Abstract
Mutations of the human desmin gene on chromosome 2q35 cause a familial or sporadic form of skeletal myopathy frequently associated with cardiac abnormalities. Skeletal and cardiac muscle from patients with primary desminopathies characteristically display cytoplasmic accumulation of desmin-immunoreactive material and myofibrillar changes. However, desmin-positive protein aggregates in conjunction with myofibrillar abnormalities are also the morphological hallmark of the large group of secondary desminopathies (synonyms: myofibrillar myopathies, desmin-related myopathies), which comprise sporadic and familial neuromuscular conditions of considerable clinical and genetic heterogeneity. Here, we will give an overview on the functional role of desmin in striated muscle as well as the main clinical, myopathological, genetic and patho-physiological aspects of primary desminopathies. Furthermore, we will discuss recent genetic and biochemical advances in distinguishing primary from secondary desminopathies.
Keywords: desminopathy, desmin-related myopathy, myofibrillar myopathy, protein aggregation, inclusion bodies, granulofilamentous material, intermediate filaments, desmin, mutations
Introduction
Protein aggregation is a well-recognized pathological feature in a wide variety of neurodegenerative diseases like Parkinson disease, various forms of dementia and amyotrophic lateral sclerosis. In analogy to this group of degenerative CNS disorders, morphological evidence of pathological protein aggregation has been established in a broad spectrum of human myopathies comprising congenital myopathies, the group of inclusion body myopathies/myositis, distal myopathies and certain limb girdle muscular dystrophies [1, 2].
Following the nomenclatorial principle of mutant proteins in certain neuromuscular disorders such as dytrophinopathies, sarcoglycanopathies, dysferlinopathy and others, respective protein abnormalities being marked by reduction or absence of these largely transsarcolemmal proteins [3], the term desminopathy has been coined for a distinct form of protein aggregate myopathy due to desmin mutations [4]. Actinopathy, myosinopathy, myotilinopathy, ZASPopathy and γ-filaminopathy are further examples of the growing list of human protein aggregate myopathies in which the underlying gene defect is represented in the name of the individual disorder [5–9].
Primary desminopathies are the best-studied disease entity among the group of human protein aggregate myopathies. In this paper, we delineate the functional role of desmin in striated muscle, summarize main clinical, myopathological, genetic and pathophysiological aspects of primary desminopathies and discuss recent genetic and biochemical advances in distinguishing primary from secondary desminopathies.
Desmin is an essential component of the extrasarcomeric cytoskeleton in striated muscle cells
Desmin, the major intermediate filament (IF) protein in skeletal and cardiac muscle cells, is a structural component of the extrasarcomeric cytoskeleton which forms a three-dimensional scaffold around myofibrillar Z-discs, thereby interlinking neighbouring myofibrils and connecting the myofibrillar apparatus to nuclei, the sub-sarcolemmal cytoskeleton and cytoplasmic organelles such as mitochondria [10–13].
The 53 kDa desmin protein has a tripartite structure comprising a central -helical coiled-coil rod domain flanked by non- α-helical head and tail domains. The central rod domain, formed by four -helical segments (1A, 1B, 2A and 2B) separated by three short polypeptide linkers (L1, L12 and L2), has been shown to play a critical role in the dimerization and further assembly of desmin polypeptides [14]. This assembly process is the structural basis for the formation of the three-dimensional desmin IF network in all mature muscle cells [15].
A milestone in our current understanding of desmin function was the generation of desmin (–/–) mice by two independent laboratories [16, 17]. Although desmin (–/–) mice are viable and fertile, they develop progressive muscle weakness and dystrophic alterations in both cardiac and skeletal muscle. Since severe structural changes with disorganization and deranged alignment of myofibrils, as well as sarcolem-mal disruption were most prominent in highly used striated muscles, it was concluded that the lack of desmin results in an increased susceptibility of muscle fibres to physical strain during muscle contraction. However, even in the absence of desmin, intermediate filament-related proteins plectin, synemin and parane-min were observed among neighbouring myofibrils and from the Z-discs of most peripheral myofibrils to the overlying sarcolemma [12]. In normal muscle, the extrasarcomeric cytoskeleton is composed of a network of various components, comprising the IF proteins desmin, synemin and syncoilin, the molecular chaperone αB-crystallin, and the multi-functional cytoskeletal linker plectin [12, 13, 18]. The pivotal role of this extrasarcomeric cytoskeleton in human skeletal muscle is further highlighted by the observation, that mutations in either desmin, αB-crystallin or plectin genes give rise to a progressive skeletal and/or cardiac myopathy [4, 7, 19].
Distal myopathy,cardiac arrhythmias,cardiomyopathy: classical criteria of primary desminopathies
Familial or sporadic primary desminopathies usually manifest in the second to the fourth decades of life with slowly progressive painless distal weakness and atrophy of the lower extremities (Fig. 1) [4]. The majority of patients exhibit an autosomal-dominant inheritance. However, rare autosomal-recessive cases as well as an increasing number of sporadic patients have been reported [4]. Although symptoms are initially restricted to distal leg muscles, weakness and atrophy affecting the upper extremities, proximal leg and trunk muscles usually evolve with disease progression. Facial weakness, if present, is usually mild and not accompanied by extraocular muscle weakness. Bulbar symptoms consisting of swallowing difficulties or dysarthria may occur in advanced stages of the disease [4].
1.
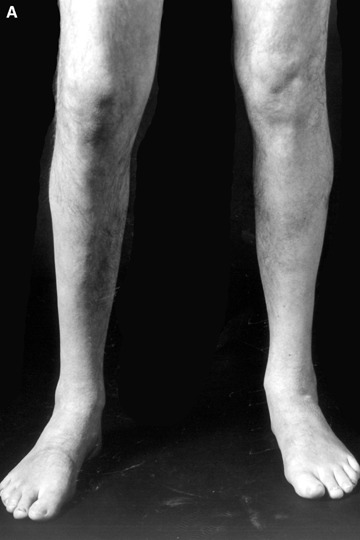
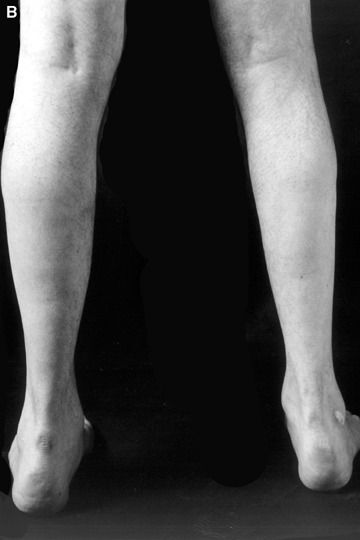
(A and B) Lower leg muscle atrophy in a German patient harbouring a heterozygous K240del desmin mutation.
Cardiac involvement comprising multiple forms of arrhythmias (conduction blocks, supraventricular and ventricular extrasystolic beats and tachycardia) or true cardiomyopathy is the second classical clinical hallmark of primary desminopathies [4, 20]. From a clinical point of view it is important to note that cardiac involvement, which affects the vast majority of patients with primary desminopathies, may either precede, coincide with or succeed skeletal muscle weakness. Furthermore, respiratory insufficiency (in the absence of cardiac involvement) along with recurrent chest infections has been reported as life-threatening disease manifestations [4].
Serum creatine kinase levels are usually only slightly elevated or even normal. Needle electromyography of clinically affected muscles characteristically reveals a myopathic pattern with small and polyphasic motor unit potentials. Nerve conduction studies usually give normal results [4].
Sub-sarcolemmal and cytoplasmic desmin-positive protein aggregates: the morphological hallmark of primary and secondary desminopathies
A broad spectrum of myopathological changes ranging from mild to severe degenerative muscle alterations has been described in genetically proven primary desminopathies [7, 20, 21]. The severity of degenerative muscle changes often mirrors the stage of disease progression in individual muscle groups. In line with the clinical presentation of a distal myopathy, muscle tissue from distal leg muscles usually show more pronounced myopathological alterations than samples from proximal muscle groups. At the light microscopic level (Figs 2 and 3), myopathic changes in conjunction with sub-sar-colemmal and/or cytoplasmic basophilic or eosinophilic inclusions, rimmed vacuoles as well as the presence of rubbed-out lesions in NADH, SDH, COX and ATPase preparations are first hints to the diagnosis. However, immunostaining and electron microscopy (EM) are essential diagnostic procedures (Fig. 4) to establish the final diagnosis of a protein aggregate myopathy. Desmin, αB-crystallin and γ-filamin antibodies have a high diagnostic value in depicting sub-sarcolemmal and cytoplasmic protein aggregates (Figs 5–7) [7, 9, 20]. However, a large series of diverse other proteins (Table 1), among them even intracellular amyloid demonstrated as Congo red positive material, has been identified in such aggregates. The entire spectrum of accruing proteins within such muscle fibres is not completely known, and the catalogue of individually encountered proteins may vary from muscle to muscle.
2.
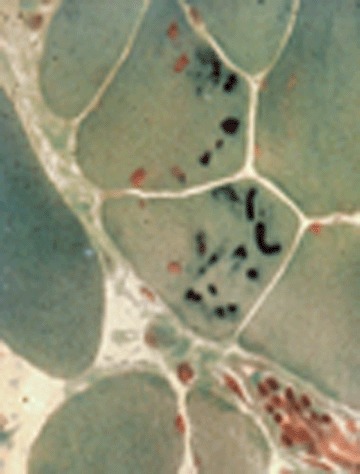
Several cytoplasmic bodies within muscle fibres. Modified Gomori trichrome.
3.
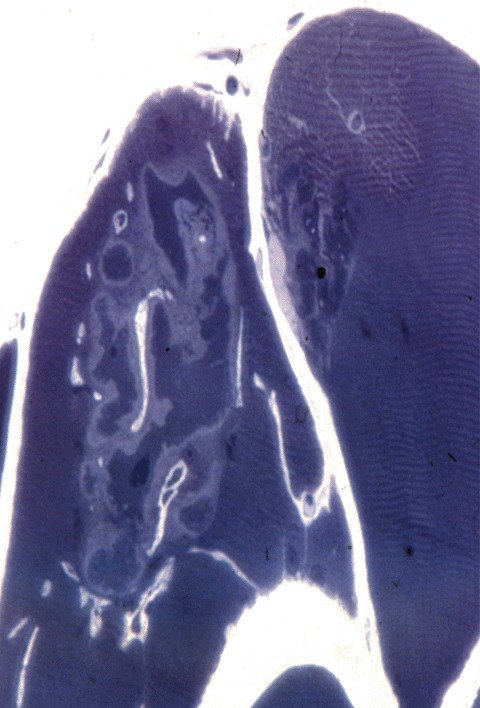
Aggregate of cytoplasmic bodies with conspicuous halos. One micrometre-thick eponembedded section, methylene blue.
4.
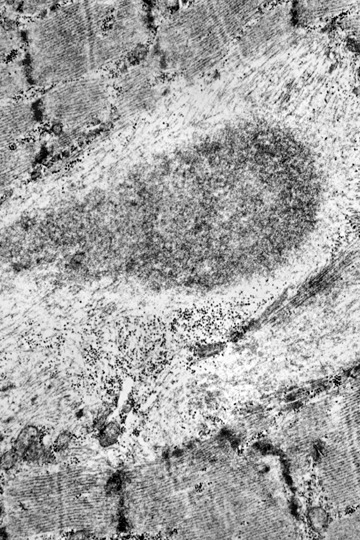
Cytoplasmic body consists of a granular electrondense core and a light halo of filaments.
5.
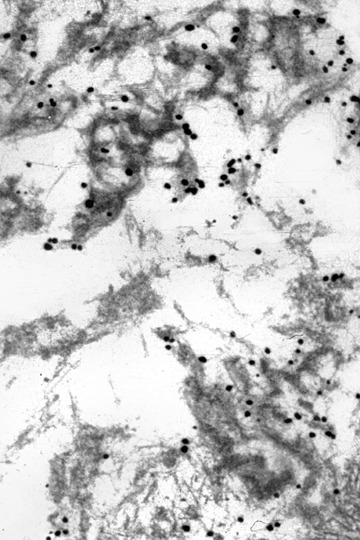
Labelling of the filamentous component of granulofilamentous material by gold-related immunoelectron microscopy using an antibody against desmin.
1.
Proteins found in relation with Desmin-related deposits
| Alzheimer types | Cyclin-dependent kinases | Cytoskeletal proteins | ||
|---|---|---|---|---|
| β-APP | CDK1 | Desmin | ||
| (N and C terminal epitopes, KPI terminal epitopes, KPI domain) | CDK2 | Vimentin | ||
| CDK3 | Nestin | |||
| CDK4 | Dystrophin | |||
| Amyloid-(residues 8-17, 17-24) | CDK5 | β-Spectrin | ||
| CDK7 | ||||
| CDK2 kinase | ||||
| p21 (CDK inhibitor) | Chaperone proteins | |||
| Ubiquitin | ||||
| α-B Crystallin | ||||
| Nuclear proteins | Sarcomeric proteins | Other proteins | ||
| Emerin | Nebulin | Cathepsin B | ||
| Lamin B | Titin | Calpain | ||
| Nuclear matrix-associated protein | Actin | Gelsolin | ||
| a-Actinin | Utrophin | |||
| Myosin fast | α-1 Antichymotrypsin | |||
| Myosin slow | N-CAM | |||
6.
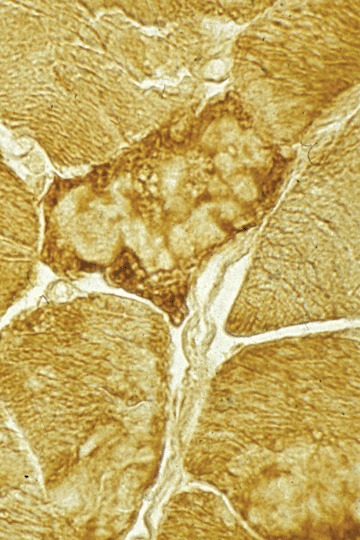
Several spheroid bodies in a muscle fibre rich in α-B crystallin.Immunohistochemistry.
7.
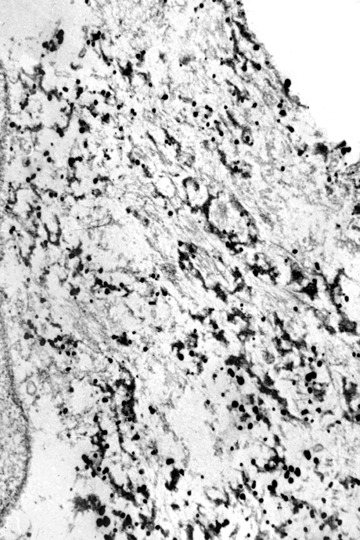
Labelling of filaments in granulofilamentous material by gold-related immunoelectron microscopy using an antibody against α-B crystallin (by courtesy of Prof. Mayer, Nottingham, U.K.).
At the EM level, myofibrillar lesions and the presence of sub-sarcolemmal and intermyofibrillar inclusions define the classical morphological hallmarks of primary desminopathies [22, 23]. Typical, though basically unspecific myofibrillar lesions comprise streaming of the Z-bands, multicore-like lesions and plain disruption of sarcomeres. Pathological protein aggregates may either take the form of cytoplasmic bodies, spheroid bodies or granulofilamentous material. Regarding the latter lesion, which is typically found underneath the plasma membrane and in the intermyofibrillar space, the granular component may be more conspicuous than the filamentous one. Cytoplasmic and spheroid bodies as well as gran-ulofilamentous material, may occur combined, even within the same muscle fibre.
When employing immunoelectron microscopy, the applied gold grains decorate filaments more than the granular material and identify different proteins such as desmin, α-B crystallin, or dystrophin (Figs 5–7). Furthermore, tubulofilamentous profiles and autophagic vacuoles, usually seen in inclusion body myositis/myopathy, may be encountered in primary desminopathies. While the above-mentioned light microscopic, immunohistochemical and ultrastructural changes are characteristic of primary desminopathies, it is important to note that they are not specific for protein aggregate myopathies due to desmin mutations. Primary desminopathies share their structural myofib-rillar and intermyofibrillar abnormalities with the large group of secondary desminopathies (synonyms: desmin-related myopathies, myofibrillar myopathies). These disorders comprise sporadic and familial neuromuscular conditions of considerable clinical and genetic heterogeneity. While part of the secondary desminopathies are due to αB-crystallin, myotilin, ZASP, γ-filamin or selenoprotein N1 mutations, a large number of secondary desminopathies are due to so far unidentified gene defects. Thus, the combined light microscopic, immunohistochemical and ultrastructural analysis of a diagnostic muscle biopsy may establish the diagnosis of protein aggregate myopathy with desmin-positive inclusions, but may not differentiate between primary and secondary desminopathies.
The spectrum of pathogenic desmin gene mutations
The human desmin protein (476 amino acids) is encoded by a single copy gene (nine exons) on chromosome 2q35. Since the first description of pathogenic desmin mutations [24], an increasing number of patients harbouring desmin mutations have been described [4, 21]. While autosomal dominant and sporadic cases of primary desminopathies are due to heterozygous mutations, homozygous as well as compound heterozygous desmin mutations have been identified in rare autosomal recessive cases [4]. Apart from a small number of patients with mutations in exons encoding the head or tail domain of the desmin protein, all other pathogenic desmin gene alterations comprising missense, deletion, splice site, insertion mutations were found in exons encoding the evolutionary highly conserved α-helical coiled-coil desmin rod domain. In particular, a significant clustering of mutations in exon 6, which encodes the C-terminal part of the 2B helix, has been described, which makes this exon a primary target for mutation analysis [4, 25].
The molecular pathogenesis of primary desminopathies: some answers gained, but even more questions raised
Cytoplasmic protein aggregates in primary desminopathies share certain aspects with the Rosenthal fibre pathology in Alexander's disease. The shared genetic background of pathological pro tein aggregates in both disorders are mutations in genes encoding cell type-specific IFs, desmin in muscle fibres and glial fibrillary acidic protein in astrocytes [26].
A number of recent studies provided important new insights in the molecular pathogenesis of primary desminopathies. The corroborative data derived from transient transfection studies indicate that the majority of desmin rod mutants are incapable of forming a de novo desmin IF network in human SW13 cells, which are completely devoid of cytoplas-mic IF proteins [20, 21, 25, 27, 28]. However, while a large number of desmin missense mutations in exons encoding the central α-helical coiled-coil rod domain have been demonstrated to induce a collapse of a pre-existing IF cytoskeleton and the formation of cytoplasmic protein aggregates [21, 25, 27], two distinct desmin disease mutants carrying small in-frame deletions in the rod domain (though unable to form a proper cytoplasmic IF network in SW13 cells) were recently reported to readily co-assemble with either preexisting vimentin (3T3 fibroblasts) or desmin (C2.7 myoblasts) and to integrate into the respective filamentous networks [28]. Variable pathogenic effects were further noted in transfected C2C12 cells using cDNA constructs carrying mutations in the non- α-helical carboxy-terminal desmin tail domain [29]. Here, three out of four desmin tail mutants were incorporated in the pre-existing desmin filament network.
A further milestone in our current understanding of the molecular pathogenesis of primary desminopathies are recent in vitro assembly studies using recombinant wild-type and mutant desmin protein. Systematic analysis of the pathogenic effects of up to now 25 distinct desmin mutations on the protein's propensity to self-assemble in vitro into IFs by the group of Bär & Hermann in Heidelberg revealed that the vast majority of desmin rod and tail mutants disturb either early, intermediate or late-stages of the IF assembly process thereby leading to pathological protein aggregates [21, 25, 27, 28]. These results point into the direction that mutated desmin-inflicted disturbances of filament-formation competence and filament-filament interactions are the key event in the molecular pathogenesis of primary desminopathies.
However, if these desmin mutants have such a toxic effect on the desmin filament system in vitro, why does it takes such a long time till the clinical effects of progressive muscle damage become apparent in vivo? A simple mechanistic explanation is further challenged by the recent observation, that certain small in-frame desmin deletion mutants exhibit assembly defects in vitro when analysed on their own, but facilitate proper filament formation when studied in one-to-one mixtures of the respective mutant protein with wild-type desmin [29]. Thus, it is unlikely that the complex human pathology is solely related to direct effects of desmin mutants on the assembly of desmin IF. As an alternative explanation, desmin mutants may interfere with the interaction with desmin-binding partners (plectin, phosphatases and kinases), thereby influencing the structural and functional organization of the extrasarcomeric cytoskeleton as well as cell signalling cascades. Furthermore, desmin mutants have been shown to impair the proteolytic function of the ubiquitin-pro-teasome system. In a recent study, the impairment of ubiquitin-proteasome system has been attributed to a defect in the entry of ubiquitinated proteins into the 20S proteasome [30]. Another contributing factor to the complex pathology may be the induction of metabolic changes due to mitochondrial dysfunction. Desmin knock-out mice as well as skeletal muscle tissue from patients with primary desminopathies display changes in the sub-cellular distribution and biochemical function of mitochondria [20]. Hence, focal disturbancies in the production of ATP may have a negative influence on the highly ATP dependent ubiquitin-proteasome system as well as the structural integrity of myofibrils. Furthermore, gender-specific effects have recently been implicated to modify the disease manifestation in primary desminopathies [31].
Diagnostic work-up to distinguish primary from secondary desminopathies
In familial and sporadic patients with distal myopathy and cardiac abnormalities manifesting between the second and fourth decades of life, direct genetic testing for desmin mutations is the diagnostic procedure of choice. In patients with less suggestive clinical phenotypes, a diagnostic muscle biopsy should always precede costly genetic testing. Once the myopathological diagnosis of a protein aggregate myopathy with desmin-positive inclusions has been established, well-founded interpretation of all clinical findings should be the basis for specific genetic testing. While childhood disease manifestation in combination with clinical signs of spine rigidity and respiratory involvement clearly stipulates selenoprotein N mutation analysis, the presence of cataracts points towards the diagnosis of αB-crystallinopathy. Though there are exceptions from the rule, disease manifestation beyond the fourth decade of life argues against a primary desminopathy. In these patients the diagnosis of a secondary desminopathy due to myotilin, ZASP or γ-filamin mutations is more likely. Since myotilin and γ-filamin mutations are clustered in one particular exon each [8, 9], direct sequencing of exon 2 (myotilin) and exon 48 (γ-filamin) should precede ZASP, desmin or αB-crystallin gene analysis. As a non-genetic approach, hsp-2D-gel electrophoresis has been reported as a diagnostic tool to differentiate primary from secondary desminopathies [32].
Treatment and clinical management of primary desminopathy patients
To date, no specific drug treatment is available for primary desminopathies. Appropriate clinical alertness to cardiac and respiratory problems is a central aspect in the management of affected patients. Since cardiac arrhythmias, conduction defects, cardiomyopathy, progressive respiratory failure and chest infections are potentially life-threatening, timely implantation of a pacemaker or defibrillation device, treatment of heart failure (or even cardiac transplantation), intermittent or permanent positive pressure ventilation and vigorous treatment of chest infections must be considered in symptomatic indi-viduals. Though excessive exercise should clearly be avoided, regular physiotherapy adjusted to the specific needs of individual patients should be advised.
Acknowledgments
RS and HHG are members of the German network on muscular dystrophies (MD-NET, research project R12, 01GM0302) funded by the German Ministry of Education and Research (BMBF, Bonn, Germany). AV was a fellow of the European Society of Neurology. Support by the European Neuromuscular Centre (ENMC), Baarn, The Netherlands (Consortium on ‘Desmin and Associated Disorders’) and the Deutsche Gesellschaft für Muskelkranke e.V. is appreciated. Mrs. A. Wöber gave invaluable editorial assistance.
References
- 1.De Bleecker JL, Engel AG, Ertl BB. Myofibrillar myopathy with abnormal foci of desmin positivity. II. Immunocytochemical analysis reveals accumulation of multiple other proteins. J Neuropathol Exp Neurol. 1996;55:563–77. doi: 10.1097/00005072-199605000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Goebel HH, Borchert A. Protein surplus myopathies and other rare congenital myopathies. Semin Pediatr Neurol. 2002;9:160–70. doi: 10.1053/spen.2002.33799. [DOI] [PubMed] [Google Scholar]
- 3.Bönnemann CG, Finkel RS. Sarcolemmal proteins and the spectrum of limb-girdle muscular dystro-phies. Semin Pediatr Neurol. 2002;9:81–99. doi: 10.1053/spen.2002.33795. [DOI] [PubMed] [Google Scholar]
- 4.Goldfarb LG, Vicart P, Goebel HH, Dalakas MC. Desmin myopathy. Brain. 2004;127:723–34. doi: 10.1093/brain/awh033. [DOI] [PubMed] [Google Scholar]
- 5.Sparrow JC, Nowak KJ, Durling HJ, Beggs AH, Wallgren-Pettersson C, Romero NB, Nonaka I, Laing NG. Muscle disease caused by mutations in the skeletal muscle alpha-actin gene (ACTA1) Neuromuscul Disord. 2003;13:519–31. doi: 10.1016/s0960-8966(03)00101-9. [DOI] [PubMed] [Google Scholar]
- 6.Oldfors A, Tajsharghi H, Darin N, Lindberg C. Myopathies associated with myosin heavy chain mutations. Acta Myol. 2004;23:90–6. [PubMed] [Google Scholar]
- 7.Selcen D, Engel AG. Mutations in myotilin cause myofibrillar myopathy. Neurology. 2004;62:1363–71. doi: 10.1212/01.wnl.0000123576.74801.75. [DOI] [PubMed] [Google Scholar]
- 8.Selcen D, Engel AG. Mutations in ZASP define a novel form of muscular dystrophy in humans. Ann Neurol. 2005;57:269–76. doi: 10.1002/ana.20376. [DOI] [PubMed] [Google Scholar]
- 9.Vorgerd M, Van Der Ven PFM, Bruchertseifer V, Löwe T, Kley RA, Schröder R, Lochmüller H, Himmel M, Koehler K, Fürst DO, Huebner A. A mutation in the dimerization domain of filamin C causes a novel type of autosomal dominant myofibril-lar myopathy. Am J Hum Genet. 2005;77:297–304. doi: 10.1086/431959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reipert S, Steinbock F, Fischer I, Bittner RE, Zenold A, Wiche G. Association of mitochondria with plectin and desmin intermediate filaments in striated muscle. Exp Cell Res. 1999;252:479–91. doi: 10.1006/excr.1999.4626. [DOI] [PubMed] [Google Scholar]
- 11.Schröder R, Warlo I, Herrmann H, Van der Ven PF, Klasen C, Blümcke I, Mundegar RR, Fürst DO, Goebel HH, Magin TM. Immunogold EM reveals a close association of plectin and the desmin cytoskeleton in human skeletal muscle. Eur J Cell Biol. 1999;78:288–95. doi: 10.1016/S0171-9335(99)80062-4. [DOI] [PubMed] [Google Scholar]
- 12.Carlsson L, Li ZL, Paulin D, Price MG, Breckler J, Robson RM, Wiche G, Thornell L-E. Differences in the distribution of synemin, paranemin, and plectin in skeletal muscles of wild-type and desmin knock-out mice. Histochem Cell Biol. 2000;114:39–47. doi: 10.1007/s004180000158. [DOI] [PubMed] [Google Scholar]
- 13.Carlsson L, Thornell L-E. Desmin-related myopathies in mice and man. Acta Physiol Scand. 2001;171:341–8. doi: 10.1046/j.1365-201x.2001.00837.x. [DOI] [PubMed] [Google Scholar]
- 14.Herrmann H, Aebi U. Intermediate filaments: molec-ular structure, assembly mechanism, and integration into functionally distinct intracellular scaffolds. Annu Rev Biochem. 2004;73:749–89. doi: 10.1146/annurev.biochem.73.011303.073823. [DOI] [PubMed] [Google Scholar]
- 15.Bär H, Strelkov SV, Sjöberg G, Aebi U, Herrmann H. The biology of desmin filaments: how do mutations affect their structure, assembly, and organisation? J Struct Biol. 2004;148:137–52. doi: 10.1016/j.jsb.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 16.Li Z, Colucci-Guyon E, Pinçon-Raymond M, Mericskay M, Pournin S, Paulin D, Babinet C. Cardiovascular lesions and skeletal myopathy in mice lacking desmin. Dev Biol. 1996;175:362–6. doi: 10.1006/dbio.1996.0122. [DOI] [PubMed] [Google Scholar]
- 17.Milner DJ, Weitzer G, Tran D, Bradley A, Capetanaki Y. Disruption of muscle architecture and myocardial degeneration in mice lacking desmin. J Cell Biol. 1996;134:1255–70. doi: 10.1083/jcb.134.5.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Price MG, Lazarides E. Expression of intermediate filament-associated proteins paranemin and synemin in chicken development. J Cell Biol. 1983;97:1860–74. doi: 10.1083/jcb.97.6.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vicart P, Caron A, Guicheney P, Li Z, Prévost M-C, Faure A, Chateau D, Chapon F, Tomé F, Dupret J-M, Paulin D, Fardeau M. A missense mutation in the alpha-B crystallin chaperone gene causes a desmin-related myopathy. Nat Genet. 1998;20:92–5. doi: 10.1038/1765. [DOI] [PubMed] [Google Scholar]
- 20.Schröder R, Goudeau B, Simon MC, Fischer D, Eggermann T, Clemen CS, Li Z, Reimann J, Xue Z, Rudnik-Schöneborn S, Zerres K, Van der Ven PF, Fürst DO, Kunz WS, Vicart P. On noxious desmin: functional effects of a novel heterozygous desmin insertion mutation on the extrasarcomeric desmin cytoskeleton and mitochondria. Hum Mol Genet. 2003;12:657–69. doi: 10.1093/hmg/ddg060. [DOI] [PubMed] [Google Scholar]
- 21.Bär H, Fischer D, Goudeau B, Kley RA, Clemen CS, Vicart P, Herrmann H, Vorgerd M, Schröder R. Pathogenic effects of a novel heterozygous R350P desmin mutation on the assembly of desmin intermediate filaments in vivo and in vitro. Hum Mol Genet. 2005;14:1251–60. doi: 10.1093/hmg/ddi136. [DOI] [PubMed] [Google Scholar]
- 22.Goebel HH, Warlo IAP. Progress in desmin-related myopathies. J Child Neurol. 2000;15:565–72. doi: 10.1177/088307380001500901. [DOI] [PubMed] [Google Scholar]
- 23.Vrabie A, Goldfarb L, Shatunov A, Nägele A, Fritz P, Kaczmarek I, Goebel HH. The enlarging spectrum of desminopathies: new morphological findings, east-ward geographic spread, novel exon 3 desmin mutation. Acta Neuropathol. 2005;109:411–7. doi: 10.1007/s00401-005-0980-1. [DOI] [PubMed] [Google Scholar]
- 24.Goldfarb LG, Park K-Y, Cervenáková S, Lee H-S, Vasconcelos O, Nagle JW, Semino-Mora C, Sivakumar K, Dalakas MC. Missense mutations in desmin associated with familial cardiac and skeletal myopathy. Nat Genet. 1998;19:402–3. doi: 10.1038/1300. [DOI] [PubMed] [Google Scholar]
- 25.Bär H, Mucke N, Kostareva A, Sjöberg G, Aebi U, Herrmann H. Severe muscle disease-causing desmin mutations interfere with in vitro filament assembly at distinct stages. Proc Natl Acad Sci USA. 2005;102:15099–104. doi: 10.1073/pnas.0504568102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li R, Johnson AB, Salomons G, Goldman JE, Naidu S, Quinlan R, Cree B, Ruyle SZ, Banwell B, D'Hooghe M, Siebert JR, Rolf CM, Cox H, Reddy A, Gutiérrez-Solana LG, Collins A, Weller RO, Messing A, Van Der Knaap MS, Brenner M. Glial fibrillary acidic protein mutations in infantile, juvenile, and adult forms of Alexander disease. Ann Neurol. 2005;57:310–26. doi: 10.1002/ana.20406. [DOI] [PubMed] [Google Scholar]
- 27.Goudeau B, Rodrigues-Lima F, Fischer D, Casteras-Simon M, Sambuughin N, De Visser M, Laforet P, Ferrer X, Chapon F, Sjoberg G, Kostareva A, Sejersen T, Dalakas MC, Goldfarb LG, Vicart P. Variable pathogenic potentials of muta-tions located in the desmin alpha-helical domain. Hum Mutat. 2006;27:906–13. doi: 10.1002/humu.20351. [DOI] [PubMed] [Google Scholar]
- 28.Bär H, Mucke N, Katus HA, Aebi U, Herrmann H. Assembly defects of desmin disease mutants carrying deletions in the alpha-helical rod domain are rescued by wild type protein. J Struct Biol. 2007;158:107–15. doi: 10.1016/j.jsb.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 29.Bär H, Goudeau B, Walde S, Casteras-Simon M, Mucke N, Shatunov A, Goldberg YP, Clarke C, Holton JL, Eymard B, Katus HA, Fardeau M, Goldfarb L, Vicart P, Herrmann H. Conspicuous involvement of desmin tail mutations in diverse cardiac and skeletal myopathies. Hum Mutat. 2007;28:374–86. doi: 10.1002/humu.20459. [DOI] [PubMed] [Google Scholar]
- 30.Liu J, Chen Q, Huang W, Horak KM, Zheng H, Mestril R, Wang X. Impairment of the ubiquitin-pro-teasome system in desminopathy mouse hearts. FASEB J. 2006;20:362–4. doi: 10.1096/fj.05-4869fje. [DOI] [PubMed] [Google Scholar]
- 31.Arias M, Pardo J, Blanco-Arias P, Sobrido MJ, Arias S, Dapena D, Carracedo A, Goldfarb LG, Navarro C. Distinct phenotypic features and gender-specific disease manifestations in a Spanish family with desmin L370P mutation. Neuromuscul Disord. 2006;16:498–503. doi: 10.1016/j.nmd.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 32.Clemen CS, Fischer D, Roth U, Simon S, Vicart P, Kato K, Kaminska A, Vorgerd M, Goldfarb L, Eymard B, Romero NB, Goudeau B, Eggermann T, Zerres K, Noegel AA, Schröder R. Hsp27-2D-gel electrophoresis is a diagnostic tool to differentiate primary desminopathies from myofibrillar myopathies. FEBS Lett. 2005;579:3777–82. doi: 10.1016/j.febslet.2005.05.051. [DOI] [PubMed] [Google Scholar]


