparison of the cysteine-containing tryptic peptides with the corresponding amino acid sequence of gamma-crystallin fraction II. Exp Eye Res. 1978 Mar;26(3):291–304. doi: 10.1016/0014-4835(78)90076-3. [DOI] [PubMed] [Google Scholar]
- Tomarev S. I., Krayev A. S., Skryabin K. G., Bayev A. A., Gause G. G., Jr The nucleotide sequence of a cloned cDNA corresponding to one of the gamma-crystallins from the eye lens of the frog Rana temporaria. FEBS Lett. 1982 Sep 20;146(2):314–318. doi: 10.1016/0014-5793(82)80942-3. [DOI] [PubMed] [Google Scholar]
- Wilbur W. J., Lipman D. J. Rapid similarity searches of nucleic acid and protein data banks. Proc Natl Acad Sci U S A. 1983 Feb;80(3):726–730. doi: 10.1073/pnas.80.3.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wistow G., Turnell B., Summers L., Slingsby C., Moss D., Miller L., Lindley P., Blundell T. X-ray analysis of the eye lens protein gamma-II crystallin at 1.9 A resolution. J Mol Biol. 1983 Oct 15;170(1):175–202. doi: 10.1016/s0022-2836(83)80232-0. [DOI] [PubMed] [Google Scholar]



